Abstract
Purpose
The present study was designed to address whether osteoblasts play a synergistic role in promoting mesenchymal stem cell (MSC) osteogenesis in a direct cell–cell contact co-culture model.
Methods
Murine C3H10T1/2 and MC3T3-E1 cell lines were mixed and plated onto 12-well culture plates and co-cultured at various ratios of initial cell densities. To compare the possible improvement on osteogenic differentiation, co-culture cells were served with or without osteogenic supplements in culture medium.
Results
Weak osteogenesis was induced in MSCs co-cultured in an untreated medium with different ratios of osteoblasts. An osteoblast-dependent increase in osteogenic gene expression of Runx2, type I collagen, and osteocalcin was observed over time. Moreover, both alkaline phosphatase (ALP) activity and calcium deposition were distinctly enhanced at levels that were proportional to the quantity of osteoblasts in the culture. The increases in mRNA expression and ALP activity were greater in co-cultures treated with osteogenic supplements than in untreated cultures. However, the production of ALP activity followed by a distinct matrix mineralization was lower in osteogenic-treated cultures containing greater numbers of osteoblasts. This suggests that a higher density of osteoblasts may lead to weak osteogenesis of MSCs by direct cell–cell contact co-culture in an untreated environment. Furthermore, additional osteogenic supplements may act synergistically with osteoblasts to accelerate matrix mineralization by reducing the process of osteogenic differentiation in osteogenic treated co-cultures.
Conclusions
The present work may improve the understanding of MSC osteogenesis and may provide benefits for regenerative medicine.
Introduction
It has been demonstrated that adult mesenchymal stem cells (MSCs) are capable of giving rise to several different cell types, including myoblasts, adipocytes, fibroblasts, chondrocytes, and osteoblasts [1–4]. MSCs exist in a highly specialized microenvironment in which their maintenance, proliferation, migration, and differentiation involve a complex interplay of many local and systematic signals between stem cells and adjacent cells [5–10]. However, very little is known about the possible interaction between bone marrow MSCs and their neighbouring cells during osteogenesis.
Osteoblasts are derived from MSCs and exist in close proximity to the bone marrow, which serves as a major source of MSCs and hematopoietic stem cells. They are located on the surface of bone, express multiple hormone receptors and various cell surface molecules, and secrete hormones and enzymes that maintain the balance between bone formation and resorption [11, 12]. Moreover, osteoblasts initiate the process of mineralization. However, it remains unclear as to whether and how osteoblasts affect the self-renewal and differentiation of another major stem cell population in bone marrow MSCs. There is little in vivo data available on this, and in vitro studies using conditioned medium or co-culture models have produced inconsistent results [13–17]. In experiments using conditioned medium, the real-time dynamic cell–cell interactions are missing and osteogenic inducers such as dexamethasone were used. Previous co-culture studies used cells from different species and did not address the effect of direct cell–cell contact between osteoblasts and MSCs, which is of great interest given the close proximity of these two cell types in vivo. In addition, in these co-culture systems, the state of differentiation of osteoblasts (which may have significant implications regarding their effectiveness) has not been sufficiently considered. Furthermore, the specific modules responsible for the stimulatory effect observed in osteoblasts in some studies were not identified.
Motivated by these issues, the present study was designed to address whether osteoblasts play a synergistic role in the promotion of MSC osteogenesis in a direct cell–cell contact co-culture model. The murine MSC cell line C3H10T1/2 and murine osteoblast cell line MC3T3-E1 (clone 14) were used to develop a direct cell–cell contact co-culture model, supplemented with or without osteogenic treatment. Cells were seeded at different ratios of initial cell densities to observe the effect of the quantity of osteoblasts on MSC osteogenesis in vitro. We found that the untreated co-cultures were able to induce weak osteogenesis in MSCs co-cultured with various ratios of osteoblasts. The osteogenic-treated co-cultures exhibited marked expression of osteogenic mRNA, such as Runx2, type I collagen, and osteocalcin. Moreover, both alkaline phosphatase (ALP) activity and calcium deposition were distinctly enhanced in these treated co-cultures at levels proportional to the quantity of osteoblasts.
Materials and methods
Cell cultures
The murine C3H10T1/2 cell line was a generous gift from Prof. Yeu Su (National Yang-Ming University, Taipei, Taiwan). The murine calvaria-derived MC3T3-E1 cell line at subclone 14, which has the osteoblastic phenotype, was purchased from ATCC. Each cell line was cultured in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum and 100 units/ml penicillin-streptomycin to enable basal cell growth. Cells were trypsinized and sub-cultured when cells reached 60−70% confluence for serial passages.
The direct cell–cell contact co-culture model
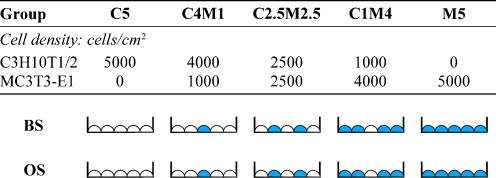
MC3T3-E1 cells (clone 14) at passages 3 and 4, and C3H10T1/2 cells at passages 8−10 were plated onto 12-well culture plates and co-cultured at various ratios of initial cell densities (Fig. 1): group B5 (pure MC3T3-E1 cells at 5,000 cells/cm2), group C1B4 (C3H10T1/2 at 1,000 cells/cm2 and osteoblasts at 4,000 cells/cm2), group C2.5B2.5 (C3H10T1/2 at 2,500 cells/cm2 and osteoblasts at 2,500 cells/cm2), group C4B1 (C3H10T1/2 at 4,000 cells/cm2 and osteoblasts at 1,000 cells/cm2), and group C5 (pure C3H10T1/2 cells at 5,000 cells/cm2). Groups B5 and C5 were used as controls. To examine whether a synergistic interaction of direct cell–cell contact and soluble osteoinducible factors exists between C3H10T1/2 and MC3T3-E1 cells, all co-cultures were divided into two groups: growth in culture medium treated with (OS) or without osteogenic (BS) supplements. For BS treatment, the culture medium used was the same as that described above in the presence of 2% FBS. For OS treatment, the medium used for BS was used, but supplemented with the following: 100 nm dexamethasone, 50 g/ml l-ascorbic acid, and 1 mM β-glycerophosphate. Half of the BS or OS medium (as appropriate) was replaced with fresh medium every two to three days.
Fig. 1.
Schema of the direct cell–cell co-culture model. C3H10T1/2 cells were co-cultured with MC3T3-E1 cells at different ratios: 5:0, 4:1, 1:1, 1:4, and 0:5. Groups C5 and M5 were used as controls. Cells were uniformly mixed before seeding onto 12-well culture plates
ALP activity assay
ALP is one of the early markers of osteoblasts. To evaluate osteogenic differentiation, ALP activity was assessed by measuring the formation of p-nitrophenol (p-NP) from p-nitrophenyl phosphate, as per the manufacturer’s instructions (Sigma). Cells were lyzed in a buffer containing 1.5 M Tris, 1 mM ZnCl2, and 1 mM MgCl2 (pH 9.0), containing 2% Triton X-100, and reacted with a phosphate substrate reagent (2 mg/ml; Sigma) in a microplate that was protected from the light. The levels of p-NP were quantified according to a p-NP standard curve based on A405 and normalized to the respective DNA content of each co-culture.
RNA isolation and reverse transcription-polymerase chain reaction
The mRNA expression of Runx2 (a critical transcriptional factor that regulates skeletogenesis), type I collagen (a major organic component that exists in bone extracellular matrix), osteocalcin (a late and specific marker of bone formation), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; as an internal control for RNA loading) in BS- or OS-treated co-cultures were determined at each time point by reverse transcriptase–polymerase chain reaction (RT-PCR) assay. Total RNA was extracted and collected by using TriZol reagent (Invitrogen) and the first-strand cDNA was synthesized by using the SuperScript First-Strand Synthesis System (Invitrogen) followed by the amplification of cDNA product using Platinum Taq DNA polymerase (Invitrogen). The sense and antisense sequences of the primers used for semiquantitative RT-PCR reactions are listed in Table 1. The reaction was performed under the following conditions: incubation at 94°C for 2 min; denaturation at 94°C for 45 s, annealing at 62°C (Runx2), 60°C (type I collagen and osteocalcin), or 52°C (GAPDH) for 30 s, and polymerization at 72°C for 60 s for 35 cycles; followed by a final extension at 72°C for 5 min. The expression of amplified products was evaluated using electrophoresis with 2% agarose gel and ethidium bromide staining. All electrophoresis images were analysed quantitatively using NIH Image J software, and normalized to their respective GAPDH values.
Table 1.
Primer sequences for RT-PCR
| Target genes | Annealing temperature (°C) | Primer sequences | Product size |
|---|---|---|---|
| Runx2 | 62 | Forward: GAC AGA AGC TTG ATG ACT CTA AAC C | 171 |
| Reverse: TCT GTA ATC TGA CTC TGT CCT TGT | |||
| Collagen Type I | 60 | Forward: GCA TGG CCA AGA AGA CAT CC | 83 |
| Reverse: CCT CGG GTT TCC ACG TCT C | |||
| Osteocalcin | 60 | Forward: ATG AGG ACC CTC TCT CTG CTC | 270 |
| Reverse: ATG AGG ACC CTC TCT CTG CTC | |||
| GAPDH | 52 | Forward: AAC TAC ATG GCT ACA TGT TCC A | 73 |
| Reverse: CCA TTC TCG GCC TTG ACT GT |
Alizarin red S staining for matrix mineralization
The matrix mineralization of co-cultures cultured in BS or OS medium was detected by histochemical staining at day 28. Cells were fixed with 60% isopropanol with 2% alizarin red (Sigma) solution (pH 4.1−4.3) for 5 min at room temperature. Matrix mineralization was visualized by staining.
Statistical analysis
All assays were repeated, with a minimum of n = 3 per group. Data are expressed as mean and standard deviation values. Statistical significance was determined using one-way ANOVA to compare means between groups, with a p value of less than 0.05 being considered significant.
Results
Osteogenic gene expression in BS- or OS-treated co-cultures at day 3
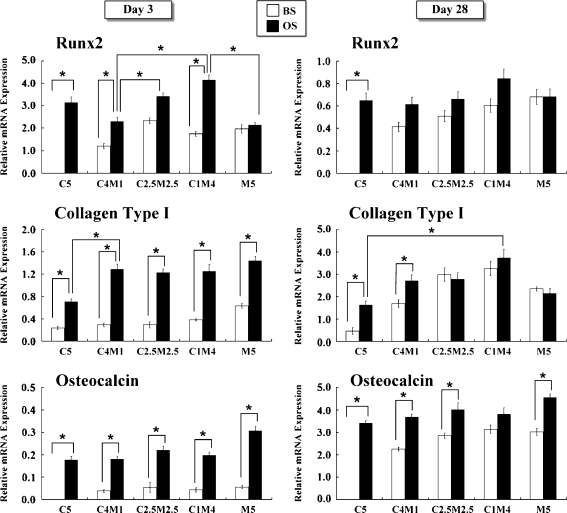
In BS-treated cultures, there was no remarkable difference in Runx2 mRNA expression between the groups at day 3 (Fig. 2). However, in OS-treated cultures, C4M1, C1M1, and C1M4 co-culture groups exhibited a significant increase in Runx2 mRNA expression in an osteoblast-cell-density-dependent manner. In these three co-cultures, a greater number of osteoblasts showed higher Runx2 mRNA expression. The expression of Runx2 mRNA was also higher in group C5 than in group M5. Type I collagen is a major organic component contained in the bone extracellular matrix. In BS-treated cultures, all groups exhibited lower type I collagen mRNA expression than OS-treated cultures, with the exception of group M5 at day 3. However, all cultures exhibited much higher type I collagen mRNA expression with the osteogenic supplement compared to those in BS-treated cultures. The osteocalcin mRNA expression did not differ significantly between the groups at day 3, irrespective of whether culturing was in BS or OS. However, the expression of osteocalcin was greatly enhanced in the OS-treated groups compared to the BS-treated control.
Fig. 2.
The expressions of Runx2, type I collagen, and osteocalcin were analysed as osteogenic markers by semiquantitative RT-PCR at days 3 and 28. C3H10T1/2 cells were co-cultured with MC3T3-E1 cells at various ratios in untreated (BS) or osteogenic-treated (OS) medium. (*p < 0.05, n = 3 for each group; data are mean and standard deviation values)
Osteogenic gene expression in BS- or OS-treated co-cultures at day 28
In 28-day cultures, all co-cultures exhibited a distinct decrease of Runx2 mRNA expression at the end of culture (Fig. 2). With the exception of group M5, Runx2 mRNA expression was higher following OS treatment than following BS treatment. Accumulation of type I collagen mRNA was observed in all groups, whether supplemented with or without osteogenic reagents. The expression of type I collagen was no more distinct in group M5 (both with or without osteogenic medium) than in the other groups.
Osteocalcin is a specific bone marker at the late stage of bone formation. After a 28-day culture, osteocalcin mRNA expression was significantly increased in all groups, but especially in the OS-treated cultures. Osteocalcin levels in group C5 were greatly enhanced by OS treatment compared to BS treatment. Most co-cultures exhibited an osteoblast-cell-density-dependent increase in the expression of Runx2, type I collagen and osteocalcin. Co-cultures with more osteoblasts exhibited higher levels of osteogenic gene expression.
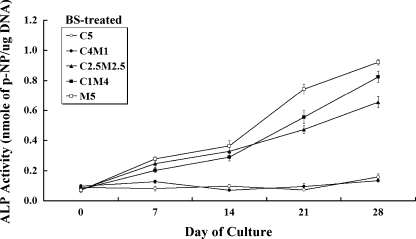
ALP activity in BS-treated co-cultures
In BS-medium culture, groups C5 and C4M1 did not exhibit a significant increase in ALP activity during the 28-day culture period (Fig. 3). However, the other three groups co-cultured with osteoblasts at higher ratios exhibited a steady increase in ALP production over time. MSCs co-cultured with fewer osteoblasts did not tend to go through the osteogenic differentiation in BS medium (i.e., without osteogenic supplements). Therefore, for MSCs, the lesser direct cell–cell contact with osteoblasts limited the contribution to the MSC osteogenic differentiation in vitro.
Fig. 3.
Alkaline phosphatase (ALP) activity in co-cultures with C3H10T1/2 and MC3T3-E1 cells maintained in BS treatment were assayed and determined spectrophotometrically during the 28-day culture period. Few alterations in ALP production were observed in groups C5 and C4M1 over time. However, groups C2.5M2.5 and C1M4 exhibited an almost linear increase in ALP production during the culture period (*p < 0.05, n = 3 for each group; data are mean and standard deviation values)
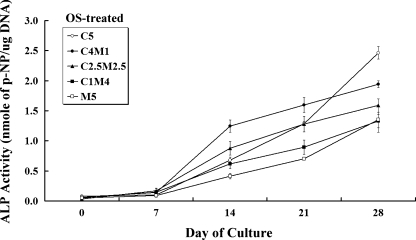
ALP activity in OS-treated co-cultures
The ALP activity from these OS-treated co-culture groups yielded contrasting results from those in BS-treated culture (Fig. 4). In the osteogenically supplemented (OS) environment, all groups exhibited a time-dependent increase in ALP production during the culture period. Interestingly, the ALP activity at the end of the culture period was highest in group C5 cultures. Group C4M1 also exhibited a remarkable time-dependent increase in ALP production compared to C5, except at day 28. In contrast, groups M5 and C1M4, which were co-cultured at higher osteoblast ratios, exhibited slightly higher ALP activities at the end of the culture period than those cultured in BS medium.
Fig. 4.
Alkaline phosphatase (ALP) activity in co-cultures with C3H10T1/2 and MC3T3-E1 cells maintained in osteogenic (OS)-treated medium were assayed and determined spectrophotometrically during the 28-day culture period. ALP activity in group C5 expressed a surprising increase, reaching its highest point at the end of the culture period. All OS-treated groups exhibited a steady increase in ALP production over time. ALP activity was overall relatively higher in the OS- than in the BS-treated co-cultures (*p < 0.05, n = 3 for each group; data are mean and standard deviation values)
Matrix mineralization and calcium deposition
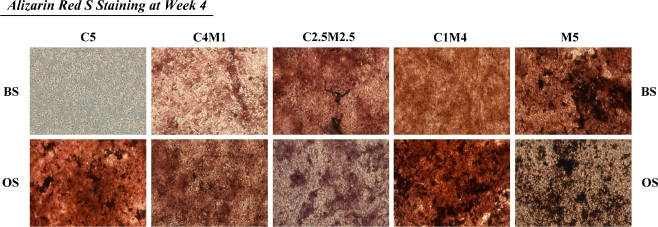
No remarkable calcium deposition was observed in group C5 after the 28-day culture period (Fig. 5) in BS-treated co-cultures. However, clear accumulation of calcium was observed in all groups cultured in the OS condition. The accumulation of calcium deposition at week 4 in either the BS or OS condition was higher in group M5 than in the other co-culture groups. Groups C4M1, C2.5M2.5, and C1M4 expressed higher calcium deposition in the OS than in the BS condition. This suggests that both the higher number of osteoblasts and the presence of osteogenic supplements in these co-culture groups enhanced the mineralization observed at the end of culture.
Fig. 5.
The matrix mineralization of all osteogenic (OS)-treated co-cultures during osteogenesis was estimated by alizarin red S staining at week 4. Calcium deposition accumulated over time, reaching its highest level at the end of the culture period. Both BS- and OS-treated M5 groups exhibited the highest levels of matrix mineralization. All groups except the BS-treated group C5 co-culture exhibited an osteoblast-dependent accumulation of calcium deposition over time. Magnification, ×50
Discussion
A monolayer co-culture model was implemented in this study, with C3H10T1/2 and MC3T3-E1 cells at various ratios of cell density plated and co-cultured in BS or OS medium, to determine whether direct cell–cell contact between osteoblasts and MSCs is critical for inducing or enhancing MSC osteogenesis. This study found a clear synergistic induction and enhancement of MSC osteogenesis in these co-culture models. The results show that co-cultures with a higher density of osteoblasts expressed greater mRNA expressions of Runx2, type I collagen, and osteocalcin, as well as ALP production in the BS-treated environment. Moreover, OS-treated co-cultures exhibited much higher expressions of most target genes, but not of ALP activity or calcium deposition. This suggests that a higher cell density of osteoblasts may lead to weak osteogenesis of MSCs by direct cell–cell contact in an environment without additional osteogenic supplements. However, additional OS treatment may promote the activity of osteoblasts as synergistic modulators to accelerate matrix mineralization by impeding osteogenic differentiation in the co-cultures.
Human bone-marrow-derived MSCs, by virtue of their capacity for self-renewal and multipotent differentiation, are considered promising candidate cells for regenerative medicine and tissue engineering applications. The multipotent differentiation capabilities of MSCs into various types of cell lineage, such as osteoblasts, chondrocytes, and adipocytes, have been characterized in numerous studies [1, 2]. Studies have used co-culture systems to further investigate the possible induction of osteogenesis of MSCs by neighbouring cells [18–21]. Osteoblasts, which are derived from bone-marrow MSCs, are the main cell type responsible for bone formation, and are one of the neighbouring cell types close to the bone marrow. Direct cell–cell contact co-culture models have produced further evidence that co-culture of MSCs and osteoblasts [17, 20, 22] or osteoblast-derived soluble factors [16, 23, 24] leads to osteogenesis. It is believed that interaction between bone-marrow MSCs and their neighbouring cells may lead to MSC osteogenesis via cell–cell contact or osteoblast-derived soluble factors. However, cells in co-cultures derived from different species, such as humans, mice, and rabbits, may give rise to different results [17].
It has been demonstrated that Runx2 is as an essential transcription factor for the induction of early osteogenic differentiation. Studies of the expression of Runx2 demonstrate that the early osteogenesis of co-cultures is highly dependent on osteoblast numbers co-cultured in an osteoinductive environment. Additional OS supplements were also critical to the overall enhancement of osteogenic gene expression compared to those in BS-treated co-cultures. The gene expression at day 28 in BS-treated co-cultures increased with the ratios of osteoblasts. Co-cultures with MSCs and osteoblasts demonstrated that direct cell–cell contact was sufficient to induce osteogenic differentiation by enhancing the gene expressions of Runx2, type I collagen, and osteocalcin. More significant and distinct osteogenic differentiation was promoted by additional osteogenic supplements.
Csaki et al. demonstrated that MSCs and osteoblasts actively search for cell–cell contact, leading to cell proliferation and osteogenic differentiation, only in monolayer co-cultures with osteoinductive treatment [14]. The quality of osteogenesis, as evidenced by protein expression, is proportional to the quantity of osteoblasts in the co-cultures, a finding that is consistent with our results. A recent study has also suggested that primary bone-derived cells promote the osteogenesis of human embryonic stem cells in a co-culture model by releasing bone morphogenetic proteins 2 and 4 [22]. These findings demonstrate that the osteogenesis of stem cells may be induced or promoted in a co-culture system through either direct cell–cell contact or secreted soluble factors derived from osteoblasts.
Our findings show that direct cell–cell contact between MSCs and osteoblasts contributed little to MSC osteogenic differentiation in the BS condition. In addition, the co-cultures required at least one osteoinductive factor to induce or enhance the osteogenic differentiation in MSCs: either an appropriate number of osteoblasts or treatment with osteogenic supplements. Culture conditions that included both a large quantity of osteoblasts and osteogenic supplements resulted in synergistic inhibition during osteogenesis by shrinking the process of osteogenic differentiation.
The untreated co-cultures with murine MSCs and osteoblasts were sufficient to induce a weak osteogenesis when cultured in BS medium without any osteogenic supplement. Furthermore, the overall level of osteogenesis in the untreated co-cultures remained lower than in the OS-treated co-cultures. This information indicates that direct cell–cell contact and soluble osteogenic factors might act as synergistic modulators to promote or inhibit osteogenic differentiation in MSCs. Understanding the possible synergistic interactions between MSCs and osteoblasts might be an alternative approach to manipulating the fates of stem cells and for differentiating lineages in cell-therapeutic strategies and regenerative medicine.
Acknowledgements
This work was supported by grants from the National Science Council, Taiwan, R.O.C. (NSC 98-2627-B-033-001). The authors also thank Prof. Yeu Su (National Yang-Ming University, Taipei, Taiwan) for generously providing the C3H10T1/2 cell line as a gift.
Footnotes
Ming-Tzu Tsai and Dan-Jae Lin contributed equally to this work.
References
- 1.Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8(3):301–316. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 3.Hao W, Dong J, Jiang M, Wu J, Cui F, Zhou D. Enhanced bone formation in large segmental radial defects by combining adipose-derived stem cells expressing bone morphogenetic protein 2 with nHA/RHLC/PLA scaffold. Int Orthop. 2010;34(8):1341–1349. doi: 10.1007/s00264-009-0946-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whu SW, Tsai CL, Hsu SH. Evaluation of human bone marrow mesenchymal stem cells seeded into composite scaffolds and cultured in a dynamic culture system for neocartilage regeneration in vitro. J Med Biol Eng. 2009;29(2):52–59. [Google Scholar]
- 5.Kratchmarova I, Blagoev B, Haack-Sorensen M, Kassem M, Mann M. Mechanism of divergent growth factor effects in mesenchymal stem cell differentiation. Science. 2005;308(5727):1472–1477. doi: 10.1126/science.1107627. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe T, Sakai D, Yamamoto Y, Iwashina T, Serigano K, Tamura F, Mochida J. Human nucleus pulposus cells significantly enhanced biological properties in a co-culture system with direct cell-to-cell contact with autologous mesenchymal stem cells. J Orthop Res. 2010;28(5):623–630. doi: 10.1002/jor.21036. [DOI] [PubMed] [Google Scholar]
- 7.Yu H, Vandevord PJ, Gong W, Wu B, Song Z, Matthew HW, Wooley PH, Yang SY. Promotion of osteogenesis in tissue-engineered bone by pre-seeding endothelial progenitor cells-derived endothelial cells. J Orthop Res. 2008;26(8):1147–1152. doi: 10.1002/jor.20609. [DOI] [PubMed] [Google Scholar]
- 8.Ozturk AM, Cila E, Kanatli U, Isik I, Senkoylu A, Uzunok D, Piskin E. Treatment of segmental bone defects in rats by the stimulation of bone marrow osteo-progenitor cells with prostaglandin E2. Int Orthop. 2005;29(2):73–77. doi: 10.1007/s00264-004-0623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen KY, Chung CM, Kuo SM, Chen YS, Yao CH. Influence of collagen I nanospheres on the growth and osteogenic difference of rat bone marrow stromal cells. J Med Biol Eng. 2009;29(6):284–289. [Google Scholar]
- 10.Jung Y, Song J, Shiozawa Y, Wang J, Wang Z, Williams B, Havens A, Schneider A, Ge C, Franceschi RT, McCauley LK, Krebsbach PH, Taichman RS. Hematopoietic stem cells regulate mesenchymal stromal cell induction into osteoblasts thereby participating in the formation of the stem cell niche. Stem Cells. 2008;26(8):2042–2051. doi: 10.1634/stemcells.2008-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borovecki F, Pecina-Slaus N, Vukicevic S. Biological mechanisms of bone and cartilage remodelling-genomic perspective. Int Orthop. 2007;31(6):799–805. doi: 10.1007/s00264-007-0408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M, Thompson DD, Paralkar VM. Prostaglandin E-2 receptors in bone formation. Int Orthop. 2007;31(6):767–772. doi: 10.1007/s00264-007-0406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ball SG, Shuttleworth AC, Kielty CM. Direct cell contact influences bone marrow mesenchymal stem cell fate. Int J Biochem Cell Biol. 2004;36(4):714–727. doi: 10.1016/j.biocel.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Csaki C, Matis U, Mobasheri A, Shakibaei M. Co-culture of canine mesenchymal stem cells with primary bone-derived osteoblasts promotes osteogenic differentiation. Histochem Cell Biol. 2009;131(2):251–266. doi: 10.1007/s00418-008-0524-6. [DOI] [PubMed] [Google Scholar]
- 15.Gerstenfeld LC, Cruceta J, Shea CM, Sampath K, Barnes GL, Einhorn TA. Chondrocytes provide morphogenic signals that selectively induce osteogenic differentiation of mesenchymal stem cells. J Bone Miner Res. 2002;17(2):221–230. doi: 10.1359/jbmr.2002.17.2.221. [DOI] [PubMed] [Google Scholar]
- 16.Heino TJ, Hentunen TA, Vaananen HK. Conditioned medium from osteocytes stimulates the proliferation of bone marrow mesenchymal stem cells and their differentiation into osteoblasts. Exp Cell Res. 2004;294(2):458–468. doi: 10.1016/j.yexcr.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Kim H, Lee JH, Suh H. Interaction of mesenchymal stem cells and osteoblasts for in vitro osteogenesis. Yonsei Med J. 2003;44(2):187–197. doi: 10.3349/ymj.2003.44.2.187. [DOI] [PubMed] [Google Scholar]
- 18.Jung Y, Wang J, Havens A, Sun Y, Wang J, Jin T, Taichman RS. Cell-to-cell contact is critical for the survival of hematopoietic progenitor cells on osteoblasts. Cytokine. 2005;32(3–4):155–162. doi: 10.1016/j.cyto.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Stahl A, Wenger A, Weber H, Stark GB, Augustin HG, Finkenzeller G. Bi-directional cell contact-dependent regulation of gene expression between endothelial cells and osteoblasts in a three-dimensional spheroidal co-culture model. Biochem Biophys Res Commun. 2004;322(2):684–692. doi: 10.1016/j.bbrc.2004.07.175. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Volloch V, Pindrus MA, Blasioli DJ, Chen J, Kaplan DL. Murine osteoblasts regulate mesenchymal stem cells via WNT and cadherin pathways: mechanism depends on cell-cell contact mode. J Tissue Eng Regen Med. 2007;1(1):39–50. doi: 10.1002/term.6. [DOI] [PubMed] [Google Scholar]
- 21.Zhang S, Wang J, Bilen MA, Lin SH, Stupp SI, Satcher RL. Modulation of prostate cancer cell gene expression by cell-to-cell contact with bone marrow stromal cells or osteoblasts. Clin Exp Metastasis. 2009;26(8):993–1004. doi: 10.1007/s10585-009-9289-0. [DOI] [PubMed] [Google Scholar]
- 22.Ahn SE, Kim S, Park KH, Moon SH, Lee HJ, Kim GJ, Lee YJ, Park KH, Cha KY, Chung HM. Primary bone-derived cells induce osteogenic differentiation without exogenous factors in human embryonic stem cells. Biochem Biophys Res Commun. 2006;340(2):403–408. doi: 10.1016/j.bbrc.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Ilmer M, Karow M, Geissler C, Jochum M, Neth P. Human osteoblast-derived factors induce early osteogenic markers in human mesenchymal stem cells. Tissue Eng A. 2009;15(9):2397–2409. doi: 10.1089/ten.tea.2008.0427. [DOI] [PubMed] [Google Scholar]
- 24.Zhou H, Mak W, Zheng Y, Dunstan CR, Seibel MJ. Osteoblasts directly control lineage commitment of mesenchymal progenitor cells through Wnt signaling. J Biol Chem. 2008;283(4):1936–1945. doi: 10.1074/jbc.M702687200. [DOI] [PubMed] [Google Scholar]