Abstract
Purpose
Bone defects resulting from tumour resection or curettage are most commonly reconstructed with autologous bone graft which is associated with limited availability and donor site morbidity. Recent research has focussed on synthetic biomaterials as bone graft substitutes. The aim of this study was to assess the safety and efficiency of a bone substitute as an alternative for autologous bone in the treatment of benign bone tumours and tumour-like lesions.
Methods
In the present study, a biphasic ceramic (60% HA and 40% β-TCP) combined with a fibrin sealant was used to reconstruct defects in 51 patients after curettage of benign bone tumours or tumour-like lesions. Patient age ranged from eight to 68 years (mean 29.7), defect size from 2 cm3 to 35 cm3 (mean 12.1), and time of follow-up from one to 56 months (mean 22.7).
Results
Radiologic analysis showed complete bony defect consolidation in 50 of 51 patients after up to 56 months. No postoperative fractures were observed. Revision surgery had to be performed in one case. Histological analysis showed new bone formation and good biocompatibility and osseointegration of the implanted material.
Conclusion
In summary, the biphasic ceramic in combination with fibrin sealant was proven an effective alternative to autologous bone grafts eliminating the risk of donor site morbidity for the patient.
Introduction
Benign bone tumours and tumour-like lesions are a heterogenic group of bone lesions. Benign bone tumours are defined as autonomic and slowly growing neoplastic lesions with no potential for metastasis with the exception of few potentially malignant entities such as osteoclastoma. Tumour-like lesions are self-limiting, non-neoplastic lesions originating from manifold tissues and potentially leading to a destruction of bone [1].
According to the World Health Organisation (WHO), benign bone tumours can be classified by the type and degree of matrix production and the predominantly residing cell type into bone-forming, cartilage-forming, connective-tissue and vascular lesions [2]. The incidence of most of these lesions is low [3]. The term “tumour-like lesion” summarizes various conditions of non-neoplastic nature originating from or affecting the bone that are manifested as solitary or multiple bone lesions. Different fibro-osseous and cystic lesions represent the most common entities in this group with non-ossifying fibromas, simple and aneurysmal bone cysts as the most frequent [3]. Many benign bone tumours and tumour-like lesions require no further treatment and can be identified radiologically [4].
Bony lesions that are accompanied by clinical symptoms or show growth over time or are of unclear radiological appearance need to be biopsied and examined histologically. After histological classification an adequate treatment is initiated. Once indicated, intralesional curettage and defect reconstruction by transplantation of cancellous bone graft is the therapy of choice for most benign bone tumours and tumour-like lesions [5].
Reported complication rates after autologous bone graft harvest from the iliac crest vary between 8% and 13% [6, 7]. In addition, autologous cancellous bone is limited in availability [8]. Nevertheless, the augmentation of bony lesions with autologous cancellous bone is the most effective treatment and therefore remains the “gold standard” [7].
Searching for an alternative to cancellous bone grafts, synthetic calcium phosphate bone substitute materials have been developed. The most widely known representatives of calcium phosphates are hydroxyapatite (HA) and β-tricalciumphosphate (β-TCP). Combinations of HA and β-TCP are considered biphasic calcium phosphates (BCP). Ceramic based biomaterials are amongst the most promising candidates for bone tissue engineering as they unite good biocompatibility with osteoinductive properties and mechanical strength. Pore size and shape of these ceramics can be tailored to aid bone ingrowth and therefore osseointegration [9, 10].
Macropores promote osteoconduction by facilitating in-growth of bone by invasion, proliferation and differentiation of osteogenic progenitor cells. The proliferating cells, supported by residing macrophages, gradually resorb and replace the ceramic material with newly formed mineralized bone [11]. Combining the bioceramic with fibrin sealant provides a mouldable composite material. The combination represents a novel approach and has been shown to have a positive effect on bone in-growth and new bone formation [10]. The fibrin based sealant acts as a bioactive matrix stimulating stem cell in-growth additionally containing growth factors and haematological proteins involved in wound healing and angiogenesis [12–14].
In the presented prospective clinical study, a biphasic synthetic bone substitute was used in combination with a fibrin matrix to fill bone defects of benign bone tumours and tumour-like lesions. The aim of this study was to assess the efficiency of the biomaterial as an alternative for autologous bone in the treatment of benign bone tumours and tumour-like lesions.
Material and methods
Over a period of three years 51 patients (age eight–69 years, mean 29.7 ± 17.0 years) presented with benign bone tumours or tumour-like lesions, located at different anatomic sites (Tables 1, 2), and were treated with a biphasic synthetic bone substitute (TricOs®, manufactured by Biomatlante, Vigneux de Bretagne, France) in combination with a diluted fibrin matrix (TissuCol®, Baxter BioScience, Vienna, Austria). The study was approved by the local ethics committee.
Table 1.
Entities, localisation and average size of benign bone tumours and tumour-like lesions
| Entity | Tibia | Fibula | Femur | Humerus | Scapula | Calcaneus | Others | Total | Size (cm3) |
|---|---|---|---|---|---|---|---|---|---|
| Osteoclastoma | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 10.5 ± 0.0 |
| Chondroblastoma | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 17.5 ± 3.5 |
| Enchondroma | 3 | 0 | 3 | 4 | 0 | 0 | 0 | 10 | 13.1 ± 5.2 |
| Chondromyxoidfibroma | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 10.5 ± 0.0 |
| Solitary cyst | 2 | 0 | 2 | 2 | 1 | 0 | 1 | 8 | 9.0 ± 5.5 |
| Calcaneal cyst | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 9 | 12.1 ± 2.9 |
| Fibrous dysplasia | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 3 | 18.7 ± 12.9 |
| Fibr. met. defect | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 2.0 ± 0.0 |
| N. o. fibroma | 3 | 1 | 2 | 0 | 0 | 0 | 0 | 7 | 9.0 ± 2.5 |
| Aneurysmatic cyst | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 3 | 14.0 ± 9.9 |
| Others | 3 | 0 | 1 | 0 | 1 | 0 | 0 | 5 | 11.7 ± 6.3 |
| Total | 14 | 2 | 11 | 9 | 2 | 10 | 2 | 51 | 12.1 ± 6.8 |
Fibr. met. fibrous metaphyseal, N.o. non-ossifying
Table 2.
Patient population and characteristics
| Patient number | Age (y) | Gender | Bone | Localisation | Diagnosis | Size (cm3) | Autologous bone graft | Complications | Follow-up (mo) | Stage |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 23 | Female | Tibia | Met/Epi | Osteoclastoma | 10.5 | Yes | None | 56 | 2 |
| 2 | 14 | Male | Calcaneus | Calcaneal cyst | 14.0 | No | None | 54 | 2 | |
| 3 | 52 | Female | Femur | Met | Enchondroma | 10.5 | Yes | Pain at donor site pelvis | 36 | 3A |
| 4 | 16 | Female | Humerus | Met/Epi | Chondroblastoma | 14.0 | No | None | 47 | 2 |
| 5 | 38 | Female | Tibia | Met | Solitary cyst | 7.0 | No | Intraosseous ganglion, revision | 48 | 2 |
| 6 | 20 | Male | Tibia | Met | Abscess, Salmonella spec. | 10.5 | No | None | 48 | 2 |
| 7 | 16 | Male | Tibia | Met | N.o. Fibroma | 10.5 | No | Pain | 36 | 3A |
| 8 | 43 | Female | Humerus | Met | Enchondroma | 17.5 | No | CRPS I | 6 | 1 |
| 9 | 46 | Female | Scapula | Solitary cyst | 10.5 | No | None | 48 | 3A | |
| 10 | 19 | Male | Femur | Dia | Fibrous dysplasia | 35.0 | No | None | 10 | 2 |
| 11 | 26 | Male | Calcaneus | Calcaneal cyst | 14.0 | No | None | 48 | 1 | |
| 12 | 68 | Female | Femur | Met | Enchondroma | 10.5 | No | None | 17 | 1 |
| 13 | 12 | Male | Calcaneus | Calcaneal cyst | 10.5 | No | None | 17 | 2 | |
| 14 | 39 | Female | Femur | Met | Solitary cyst | 7.0 | No | None | 16 | 1 |
| 15 | 9 | Male | Calcaneus | Calcaneal cyst | 10.5 | No | None | 1 | 1 | |
| 16 | 50 | Male | Tibia | Met | Solitary cyst | 7.0 | No | None | 26 | 2 |
| 17 | 43 | Female | Calcaneus | Calcaneal cyst | 14.0 | Yes | None | 25 | 1 | |
| 18 | 30 | Male | Calcaneus | Calcaneal cyst | 10.5 | No | None | 23 | 1 | |
| 19 | 30 | Male | Calcaneus | Calcaneal cyst | 10.5 | No | None | 22 | 2 | |
| 20 | 11 | Female | Calcaneus | Chondromyxoidfibroma | 10.5 | No | None | 24 | 1 | |
| 21 | 13 | Female | Tibia | Met | N.o. fibroma | 7.0 | No | None | 22 | 3A |
| 22 | 16 | Male | Tibia | Met | Fibrous cortical defect | 2.0 | No | None | 12 | 3A |
| 23 | 35 | Female | Calcaneus | Calcaneal cyst | 7.0 | No | None | 2 | 2 | |
| 24 | 57 | Male | Femur | Met | Fibrous dysplasia | 17.5 | No | None | 25 | 2 |
| 25 | 57 | Male | Scapula | Solitary cyst | 2.0 | No | None | 1 | 1 | |
| 26 | 41 | Female | Humerus | Met | Enchondroma | 21.0 | No | None | 37 | 2 |
| 27 | 14 | Female | Fibula | Met | Aneurysmatic bone cyst | 7.0 | No | Temp. paralysis peroneal nerve | 12 | 2 |
| 28 | 16 | Male | Tibia | Met | Enchondroma | 7.0 | No | None | 13 | 2 |
| 29 | 35 | Male | Tibia | Met | Intraosseous ganglion | 17.5 | No | Wound healing | 17 | 2 |
| 30 | 58 | Male | Femur | Met | Solitary cyst | 17.5 | No | None | 35 | 1 |
| 31 | 26 | Female | Humerus | Dia | Fibrous dysplasia | 3.5 | No | None | 30 | 2 |
| 32 | 15 | Male | Tibia | Met | N.o. fibroma | 10.5 | No | None | 29 | 3A |
| 33 | 47 | Female | Humerus | Met | Enostoma | 10.5 | No | None | 25 | 1 |
| 34 | 9 | Female | Calcaneus | Calcaneal cyst | 17.5 | No | None | 25 | 1 | |
| 35 | 26 | Female | Tibia | Dia | Enchondroma | 14.0 | No | None | 2 | 2 |
| 36 | 55 | Female | Tarsus | Os cunei. | Solitary cyst | 3.5 | No | None | 4 | 2 |
| 37 | 14 | Female | Femur | Met | N.o. fibroma | 10.5 | No | None | 15 | 2 |
| 38 | 18 | Female | Pelvis | Os pubis | Aneurysmatic bone cyst | 28.0 | No | None | 28 | 2 |
| 39 | 13 | Male | Fibula | Met | N.o. fibroma | 10.5 | No | None | 13 | 2 |
| 40 | 11 | Male | Femur | Dia | Aneurysmatic bone cyst | 7.0 | No | None | 13 | 2 |
| 41 | 8 | Female | Femur | Met | N.o. fibroma | 3.5 | No | None | 12 | 2 |
| 42 | 56 | Female | Tibia | Met | Osteonecrosis | 7.0 | Yes | None | 24 | 1 |
| 43 | 48 | Female | Femur | Met | Enchondroma | 5.0 | No | None | 12 | 2 |
| 44 | 15 | Female | Humerus | Met | Fibrous cortical defect | 2.0 | No | None | 6 | 2 |
| 45 | 54 | Male | Humerus | Met | Solitary cyst | 17.5 | No | None | 12 | 2 |
| 46 | 44 | Male | Humerus | Met | Enchondroma | 21.0 | No | None | 13 | 1 |
| 47 | 21 | Female | Tibia | Dia | Enchondroma | 10.5 | No | None | 7 | 2 |
| 48 | 48 | Female | Femur | Met | Enchondroma | 14.0 | No | None | 27 | 2 |
| 49 | 16 | Male | Tibia | Met/Epi | Chondroblastoma | 21.0 | Yes | None | 24 | 1 |
| 50 | 13 | Female | Femur | Met | Lipoma | 24.0 | No | None | 26 | 1 |
| 51 | 11 | Male | Tibia | Met | N.o. fibroma | 10.5 | No | None | 26 | 2 |
Met metaphysis, Epi epiphysis, Dia diaphysis, Stage radiographic stage at final follow-up, N.o. non-ossifying
TricOs® is a biphasic calcium phosphate ceramic (60% HA and 40% ß-TCP). The material consists of granules of 1–2 mm in diameter. The granules have a surface structure with macropores within a range of 300–600 μm and micropores (<10 μm) accounting for an interconnective overall porosity of 70–80% [15]. Each application-syringe contains 3.5 cm3 of granules. Prior to use the ceramic is moistened with 1 ml of distilled water.
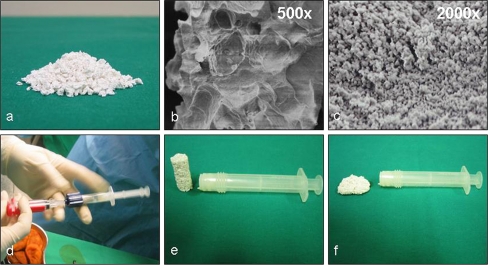
TissuCol® Duo S 1 ml Immuno (Baxter BioScience, Vienna, Austria) is a biological two-component fibrin sealant consisting of two frozen ready-to-use syringes. Each unit contains 70–110 mg of fibrinogen and 500 units of human thrombin. Prior to application the thrombin was defrosted and diluted with a 20 mM calcium-chloride solution (St. Vincenz Krankenhaus, Limburg) to obtain a thrombin solution of 5 units/ml. The fibrin sealant was deposited onto the ceramic granules. After approximately 2 min the fibrin was cross-linked and the composite was ready for use (Fig. 1).
Fig. 1.
a Macroscopic appearance of biphasic calcium phosphate granules. b SEM images of bone substitutes macrostructure and microstructure (c). d The material is moistened with 1 ml aqua dest./3.5 cm3 and the diluted fibrin sealant is added to form a mouldable mass (e, f) that can easily be applied to a lesion
The lesions treated were a heterogeneous group of entities (Table 2). In all cases the cortical bone was fenestrated and intralesional curettage was performed. Samples for histological assessment were obtained. Afterwards, the defects were completely filled with the composite material (see Figs. 2, 3, 4). Autologous cancellous bone graft was applied in five cases. The decision to use autologous bone graft was made when the lesion was in close contact with the joint.
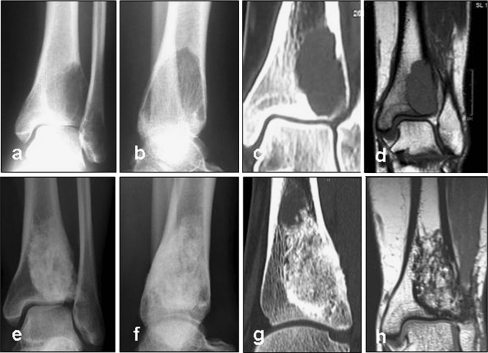
Fig. 2.
X-ray (a, b) and CT (c, d) images of a 24-year-old female patient diagnosed with an osteoclastoma of the distal tibia. The patient experienced pain when weight-bearing. TricOs bone substitute material was applied in combination with autologous cancellous bone from the iliac crest at a rate of 1:5. The defect had a volume of 10.5 cm3. At 18 months post surgery, CT scans were performed. The lesion was completely filled with newly formed tissue reminiscent of healthy cancellous bone. A sclerotic margin was observed. After 56 months the bone substitute material was radiographically still detectable. Resorption was determined as a stage 2 (e–g). Also, MRI was performed after 56 months to rule out recurrence of the osteoclastoma. A hypointensive zone was seen in the CT images corresponding to the sclerotic margin (h)
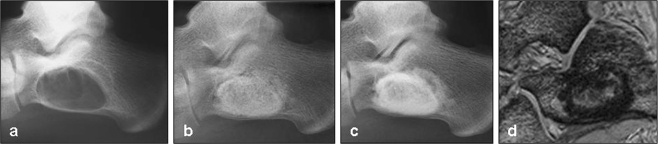
Fig. 3.
X-ray and MRI images of a 16-year-old male suffering from a large calcaneal cyst (a). Intralesional curettage was performed and the lesion filled with 14 cm3 TricOs®. Radiographs showed complete defect filling postoperatively (b), and after 54 months postoperatively (c). Over time radiolucency decreased especially in the marginal area of the lesion. No signs of osteolysis were evident. The resorption was classified as a stage 2. High dissolution MRI was performed after 12 months postoperatively to assess defect filling. In T1-weighted images (d) the margin of the filled lesion showed no signal intensity. The patient had fully recovered six weeks after surgery and was free of pain
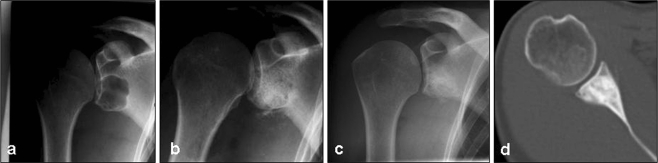
Fig. 4.
X-rays and CT image of a 47-year-old female patient with a juvenile cyst of the glenoid (a). The lesion was filled with 7 cm3 of TricOs® (b). Radiologic controls and CT scans showed complete defect filling after 48 months (c, d), the resorption process of the ceramic was staged 3A. Radiologically, the implanted material was still detectable
Radiological evaluation was performed six weeks, and at three, six, 12 and 24 months postoperatively. Decisions on the necessity of and indication for further follow-up visits beyond 24 months were made on an individual basis.
CT or MRI scans were performed 12 months postoperative to rule out recurrence of benign bone tumours. Radiographic findings were described according to the classification system proposed by Matsumine et al. (stage 1 clear margin, stage 2 hazy margin, stage 3 absorbed margin: stage 3A absorption < 50% and stage 3B absorption > 50%) [16]. Radiographs were evaluated regarding to defect consolidation, osseointegration and resorption of the transplanted material.
Histological examination was performed after fixation in 4% paraformaldehyde, and the sample was embedded in Glycol-PMMA. Samples of 30-μm thickness were prepared using a circular diamond saw (Leitz, Wetzlar, Germany) and sectioned using a hard tissue microtome (Reichert-Jung 2050, Supercut, Cambridge Instruments, Austria). One section was polished and sputter-coated prior to visualization with a 20 kV scanning electron microscope (Jeol JSM 6300, Japan). Histological sections of 10 μm were also stained with Movat’s pentachrome and viewed using a light or polarized light microscope.
For statistical analysis univariate analysis was performed using the Mann-Whitney U-test for nonparametric data. A p-value of less than 0.05 was considered significant.
Results
The size of the lesions treated ranged from 2–35 cm3 (mean 11.8 ± 6.6 cm3) measured as the amount of synthetic bone substitute applied. The average time of follow-up was 22.7 ± 14.3 months (range one–56 months). Histological analysis revealed one osteoclastoma, two chondroblastomas, ten enchondromas and one chondromyxoidfibroma. The group of tumour-like lesions consisted of eight solitary cysts, nine calcaneal cysts, three fibrous dysplasias, two fibrous metaphyseal defects, seven non-ossifying fibromas, three aneurysmatic cysts and five entities that were classified as others (one lipoma, one osteonecrosis, one bone abscess, one intraosseous ganglion, one enostoma).
All but one patient showed complete bone defect filling in radiological controls at the time of final follow-up. In 17 cases sclerosis of the outer margin was observed (stage 1), and in 28 cases a hazy margin was observed (stage 2) (Fig. 2, 3). In six cases a partial resorption of less than 50% was seen classified as a stadium 3A according to Matsumine et al. (Fig. 4) [16]. In all cases an overall decrease in radiolucency over time was found. Only incomplete resorption of the transplanted material was observed as determined by X-ray analysis, and CT or MRI scans, even after up to 56 months. No postoperative fractures occurred. Clinical and radiological follow-up indicate no signs of inflammatory tissue reactions or osteolysis.
In MRI scans the biomaterial showed no signal intensity in T1- and T2-weighted images. No case of tumour recurrence was observed during the period of follow-up. One patient, initially with a solitary cyst of the distal tibia developed an intraosseous ganglion and led to a failure. In this case revision surgery was performed 37 months postoperatively and autologous cancellous bone graft from the iliac crest was applied to the defect. A biopsy was obtained which showed that the bone substitute material was still present, being well integrated with the native bone. The histological examination of this case showed unmineralized osteoid, mineralized plexiform bone and remodelling with the formation of haversian systems. No signs of inflammation or adverse reactions to the transplanted material were observed while the material itself was not completely resorbed (Fig. 5).
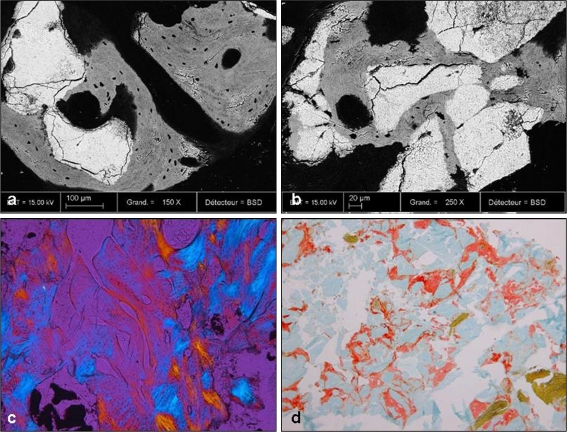
Fig. 5.
a, b Backscattered scanning electron microscopy (BSEM) demonstrating remnants of the transplanted bone substitute (white) material and new bone formation in grey and black. New bone formation is in close apposition to the biomaterial indicating good osseointegration. c Polarized light microscopy (10x) showed both residual granules and newly formed bone with haversian system. d In PMMA sections stained for Movat’s pentachrome (10x) new mineralized bone (green) and some unmineralized osteoid (red) as well as residual granules (light blue) were seen gradually being resorbed by osteoclastic cells
Minor complications were observed in five cases (9.8%). None of the complications were attributed to the applied biomaterial. Longitudinal bone growth was not disturbed.
Statistical analysis showed a significantly higher stage of resorption in smaller defects (≤ 10.5 cm3; p = 0.045). The data do not indicate a significant difference in the resorption stage between gender, age, type of bone, localisation or time of follow-up. The application of autologous bone did not show a significant difference in the stage of resorption (Table 3).
Table 3.
Relationship of radiographic staging at final follow-up contingent on gender, age, defect size, bone type, defect localisation, application of autologous bone graft and time of follow-up
| Characteristics | Number of patients | Radiographic stage at final follow-up | p-value | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3A | 3B | |||
| Total | 51 | 17 | 28 | 6 | 0 | |
| Gender | ||||||
| F | 28 | 9 | 16 | 3 | 0 | |
| M | 23 | 7 | 13 | 3 | 0 | 0.831 |
| Age (years) | ||||||
| < 20 | 22 | 5 | 13 | 4 | 0 | |
| > 20 | 29 | 11 | 16 | 2 | 0 | 0.147 |
| Size | ||||||
| ≤ 10.5 cm3 | 33 | 8 | 19 | 6 | 0 | |
| > 10.5 cm3 | 18 | 8 | 10 | 0 | 0 | 0.045* |
| Type of bone | ||||||
| Long bone | 37 | 9 | 23 | 5 | 0 | |
| None long bone | 14 | 7 | 6 | 1 | 0 | 0.095 |
| Autologous bone graft | ||||||
| Yes | 5 | 3 | 1 | 1 | 0 | |
| No | 46 | 13 | 28 | 5 | 0 | 0.371 |
| Localisation | ||||||
| Diaphysis | 5 | 0 | 5 | 0 | 0 | |
| Metaphysis | 29 | 8 | 16 | 5 | 0 | |
| Metaphysis/Epiphysis | 3 | 1 | 2 | 0 | 0 | |
| Calcaneus | 10 | 6 | 4 | 0 | 0 | |
| Others | 4 | 1 | 2 | 1 | 0 | 0.803 |
| Time of follow-up | ||||||
| < 24 months | 26 | 7 | 17 | 2 | 0 | |
| > 24 months | 25 | 9 | 12 | 4 | 0 | 0.957 |
*p < 0.05
Discussion
The ideal bone substitute material should be osteoinductive, osteoconductive and should be subject to complete resorption during the process of new bone formation. A well-balanced equilibrium between biomaterial degradation and new bone formation is desirable. The described scaffold requirements may vary depending on type, size and anatomic location of defects.
Synthetic bone substitutes such as calcium phosphate ceramics reveal good results in various clinical applications [16–18]. Only few reports are available regarding the clinical application of such ceramics in the treatment of bone defects resulting from benign tumours or tumour-like lesions and long-term outcomes [16, 18, 19].
Bioceramics that are often presented in the form of granules may be difficult to handle. The combination of a biphasic ceramic with a fibrin matrix as a composite represents a new approach to improve the properties and the handling of ceramic-based bone substitute materials. Furthermore, this specific combination was shown to have positive effects on the material’s osseointegration and bone formation in general [8, 14, 20, 21]. The addition of a fibrin matrix increases the malleability of the composite [13]. Moreover, fibrin acts as a bioactive matrix facilitating stem cell in-growth as the sealant contains growth factors and haematological proteins involved in wound healing and angiogenesis [12–14]. This positive effect is dependent on the density of the fibrin network that can be influenced by varying the thrombin concentration [14, 21].
Calcium phosphate ceramics provide a surface chemistry that facilitates protein absorption, e.g. the absorption of circulating bone morphogenetic proteins, and have been shown to have osteoinductive properties [22]. The osteoinductive properties of such calcium phosphate based ceramics, however, are by no means comparable to the osteoinductive properties of potent bone growth stimulating agents such as BMP-2 or BMP-7 and other growth factors. As a result, the osteoinductive effect of these ceramics greatly depends on the local microenvironment provided by the host and therefore the delivery of osteoinductive mediators such as bone morphogenetic proteins, transforming growth factors and platelet-derived growth factor secreted by residing hematopoetic cells and stem cells/progenitor cells of the surrounding bone [19].
In contrast to the results described by Uchida et al. [23] in the present study, no radiolucent areas adjacent to the implanted bone substitute were observed. Similarly, Uchida et al. have used a biphasic ceramic consisting of 70% HA and 30% ß-TCP. The applied granules, however, were of larger diameter. Nevertheless, the results of this particular study showed comparable outcomes in terms of osseointegration and increasing radiologic density over time, an effect that was attributed to new bone formation around and within the implanted bone substitute material [23].
Schindler et al. [19] have applied a bone substitute material containing 65% HA and 35% calcium sulphate hemihydrate in combination with autologous venous blood in 13 patients with osteoclastomas or aneurysmatic bone cysts. In this study, a radiolucent zone surrounding the graft material was also observed and interpreted as a sign of graft resorption [19]. The absence of such a radiolucent zone in defects treated in the present study could be indicative of improved new bone formation mediated by the novel bone substitute material. The radiologically detectable sclerotic defect margin present in some of the examined cases as well as the decrease in radiolucency can be explained by a summation effect of new bone formation and the bone substitute material that is still in place. This is in line with our findings of MRI scans that show a hypodense margin in T2-weighted as well as in T1-weighted images at the corresponding anatomic site. Radiologically this hypodense margin has similarities with compact bone.
Hirata et al. [24] reported on 53 patients with benign bone tumours and tumour-like lesions that were treated with curettage. Defects were filled with pure ß-TCP. Complete resorption of the transplanted material was observed in 20 cases after a mean follow-up time of 12.7 months and an average defect size of 9 cm3. Incomplete resorption was observed in 30 cases [24].
After a follow-up period of up to 56 months, the clinical and radiological results after transplantation of our biphasic calcium phosphate fibrin composite are encouraging. No adverse effects or biological complications had occurred that were attributed to the biomaterial. Minor complications were seen in five patients (9.8%). However, in one case of an osseous cyst of the distal tibia surgical revision had to be performed. Intraoperatively, a connection of the cyst to the joint was revealed that had caused an intraosseous ganglion. In the biopsy taken from within the ganglion during revision, newly formed bone was evident while residues of the transplanted biomaterial were still present. The unsuccessful treatment in this particular case can be explained by the constant flow of synovial fluid between cyst and joint space that negatively influenced bone formation and in-growth.
Even though there are no reports on adverse effect after application of fibrin glue it has to be stated that the product contains human plasma proteins and thrombin as well as bovine aprotinin. As a result, there is a low risk of disease transmission. Substituting the fibrin matrix with autologous platelet rich plasma (PRP) could eliminate this risk and provide both autologous thrombin and growth factors. However, the amount of growth factors and the concentration of thrombin, which may be decisive factors influencing clinical outcomes, are subject to great variation in the case of PRP application. The combination of ceramic-based biomaterials with bone marrow aspirates [25, 26], growth factors or ex vivo expanded osteogenic cells is also conceivable.
The significantly higher degree of resorption in smaller lesions (< 10.5 cm3) is in line with the hypothesis that smaller lesions contain less biomaterial leading to a smaller surface area. This facilitates osseointegration and resorption by ingrowing cells. In contrast to the study of Matsumine et al. [16] we did not find a significant difference regarding the resorption in males and females and younger patients.
Limitations of our study include a relatively short follow-up period; long-term follow-up may be required to define differences in resorption contingent on the age of the patient, type of bone, localisation within the bone and time of follow-up. Additional, more objective methods of analysis would be desirable to assess material resorption more objectively and to avoid bias.
In summary, the biomaterial represents an easy-to-handle alternative to autologous cancellous bone grafts for the treatment of benign bone tumours and tumour-like lesions. Best long-term results, determined as resorption stage 3A, were obtained in small lesions (≤ 10.5 cm3) at metaphyseal sites.
Acknowledgments
The authors would like to acknowledge Maurice Bagot d’Arc, Baxter BioSciences for his advice and support and for critically proof reading the manuscript.
Conflict of interest The applied biomaterial was sponsored by Baxter BioScience, Vienna, Austria. There are no financial relationships with Baxter BioScience and the authors. The authors declare that they have no conflict of interest.
References
- 1.Windhager R KN, Leithner A (2006) Benigne Knochentumoren und tumorähnliche Läsionen, vol 154. Monatsschrift Kinderheilkunde, Springer Medizin
- 2.Schajowicz F, Sissons HA, Sobin LH. The World Health Organization’s histologic classification of bone tumors. A commentary on the second edition. Cancer. 1995;75(5):1208–1214. doi: 10.1002/1097-0142(19950301)75:5<1208::AID-CNCR2820750522>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 3.Woertler K. Benign bone tumors and tumor-like lesions: value of cross-sectional imaging. Eur Radiol. 2003;13(8):1820–1835. doi: 10.1007/s00330-003-1902-z. [DOI] [PubMed] [Google Scholar]
- 4.Drunen B, Freyschmidt J. Standardized procedure for suspected bone tumor. Chirurg. 2002;73(12):1153–1161. doi: 10.1007/s00104-002-0591-5. [DOI] [PubMed] [Google Scholar]
- 5.Schaser KD, Bail HJ, Haas NP, Melcher I. Treatment concepts of benign bone tumors and tumor-like bone lesions. Chirurg. 2002;73(12):1181–1190. doi: 10.1007/s00104-002-0584-4. [DOI] [PubMed] [Google Scholar]
- 6.Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3(3):192–195. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Jager M, Westhoff B, Wild A, Krauspe R (2005) Bone harvesting from the iliac crest. Orthopade 34(10):976–982 [DOI] [PubMed]
- 8.Guehennec L, Layrolle P, Daculsi G. A review of bioceramics and fibrin sealant. Eur Cell Mater. 2004;8:1–10. doi: 10.22203/ecm.v008a01. [DOI] [PubMed] [Google Scholar]
- 9.Gauthier O, Bouler JM, Aguado E, Pilet P, Daculsi G. Macroporous biphasic calcium phosphate ceramics: influence of macropore diameter and macroporosity percentage on bone ingrowth. Biomaterials. 1998;19(1–3):133–139. doi: 10.1016/S0142-9612(97)00180-4. [DOI] [PubMed] [Google Scholar]
- 10.Guehennec L, Goyenvalle E, Aguado E, Pilet P, Bagot D’Arc M, Bilban M, Spaethe R, Daculsi G. MBCP biphasic calcium phosphate granules and tissucol fibrin sealant in rabbit femoral defects: the effect of fibrin on bone ingrowth. J Mater Sci Mater Med. 2005;16(1):29–35. doi: 10.1007/s10856-005-6443-3. [DOI] [PubMed] [Google Scholar]
- 11.Schilling AF, Linhart W, Filke S, Gebauer M, Schinke T, Rueger JM, Amling M. Resorbability of bone substitute biomaterials by human osteoclasts. Biomaterials. 2004;25(18):3963–3972. doi: 10.1016/j.biomaterials.2003.10.079. [DOI] [PubMed] [Google Scholar]
- 12.Amrani DL, Diorio JP, Delmotte Y. Wound healing. Role of commercial fibrin sealants. Ann NY Acad Sci. 2001;936:566–579. doi: 10.1111/j.1749-6632.2001.tb03545.x. [DOI] [PubMed] [Google Scholar]
- 13.Jegoux F, Goyenvalle E, Bagot D’arc M, Aguado E, Daculsi G. In vivo biological performance of composites combining micro-macroporous biphasic calcium phosphate granules and fibrin sealant. Arch Orthop Trauma Surg. 2005;125(3):153–159. doi: 10.1007/s00402-004-0748-4. [DOI] [PubMed] [Google Scholar]
- 14.Nihouannen D, Saffarzadeh A, Aguado E, Goyenvalle E, Gauthier O, Moreau F, Pilet P, Spaethe R, Daculsi G, Layrolle P. Osteogenic properties of calcium phosphate ceramics and fibrin glue based composites. J Mater Sci Mater Med. 2007;18(2):225–235. doi: 10.1007/s10856-006-0684-7. [DOI] [PubMed] [Google Scholar]
- 15.Daculsi G, LeGeros RZ, Nery E, Lynch K, Kerebel B. Transformation of biphasic calcium phosphate ceramics in vivo: ultrastructural and physicochemical characterization. J Biomed Mater Res. 1989;23(8):883–894. doi: 10.1002/jbm.820230806. [DOI] [PubMed] [Google Scholar]
- 16.Matsumine A, Myoui A, Kusuzaki K, Araki N, Seto M, Yoshikawa H, Uchida A. Calcium hydroxyapatite ceramic implants in bone tumour surgery. A long-term follow-up study. J Bone Joint Surg Br. 2004;86(5):719–725. doi: 10.1302/0301-620X.86B5.14242. [DOI] [PubMed] [Google Scholar]
- 17.Bagot d’Arc M, Daculsi G, Emam N. Biphasic ceramics and fibrin sealant for bone reconstruction in ear surgery. Ann Otol Rhinol Laryngol. 2004;113(9):711–720. doi: 10.1177/000348940411300907. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto T, Onga T, Marui T, Mizuno K. Use of hydroxyapatite to fill cavities after excision of benign bone tumours. Clinical results. J Bone Joint Surg Br. 2000;82(8):1117–1120. doi: 10.1302/0301-620X.82B8.11194. [DOI] [PubMed] [Google Scholar]
- 19.Schindler OS, Cannon SR, Briggs TW, Blunn GW. Composite ceramic bone graft substitute in the treatment of locally aggressive benign bone tumours. J Orthop Surg (Hong Kong) 2008;16(1):66–74. doi: 10.1177/230949900801600116. [DOI] [PubMed] [Google Scholar]
- 20.Nihouannen D, Daculsi G, Saffarzadeh A, Gauthier O, Delplace S, Pilet P, Layrolle P. Ectopic bone formation by microporous calcium phosphate ceramic particles in sheep muscles. Bone. 2005;36(6):1086–1093. doi: 10.1016/j.bone.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 21.Nihouannen D, Goyenvalle E, Aguado E, Pilet P, Bilban M, Daculsi G, Layrolle P. Hybrid composites of calcium phosphate granules, fibrin glue, and bone marrow for skeletal repair. J Biomed Mater Res A. 2007;81(2):399–408. doi: 10.1002/jbm.a.31058. [DOI] [PubMed] [Google Scholar]
- 22.Paul W, Sharma CP. Ceramic drug delivery: a perspective. J Biomater Appl. 2003;17(4):253–264. doi: 10.1177/0885328203017004001. [DOI] [PubMed] [Google Scholar]
- 23.Uchida A, Araki N, Shinto Y, Yoshikawa H, Kurisaki E, Ono K. The use of calcium hydroxyapatite ceramic in bone tumour surgery. J Bone Joint Surg Br. 1990;72(2):298–302. doi: 10.1302/0301-620X.72B2.2155908. [DOI] [PubMed] [Google Scholar]
- 24.Hirata M, Murata H, Takeshita H, Sakabe T, Tsuji Y, Kubo T. Use of purified beta-tricalcium phosphate for filling defects after curettage of benign bone tumours. Int Orthop. 2006;30(6):510–513. doi: 10.1007/s00264-006-0156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arinzeh TL. Mesenchymal stem cells for bone repair: preclinical studies and potential orthopedic applications. Foot Ankle Clin. 2005;10(4):651–665. doi: 10.1016/j.fcl.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 26.El-Adl G, Mostafa MF, Enan A, Ashraf M. Biphasic ceramic bone substitute mixed with autogenous bone marrow in the treatment of cavitary benign bone lesions. Acta Orthop Belg. 2009;75(1):110–118. [PubMed] [Google Scholar]