Abstract
Purpose
Whether neoadjuvant chemotherapy safely allows close margins in osteosarcoma patients is still unknown. This study investigates the impact of close margins on local recurrence (LR) and overall survival (OS) for osteosarcoma patients treated with neoadjuvant chemotherapy.
Methods
We retrospectively reviewed 47 cases of conventional osteosarcoma who were treated at our institution. Patient and treatment factors such as age, gender, MSTS stage, tumour site, surgery type, pathological type, tumour size, surgical margin, tumour necrosis rate, chemotherapy regimens and cycles were recorded. A close margin was defined as tumour present less than 5 mm from the closest resection margin. The average followup was 87.6 months (range, 25–135 months).
Results
Twenty-five patients were alive, 22 patients had died, and eight had LR. Twenty-eight patients had wide margins, seven had positive margins and 12 had close margins. Positive margins had a greater risk of LR (57.1%) than wide margins and close margins. There was no difference in LR (8.3% vs 10.7%) between close margins and wide margins. Margin status was not correlated with OS.
Conclusion
Compared with wide margins, close margins did not lead to increased local recurrence in our study group. Whether close margins, as defined in our study, are just as acceptable as wide margins in terms of patient outcomes for osteosarcoma patients with neoadjuvant chemotherapy needs to be further confirmed in the future.
Introduction
Today, using a multi-modal approach consisting of preoperative (“neoadjuvant”) systemic polychemotherapy followed by local surgical therapy and then postoperative (“adjuvant”) chemotherapy, long-term, disease-free survival can be achieved in 60–70% of patients with osteosarcoma [1]. Most patients during this period have been treated with limb salvage surgery instead of amputation to retain function and improve their quality of life. Many studies [2–9] have defined and reported surgical margin status of osteosarcoma patients according to Enneking’s classification [10]. Although several studies showed that inadequate margins (including intralesional margins and marginal margins based on Enneking’s classification) were correlated with a high risk of local recurrence [3–9], one study demonstrated no local recurrence at mean follow-up of 97 months in patients with osteosarcoma around the knee joint who received intentional marginal excision in conjunction with caffeine-potentiated chemotherapy [11]. The best margin width for osteosarcoma is still unclear and remains controversial. In one study including 837 patients, the safe margin was advised as 2 cm wide when the preoperative modality is effective [12], but another earlier study failed to detect the difference in LR between margins less than 2 mm and greater than 2 mm in osteosarcoma patients [13]. Several studies have shown that histological response to chemotherapy was closely associated with LR [4, 5, 7, 8], but other studies did not confirm this [3, 6, 9, 14]. Whether neoadjuvant chemotherapy allows limb salvage with close margins is not yet completely clear, even though chemotherapy can potentially kill clinically undetectable micro satellite or skip lesions around the tumour.
In the light of this, following IRB approval, we retrospectively reviewed our experience with osteosarcoma specifically looking at the impact of surgical margins on local recurrence and overall survival.
Patients and materials
From 1999 to 2008, 67 consecutive osteosarcoma patients were treated at our institution. This included 51 conventional osteosarcoma, four extraskeletal osteosarcoma, seven radiation-induced osteosarcoma, three parosteal osteosarcoma, one Pagetoid osteosarcoma, and one multifocal osteosarcoma. All diagnoses were confirmed histologically. Patients lost to follow-up or without preoperative chemotherapy and those with unconventional types were excluded from this study. The remaining 47 consecutive patients met the inclusion criteria. This group included 28 males and 19 females ranging in age from 12 to 76 years (median, 22 years), and the MSTS stages were IIB (40) and IIIB (7). The pathological types included: 38 osteoblastic, eight chondroblastic and one fibroblastic. The location of osteosarcoma included: 26 distal femur, five proximal tibia, five pelvic and acetabulum, two humerus, two proximal femur, two distal tibia, two fibular, one whole femur, one rib and one radius.
All patients had two to six cycles of preoperative chemotherapy following biopsy. The three-drug regimen of cisplatin, adriamycin and high dose methotrexate was used in 31 patients (31/47: 66%). Other regimens were used in the other 16 patients preoperatively, including a two-drug regimen of cisplatin (or carboplatin) and adriamycin in eight patients, a three-drug regimen of ifosfamide, adriamycin and methotrexate in three patients, a two-drug regimen of ifosfamide and etoposide in one patient, a combination of a three-drug regimen of cytoxan, adriamycin, vincristine with a two-drug regimen of ifosfamide and etoposide in one patient, and a combination of a three-drug regimen (cisplatin, adriamycin and methotrexate) with other regimens in three patients. After surgical healing, 42 patients underwent postoperative chemotherapy, whereby a three-drug regimen of cisplatin, adriamycin and high dose methotrexate was used in 22 patents, and other regimens were used in 20 patients postoperatively, including two- or three-drug regimens of ifosfamide and etoposide with or without adriamycin in eight patients, a two-drug regimen of cisplatin (or carboplatin) and adriamycin in seven patients, a two-drug regimen of ifosfamide and adriamycin in one patient, a one-drug regimen of ifosfamide in one patient, and a combination of a three-drug regimen (cisplatin, adriamycin and methotrexate) and other regimens in three patients. The chemotherapy was individually based on protocols in use at our institution and each medical oncologist’s preferences; 22 patients had a regimen change because of toxicity of chemotherapy drugs or poor histological response. Five patients did not have postoperative chemotherapy; among them, one patient had six cycles of preoperative chemotherapy, the other four patients had no postoperative chemotherapy for delayed healing, infection or life-threatening toxicity. All patients were divided into two groups: the complete chemotherapy group was defined as the group of patients who completed five or more cycles of chemotherapy, and the incomplete chemotherapy group was defined as the group of patients who received less than five cycles of chemotherapy.
Definitive surgery was performed within four weeks following completion of the preoperative chemotherapy. Forty-one patients underwent limb salvage surgery and six patients had amputation including two external hemipelvectomy, two below knee amputation, one hip disarticulation, and one above knee amputation. A wide resection with prosthesis reconstruction was performed in 33 cases, and without reconstruction in eight cases. Patients were followed up with both imaging studies and physical examination. Given that most recurrences of osteosarcoma occur within two years after surgery [13], the minimum follow-up was 25 months, and the follow-up time ranged from 25 to 135 months (mean 87.6 months, median 92 months). All patients were followed up every three months for the first two years with X-ray of the tumour site, MRI of the tumour site and CT of the chest. This was repeated every six months for a duration of five years and once per year until ten years. Physical examinations were performed carefully at each visit.
The specimen is inked in six colours according to the surgeon involved. The specimen (bone) is cut into thin sections in the direction of long axis of the tumour, with surrounding bone and soft tissue. Multiple complete slices may be needed in order to find the gross closest margin. Tissue from all margins are submitted for microscopic evaluation. We report a positive margin when the tumour is on the inked margin; when the margin is negative, we measure the distance of the tumour to the closest margin microscopically on the section when the distance is within 1 cm. A positive margin is defined as tumour present at an inked surgical margin. A close margin is defined as the tumour present less than 5 mm from the closest resection margin, though the resection margin was clear of tumour; a wide margin is defined as no tumour present within 5 mm of the closest resection margin. Histological response to preoperative chemotherapy is graded as “good” (90% or more tumour necrosis) or “poor” (less than 90% tumour necrosis). The tumour size is the largest diameter measured in the resection specimen in 44 patients, in two patients it was not available in the pathological reports, but it was estimated according to the largest diameter in the preoperative MRI reports. No record of tumour size was available in one patient.
Statistics All clinical and pathological factors including age (younger or older than 22 years old), gender, tumour site (trunk or extremities), MSTS stage (2B or 3B), surgery type (amputation or resection), pathological type (osteoblastic or other), tumour size (<8.2 cm versus ≥ 8.2 cm), margin status (wide, close or positive), histological response (good or poor), chemotherapy regimens (the three drug regimen including cisplatin, adriamycin and methotrexate or other regimens), and completed cycles of chemotherapy (less than five cycles of chemotherapy versus five or more cycles of chemotherapy) were first investigated by univariate techniques; chi-square test and ANOVA were used when appropriate. The time zero was the date of diagnosis (biopsy date). The local recurrence-free survival (LRFS) and overall survival (OS) were calculated by Kaplan-Meier (KM) method with log-rank test. Cox proportional hazards model was used for multivariate analysis to identify factors predictive of local recurrence (LR); factors with P≤0.10 in univariate analysis and some selected covariates were included in multivariate analysis. P≤0.05 was considered statistically significant. Marginal statistical significance was defined as 0.05< P≤0.10. The SPSS software was used for all statistical analysis.
Results
Twenty-five patients were alive and 22 patients died of tumour. The OS rate was 59.7% at five-year follow-up. Eight patients developed LR, among them, one patient was alive with no evidence of disease, and the other seven patients died of disease. The time to LR ranged from eight to 54 months (median 17 months). LR occurred in one IIIB and seven IIB patients. Two patients had LR alone, while five patients experienced simultaneous LR and metastasis (lung in three cases, lung and bone in one case and multiple bone in one case). Fourteen of 40 IIB patients developed metastasis alone (lung in nine cases, lung and bone in two cases, lung and lymph node in one case, multiple bone in one case, tongue and chest in one case). The median time to metastasis was 18 months (2–98 months). The tumour necrosis rate ranged from 1% to 100% (median 70%). Defining good response as greater than 90% necrosis, 12 patients achieved good histological response and 35 patients had poor response. Wide margins were achieved in 28 patients, close margins and positive margins in 12 and seven patients, respectively. The median tumour size was 8.2 cm (2.5–32.5 cm).
The distribution of patients and tumour characteristics was not significantly different among resections with positive, close or wide margins (Table 1). Patients with a positive margin had larger tumours than those with close and wide margin, and this approached statistical significance (P = 0.060, Table 2).
Table 1.
Distribution of patient, tumour, and treatment characteristics by margin of resection
| Factor | Patient group | Positive margin | Close margin | Wide margin | P value |
|---|---|---|---|---|---|
| (N = 7) n (%) | (N = 12) n (%) | (N = 28) n (%) | |||
| Age | < 22 | 4 (17.4) | 3 (13) | 16 (69.6) | 0.158 |
| ≥ 22 | 3 (12.5) | 9 (37.5) | 12 (50) | ||
| Gender | Male | 3 (10.7) | 7 (25) | 18 (64.3) | 0.583 |
| Female | 4 (21.1) | 5 (26.3) | 10 (52.6) | ||
| Tumour site | Extremities | 5 (12.2) | 12 (29.3) | 24 (58.5) | 0.184 |
| Trunk | 2 (33.3) | 0 (0) | 4 (66.7) | ||
| MSTS stage | IIB | 6 (15) | 9 (22.5) | 25 (62.5) | 0.508 |
| IIIB | 1 (14.3) | 3 (42.9) | 3 (42.9) | ||
| Surgery type | Amputation | 2 (33.3) | 0 (0) | 4 (66.7) | 0.184 |
| Resection | 5 (12.2) | 12 (29.3) | 24 (58.5) | ||
| Pathological type | Osteoblastic | 4 (10.5) | 10 (26.3) | 24 (63.2) | 0.221 |
| Other type | 3 (33.3) | 2 (22.2) | 4 (44.4) | ||
| Preop chemo | CDDP, ADRIA, MTX | 4 (12.9) | 8 (25.8) | 19 (61.3) | 0.865 |
| Other regimens | 3 (18.8) | 4 (25) | 9 (56.3) | ||
| Size (8.2 cm cut-off) | <8.2 cm | 1 (4.5) | 7 (31.8) | 14 (63.6) | 0.145 |
| ≥8.2 cm | 6 (25) | 5 (20.8) | 13 (54.2) | ||
| Histological response | Good (≥90%) | 2 (16.7) | 1 (8.3) | 9 (75) | 0.280 |
| Poor (<90%) | 5 (14.3) | 11 (31.4) | 19 (54.3) |
Preop chemo preoperative chemotherapy regimens, CDDP cisplatin, ADRIA adriamycin, MTX methotrexate
Table 2.
Tumour size and necrosis rate versus margin of resection
| Characteristics | Positive margin, mean ± SD | Close margin, mean ± SD | Wide margin, mean ± SD | P value |
|---|---|---|---|---|
| Tumour size | 13.129 ± 8.894 | 8.833 ± 3.956 | 8.259 ± 3.448 | 0.060 |
| Necrosis rate (%) | 64.29 ± 30.059 | 60.50 ± 25.051 | 68.32 ± 23.789 | 0.658 |
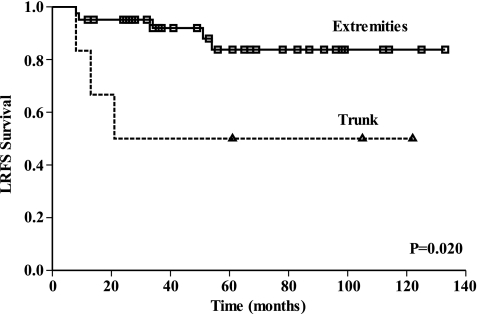
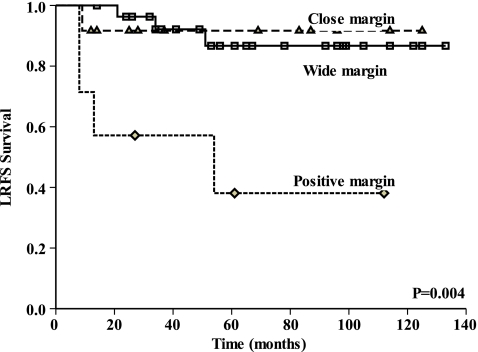
Univariate analysis showed that age, gender, MSTS stage, tumour size, surgery type, pathological type, histological response, preoperative chemotherapy regimens and completed cycles of chemotherapy were not statistically correlated with LRFS (Table 3). Tumours in the trunk had a higher LR rate of 50% compared with 12.2% for extremities (P = 0.020, Fig. 1). Positive margins showed a higher LR rate of 57.1%, compared with wide margins (10.7%) or close margins (8.3%) (P = 0.004). No statistical difference was found between the close and wide margins (Fig. 2). Along with tumour site and margin status, histological response was selected to enter the multivariate analysis. The result identified two independent factors predictive of LR: positive margin (P = 0.008) and trunk location of tumour (P = 0.043) (Table 4). Close margins did not increase the risk of local recurrence in multivariate analysis. Kaplan-Meier analysis with log rank test showed that margin status did not correlate with OS in our study group (P = 0.319).
Table 3.
Univariate analysis on local recurrence (LR) rate among various patient groups
| Factor | Group | Number of LR | LR rate (%) | P |
|---|---|---|---|---|
| Age | <22 | 4/23 | 17.4 | 0.959 |
| ≥22 | 4/24 | 16.7 | ||
| Gender | Male | 4/28 | 14.3 | 0.516 |
| Female | 4/19 | 21.1 | ||
| Tumour site | Extremities | 5/41 | 12.2 | 0.020a |
| Trunk | 3/6 | 50 | ||
| MSTS stage | IIB | 7/40 | 17.5 | 0.908 |
| IIIB | 1/7 | 14.3 | ||
| Surgery type | Amputation | 2/6 | 33.3 | 0.216 |
| Resection | 6/41 | 14.6 | ||
| Pathological type | Osteoblastic | 6/38 | 15.8 | 0.69 |
| Other type | 2/9 | 22.2 | ||
| Tumour size | <8.2 | 2/22 | 9.1 | 0.295 |
| ≥8.2 | 5/24 | 20.8 | ||
| Histological response | Good | 1/12 | 8.3 | 0.335 |
| Poor | 7/35 | 20 | ||
| Margin status | Wide | 3/28 | 10.7 | 0.004a |
| Close | 1/12 | 8.3 | ||
| Positive | 4/7 | 57.1 | ||
| Chemo regimens | CDDP, ADRIA, MTX | 2/19 | 10.5 | 0.358 |
| Other regimens | 6/28 | 21.4 | ||
| Completed cycles of chemo | < 5 cycles | 3/12 | 25 | 0.222 |
| ≥ 5 cycles | 5/35 | 14.3 |
Chemo chemotherapy, CDDP cisplatin, ADRIA adriamycin, MTX methotrexate
a Statistically significant
Fig. 1.
This Kaplan-Meier curve shows LRFS for patients grouped by tumour site. Tumours in the trunk had a higher local recurrence (LR) rate of 50% compared with 12.2% for extremity tumours (P = 0.020)
Fig. 2.
This Kaplan-Meier curve shows LRFS for patients grouped by margin status. Positive margins showed a higher LR of 57.1%, compared with 10.7% for wide margins and 8.3% for close margins (P = 0.004), no statistical difference was found between close margins and wide margins
Table 4.
Multivariate analysis on local recurrence-free survival (LRFS)
| Factor | Group | P value | Relative risk | 95% confidence interval for relative risk |
|---|---|---|---|---|
| Tumour site | Extremities | 1 | ||
| Trunk | 0.043a | 5.191 | 1.056–25.515 | |
| Histological response | Good | |||
| Poor | NS | |||
| Margin status | Wide | 1 | ||
| Close | NS | |||
| Positive | 0.008a | 8.440 | 1.755 – 40.579 |
NS not statistically significant
a Statistically significant
Discussion
An earlier study has shown that close margins and positive margins were associated with poor outcomes in soft tissue sarcoma [15]. For osteosarcoma, little data is available about the relation of close margins and LR for osteosarcoma. Although neoadjuvant chemotherapy is potentially capable of killing clinically undetectable microsatellite lesions outside the tumour, whether it safely allows close surgical margins is still unknown. One study failed to detect the difference in LR between margins less than 2 mm and greater than 2 mm in osteosarcoma patients [13]. In our study, no statistical difference was found for LR between close margins (8.3%) and wide margins (10.7%), but the positive margin group showed a higher rate of LR (57.1%) than the other two groups (P = 0.004) in univariate analysis, and a positive margin was a negative prognostic factor for LR in multivariate analysis (P = 0.008, relative risk 8.44, 95% CI 1.755–40.579). While close margins did not increase the risk of LR, this result was possibly due to the effect of preoperative chemotherapy in killing microsatellite lesions beyond the primary tumour. Several studies have shown that histological response to chemotherapy is closely associated with LR [4, 5, 7, 8], but other studies did not confirm this [3, 6, 9, 14]. In our study, the 8.3% LR rate for patients with good histological response was lower than 20% for those with poor response, but the difference was not statistically significant. Inclusion of more patients is necessary for further study on the impact of histological response on LR in the future.
Tumours in the trunk (including pelvis and rib) had a higher percentage of LR at 50% compared with extremity at 12.2% (P = 0.020) in our study. Multivariate analysis showed that trunk location independently predicted high risk of LR (P = 0.043). Because only a small percentage of patients in our study group had tumours in pelvis and trunk, the 95% CI was broad (1.056–25.515). Similar results for LR have been reported from 35% to 62% in the pelvis [16–18], which is much higher than extremity osteosarcomas.
In our study, positive margins seemed to be associated with larger tumours (13.129 ± 8.894 cm) compared with close margins (8.833 ± 3.956 cm) and wide margins (8.259 ± 3.448 cm), and this approached statistical significance (P = 0.060). However, tumour size was not correlated with LR (P = 0.295). A review of the literature found no agreement on the correlation of tumour size and LR [4, 8]. One study showed no association between tumour size and LR in 1,126 patients with non-metastatic osteosarcoma of the extremities [4]. But another study found close relation of the margin adequacy and LR [8]. The different results may possibly be due to different methodologies used in measuring tumour size. Further study, using more accurate methods such as three-dimensional technique [19] is needed to explore the effect of tumour size on LR of osteosarcoma.
There is still controversy on the correlation of surgical margins and survival of osteosarcoma in different studies [3, 20, 21]. Inadequate margins (including intralesional margin and marginal margin based on Enneking’s classification [10]) were found to be associated with poor event-free survival in a study including 789 patients with extremity osteosarcomas [3]. Macroscopic residual tumour independently increased risk of death in another study including 1,702 cases of osteosarcomas [20]. But one study [21] found that adequacy of surgical margins was not significantly associated with DFS or OS. Our result also showed no significant correlation between margin status and OS using Kaplan-Meier method. Collectively, whether surgical margins correlate with survival in osteosarcoma patients remains controversial, which indicates the need for further retrospective studies to evaluate the relationship of margin width and survival in osteosarcoma patients in the future.
It should be noted that even in patients with positive margins, three patients (42.9%) did not develop LR, but achieved long-term survival, and two of them had non-viable tumour in the resection margin due to chemotherapy induced tumour death. Whether this kind of margin could decrease the LR and improve survival for osteosarcoma patients with neoadjuvant chemotherapy needs further study in the future.
Our study showed that pathological type was not statistically correlated with LRFS. The predominant cell type is osteoblastic (38 patients in our study), while only nine patients had the nonosteoblastic type. One earlier study reported different expression levels of gene involved in chemotherapy resistance and angiogenesis in osteoblastic osteosarcoma samples compared with nonosteoblastic types [22]. Whether this difference in gene expression correlates with local recurrence is unknown. Further study is needed to evaluate the relation of histological type and local recurrence by including more patients.
There are several limitations in this study. First, it was a retrospective study. Second, it is limited in patient numbers because of low incidence of this disease in the whole population. The early death of some patients may decrease the LR because these patients did not survive long enough to develop LR; thus, further study should include more patients and use landmark analysis to reduce this bias. Moreover, subgroup analysis for nonmetastatic or extremity osteosarcoma was not tried in this study because such analysis would inevitably further reduce the statistical power. The pathologically examined margins may not necessarily reflect operative margins. After removing tumour from the body, soft tissues around the tumour tend to shrink to the tumour surface, leading to underestimation of the real margin width. Moreover, formalin fixation could interfere with the clinical assessment of the tumour-free margin [23].
In conclusion, compared with wide margins, close margins did not lead to increased LR in our study. Similarly, there was no statistically significant difference in OS between these two groups. Whether close margins, as defined in our study, are just as acceptable as wide margins in terms of patient outcomes for osteosarcoma patients with neoadjuvant chemotherapy should be further evaluated in the future.
Acknowledgments
We thank Linda D. Pickron for assistance with collecting all the medical charts for this research.
Footnotes
This work was performed in the Department of Orthopaedic Surgery, Pennsylvania Hospital, University of Pennsylvania, Philadelphia, PA.
Each author certifies that his or her institution has approved or waived approval for the human protocol for this investigation and that all investigators were conducted in conformity with ethical principles for research. This study was approved by the Institutional Review Board.
Xin Li received funding in the form of a visiting scholarship from the China Scholarship Council; Vincent M. Moretti and Adedayo O. Ashana received funding in the form of a research fellowship from Stryker Orthopaedics, Mahwah, NJ. One of the authors (RDL) is a consultant for Stryker Orthopaedics, Mahwah, NJ.
References
- 1.Carrle D, Bielack SS. Current strategies of chemotherapy in osteosarcoma. Int Orthop. 2006;30:445–451. doi: 10.1007/s00264-006-0192-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Funovics PT, Edelhauser G, Funovics MA, Laux C, Berzaczy D, Kubista B, Kotz RI, Dominkus M (2011) Pre-operative serum C-reactive protein as independent prognostic factor for survival but not infection in patients with high-grade osteosarcoma. Int Orthop Jan 21 [Epub ahead of print] doi:10.1007/s00264-011-1208-8 [DOI] [PMC free article] [PubMed]
- 3.Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy. Cancer. 2006;106:1154–1161. doi: 10.1002/cncr.21724. [DOI] [PubMed] [Google Scholar]
- 4.Bacci G, Forni C, Longhi A, Ferrari S, Mercuri M, Bertoni F, Serra M, Briccoli A, Balladelli A, Picci P. Local recurrence and local control of non-metastatic osteosarcoma of the extremities: a 27-year experience in a single institution. J Surg Oncol. 2007;96:118–123. doi: 10.1002/jso.20628. [DOI] [PubMed] [Google Scholar]
- 5.Bacci G, Ferrari S, Longhi A, Perin S, Forni C, Fabbri N, Salduca N, Versari M, Smith KVJ. Pattern of relapse in patients with osteosarcoma of the extremities treated with neoadjuvant chemotherapy. Eur J Cancer. 2001;37:32–38. doi: 10.1016/S0959-8049(00)00361-0. [DOI] [PubMed] [Google Scholar]
- 6.Bacci G, Briccoli A, Ferrari S, Longhi A, Mercuri M, Capanna R, Donati D, Lari S, Forni C, DePaolis M. Neoadjuvant chemotherapy for osteosarcoma of the extremity: long-term results of the Rizzoli’s 4th protocol. Eur J Cancer. 2001;37:2030–2039. doi: 10.1016/S0959-8049(01)00229-5. [DOI] [PubMed] [Google Scholar]
- 7.Picci P, Sangiorgi L, Rougraff BT, Neff JR, Casadei R, Campanacci M. Relationship of chemotherapy-induced necrosis and surgical margins to local recurrence in osteosarcoma. J Clin Oncol. 1994;12:2699–2705. doi: 10.1200/JCO.1994.12.12.2699. [DOI] [PubMed] [Google Scholar]
- 8.Grimer RJ, Taminiau AM, Cannon SR. Surgical outcomes in osteosarcoma. J Bone Joint Surg Br. 2002;84:395–400. doi: 10.1302/0301-620X.84B3.12019. [DOI] [PubMed] [Google Scholar]
- 9.Ferrari S, Bacci G, Picci P, Mercuri M, Briccoli A, Pinto D, Gasbarrini A, Tienghi A, Brach del Prever A. Long-term follow-up and post-relapse survival in patients with non-metastatic osteosarcoma of the extremity treated with neoadjuvant chemotherapy. Ann Oncol. 1997;8:765–771. doi: 10.1023/A:1008221713505. [DOI] [PubMed] [Google Scholar]
- 10.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106–120. [PubMed] [Google Scholar]
- 11.Hayashi K, Tsuchiya H, Yamamoto N, Takeuchi A, Tomita K. Functional outcome in patients with osteosarcoma around the knee joint treated by minimised surgery. Int Orthop. 2008;32(1):63–68. doi: 10.1007/s00264-006-0289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawaguchi N, Ahmed AR, Matsumoto S, Manabe J, Matsushita Y. The concept of curative margin in surgery for bone and soft tissue sarcoma. Clin Orthop Relat Res. 2004;419:165–172. doi: 10.1097/00003086-200402000-00027. [DOI] [PubMed] [Google Scholar]
- 13.Bispo Júnior RZ, Camargo OP. Prognostic factors in the survival of patients diagnosed with primary non-metastatic osteosarcoma with a poor response to neoadjuvant chemotherapy. Clinics. 2009;64:1177–1186. doi: 10.1590/S1807-59322009001200007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bacci G, Mercuri M, Longhi A, Ferrari S, Bertoni F, Versari M, Picci P. Grade of chemotherapy-induced necrosis as a predictor of local and systemic control in 881 patients with non-metastatic osteosarcoma of the extremities treated with neoadjuvant chemotherapy in a single institution. Eur J Cancer. 2005;41:2079–2085. doi: 10.1016/j.ejca.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 15.Bree R, Valk P, Kuik DJ, Diest PJ, Doornaert P, Buter J, Eerenstein SE, Langendijk JA, Waal I, Leemans CR. Prognostic factors in adult soft tissue sarcomas of the head and neck: a single-centre experience. Oral Oncol. 2006;42:703–709. doi: 10.1016/j.oraloncology.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Ham SJ, Kroon HM, Schraffordt Koops H, Hoekstra HJ. Osteosarcoma of the pelvis-oncological results of 40 patients registered by the Netherlands Committee on Bone Tumours. Eur J Surg Oncol. 2000;26:53–60. doi: 10.1053/ejso.1999.0741. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs B, Hoekzema N, Larson DR, Inwards CY, Sim FH. Osteosarcoma of the pelvis: outcome analysis of surgical treatment. Clin Orthop Relat Res. 2009;467:510–518. doi: 10.1007/s11999-008-0495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozaki T, Flege S, Kevric M, Lindner N, Maas R, Delling G, Schwarz R, Hochstetter AR, Salzer-Kuntschik M, Berdel WE, Jürgens H, Exner GU, Reichardt P, Mayer-Steinacker R, Ewerbeck V, Kotz R, Winkelmann W, Bielack SS. Osteosarcoma of the pelvis: experience of the Cooperative Osteosarcoma Study Group. J Clin Oncol. 2003;21:334–341. doi: 10.1200/JCO.2003.01.142. [DOI] [PubMed] [Google Scholar]
- 19.Shin KH, Moon SH, Suh JS, Yang WI. Tumor volume change as a predictor of chemotherapeutic response in osteosarcoma. Clin Orthop Relat Res. 2000;376:200–208. doi: 10.1097/00003086-200007000-00027. [DOI] [PubMed] [Google Scholar]
- 20.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jürgens H, Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. doi: 10.1200/JCO.20.3.776. [DOI] [PubMed] [Google Scholar]
- 21.Ford S, Saithna A, Grimer RJ, Picci P. Comparison of the outcome of conventional osteosarcoma at two specialist international orthopaedic oncology centres. Sarcoma. 2004;8:13–18. doi: 10.1080/13577140410001679202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubista B, Klinglmueller F, Bilban M, Pfeiffer M, Lass R, Giurea A, Funovics PT, Toma C, Dominkus M, Kotz R, Thalhammer T, Trieb K, Zettl T, Singer CF (2010) Microarray analysis identifies distinct gene expression profiles associated with histological subtype in human osteosarcoma. Int Orthop Mar 26 [Epub ahead of print] doi:10.1007/s00264-010-0996-6 [DOI] [PMC free article] [PubMed]
- 23.Docquier PL, Paul L, Cartiaux O, Lecouvet F, Dufrane D, Delloye C, Galant C. Formalin fixation could interfere with the clinical assessment of the tumor-free margin in tumor surgery: magnetic resonance imaging-based study. Oncology. 2010;78(2):115–124. doi: 10.1159/000306140. [DOI] [PubMed] [Google Scholar]