Abstract
Purpose
The study was performed to evaluate the ways of application, image quality (IQ) and radiation exposure resulting from introduction of a prospectively ECG-triggered high-pitch cardiac CTA acquisition mode into routine clinical practice.
Materials and Methods
42 prospectively triggered cardiac CTAs were acquired in 34 patients (11 female, 23 male, mean age 56±15 years) using a high-pitch mode (pitch 3.4) on a dual source CT (DefinitionFLASH, Siemens, Germany). In 8 of these patients with higher heart rates or occasional premature ventricular contractions (PVCs) two immediately subsequent CTAs were performed (“double flash protocol”). Subjective IQ was assessed for coronary arteries using a four-point scale (1=unevaluable –4=excellent). Contrast-to-noise ratio (CNR) was measured in nine locations. CT-Dose-Index and Dose-length-product were obtained, patient’s effective dose was calculated.
Results
Mean effective doses were 2.6±1.4 mSv (range 1.1–6.4) for the entire cardiac examination and 1.4±0.7 mSv (0.4–3.1) for individual high-pitch cardiac CTA. Z-coverage ranged from 9.9cm in a native coronary CTA to 31.4cm in a bypass graft case. Overall subjective IQ was good to excellent (mean score: 3.5) with 1.5 % unevaluable coronary segments. The “double flash protocol” resulted in a fully diagnostic CT study in all cases just after taking both scans into consideration. Mean CNR of all locations was 19.7±2.6.
Conclusion
Prospectively ECG-triggered high-pitch mode cardiac CTA is a feasible and promising technique in clinical routine, allowing for evaluation of the coronaries at good to excellent IQ providing high CNR and minimal radiation doses. The “double flash protocol” might become a more robust tool in patients with elevated heart rates or PVCs.
Keywords: cardiac CT, Flash, dose, pitch, image quality
Introduction
Contrast-enhanced multi-detector computed tomography (MDCT) permits reliable non-invasive evaluation of the coronary arteries (1). Improvements in scanner technology over the last decade have resulted in excellent spatial and temporal resolution and a concurrent considerable increase of cardiac CT examinations in clinical practice. Using 4-detector row MDCT, 75% of the coronary segments could be visualized without artifacts, on average (2–4). With the introduction of 16- and 64-row MDCT, major improvements of image quality were achieved with adaequate visualization of up to 97% of coronary segments (5–6). Dual source CT (DSCT) was introduced in 2005, providing high temporal resolution (83 msec) by using only one-quarter of the gantry rotation to acquire data for one cross-sectional image. This advance allowed diagnostic image quality at increased heart rates. Cardiac-CTA can now exclude coronary artery stenosis with a high negative predictive value and therefore avoid unnecessary invasive angiography (7–8). Cardiac CTA is frequently performed using retrospective ECG gating combined with a very low pitch (0.14–0.4), which can result in high radiation exposures exceeding 30 mSv (9). Radiation dose due to cardiac CTA has become of increasing concern in clinical practice (10) and in the lay press, and multiple technological advances have allowed for reduction of radiation exposure from coronary CTA. The simple use of a tailored tube potential (kV) appropriate to patient’s body mass index (BMI) or thorax size has a great impact in the overall radiation dose (up to 45% reduction) (11). ECG-based tube current modulation can be applied to limit the phase interval of maximum x-ray exposure, thereby reducing the effective dose by approx. 40 % (12). In patients with a low heart rate, radiation exposure can be further reduced by using a prospectively triggered axial acquisition (“step and shoot”). Average radiation doses of less than 5 mSv have been reported for prospective scan modes (13–15).
Latest generation of DSCT scanners has introduced a new scan mode, a prospectively ECG-triggered helical data acquisition with very high pitch values of more than 3.0. This technique enables acquisition of the entire volumetric data set of the heart within a fraction of a single cardiac cycle. The high pitch allows acquisition with very low radiation exposure (<1 mSv) in patients with low, regular heart rates (16,17).
Accurate and consistent visualization of the coronary arteries at high image quality is critical for cardiac CTA. In this study we aimed to describe initial results of prospectively ECG-triggered high-pitch mode cardiac CTA in clinical routine practice in a tertiary care center with respect to image quality and radiation exposure.
Materials and Methods
Patients
In this retrospective study, which was approved by the institutional review board, we analyzed the first 42 contrast enhanced prospectively ECG-triggered cardiac CTAs in 34 consecutive patients performed with a high-pitch mode in an academic medical center between April and May 2010. Study patients were all referred for cardiac CTA, 11 were female, 23 were male (mean age: 56 ± 15 years). Mean BMI was 27 (range 20 – 39). Indication for coronary CTA were noted. Patients undergoing pulmonary vein imaging were excluded. Patients referred for evaluation of coronary bypass grafts (n=2), or abnormalities of the ascending aorta (n=2), however, were included. A heart rate of 60 bpm or lower and a sinus rhythm was aspired. If necessary, patients’ heart rate was controlled with intravenous (iv) beta-blocker (Metoprolol, Bedford Laboratories, Bedford, OH, USA) immediately before the scan. Patients with a heart rate higher than 60 bpm or patients who had an occasional isolated premature ventricular contraction (PVC) prior to the scan, were not excluded from the use of a high pitch mode. Patients with irregular rhythms or heart rate higher than 100 bpm were excluded from high-pitch mode scanning and were not included in the study. Thirty-three patients received two 0.3 mg tablets of sublingual nitroglycerine (Nitrostat, Pfizer, New York, NY, USA) sublingually prior to coronary CT angiography.
MDCT scan protocol
Cardiac CTA was performed on a second-generation DSCT multidetector system (Definition FLASH, Siemens Medical Solutions, Forchheim, Germany) with two sets of x-ray tubes and detector arrays. Each array enables data acquisition with 64 detector rows. In combination with a z-flying focal spot simultaneous data acquisition of up to 128 slices (2 × 64) is performed (17). The scanner technology enables a prospectively ECG-triggered high-pitch (3.4) spiral acquisition (FLASH Spiral Cardio, Siemens Healthcare, Forchheim, Germany). The imaging protocol included anterior-posterior and lateral scout images, a non-contrast scan to assess calcium score if clinically indicated (32 of 34 patients), and a timing bolus scan using 20 ml of iodinated contrast at an injection rate of 5–7 ml/s (Iopamidol 370 mg/ml, Isovue 370, Bracco Diagnostics, Princeton, NJ USA) followed by a 20 ml saline flush at the same flow rate (table 1). Coronary CTA was obtained after the administration of approximately 50–70 ml of contrast at a rate of 5–7 ml/s, followed by 40 ml saline flush at the same flow rate. Scan parameters included a 64 × 0.6 mm collimation, gantry rotation time of 280 ms, pitch of 3.4, tube voltage of 80–120 kV (weight-based nomogram), and a tube current of 312–370 mAs/rotation (scout-based automatic reference tube current selection – CareDose 4D, Siemens Siemens Medical Solutions, Forchheim, Germany). The z-axis field of view extended from the carina or pulmonary artery segment down to the diaphragm for native coronary CTA, and in patients with suspicious mediastinal mass, aortic disease, or coronary artery bypass graft surgery, the z-coverage was extended (Table 1). The ECG triggered image acquisition started at 60% of the RR interval. Axial images were reconstructed with a slice thickness of 0.75 mm at a reconstruction increment of 0.5 mm (17).
Table 1.
Patients’ characteristics, radiation dose and contrast application in 42 cardiac high pitch CTAs of 34 patients: A second high –pitch CTA was applied in 8 patients (n=7 “double flash protocol”, n=1 second contrast injection), an additional delayed scan in 5 patients.
| Patient No. |
Age | Gender | BMI | Mean heart rate |
Second scan | Amount of contrast (cc) |
Flow rate (cc/s) |
CTDI CTA | DLP CTA only |
Effective dose CTA only (mSv) |
Effective dose total (mSv) |
DLP total (mGyxcm) |
z- coverage (cm) |
Chest diameter (cm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 46 | f | 25 | 62 | no | 70 | 5 | 2.5 | 46 | 0.6 | 1.1 | 76 | 13.6 | 29.1 |
| 2 | 45 | m | 24 | 72 | no | 60 | 5 | 3.3 | 119 | 1.7 | 2.7 | 192 | 31.4 | 32.8 |
| 3 | 62 | f | 23 | 60 | no | 65 | 5 | 2.9 | 48 | 0.7 | 1.2 | 84 | 12.0 | 34.5 |
| 4 | 49 | m | 30 | 51 | no | 70 | 5 | 7.2 | 129 | 1.8 | 2.7 | 193 | 13.1 | 46.5 |
| 5 | 61 | m | 26 | 53 | no | 70 | 5 | 6.4 | 100 | 1.4 | 2.0 | 143 | 10.8 | 39.7 |
| 6 | 60 | m | 39 | 52 | delayed scan | 70 | 6 | 7.4 | 213 | 3.0 | 3.7 | 263 | 23.9 | 38.0 |
| 7 | 59 | m | 25 | 52 | no | 65 | 7 | 3.1 | 57 | 0.8 | 1.3 | 92 | 13.5 | 32.2 |
| 8 | 66 | f | 30 | 59 | CTA, twice contrast | 65 | 6 | 5.3 | 83 | 1.2 | 2.7 | 196 | 10.1 | 34.1 |
| 65 | 6 | 5.6 | 84 | 1.2 | 10.1 | |||||||||
| 9 | 41 | m | 27 | 67 | double flash protocol | 90 | 6 | 5.9 | 98 | 1.4 | 3.3 | 239 | 11.7 | 36.9 |
| 5.9 | 100 | 1.4 | 11.7 | |||||||||||
| 10 | 57 | m | 25 | 49 | no | 65 | 6 | 3.2 | 53 | 0.7 | 1.2 | 87 | 11.7 | 33.4 |
| 11 | 6 | f | 96 | no | 20 | 2 | 1.4 | 21 | 0.3 | 0.7 | 49 | 9.9 | 23.0 | |
| 12 | 76 | m | 29 | 62 | delayed scan | 65 | 6 | 6.7 | 191 | 2.7 | 3.6 | 260 | 24.0 | 32.9 |
| 13 | 58 | f | 22 | 60 | delayed scan | 65 | 6 | 2.5 | 48 | 0.7 | 2.1 | 152 | 14.6 | 27.8 |
| 14 | 57 | f | 31 | 58 | no | 60 | 6 | 7.4 | 115 | 1.6 | 2.4 | 169 | 10.8 | 43.5 |
| 15 | 78 | f | 30 | 57 | no | 60 | 6 | 6.9 | 117 | 1.6 | 2.5 | 180 | 12.0 | 45.4 |
| 16 | 61 | m | 24 | 50 | no | 60 | 6 | 3.0 | 56 | 0.8 | 1.4 | 97 | 13.7 | 36.8 |
| 17 | 65 | m | 26,1 | 58 | delayed scan | 60 | 6 | 6.3 | 160 | 2.2 | 5.4 | 389 | 20.5 | 33.6 |
| 18 | 38 | m | 24 | 58 | no | 65 | 7 | 3.1 | 51 | 0.7 | 1.1 | 77 | 11.5 | 31.9 |
| 19 | 61 | f | 31 | 57 | no | 65 | 5 | 5.3 | 93 | 1.3 | 1.8 | 127 | 12.6 | 33.2 |
| 20 | 43 | m | 25 | 59 | no | 65 | 5 | 3.4 | 63 | 0.9 | 1.4 | 98 | 13.9 | 37.8 |
| 21 | 56 | m | 30 | 66 | double flash protocol | 65 | 6 | 5.9 | 117 | 1.6 | 3.9 | 280 | 15.1 | 41.0 |
| 6 | 5.8 | 114 | 1.6 | |||||||||||
| 22 | 77 | f | 26 | 57 | double flash protocol | 100 | 5 | 2.8 | 48 | 0.7 | 2.5 | 178 | 12.0 | 29.8 |
| and delayed scan | 2.8 | 48 | 0.7 | |||||||||||
| 23 | 44 | m | 28 | 59 | double flash protocol | 90 | 6 | 6.0 | 110 | 1.5 | 3.8 | 268 | 13.7 | 35.7 |
| 6.0 | 113 | 1.6 | ||||||||||||
| 24 | 65 | m | 26 | 56 | double flash protocol | 100 | 7 | 4.6 | 84 | 1.2 | 3.0 | 212 | 13.5 | 35.2 |
| 4.6 | 84 | 1.2 | ||||||||||||
| 25 | 49 | m | 30 | 58 | double flash protocol | 110 | 7 | 6.5 | 200 | 2.8 | 6.4 | 457 | 25.9 | 38.1 |
| 6.5 | 199 | 2.8 | ||||||||||||
| 26 | 37 | m | 29 | 64 | double flash protocol | 100 | 6 | 6.8 | 223 | 3.1 | 5.8 | 413 | 28.2 | 37.3 |
| 6.4 | 132 | 1.8 | 15.6 | |||||||||||
| 27 | 67 | m | 26 | 54 | no | 65 | 6 | 4.5 | 76 | 1.1 | 1.6 | 116 | 12.0 | 32.9 |
| 28 | 80 | m | 25 | 60 | no | 65 | 6 | 3.6 | 66 | 0.9 | 1.5 | 104 | 13.5 | 36.6 |
| 29 | 42 | m | 20 | 56 | no | 65 | 6 | 1.4 | 25 | 0.4 | 2.0 | 141 | 13.1 | 30.9 |
| 30 | 72 | m | 31 | 55 | no | 65 | 6 | 6.6 | 116 | 1.6 | 2.3 | 164 | 12.9 | 38.7 |
| 31 | 48 | f | 32 | 56 | no | 60 | 6 | 7.0 | 127 | 1.8 | 2.4 | 174 | 13.3 | 36.8 |
| 32 | 67 | m | 22 | 51 | no | 70 | 7 | 2.9 | 61 | 0.9 | 1.3 | 92 | 15.8 | 29.2 |
| 33 | 44 | f | 24 | 64 | no | 50 | 7 | 2.9 | 54 | 0.8 | 1.1 | 79 | 13.5 | 33.2 |
| 34 | 47 | m | 38 | 62 | no | 65 | 7 | 7.4 | 135 | 1.9 | 4.7 | 339 | 13.3 | 36.7 |
| mean | 55.4 | 27.4 | 59.1 | 68.9 | 5.9 | 4.9 | 99.5 | 1.4 | 2.5 | 181.8 | 14.8 | 35.2 | ||
| SD | 14.9 | 4.2 | 8.3 | 16.2 | 0.9 | 1.8 | 51.1 | 0.7 | 1.4 | 102.8 | 5.2 | 5.2 |
A total of 42 CTAs in 34 patients were evaluated. In 8 patients two prospectively triggered high-pitch acquisitions were performed. In 7 out of the 8 patients a second CTA was acquired immediately during the same contrast injection timed for the same point (60%) in the cardiac cycle (“double flash protocol”) (table 1). In these cases, the delay time between the two acquisitions was set to 4 seconds, the minimal allowable time on this system. Therefore the total scan time was longer and the total contrast volume was increased by 25 to 35 cc compared to single high pitch CTA acquisition ([4 seconds + ~1second scan] × flow rate). This protocol was used in patients with regular heart rates > 60 bpm or in whom occasional PVCs were noted prior to the scan. In 1 of the 8 patients, a second injection of contrast agent was performed and second scan was acquired separately. In five patients with low rates and regular rhythms a delayed scan was performed to ameliorate the evaluation mediastinal or cardiac masses and aortic abnormalities (table 1).
Image Analysis
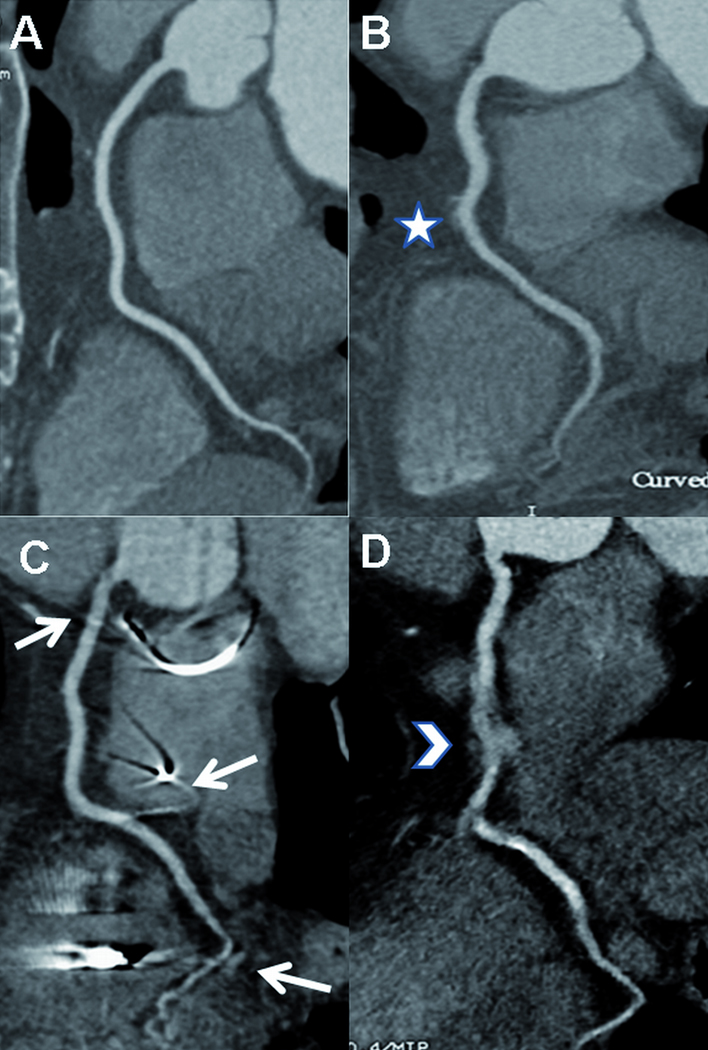
Data was transferred to an off-line workstation (Multimodality Workplace, Siemens, Erlangen, Germany) and image quality and CNR were evaluated by two independent experienced cardiac imagers (P.K., C.A.R., 5 and 3 years of experience in cardiac CT). Based on the 18-segment model of the Society of Cardiovascular Computed Tomography (18) subjective image quality was assessed for every segment using a four-point scale (1 = unevaluable; 2 = moderate image quality with artifacts, but evaluable concerning the presence of stenoses; 3 = good image quality with minimal artifacts, but fully evaluable coronary vessel structures; 4 = excellent image quality without artifacts). Typical examples for each grade (1–4) are given in figure 1. The two readers rated the image quality independently with a subsequent consent read.
Figure 1.
Subjective image quality. The figure demonstrates typical images for each grade (1–4) regarding the right coronary artery (RCA).
A. Excellent image quality (grade 4). B. Good image quality (grade 3) with slight blurring artifacts (*). C. Moderate image quality (grade 2) due to beam hardening artefacts of pacemaker leads (arrows). D. Severe blurring artifacts (arrowhead) cause non diagnostic image quality (grade 1) of the mid portion of the vessel.
An objective measure of scan quality was then performed as it has been previously published (6). The following measurements were obtained by one reader using 0.75 mm axial images. Circular regions of interest (as large as possible, 2–4 mm2) were drawn in the lumen of the coronary arteries and the adjacent epicardial fatty tissue to measure the contrast-to-noise ratio (CNR) in nine locations: left main coronary artery (LM), proximal and distal (distal to the second diagonal branch) left anterior descending coronary artery (LAD), proximal first diagonal branch (D1), proximal and distal left circumflex coronary artery (LCX), first obtuse marginal branch (OM1), proximal and distal (proximal to the origin of the posterior descending coronary artery) right coronary artery (RCA).
A circular region of interest (100 mm2) was placed in the contrast enhanced lumen of the aortic root to measure image noise by determining the standard deviation of CT attenuation (19,20). Signal-to-noise ratio (SNR) was determined for all 9 coronary locations by dividing CT attenuation of the coronary lumen and the image noise.
CNR was calculated by the following formula as described previously (6): contrast-to-noise ratio = (CT attenuation coronary lumen − CT attenuation adjacent tissue) / image noise.
Using the lateral scout view, antero-posterior chest diameters of every patient were measured at the level of the right upper lobe bronchus as well as 5 cm and 10 cm below. On the PA scout view, left-to-right chest diameters were measured at the level of the carina as well as 5 cm and 10 cm below to study the influence of the chest size on the parameters of image quality. The mean values of the left-to-right and antero-posterior chest diameters were used to estimate the chest size.
Radiation dose
CT Dose Index (CTDIvol) and Dose-length product (DLP) were obtained for all scans using the Dose Exposure Record generated by the scanner. Additionally the patient’s effective dose (mSv) was estimated using the DLP method with a conversion factor k=0.014 for adult and k=0.048 for pediatric patients (21–23).
Statistical analysis
The statistical analysis was performed using commercially available software (SPSS, 12.0, Inc., Chicago, IL, USA; Microsoft Excel, Redmond, WA, USA). Continuous data are expressed as mean ± SD. Differences of CNR among different coronary locations were examined using one-way analysis of variance (ANOVA). A two tailed p-value < 0.05 was considered statistically significant.
Intraclass correlation coefficient (ICC) was used for interobserver agreement of subjective image quality. The coefficient represents concordance, where 1 is perfect agreement and 0 is no agreement at all. In a subgroup analysis quantitative parameters of image quality (noise and CNR) were compared in patients with a heart rate of > 60 bpm versus <60 bpm and in patients with a BMI of > 30 versus < 30 (t-test). Linear regression analysis was preformed to explore the influence of the chest diameters on the measured CNR.
Results
Patient characteristics
Patient characteristics are listed in Table 1. The most frequent indication for coronary CTA was recurrent atypical or anginal chest pain (n=20), followed by a positive or non conclusive stress test (n=5), evaluation for a mediastinal or cardiac mass (n=3), for patency of bypass grafts (n=2), coronary or cardiac anomalies (n=2) and evaluation of the ascending aorta (n=2). A family history of coronary artery disease (CAD) was reported in n=6 patients. Mean heart rate during the scan was 59 ± 8.5 bpm (range 49 – 96 bpm). A mean dose of 11.6 ± 8.1 mg iv metoprolol was administered to 24 (70%) of the patients. A mean flow rate of 5.7 ± 1.2 ml/s for a mean dose of 69 ± 18 ml of contrast agent was injected (injections were tailored to body habitus and scan time).
Radiation Dose
Mean z-axis scan length was 14.8 ± 5.2 cm. Mean total DLP to the 34 patients was 181.8 ± 103 mGy cm, corresponding to an estimated effective dose of 2.5 ± 1.4 mSv (range 1.1–6.4). Regarding the 42 single prospectively triggered high-pitch CTA acquisitions, mean DLP was 99.5 ± 51.1 mGy-cm corresponding to an estimated effective dose of 1.4 ± 0.7 mSv (range 0.4–3.1). Mean effective dose for a single high-pitch CTA exclusively of native coronary arteries (after exclusion of the studies for bypass graft, aortic artery and mediastinal mass evaluation) was 1.1 ± 0.4 mSv (range 0.4–1.9). Patients with a BMI < 25 demonstrated a mean effective dose of 1.6 ± 0.6 mSv cm for entire examination and 0.8 ± 0.4 mSv for the contrast-enhanced CTA alone (range 0.4 – 1.7). Regarding the 8 patients who underwent two prospectively triggered high-pitch mode CTAs and the 5 patients who got a delayed scan, mean DLP of the whole examination was 275.6 ± 96.1 mGy cm corresponding to an estimated effective dose of 3.9 ± 1.4 mSv (range 2.1–6.4).
Subjective image analysis
A total of 584 coronary artery segments were analyzed. 396 segments (68%) had an image quality score of 4 (“excellent”), 121 segments (20 %) a score of 3 (“good”), 47 segments (8%) a score of 2 (“moderate”), and 20 segments (3%) were scored as “unevaluable”. Mean rating score for all patients and segments was 3.5 (“good” to “excellent”). An example is given in Figure 2. Unevaluable segments were observed in n=8 patients. The predominant reasons given by the observers were extensive motion, noise, and streak artifacts due to extensive calcifications. However, 4 of these 8 patients belonged to the subgroup that was examined by the “double flash protocol”. In these patients, all segments were evaluable after taking both scans into account. Nine segments (1.5 %) on 4 patients without “double flash protocol” remained unevaluable. Six of these 9 segments were small distal branches with little clinical relevance in the individual patients. The two radiologists demonstrated a good agreement regarding subjective image quality (ICC-coefficient of 0.82).
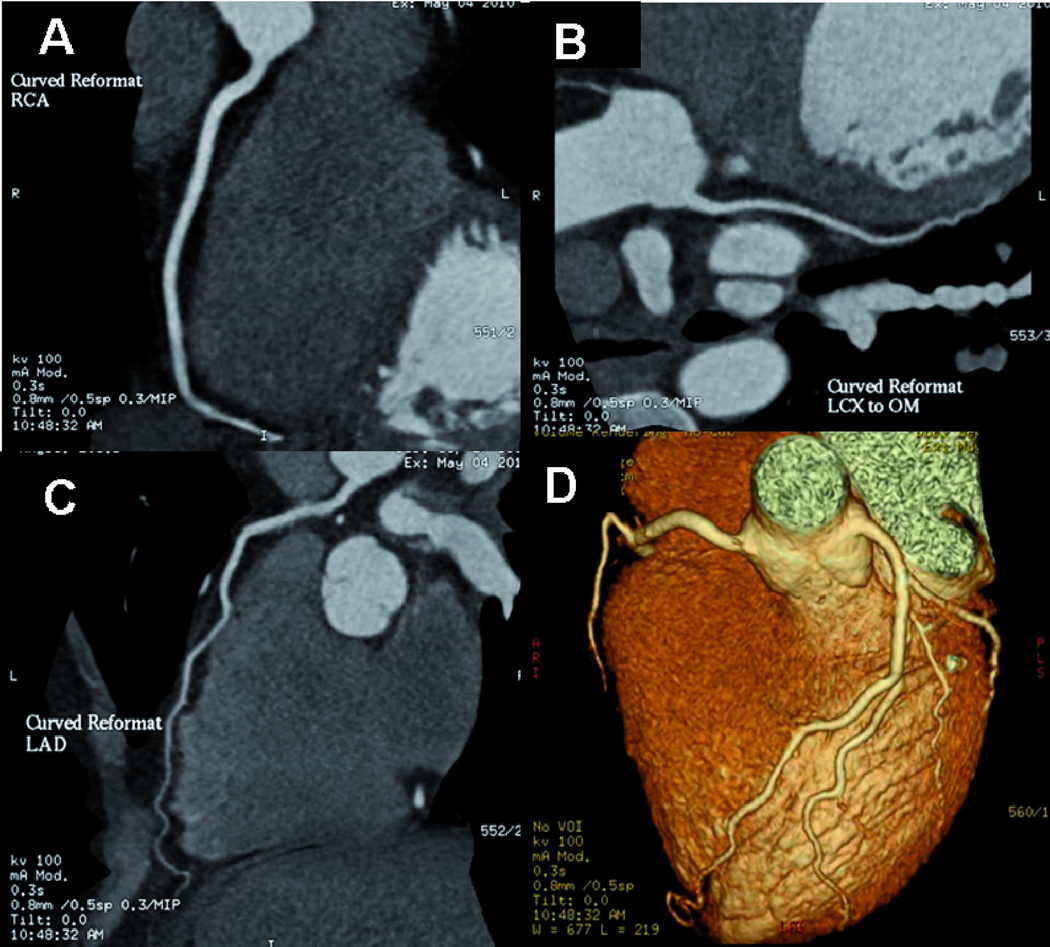
Figure 2.
Images of excellent image quality using the “flash protocol”.
Prospective high pitch mode cardiac CTA (pitch 3.4, 65 ml at 7ml/s) of a 44 year-old female with atypical chest pain, an averaged heart rate of 64 bpm and BMI 24. Curved reformations of the right coronary artery (A), left main and circumflex artery (B), left main and left anterior descending artery (C) and volume rendering reformation (D) demonstrate excellent image quality. Effective dose was 0.8 mSv for the CTA alone, 1.1 mSv for the whole cardiac exam.
Image noise, signal-to-noise and contrast-to-noise ratio measurements
The mean CNR and SNR of all measured locations was 19.9 ± 4.6 and 16.2 ± 2.6. Mean CNR and SNR of the nine examined regions of the coronary arteries are shown in Table 2. CNR was significantly lower in the distal LCX as compared to the proximal LCX (p < 0.04) and the LM (p < 0.03) as well as in the distal LAD compared to the LM (p < 0.03). In the RCA, however, differences of CNR between the proximal and distal vessel were not significant. Concerning the CNR in the proximal and distal segments of the different coronary arteries, no significant differences were obtained. In a subgroup analysis of patients with a heart rate of > 60 bpm versus <60 bpm, the slight differences in image noise (28.4 versus 27.9) and mean CNR (17.7 versus 20.5) were not statistically significant. In a subgroup analysis of patients with a BMI > 30 versus those with a BMI < 30, mean image noise (28.7 versus 25.8) was slightly higher and mean CNR was slightly lower (18.9 versus 20.0); however, differences were again not significant. The estimated size of the chest had no significant influence on the CNR.
Table 2.
Coronary CNR and SNR measurements in 34 patients. Mean values and standard deviations (SD). LM: left main coronary artery, LAD: left anterior descending coronary artery, D1: proximal first diagonal branch, LCX: left circumflex coronary artery, OM1: first obtuse marginal branch, RCA: right coronary artery.
| Coronary artery (location of measurement) | mean CNR | SDCNR | mean SNR | SDSNR |
|---|---|---|---|---|
| LM | 21.6 | 12.0 | 17.5 | 9.0 |
| LAD proximal | 25.1 | 32.6 | 22.0 | 32.3 |
| LAD distal | 16.8 | 5.5 | 13.3 | 5.1 |
| D1 | 18.9 | 10.3 | 15.2 | 10.2 |
| LCX proximal | 20.0 | 6.8 | 17.0 | 6.5 |
| LCX distal | 16.9 | 5.9 | 13.8 | 5.9 |
| OM 1 | 17.6 | 6.8 | 14.4 | 6.6 |
| RCA proximal | 20.6 | 7.1 | 17.3 | 6.6 |
| RCA distal | 19.6 | 7.3 | 15.8 | 6.6 |
Discussion
Radiation dose is of increasing concern in radiology. As radiation dose at cardiac CT is closely related to the retrospectively-gated helical pitch value, or scan overlap at axial, sequential scanning, second-generation DSCT prospectively ECG-triggered high-pitch spiral coronary angiography offers a unique opportunity to achieve maximal dose reduction while preserving fully diagnostic image quality. DSCT is necessary for this technique as rapid table motion leads to gaps in the trajectory of the first detector. A second detector is needed to fill these gaps. Radiation exposure is very low using this technique since slice overlap (and therefore redundant exposure) is avoided. Using a pitch value of 3.4, data acquisition of the entire z-axis of the adult heart within a fraction of one cardiac cycle (approximately 260 ms) is possible. Whereas in conventional prospective triggered axial acquisition protocols (“step and shoot”) some redundant radiation exposure is still applied at slab interfaces, redundant radiation exposure is nearly eliminated in a high-pitch protocol and occurs only at the beginning and end of the scanned volume.
However, dose reduction must not compromise image quality. Non-diagnostic scans because of ‘too little radiation’ are a worst-case scenario as they expose the patient to radiation without the hoped for benefit. Several studies have recently described the use of this novel technique (16–17, 24–26). Studies by Lell et al. (16) and Achenbach et al. (17) have shown that prospectively ECG-triggered high-pitch spiral acquisition for cardiac CTA is feasible and provides excellent image quality at a consistent dose below 1.0 mSv in carefully selected patients with a low and regular heart rate (< 60 bpm) and a body weight of less than 100kg (16). We retrospectively analyzed the initial 34 patients in which this technique was applied in clinical routine at a tertiary academic center. In contrast to the previous studies, we included all patients scanned on our service regardless of body habitus and with much less rigorous heart rate and rhythm restrictions. Our data show that cardiac CTA using this mode is technically feasible in a less strict clinical routine setting, resulting in an average “good to excellent” image quality and a high CNR at a reasonably low mean radiation exposure. Our series demonstrated a slightly higher mean radiation dose of 2.5 mSv for all patients and 1.4 mSv for a single-acquisition CTA in contrast to prior publications (16,17). Reasons for these differences include a wider range in BMI and z-axis coverage, particularly in patients who were referred for evaluation of bypass grafts or aortic abnormalities, as the z-coverage was larger in these patients. After exclusion those studies mean effective dose for a single coronary CTA applied 1.1 ± 0.4 mSv, which is in the same range as reported in the above mentioned studies. The percentage of unevaluable segments and subjective image quality analysis was higher in the present study (1.5%) compared to the data presented by Lell et al. (16) (0.6 %). But the majority of non-diagnostic segments were small, distal and of little clinical relevance. A wide range of heart rates (range 49 – 66, and one pediatric patient at 96) and BMI in our study population explain these differences, as image quality was diminished by coronary motion and noise. Another important factor is excessive calcifications of the coronary arteries in bypass graft cases, which lead to “unevaluable” segments due to blooming and streak artifacts.
Compared with studies reporting on DSCT of coronary arteries with standard axial “step and shoot” technique the prospective high pitch mode technique enables another dose reduction of approximately 40–50 % (13–15). Earls et al. report a mean effective dose of 2.8 mSv (DLP 170, conversion factor 0.017) for a prospectively ECG-triggered single source coronary CTA, whereas an effective dose of 1.4 mSv (DLP 99, conversing factor 0.014) was found in the here presented study (15). As the radiation dose of a high pitch mode spiral-CTA is very low, a protocol consisting of two scans immediately one after another (“double flash mode”) might be an opportunity to obtain diagnostic image quality despite a patient’s elevated heart rate or occasional PVCs. In comparison to increased “padding” in prospectively triggered single source CT, which allows assessment of the heart in additional phases within the same cardiac cycle (27), the “double flash protocol” performed in this study concentrates on the same phase (60%) at different cardiac cycles. In our small cohort, this protocol led to diagnostic image quality of all coronary segments after interpreting both acquisitions. The patients, in whom several coronary segments would have been unevaluable after a single scan, were actually diagnostic due to this protocol. Therefore, this technique might obviate a second retrospectively ECG-gated cardiac scan with a second injection of contrast, yet still results in low total radiation exposure.
Limitations
Our study has some major limitations. In a retrospective analysis of clinical data, a systemical bias cannot be excluded. A relatively small number of patients was included in this initial study, although this number is similar to comparable previously published series (16, 17). The CT-protocol was not completely identical in every patient, as amount of contrast, flow rate of contrast and z-coverage were adjusted individually in every patient. The study population itself was inhomogeneous with regard to heart rate, BMI, calcium score, and thoracic diameters but reflects consecutive patients in clinical routine of a tertiary care academic hospital. A systematic comparison of cardiac CTA and invasive coronary angiography was not performed, so diagnostic accuracy versus a gold standard is not available. Radiation doses were calculated and not directly measured in our analysis.
Conclusion
Prospectively ECG-triggered high-pitch mode coronary CTA is a feasible and promising technique. When applied in clinical routine, evaluation of the coronary arteries is possible at good to excellent image quality, with high CNR and very low radiation exposure. The “double flash protocol” may be a promising technique for patients with elevated heart rates > 60 bpm or with occasional PVCs, who might otherwise not receive a diagnosis with a single acquisition in this mode. Further studies are necessary to validate these findings.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hendel RC, Patel MR, Kramer CM, et al. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006;48(7):1475–1497. doi: 10.1016/j.jacc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Nieman K, Oudkerk M, Rensing BJ, et al. Coronary angiography with multi-slice computed tomography. Lancet. 2001;357(9256):599–603. doi: 10.1016/S0140-6736(00)04058-7. [DOI] [PubMed] [Google Scholar]
- 3.Gerber TC, Kuzo RS, Lane GE, et al. Image quality in a standardized algorithm for minimally invasive coronary angiography with multislice spiral computed tomography. J Comput Assist Tomogr. 2003;27(1):62–69. doi: 10.1097/00004728-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Achenbach S, Giesler T, Ropers D, et al. Comparison of image quality in contrast-enhanced coronary–artery visualization by electron beam tomography and retrospectively electrocardiogram-gated multislice spiral computed tomography. Invest Radiol. 2003;38(2):119–128. doi: 10.1097/00004424-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Maruyama T, Yoshizumi T, Tamura R, et al. Comparison of eightversus 16-slice multidetector-row computed tomography for visibility and image quality of coronary segments. Am J Cardiol. 2004;94:1539–1543. doi: 10.1016/j.amjcard.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 6.Ferencik M, Nomura CH, Maurovich-Horvat P, Hoffmann U, Pena AJ, Cury RC, Abbara S, Nieman K, Fatima U, Achenbach S, Brady TJ. Quantitative parameters of image quality in 64-slice computed tomography angiography of the coronary arteries. Eur J Radiol. 2006;57:373–379. doi: 10.1016/j.ejrad.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 7.Mowatt G, Cook JA, Hillis GS, Walker S, Fraser C, Jia X, Waugh N. 64-Slice computed tomography angiography in the diagnosis and assessment of coronary artery disease: systematic review and meta-analysis. Heart. 2008;94(11):1386–1393. doi: 10.1136/hrt.2008.145292. [DOI] [PubMed] [Google Scholar]
- 8.Fang XM, Chen HW, Hu XY, Bao J, Chen Y, Yang ZY, Buckley O, Wu XQ. Dual-source CT coronary angiography without heart rate or rhythm control in comparison with conventional coronary angiography. Int J Cardiovasc Imaging. 2010;26(3):323–331. doi: 10.1007/s10554-009-9527-1. [DOI] [PubMed] [Google Scholar]
- 9.Hausleiter J, Meyer T, Hermann F, Hadamitzky M, Krebs M, Gerber TC, McCollough C, Martinoff S, Kastrati A, Scho¨mig A, Achenbach S. Estimated radiation dose associated with cardiac CT angiography. JAMA. 2009;301:500–507. doi: 10.1001/jama.2009.54. [DOI] [PubMed] [Google Scholar]
- 10.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298:317–323. doi: 10.1001/jama.298.3.317. [DOI] [PubMed] [Google Scholar]
- 11.Feuchtner GM, Jodocy D, Klauser A, Haberfellner B, Aglan I, Spoeck A, Hiehs S, Soegner P, Jaschke W. Radiation dose reduction by using 100-kV tube voltage in cardiac 64-slice computed tomography: A comparative study. Eur J Radiol. 2009;75:e51–e56. doi: 10.1016/j.ejrad.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Hausleiter J, Meyer T, Hadamitzky M, et al. Radiation dose estimates from cardiac multislice computed tomography in daily practice: impact of different scanning protocols on effective dose estimates. Circulation. 2006;113:1305–1310. doi: 10.1161/CIRCULATIONAHA.105.602490. [DOI] [PubMed] [Google Scholar]
- 13.Shuman WP, Branch KR, May JM, Mitsumori LM, Lockhart DW, Dubinsky TJ, Warren BH, Caldwell JH. Prospective versus retrospective ECG gating for 64-detector CT of the coronary arteries: comparison of image quality and patient radiation dose. Radiology. 2008;248(2):431–437. doi: 10.1148/radiol.2482072192. [DOI] [PubMed] [Google Scholar]
- 14.Stolzmann P, Leschka S, Scheffel H, et al. Dual-source CT in step-and-shoot mode: noninvasive coronary angiography with low radiation dose. Radiology. 2008;249:71–80. doi: 10.1148/radiol.2483072032. [DOI] [PubMed] [Google Scholar]
- 15.Earls JP, Berman EL, Urban BA, et al. Prospectively gated transverse coronary CT angiography versus retrospectively gated helical technique: improved image quality and reduced radiation dose. Radiology. 2008;246:742–753. doi: 10.1148/radiol.2463070989. [DOI] [PubMed] [Google Scholar]
- 16.Lell M, Marwan M, Schepis T, Pflederer T, Anders K, Flohr T, Allmendinger T, Kalender W, Ertel D, Thierfelder C, Kuettner A, Ropers D, Daniel WG, Achenbach S. Prospectively ECG-triggered high-pitch spiral acquisition for coronary CT angiography using dual source CT: technique and initial experience. Eur Radiol. 2009;19:2576–2583. doi: 10.1007/s00330-009-1558-4. [DOI] [PubMed] [Google Scholar]
- 17.Achenbach S, Marwan M, Ropers D, Schepis T, Pflederer T, Anders K, et al. Coronary computed tomography angiography with a consistent dose below 1 mSv using prospectively electrocardiogram-triggered high-pitch spiral acquisition. Eur Heart J. 2010;31:340–346. doi: 10.1093/eurheartj/ehp470. [DOI] [PubMed] [Google Scholar]
- 18.Raff GL, Abidov A, Achenbach S, Berman DS, Boxt LM, Budoff MJ, Cheng V, DeFrance T, Hellinger JC, Karlsberg RP. Society of Cardiovascular Computed Tomography, SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr. 2009;3:122–136. doi: 10.1016/j.jcct.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Ferencik M, Moselewski F, Ropers D, et al. Quantitative parameters of image quality in multidetector spiral computed tomographic coronary imaging with submillimeter collimation. Am J Cardiol. 2003;92:1257–1262. doi: 10.1016/j.amjcard.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Achenbach S, Giesler T, Ropers D, Ulzheimer S, Anders K, Wenkel E, Pohle K, Kachelriess M, Derlien H, Kalender WA, Daniel WG, Bautz W, Baum U. Comparison of image quality in contrast-enhanced coronary-artery visualization by electron beam tomography and retrospectively electrocardiogram-gated multislice spiral computed tomography. Invest Radiol. 2003;38:119–128. doi: 10.1097/00004424-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Bongartz G, Golding SJ, Jurik AG, et al. [Accessed 05/2010];European guidelines for multislice computed tomography: Appendix C. Funded by the European Commission. Contract No FIGM-CT2000-20078-CT-TIP. 2004 http://www.drs.dk/guidelines/ct/quality/htmlindex.htm.
- 22.Mayo JR, Aldrich J, Muller NL. Radiation exposure at chest CT: a statement of the Fleischner society. Radiology. 2003;228:15–21. doi: 10.1148/radiol.2281020874. [DOI] [PubMed] [Google Scholar]
- 23.Fujii K, Aoyama T, Yamauchi-Kawaura C, et al. Radiation dose evaluation in 64-slice CT examinations with adult and paediatric anthropomorphic phantoms. Br J Radiol. 2009;82:1010–1018. doi: 10.1259/bjr/13320880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goetti R, Leschka S, Baumüller S, et al. Low dose high-pitch spiral acquisition 128-slice dual-source computed tomography for the evaluation of coronary artery bypass graft patency. Invest Radiol. 2010 Jun;45:324–330. doi: 10.1097/RLI.0b013e3181dfa47e. [DOI] [PubMed] [Google Scholar]
- 25.Alkadhi H, Stolzmann P, Desbiolles L, et al. Low-dose, 128-slice, dual-source CT coronary angiography: accuracy and radiation dose of the high-pitch and the step-and-shoot mode. Heart. 2010 Jun;96:933–938. doi: 10.1136/hrt.2009.189100. [DOI] [PubMed] [Google Scholar]
- 26.Leschka S, Stolzmann P, Desbiolles L, et al. Diagnostic accuracy of high-pitch dual-source CT for the assessment of coronary stenoses: first experience. Eur Radiol. 2009;19:2896–2903. doi: 10.1007/s00330-009-1618-9. [DOI] [PubMed] [Google Scholar]
- 27.LaBounty TM, Leipsic J, Min JK, et al. Effect of Padding Duration on Radiation Dose and Image Interpretation in Prospectively ECG-Triggered Coronary CT Angiography. AJR. 2010;194:933–937. doi: 10.2214/AJR.09.3371. [DOI] [PubMed] [Google Scholar]