Abstract
All metazoan cells produce and/or interact with tissue-specific extracellular matrices (ECMs). Such ECMs play important structural roles not only in connective tissues, but in all tissues in which they provide support and anchorage for cells. However, in addition to such structural roles, it has become increasingly clear that the tissue-specific microenvironments formed by the ECM play instructional roles that inform the proper phenotypes and functional behaviors of specialized cell types and, recent in vivo and in vitro studies suggest that ECM components also affect metabolic function. This review summarizes data that provide insights into roles of ECM in informing the proper development and functioning of highly specialized cells of metabolic tissues, such as adipocytes and islet β cells.
Cells too are products of their environment
Tissues are far more than collections of cells, as a major portion of tissues is composed of what lies between cells, and the majority of what lies between cells is the extracellular matrix (ECM). In fact, the most abundant proteins of the body are not intracellular or cell surface proteins, rather they are the collagens, a single subgroup of ECM proteins that alone represents 30% of the protein mass of the human body [1]. There are at least 28 different collagen types [1] that together provide frameworks underlying the two major categories of ECM: 1) interstitial or stromal ECM, found between cells not closely juxtaposed to one another and characterized by fibrillar supramolecular structures; and 2) basement membrane (BM), a sheet-like ECM associated with epithelia and various specialized cell types, including adipocytes (Figure 1), myofibers, and cells of the endocrine pancreas. Other major ECM proteins include the highly glycosylated proteoglycans, found both in interstitial ECM and BM, the stromal protein fibronectin (FN) (Table 1), and the laminin (LN) family of BM proteins [2]. Importantly, ECMs are not limited to structural roles, but are instructional as well, affecting cellular differentiation, behavior and function. One way that cells are instructed by, or communicate with ECM, is via specific ECM receptors that include the integrins, dystroglycan, the discoidin domain receptors, and LAIR-1 [3–5]. In addition, ECM is now known to bind and to serve as a reservoir for growth factors that affect cell behaviors, and to act in regulating the activity of such growth factors [6–9]. Key to the influences of ECMs in modulating cell behavior is their dynamic nature which allows remodeling throughout life, and particularly during development, wound healing, and in various pathological processes. Important to such remodeling are the degradative roles of various extracellular proteinases.
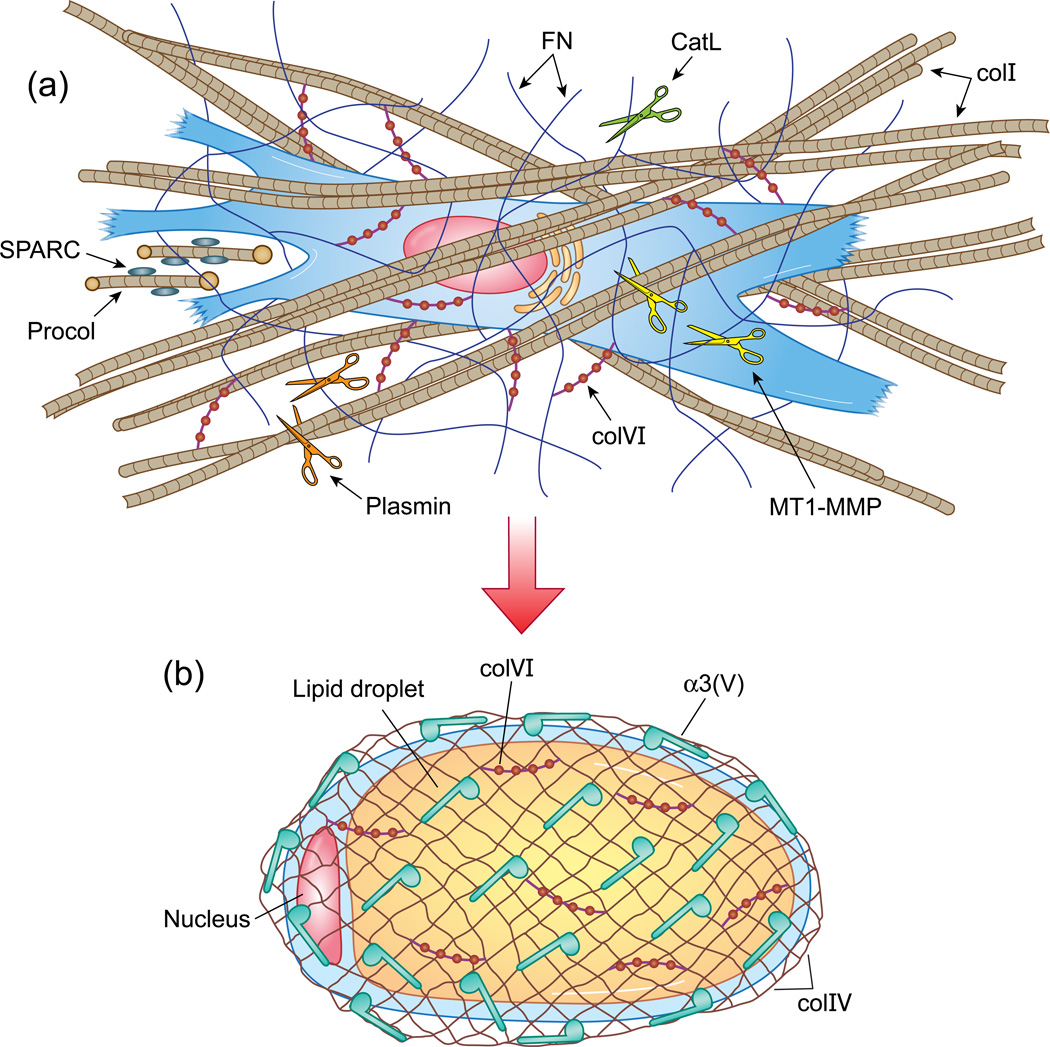
Figure 1.
Transition from preadipocyte to adipocyte, with associated ECMs. Representative drawings of a fibroblastic preadipocyte (a), a mature adipocyte (b), and the ECMs characteristic of each cell type. (a) A preadipocyte associates with a stromal ECM comprising colI-containing fibrils (tan cross-banded strands), FN fibrils (thin blue wavy lines); and colVI microfilaments (red beaded structures). The latter may serve to link other ECM components (e.g. colI and FN) to each other, and to cell surfaces. The proteinases MT1-MMP, CatL, and plasmin are represented by gold, green, and orange scissors, respectively. MT1-MMP and plasmin cleave both colI and FN, whereas CatL is thought to cleave FN, but not colI. The fibroblastic cell secretes colI procollagen precursors (Procol), which have small NH2-terminal and larger COOH-terminal propeptides (yellow circles) that are cleaved off to yield the triple helical monomers that self-associate to form colI fibrils. Blue ovals adhered to the procollagen represent SPARC, which is thought to be involved in modulating colI biosynthesis and ECM deposition. (b) An adipocyte containing a large internal lipid droplet is shown enveloped by a net-like colIV framework, which forms the structural backbone of all basement membranes. Other major BM proteins, such as LNs, are not shown. Blue musical note-shapes represent colV α1(V)α2(V)pNα3(V) heterotrimers, with the rounded part of the structure representing the pNα3(V) NH2-terminal globular domain, hypothesized to interact with cell surface components. Macromolecular structures formed by or incorporating α1(V)α2(V)pNα3(V) heterotrimers are as yet unknown. ColVI microfilaments (red beaded structures) found in the stromal ECM of fibroblastic cells (a), is also found associated with BM of highly specialized cells, such as adipocytes, and myofibers, and binds colIV, as shown (b). ColI, FN, colVI, and colIV can all interact with cell surfaces via integrin receptors, which are not represented in the figures. pNα3(V) chains are hypothesized to interact with as yet unknown cell surface components.
Table 1.
ECM components implicated in effects on metabolic tissues.
| Component | Composition | Macromolecular Structure |
Functions | References |
|---|---|---|---|---|
| Fibronectin (FN) | Dimers, noncollagenous 250 kDa multidomain subunits | Dense stromal fibrillar meshwork | Cell adhesion, migration, morphology, and differentiation | [19, 20] |
| Laminin (LN) | Trimers of noncollagenous α,β, and γ chains | Layered sheets within BM | Cell adhesion, migration, morphology, and differentiation | [71] |
| Collagen type I (ColI) | α1(I)2α2(I) heterotrimers | Stromal fiber-forming collagen | Tissue rigidity and tensile strength | [72] |
| Collagen type IV (ColIV) | α1(IV)2α2(IV)a heterotrimers | Network structures within BM | Structural backbone stabilizing BMs | [71] |
| Collagen type V (ColV) | α1(V)α2(V)α3(V) heterotrimersb | Pericellular, structure unknown | Affects adipocyte and β cell function | [59, 60] |
| Collagen type VI (ColVI) | α1(VI)α2(VI)α3(VI)c heterotrimers | Stromal beaded microfilaments | Links cells and ECM components, tissue rigidity | [45–47] |
| Secreted Protein Acidic and Rich in Cysteine (SPARC) | Matricellular monomer | Bound to collagens | Affects collagen biosynthesis/assembly, adipocyte maturation, cell signaling | [49, 54, 73] |
α3(IV)α4(IV)α5(IV) and α5(IV)2α6(IV) ColIV variants are found in some tissues [71].
A more widely distributed α1(V)2α2(V) heterotrimeric colV form is incorporated within colI fibrils and affects colI fibril geometry [74].
α4(VI), α5(VI), and α6(VI) chains are thought to substitute for the α3(VI) chains in some tissues [75].
One obvious way in which ECM can affect metabolic tissues is via fibrosis, in which parenchyma are replaced by a collagen I (colI)-rich ECM, with consequent diminishing of functional capacity. An example is fibrosis/cirrhosis of the liver [10]. In addition, inflammation-induced collagen deposition in skeletal muscle may contribute to increased insulin-resistance in this tissue, via interactions between collagens and endothelial cell α2β1 integrin receptors, which affect the nature of the physical barrier between muscle and vasculature [11]. The studies described below present findings that suggest other cellular mechanisms whereby ECM can affect metabolic function. The studies focus either on ECM components themselves, or on proteinases thought to exert metabolic effects, at least in part, via effects on ECM and have reported findings relevant to pancreatic islets and adipocytes.
ECM and islet β cell function
Pancreatic islets are densely vascularized. In mice, in which β cells do not form their own BM but instead are in contact with the BM laid down by islet endothelial cells, signals affecting β cell function and proliferative potential involve interactions between β cell surface β1-integrins and LNs of the endothelial BM [12]. In contrast to murine islets, human islets have, in addition to an endothelial BM, a second, separate BM that surrounds the endocrine cells themselves [13]. As in murine islet BM, the BM surrounding human islet endocrine cells includes α5 chain-containing LN, to which β cells appear to bind via β1-integrins, although LN binding by β cells also appears to involve the human β cell surface glycoprotein Lutheran [13]. Various cell culture experiments have demonstrated that BM components LN and colIV have important effects on rodent and human β cells, including enhancement of insulin gene transcription, insulin secretion, and β cell survival rates and proliferation [14]. Gaining a better understanding of such effects could positively impact efforts to improve the in vitro culturing and expansion of functional islets, and the success rates of islet transplantation.
It will be seen below that the non-BM, stromal ECM components FN and collagen VI (colVI), play marked roles in adipocyte biology. We thus note here that FN has been reported as an ECM component of pre-natal pancreatic islets [15] and exogenous FN can affect behaviors of isolated islets and β-cells in culture, albeit with variable effects, depending on the study [16]. It thus remains unclear the extent to which interactions with FN might affect β-cell function in vivo. Similarly, we note that ColVI has been reported in the ECM capsule at the islet-exocrine pancreas interface [17, 18], although it is unknown whether it plays any role in affecting islet function.
ECM in adipocyte remodeling, differentiaton and maturation
Fibronectin: a key ECM component affecting adipocytic differentiation
FN, a major ECM glycoprotein, forms fibrillar structures and participates in organizing the assembly of various other ECM components [19]. FN-integrin interactions, which can be important for cell-ECM adhesion, can generate inside-out signaling, involved in cell-dependent formation of FN fibrils [19], and outside-in signaling that induces signaling cascades and expression of select genes, and affects formation of the actin-containing cytoskeleton [20]. FN-integrin interactions can thus profoundly affect cell morphology, differentiation, and behavior. FN levels decrease during adipogenic differentiation of 3T3-L1 cells, during which FN-rich preadipocyte stromal ECM is replaced by a colIV- and LN-containing BM, characteristic of mature adipocytes [21] (Figure 1). Moreover, culturing 3T3-L1 cells on exogenous FN inhibits adipogenic differentiation, yielding reduced expression of lipogenic enzymes, and interfering with differentiation-dependent decreases in actin assembly [22]. In fact, as cytochalasin D, which disrupts actin filaments, can reverse the inhibitory effects of FN on adipogenesis, some portion of the adipogenic program may depend on disassembly of the actin-containing cytoskeleton, consequent to loss of FN [22]. However, Cytochalasin D also disrupts FN binding to cell receptors [23], which could play a role in the drug’s ability to reverse FN’s effects on adipogeneisis. Thus, receptor-FN interactions promote the fibroblastic preadipocyte phenotype, whereas interactions between cell surface receptors and components of the BM that replaces FN-rich stromal ECM may promote adipogenesis.
Effects of the pericellular collagenase MT1-MMP on adipocyte maturation
Unlike most MMPs (matrix metalloproteinases), which are secreted as soluble forms, membrane type 1-MMP (MT1-MMP, also known as MMP-14) belongs to a small group of plasma membrane-tethered MMPs with activities confined to the pericellular space [24]. MT1-MMP is capable of degrading many ECM components [25], and can operate as the predominant pericellular collagenase in mesenchyme-derived cells [26, 27]. Ability to remodel pericellular ECM gives MT1-MMP the ability to alter behaviors of various cell types [25]. MT1-MMP-null mice have a lipodystrophic phenotype specific to white adipose tissue (WAT), characterized by “mini-adipocytes” with reduced lipid content [28]. Although MT1-MMP-null adipocytes have normal expression levels of the essential adipogenic transcription factor PPARγ, there are reductions in expression of genes linked to carbohydrate and fatty acid metabolism, and in expression of other transcription factors involved in adipogenesis [28]. Thus, MT1-MMP-null preadipocytes differentiate in vivo to some degree, but seem unable to fully mature. In contrast to the in vivo phenotype, MT1-MMP-null preadipocytes cultured on plastic or atop collagen gels develop normally, whereas MT1-MMP-null preadipocytes cultured within 3-D colI gels, or transplanted into wild-type recipient mice show impaired ability to fully mature [28]. This cell-autonomous inability to fully mature, accompanied by inability to degrade surrounding colI, is consistent with a model in which absence of MT1-MMP-dependent remodeling of colI-rich ECM impairs the hypertrophic response necessary to complete maturation. In support of this model, decreasing colI concentrations in 3-D gels increases ability of MT1-MMP-null preadipocytes to differentiate, whereas increasing colI concentrations in 3-D gels interferes with full maturation of even wild type preadipocytes [28]. It should be noted that MT1-MMP is also capable of degrading FN, either directly (Figure 1) or via activation of MMP2 [24, 29], thus suggesting another mechanism whereby MT1-MMP may affect adipogenesis.
MT1-MMP plays roles not only in WAT development, but in functioning of adult WAT, as adult heterozygous MT1-MMP +/− mice, employed due to the early morbidity/mortality of MT1-MMP-nulls [30], show reduced remodeling of WAT colI ECM in response to a high-fat diet, with concomitant protection against diet-induced obesity [31]. Interestingly, single nucleotide polymorphisms in the human MT1-MMP gene have been positively associated with female, but not male, obesity; and with male, but not female diabetic traits [31], thus implicating MT1-MMP variants as gender-specific genetic modifiers in human predisposition to obesity and diabetes.
Roles for plasmin in ECM-remodeling associated with adipogenesis
Decreased FN and colI levels, accompanying adipocytic differentiation of 3T3-L1 cells, result from decreased synthesis [32] and increased degradation of both proteins. Interestingly, plasmin inhibition interferes with FN degradation in differentiating 3T3-L1 cells, while, in vitro, plasmin can cleave FN to produce the same size FN fragments produced by differentiating 3T3-L1 cells [33]. Moreover, treatment with a plasmin inhibitor blocks 3T3-L1 differentiation, including blocked induction of adipocyte-specific genes, such as C/EBPβ and PPARγ, while depletion of the plasmin precursor plasminogen also impairs 3T3-L1 adipogenesis [33]. Consistent with in vivo roles for plasmin in adipogenesis, serine proteinase inhibitors reduce adipogenesis and, conversely, increase both FN and colI deposition during mammary-gland involution in mice, while plasminogen-null mice are leaner than wild-type controls, and show impaired adipogenesis and increased colI deposition in mammary tissue [33]. Effects of plasmin inhibition/ablation on collagen deposition suggest that plasmin cleavage of colI might also play a role in adipogenesis (Figure 1), although plasmin’s role in colI cleavage may be indirect, as plasmin has been shown by a number of studies to be capable of activating MMPs, which play important roles in collagen remodeling [34, 35]. Kallikrein has been implicated as the plasminogen activating serine protease required for adipogenesis, while kallakrein activation of plasminogen appears to be enhanced by FN, thus suggesting a positive feedback loop in the degradation of FN-rich ECM [33].
Roles for cathepsins in ECM-remodeling associated with WAT development and function
Levels of the proteinase cathepsin L (CatL) increase in the late differentiation stages of primary preadipocytes, CatL inhibitors interfere with this differentiation, and CatL overexpression fosters 3T3-L1 adipogenesis [36]. Cathepsins are found primarily in lysosomes/endosomes [37], but can be released from cells and participate in ECM degradation [38]. In fact, CatL can degrade FN in vitro, while specific CatL inhibition reverses the decreasing FN levels associated with differentiation of primary preadipocytes [36]. CatL inhibition in preadipocytes also yields accumulation of the insulin and insulin growth factor-1 receptors (IR and IGF-1R), normally degraded in lysosomes/endosomes, although this accumulation did not increase adipogenic potential, thus suggesting retained FN-ECM to have an overriding influence on adipogenesis [36].
In vivo, CatL-null mice have lowered serum insulin and glucose levels and increased glucose tolerance, suggestive of increased peripheral glucose utilization, and, on a high fat diet, CatL-null mice are protected against diet-induced obesity and insulin resistance [36]. Effects on peripheral tissues are not limited to WAT, as CatL-null skeletal muscle shows increased glucose uptake and increased FN levels. Pharmacological inhibition of CatL in ob/ob mice causes reductions in body weight gain and serum insulin levels, and reduced glucose intolerance, accompanied by increased muscle FN, IR, and Glut-4 levels, suggesting CatL as a potential target for therapeutic interventions [36]. Relevance to human conditions is suggested by observations that human serum CatL levels are increased in diabetic patients, and in nondiabetic obese human subjects, compared to lean non-diabetic individuals [36].
It should be noted that CatL effects on adipogenesis are unlikely to involve colI degradation, as CatL appears relatively ineffective at cleaving native collagenous domains [39]. In contrast, cathepsin K (CatK), which has been reported to be upregulated in the WAT of rodent models of obesity and in obese humans [40], is known to have a collagenolytic activity capable of degrading colI [41]. Thus, the observation that CatK-null mice in the post weaning period and CatK-null mice exposed to a high fat diet show reduced adiposity compared to wild type controls [42], suggests that CatK collagenolytic activity may play a role in regulating the development and functioning of WAT.
Collagen VI may normally act to impede adipocyte hypertrophy/function
It has been suggested that increased expression of collagens I-VI is a response of metabolically-stressed adipocytes, designed to counteract hypertrophy in obese or diabetic states, but that the increased rigidity imparted to WAT by such responses further exacerbates adipocyte dysfunction [43]. This seemingly fibrotic response may involve reciprocal interactions between metabolically-stressed adipocytes and WAT-resident macrophages that shift macrophages to an M2-like phenotype characterized by secretion of relatively high levels of TGFβ, which in turn induce expression and deposition of collagens [44]. One of these induced collagens, colVI [43, 44], appears to be a major adipocyte secretory protein [43]. ColVI is a nonfibrillar collagen, distributed widely in tissues as α1(VI)α2(VI)α3(VI) heterotrimers that form thin microfilaments, which bind various other ECM components, including collagens I, II and IV, and FN [45] (Figure 1). In addition, the triple helical domains of the three colVI chains contain multiple Arg-Gly-Asp triplets that bind cell surface integrins [46], thus linking cells to the ECM. Defects in any of the colVI chains can underlie the heritable muscle disorders Bethlem myopathy and Ullrich congenital muscular dystrophy [45], perhaps due to disrupted myofiber-BM links [47]. Interestingly, crossing leptin-deficient ob/ob mice onto a colVI-null background improves their metabolic profile, including improvements in fasting glucose levels, glucose tolerance, lipid metabolism, and resolution of pancreatic β-cell hyperplasia, perhaps due to increased insulin-sensitivity in peripheral tissues [43]. Improved metabolic phenotype, compared to wild type, is also achieved when colVI-null mice on an otherwise normal genetic background are placed on a high fat diet. Surprisingly, although improved adipocytic function usually correlates with decreased cell size, ob/ob/colVI-null adipocytes are larger than those of ob/ob mice with normal colVI alleles. Similarly, on a high fat diet, adipocytes of colVI-null mice on an otherwise normal genetic background are larger than those of wild type counterparts [43]. Thus, decreased ECM rigidity, resulting from colVI ablation, may facilitate hypertrophic maturation of adipocytes, thereby facilitating function.
SPARC plays multiple anti-adipogenic roles
Matricellular proteins are ECM-associated, and contribute to cell-ECM interactions, but not to ECM structural properties, [48]. Ablation of the matricellular protein SPARC (Secreted Protein Acidic and Rich in Cysteine) yields mice with both increased adipocyte numbers and average adipocyte size, but without increased body weight [49], perhaps due to counterbalancing loses in bone [50], muscle, and other connective tissues [49]. SPARC-null mice show increasing adiposity upon aging, although serum glucose, insulin, cholesterol, high-density lipoproteins and triglycerides levels remain normal [49]. Most SPARC in fat pads appears to be produced by preadipocytes, although the time course of increasing adiposity in SPARC-null mice upon aging suggests deficits in adipocyte maturation, rather than in preadipocyte differentiation. Interestingly, SPARC-null fat pads have decreased colI content [49], perhaps related to roles of SPARC in modulating the growth and deposition of colI fibrils [51] (Figure 1). Thus, decreased ECM rigidity due to decreased colI may provide a more permissive microenvironment for the hypertrophic maturation of adipocytes in SPARC-null WAT. However, distributions of BM proteins colIV and laminin are altered in SPARC-null mouse lens capsule [52], suggesting that alterations to adipocyte BM may also contribute to the SPARC-null phenotype.
Concomitant with the adipogenic switch from FN-rich stromal ECM to laminin-rich BM is a switch from expression of α5 to α6 integrins [53], which bind FN and LN, respectively. SPARC appears to enhance both FN deposition, perhaps via effects on FN folding, and expression of the α5 chain of the integrin FN receptor [54], the latter perhaps being a downstream effect of SPARC-induced reduction in PPARγ expression [54], as PPARγ can inhibit transcription of the α5 gene [55]. SPARC can also inhibit expression of the LN α1 chain and of the α6 chain of the integrin LN receptor [54]. The latter inhibition, apparently mediated via SPARC activation of integrin-linked kinase (ILK) and subsequent β-catenin stabilization [54], appears to favor adipogenic differentiation over preadipocyte proliferation [53]. Thus, SPARC may affect adipogenesis by interfering with conversion from stromal ECM to BM, while fostering cellular interactions with the former and impairing interaction with the latter. SPARC may also inhibit adipogenesis by predisposing mesenchymal precursor cells to osteoblastic, rather than adipogenic, differentiation pathways [54], thus correlating with the osteopenia and increased adiposity found in SPARC-null mice.
In regard to the apparently manifold anti-adipogenic roles of SPARC, directed in large part via its effects on ECM, it is of interest that expression of SPARC can itself be influenced by fat mass, and by leptin, insulin and glucose levels [56]. Thus, SPARC may be an important intermediate in regulating adipose-ECM interactions in response to key metabolic readouts.
Collagen V: a minor collagen with manifold effects on glucose metabolism
Collagen V (colV) is a low-abundance, minor fibrillar collagen, broadly distributed in tissues as α1(V)2α2(V) heterotrimers, which can incorporate into fibrils of the much more abundant colI, and are involved in regulating the geometry of the resulting heterotypic fibrils [57]. Consequently, mutations in genes for either the α1(V) or α2(V) chain can underlie the human connective tissue disorder classic Ehlers-Danlos syndrome, characterized by abnormal collagen fibrils with diminished tensile strength [58]. However, colV also occurs as relatively uncharacterized α1(V)α2(V)α3(V) heterotrimers, which have a relatively limited tissue distribution [59]. Interestingly, like colVI, the α3(V) chain is expressed at highest levels in WAT [60]. Consistent with an adipocyte-specific dimension to α3(V) function, α3(V) expression is not detected in preadipocytic 3T3-L1 cells, but is induced upon adipogenic differentiation, whereas α1(V) chains are produced by both differentiated and non-differentiated 3T3-L1 cells [60]. Unlike mice with mutated α1(V) and α2(V) chain genes, mice null for the α3(V) gene Col5a3 [60] do not have an obvious Ehlers-Danlos-like phenotype, but instead have subtle changes in adiposity, including thinning of the adipocyte-rich hypodermal layer. In addition, Col5a3-null mice are glucose-intolerant, insulin-resistant, and hyperglycemic [60]. The α3(V) chain is also found in skeletal muscle, juxtaposed to the sarcolemma, and within pancreatic islets [60], in which it co-localizes with α and β cells. In addition, immortalized murine α, β, and islet endothelial cells each produce a novel ECM lacking colI, with which colV is thought to normally associate [57]. This novel ECM contains colV that comprises α1(V), α2(V), and pNα3(V) chains. This latter form of α3(V) chains retains NH2-terminal globular sequences previously thought to be cleaved by extracellular proteinases [61]. WAT, skeletal muscle and islets are all affected in Col5a3−/− mice.
Col5a3−/− adipocytes and skeletal muscle both show deficits in GLUT4 translocation to plasma membranes and in glucose uptake. As impaired glucose uptake can be the most significant rate-limiting defect in insulin resistance [62], these deficits may be of central importance to insulin-resistance in Col5a3−/− mice. Reduced phospho-Akt and IRS2 (insulin receptor substrate 2) levels are also found in Col5a3−/− WAT and skeletal muscle, although the significance of these findings is unclear, as differences were not detected in levels of some other insulin/IGF-1 signaling components, and as defects in upstream insulin/IGF-1 signaling components may fail to be transmitted further downstream, and/or be the consequence rather than cause of insulin resistance [63].
Col5a3−/− mice are also hypoinsulinemic and have decreased islet numbers, with residual islets deficient in their proliferative potential and ability to produce insulin. Insulin/IGF-I signaling can affect β cell mass and function [64] and, as in WAT and skeletal muscle, reproducible decreases are found in Col5a3−/− islet phospho-Akt and IRS2 levels. Interestingly, the levels of the transcription factor Pdx1, important to β cell differentiation and function, are reduced in Col5a3−/− islets. Islets also show unusually high apoptotic indices in response to the β cell toxin streptozotocin, and upon aging, phenomena perhaps related to decreased IRS2 and Pdx1 levels, as both have been implicated in anti-apoptotic activity [65, 66]. In Col5a3−/− newborns, islet numbers and plasma insulin levels are already decreased [60], suggesting these defects to be due to developmental defects/delay rather than to progressive postnatal loss. However, plasma glucose levels in Col5a3−/− newborns are similar to wild type, consistent with the assertion that β cell dysfunction can occur well before hyperglycemia, in progression toward type 2 diabetes [67]. By one year of age, Col5a3−/− fasting plasma glucose is near diabetic levels, perhaps attributable to the relatively severe insulin resistance in WAT and skeletal muscle of 1-year-old Col5a3−/− mice, and to failure of deficient β cells to meet the demand created by insulin resistance. Thus, despite subtle changes in fat mass, Col5a3−/− mice have pronounced deficits in metabolic functions of at least three tissues in which α3(V) chains are normally expressed. As discussed below, α3(V) chains, unlike colI and colVI, do not appear to affect cells via effects on the rigidity of ECM collagenous meshworks, perhaps explaining the somewhat opposite effects on adipocyte metabolic behavior obtained by ablation of α3(V) chains and colVI. Rather, α3(V) chains may exert their effects on cellular behavior via direct interactions with cell surface components.
Concluding remarks
β cells appear able to function and proliferate fully only in the context of a specific microenvironment composed of islet BM, which affects β cells via outside-in signaling involving integrins and other cell surface receptors. Such interactions may involve both direct ECM-cell interactions, as well as ECM regulated presentation of soluble growth factors to cells. For adipocytes, a common theme seems to underlie effects of ablating MT1-MMP or colVI. In both cases, effects on adipocyte morphology and function appear to be secondary to effects on ECM rigidity, and the extent to which it restricts or is permissive to the hypertrophy attending complete adipocytic differentiation/maturation and functionality. SPARC ablation may similarly affect adipocyte size and function by affecting ECM rigidity, although additional mechanisms appear to be involved.
Aside from ECM rigidity, adipocyte differentiation involves a shift from the FN-rich stromal ECM of preadipocytes, in which FN provides outside-in signaling that inhibits differentiation, to the BM of mature adipocytes (Figure 1). MT1-MMP and plasmin may both affect adipogenesis by degrading both colI, thus decreasing ECM rigidity, and FN, thereby more directly affecting adipogenic differentiation. Roles of CatL in WAT development and metabolism seem more complex, as CatL inhibition/ablation interferes with adipogenic differentiation, while appearing to increase glucose utilization by both WAT and skeletal muscle, and to increase glucose tolerance and insulin sensitivity in whole animals. However, while decreased adipocytic differentiation may be due to failure to sufficiently remodel FN-rich ECM, increases in metabolic function may be due to concurrent accumulation of uncleaved IR, IGF1R and Glut-4 in peripheral tissues. Additional, as yet uncharacterized CatL functions in these and/or other tissues may also be involved.
The pericellular distribution of α3(V) chains in WAT, skeletal muscle, and islets, and its appearance in the absence of colI, suggest that α3(V)-containing colV is not a stromal ECM component in these tissues. Moreover, as α3(V) ablation does not result in larger adipocytes, α3(V) chains do not appear to contribute to ECM rigidity. Instead, pericellular distribution may provide the large, highly charged pNα3(V) NH2-terminal domain, with opportunities for interactions with cell surface components (Figure 1), thus affecting cellular function and maturation via outside-in signaling. Interestingly, some early studies reported that colV can occur as a pericellular/BM-associated form [68, 69]. Thus, pNα3(V)-containing colV may associate with islet BM components, important to the survival and function of β cells, and with the BMs that invest adipocytes and myofibers. Survival and function of β cells encapsulated in immunoprotective gels for transplantation in the treatment of insulin-dependent diabetes can be enhanced by including ECM components [70]. Thus, pNα3(V)-containing colV may be a candidate for such approaches. Similarly, manipulating the expression levels of various ECM components in tissues may represent a future approach towards therapeutic interventions in metabolic disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004978. a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosman FT, Stamenkovic I. Functional structure and composition of the extracellular matrix. J Pathol. 2003;200:423–428. doi: 10.1002/path.1437. [DOI] [PubMed] [Google Scholar]
- 3.Leitinger B. Transmembrane Collagen Receptors. Annu Rev Cell Dev Biol. 2011 doi: 10.1146/annurev-cellbio-092910-154013. [DOI] [PubMed] [Google Scholar]
- 4.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 5.Kanagawa M, Toda T. The genetic and molecular basis of muscular dystrophy: roles of cell-matrix linkage in the pathogenesis. J Hum Genet. 2006;51:915–926. doi: 10.1007/s10038-006-0056-7. [DOI] [PubMed] [Google Scholar]
- 6.Ramirez F, Rifkin DB. Extracellular microfibrils: contextual platforms for TGFbeta and BMP signaling. Curr Opin Cell Biol. 2009;21:616–622. doi: 10.1016/j.ceb.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zacchigna L, et al. Emilin1 links TGF-beta maturation to blood pressure homeostasis. Cell. 2006;124:929–942. doi: 10.1016/j.cell.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Y, et al. Type IIA procollagen containing the cysteine-rich amino propeptide is deposited in the extracellular matrix of prechondrogenic tissue and binds to TGF-beta1 and BMP-2. The Journal of cell biology. 1999;144:1069–1080. doi: 10.1083/jcb.144.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, et al. Type IV collagens regulate BMP signalling in Drosophila. Nature. 2008;455:72–77. doi: 10.1038/nature07214. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 11.Kang L, et al. Diet-induced muscle insulin resistance is associated with extracellular matrix remodeling and interaction with integrin alpha2beta1 in mice. Diabetes. 2011;60:416–426. doi: 10.2337/db10-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nikolova G, et al. The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev Cell. 2006;10:397–405. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Otonkoski T, et al. Unique basement membrane structure of human pancreatic islets: implications for beta-cell growth and differentiation. Diabetes Obes Metab. 2008;10 Suppl 4:119–127. doi: 10.1111/j.1463-1326.2008.00955.x. [DOI] [PubMed] [Google Scholar]
- 14.Kragl M, Lammert E. Basement membrane in pancreatic islet function. Adv Exp Med Biol. 2010;654:217–234. doi: 10.1007/978-90-481-3271-3_10. [DOI] [PubMed] [Google Scholar]
- 15.Wang R, et al. Role for beta1 integrin and its associated alpha3, alpha5, and alpha6 subunits in development of the human fetal pancreas. Diabetes. 2005;54:2080–2089. doi: 10.2337/diabetes.54.7.2080. [DOI] [PubMed] [Google Scholar]
- 16.Stendahl JC, et al. Extracellular matrix in pancreatic islets: relevance to scaffold design and transplantation. Cell transplantation. 2009;18:1–12. doi: 10.3727/096368909788237195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes SJ, et al. Characterisation of collagen VI within the islet-exocrine interface of the human pancreas: implications for clinical islet isolation? Transplantation. 2006;81:423–426. doi: 10.1097/01.tp.0000197482.91227.df. [DOI] [PubMed] [Google Scholar]
- 18.Meyer T, et al. Extracellular matrix proteins in the porcine pancreas: a structural analysis for directed pancreatic islet isolation. Transplant Proc. 1998;30:354. doi: 10.1016/s0041-1345(97)01302-x. [DOI] [PubMed] [Google Scholar]
- 19.Singh P, et al. Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol. 2010;26:397–419. doi: 10.1146/annurev-cellbio-100109-104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyamoto S, et al. Fibronectin and integrins in cell adhesion, signaling, and morphogenesis. Ann N Y Acad Sci. 1998;857:119–129. doi: 10.1111/j.1749-6632.1998.tb10112.x. [DOI] [PubMed] [Google Scholar]
- 21.Smas CM, Sul HS. Control of adipocyte differentiation. Biochem J. 1995;309(Pt 3):697–710. doi: 10.1042/bj3090697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spiegelman BM, Ginty CA. Fibronectin modulation of cell shape and lipogenic gene expression in 3T3-adipocytes. Cell. 1983;35:657–666. doi: 10.1016/0092-8674(83)90098-3. [DOI] [PubMed] [Google Scholar]
- 23.Ali IU, Hynes RO. Effects of cytochalasin B and colchicine on attachment of a major surface protein of fibroblasts. Biochim Biophys Acta. 1977;471:16–24. doi: 10.1016/0005-2736(77)90388-1. [DOI] [PubMed] [Google Scholar]
- 24.Nagase H, et al. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Itoh Y, Seiki M. MT1-MMP: a potent modifier of pericellular microenvironment. J Cell Physiol. 2006;206:1–8. doi: 10.1002/jcp.20431. [DOI] [PubMed] [Google Scholar]
- 26.Chun TH, et al. MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix. The Journal of cell biology. 2004;167:757–767. doi: 10.1083/jcb.200405001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabeh F, et al. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. The Journal of cell biology. 2004;167:769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chun T-H, et al. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell. 2006;125:577–591. doi: 10.1016/j.cell.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 29.Ohtake Y, et al. Multifunctional roles of MT1-MMP in myofiber formation and morphostatic maintenance of skeletal muscle. Journal of cell science. 2006;119:3822–3832. doi: 10.1242/jcs.03158. [DOI] [PubMed] [Google Scholar]
- 30.Holmbeck K, et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 31.Chun TH, et al. Genetic link between obesity and MMP14-dependent adipogenic collagen turnover. Diabetes. 2010;59:2484–2494. doi: 10.2337/db10-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bortell R, et al. TGF beta 1 prevents the down-regulation of type I procollagen, fibronectin, and TGF beta 1 gene expression associated with 3T3-L1 pre-adipocyte differentiation. J Cell Biochem. 1994;54:256–263. doi: 10.1002/jcb.240540214. [DOI] [PubMed] [Google Scholar]
- 33.Selvarajan S, et al. A plasma kallikrein-dependent plasminogen cascade required for adipocyte differentiation. Nature cell biology. 2001;3:267–275. doi: 10.1038/35060059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baramova EN, et al. Involvement of PA/plasmin system in the processing of pro-MMP-9 and in the second step of pro-MMP-2 activation. FEBS Lett. 1997;405:157–162. doi: 10.1016/s0014-5793(97)00175-0. [DOI] [PubMed] [Google Scholar]
- 35.Davis GE, et al. Matrix metalloproteinase-1 and -9 activation by plasmin regulates a novel endothelial cell-mediated mechanism of collagen gel contraction and capillary tube regression in three-dimensional collagen matrices. Journal of cell science. 2001;114:917–930. doi: 10.1242/jcs.114.5.917. [DOI] [PubMed] [Google Scholar]
- 36.Yang M, et al. Cathepsin L activity controls adipogenesis and glucose tolerance. Nature cell biology. 2007;9:970–977. doi: 10.1038/ncb1623. [DOI] [PubMed] [Google Scholar]
- 37.Roberts R. Lysosomal cysteine proteases: structure, function and inhibition of cathepsins. Drug News Perspect. 2005;18:605–614. doi: 10.1358/dnp.2005.18.10.949485. [DOI] [PubMed] [Google Scholar]
- 38.Punturieri A, et al. Regulation of elastinolytic cysteine proteinase activity in normal and cathepsin K-deficient human macrophages. J Exp Med. 2000;192:789–799. doi: 10.1084/jem.192.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lecaille F, et al. The S2 subsites of cathepsins K and L and their contribution to collagen degradation. Protein Sci. 2007;16:662–670. doi: 10.1110/ps.062666607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiellini C, et al. Identification of cathepsin K as a novel marker of adiposity in white adipose tissue. J Cell Physiol. 2003;195:309–321. doi: 10.1002/jcp.10253. [DOI] [PubMed] [Google Scholar]
- 41.Garnero P, et al. The collagenolytic activity of cathepsin K is unique among mammalian proteinases. J Biol Chem. 1998;273:32347–32352. doi: 10.1074/jbc.273.48.32347. [DOI] [PubMed] [Google Scholar]
- 42.Funicello M, et al. Cathepsin K null mice show reduced adiposity during the rapid accumulation of fat stores. PLoS One. 2007;2 doi: 10.1371/journal.pone.0000683. e683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khan T, et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spencer M, et al. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. American journal of physiology. 2010;299:E1016–E1027. doi: 10.1152/ajpendo.00329.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maraldi NM, et al. Collagen VI myopathies: from the animal model to the clinical trial. Adv Enzyme Regul. 2009;49:197–211. doi: 10.1016/j.advenzreg.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 46.van der Rest M, Garrone R. Collagen family of proteins. FASEB J. 1991;5:2814–2823. [PubMed] [Google Scholar]
- 47.Kuo HJ, et al. Type VI collagen anchors endothelial basement membranes by interacting with type IV collagen. J Biol Chem. 1997;272:26522–26529. doi: 10.1074/jbc.272.42.26522. [DOI] [PubMed] [Google Scholar]
- 48.Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14:608–616. doi: 10.1016/s0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 49.Bradshaw AD, et al. SPARC-null mice exhibit increased adiposity without significant differences in overall body weight. Proc Natl Acad Sci U S A. 2003;100:6045–6050. doi: 10.1073/pnas.1030790100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delany AM, et al. Osteopenia and decreased bone formation in osteonectin-deficient mice. J Clin Invest. 2000;105:915–923. doi: 10.1172/JCI7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rentz TJ, et al. SPARC regulates processing of procollagen I and collagen fibrillogenesis in dermal fibroblasts. J Biol Chem. 2007;282:22062–22071. doi: 10.1074/jbc.M700167200. [DOI] [PubMed] [Google Scholar]
- 52.Yan Q, et al. Alterations in the lens capsule contribute to cataractogenesis in SPARC-null mice. Journal of cell science. 2002;115:2747–2756. doi: 10.1242/jcs.115.13.2747. [DOI] [PubMed] [Google Scholar]
- 53.Liu J, et al. Changes in integrin expression during adipocyte differentiation. Cell metabolism. 2005;2:165–177. doi: 10.1016/j.cmet.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 54.Nie J, Sage EH. SPARC inhibits adipogenesis by its enhancement of beta-catenin signaling. J Biol Chem. 2009;284:1279–1290. doi: 10.1074/jbc.M808285200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han S, et al. Peroxisome proliferator-activated receptor-gamma ligands inhibit alpha5 integrin gene transcription in non-small cell lung carcinoma cells. Am J Respir Cell Mol Biol. 2005;32:350–359. doi: 10.1165/rcmb.2004-0345OC. [DOI] [PubMed] [Google Scholar]
- 56.Kos K, et al. Regulation of the fibrosis and angiogenesis promoter SPARC/osteonectin in human adipose tissue by weight change, leptin, insulin, and glucose. Diabetes. 2009;58:1780–1788. doi: 10.2337/db09-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wenstrup RJ, et al. Murine model of the Ehlers-Danlos syndrome. col5a1 haploinsufficiency disrupts collagen fibril assembly at multiple stages. J Biol Chem. 2006;281:12888–12895. doi: 10.1074/jbc.M511528200. [DOI] [PubMed] [Google Scholar]
- 58.Malfait F, et al. Clinical and genetic aspects of Ehlers-Danlos syndrome, classic type. Genet Med. 2010;12:597–605. doi: 10.1097/GIM.0b013e3181eed412. [DOI] [PubMed] [Google Scholar]
- 59.Imamura Y, et al. The pro-alpha3(V) collagen chain. Complete primary structure, expression domains in adult and developing tissues, and comparison to the structures and expression domains of the other types V and XI procollagen chains. J Biol Chem. 2000;275:8749–8759. doi: 10.1074/jbc.275.12.8749. [DOI] [PubMed] [Google Scholar]
- 60.Huang G, et al. alpha3(V) Collagen is critical for glucose homeostasis in mice due to effects in pancreatic islets and peripheral tissues. J Clin Invest. 2011;121:769–783. doi: 10.1172/JCI45096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gopalakrishnan B, et al. Biosynthetic processing of the Pro-α1(V)Pro-α2(V)Pro-α3(V) procollagen heterotrimer. The Journal of biological chemistry. 2004;279:30904–30912. doi: 10.1074/jbc.M402252200. [DOI] [PubMed] [Google Scholar]
- 62.Petersen KF, Shulman GI. Etiology of insulin resistance. The American journal of medicine. 2006;119:S10–S16. doi: 10.1016/j.amjmed.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoehn KL, et al. IRS1-independent defects define major nodes of insulin resistance. Cell metabolism. 2008;7:421–433. doi: 10.1016/j.cmet.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Assmann A, et al. Growth factor control of pancreatic islet regeneration and function. Pediatr Diabetes. 2009;10:14–32. doi: 10.1111/j.1399-5448.2008.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sachdeva MM, et al. Pdx1 (MODY4) regulates pancreatic beta cell susceptibility to ER stress. Proc Natl Acad Sci U S A. 2009;106:19090–19095. doi: 10.1073/pnas.0904849106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Venieratos PD, et al. High glucose induces suppression of insulin signalling and apoptosis via upregulation of endogenous IL-1beta and suppressor of cytokine signalling-1 in mouse pancreatic beta cells. Cell Signal. 2010;22:791–800. doi: 10.1016/j.cellsig.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 67.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 68.Gay S, et al. The collagenous exocytoskeleton of smooth muscle cells. Collagen and related research. 1981;1:377–384. doi: 10.1016/s0174-173x(81)80014-3. [DOI] [PubMed] [Google Scholar]
- 69.Konomi H, et al. Localization of type V collagen and type IV collagen in human cornea, lung, and skin. Immunohistochemical evidence by anti-collagen antibodies characterized by immunoelectroblotting. The American journal of pathology. 1984;116:417–426. [PMC free article] [PubMed] [Google Scholar]
- 70.Weber LM, et al. Cell-matrix interactions improve beta-cell survival and insulin secretion in three-dimensional culture. Tissue engineering. 2008;14:1959–1968. doi: 10.1089/ten.tea.2007.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yurchenco PD. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kivirikko KI. Collagens and their abnormalities in a wide spectrum of diseases. Ann Med. 1993;25:113–126. doi: 10.3109/07853899309164153. [DOI] [PubMed] [Google Scholar]
- 73.Bradshaw AD. The role of SPARC in extracellular matrix assembly. J Cell Commun Signal. 2009;3:239–246. doi: 10.1007/s12079-009-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fichard A, et al. Another look at collagen V and XI molecules. Matrix Biol. 1995;14:515–531. doi: 10.1016/s0945-053x(05)80001-0. [DOI] [PubMed] [Google Scholar]
- 75.Gara SK, et al. Differential and restricted expression of novel collagen VI chains in mouse. Matrix Biol. 2011;30:248–257. doi: 10.1016/j.matbio.2011.03.006. [DOI] [PubMed] [Google Scholar]