Abstract
Ultraviolet (UV) irradiation is the most important factor contributing to the development of skin cancer. The use of chemopreventive agents, especially naturally occurring plant products, to prevent skin cancer caused by UV might an effective therapeutic or preventive intervention. Using in silico virtual screening of the Chinese Medicine Library, we identified norathyriol as a potential ERK2 inhibitor. Norathyriol is a metabolite of mangiferin, which is found in mango, Hypericum elegans, and Tripterospermum lanceolatum, and has potent anticancer-promoting activity. Here, we show that norathyriol inhibits ERK1/2 kinase activities and attenuates UVB-induced phosphorylation of the mitogen-activated protein kinase (MAPK) cascades. Direct binding of norathyriol with ERK2 was confirmed by a co-crystal structure. The norathyriol xanthone moiety acts as an adenine mimeric and anchors the compound by hydrogen bonds to the hinge region of the protein ATP-binding site. Norathyriol inhibited cell growth in mouse skin epidermal JB6 P+ cells by inducing G2-M phase arrest. Mouse skin tumorigenesis data clearly showed that treatment with norathyriol significantly suppressed solar UV-induced skin carcinogenesis in vivo. Results also indicated that norathyriol exhibits a potent chemopreventive activity through the inhibition of transcription factor AP-1 and NF-κB by targeting ERKs in UV-induced skin carcinogenesis.
Keywords: cancer, skin cancer, solar UV, UVB, chemoprevention, norathyriol, MAPK, ERK, ERK2 crystal structure
Introduction
Skin cancer is the most common cancer in Americans, and its incidence is rising dramatically (1–2). Although many environmental and genetic factors contribute to the development of skin cancer, the most important factor is chronic exposure of the skin to solar ultraviolet (UV) radiation (3–4). The solar UV spectrum can be divided into three subtypes, each of which has distinct biological effects: UVA (320–400 nm, long wave), UVB (280–320 nm, mid wave), and UVC (200–280 nm, short wave). Although UVC radiation is largely absorbed by stratospheric ozone, UVA and UVB reach the surface of the earth. UVA (90–95% of the total solar UV radiation), is considered as the “aging ray” that can lead to benign tumor formation as well as malignant cancers through the generation of reactive oxygen species (3). However, UVB (5% of the total solar UV radiation) is mainly responsible for a variety of skin diseases, including non-melanoma and melanoma skin cancers (1, 3, 5). It acts as a tumor initiator, tumor promoter and co-carcinogen by induction of oxidative stress, DNA damage and immunosuppression (2–3). UVB is recognized as a complete carcinogen with relevance to human skin cancer (2) and a potent inducer of mitogen-activated protein kinases (MAPKs) (1, 6–7). MAPKs are serine-threonine kinases that control fundamental cellular processes such as growth, proliferation, differentiation, migration and apoptosis (8–10). The mammalian MAPK family consists of extracellular signal-regulated kinases (ERKs), c-Jun NH2-terminal kinases (JNKs; also known as stress-activated protein kinases or SAPKs) and p38. Among the MAPK family, the ERKs cascade has been a focus of cancer chemoprevention because of its importance in carcinogenesis. Abnormalities in the ERKs pathway play a critical role in the development and progression of cancer and have been reported in approximately one-third of all human cancers (8). Therefore, targeting UV-induced ERKs might be an effective strategy for preventing skin tumorigenesis.
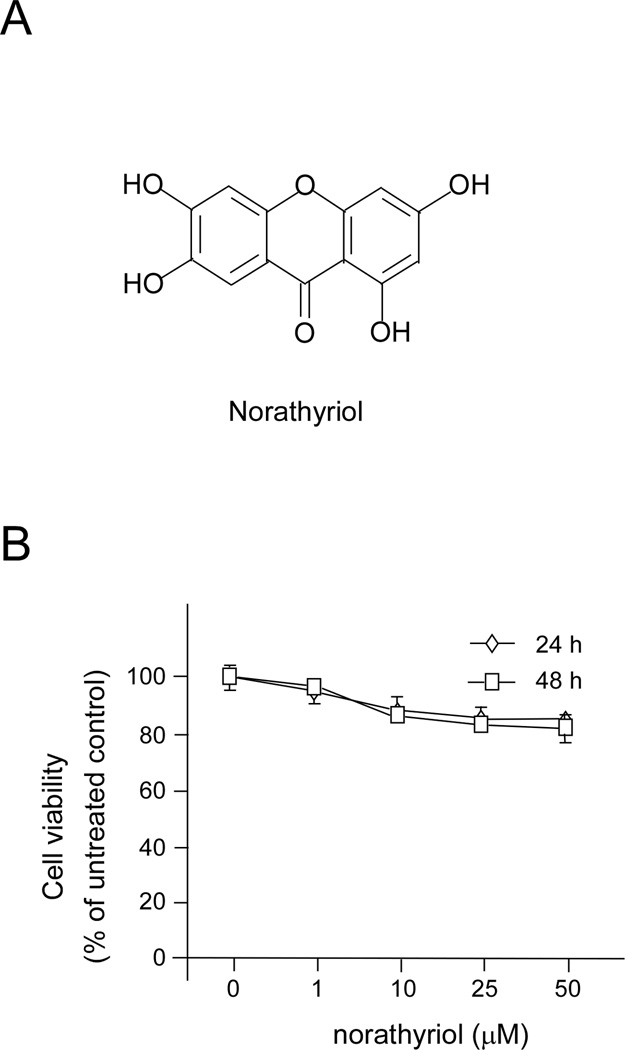
Norathyriol (1, 3, 6, 7-tetrahydroxy-9H-xanthen-9-one), an aglycone of a xanthone C-glycoside mangiferin, is isolated from several plants including mango, Hypericum elegans, and Tripterospermum lanceolatum (Figure 1A). Norathyriol is known to possess antioxidant, anti-inflammatory and antitumor properties (11–13). This compound has multiple targets and inhibits the transactivation of peroxisome proliferator-activated receptor isoforms (14), ABCB1/P-glycoprotein activity (11), protein kinase C activity (13), and cyclooxygenase and lipoxygenase activity (12). Norathyriol was reported to inhibit platelet aggregation (15), relax the rat thoracic aorta (16), and inhibit cutaneous plasma extravasation (17). In addition, norathyriol induces Ca2+ release from the sarcoplasmic reticulum of skeletal muscle (18), and inhibits the PMA-induced neutrophil respiratory burst and aggregation (19–20).
Figure 1. Norathyriol has no cytotoxity in JB6 cells.
(A) Chemical structure of norathyriol. (B) Cells were treated with norathyriol (0–50 µM), or its vehicle, DMSO, as a negative control, in 5% FBS/MEM for 24 or 48 h. Cell viability was determined by MTS assay and data are represented as means ± ± S.E.
Here, we report that norathyriol inhibits ERK 1 and 2 activities and suppresses UVB-induced ERKs signaling in JB6 P+ mouse skin epidermal cells. In a mouse skin tumorigenesis model, norathyriol strongly suppresses solar UV-induced mouse skin tumor numbers and volume.
Materials and methods
Chemicals
Eagle’s minimum essential medium (MEM), basal medium Eagle was purchased from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) was purchased from Gemini Bio-products (Calabasa, CA). The antibodies against phosphorylated ERKs (Tyr-202/Tyr-204), total ERKs, phosphorylated c-Jun N-terminal kinases (Thr-183/Tyr-185), total c-Jun N-terminal kinases, phosphorylated p90RSK (Thr-359/Ser-363), total p90RSK, phosphorylated p38 (Thr-180/Tyr-182), total p38, cyclin B1, phosphorylated Cdk1 (Tyr15) and total Cdk1 were purchased from Cell Signaling Biotechnology (Beverly, MA). The antibody against β-actin was purchased from Sigma-Aldrich (St. Louis, MO). The protein assay kit was from Bio-Rad (Hercules, CA), and the CellTiter96 Aqueous One Solution Cell Proliferation Assay Kit and the luciferase assay substrate were purchased from Promega (Madison, WI). The active ERK1 and ERK2 kinases were obtained from Upstate Biotechnology (Lake Placid, NY). CNBr-Sepharose 4B and [γ-32P] ATP were purchased from GE Healthcare (Piscataway, NJ).
In silico virtual screening
Molecular docking-based virtual screening was employed to screen the Chinese Medicine Library. The Glide software program (Schrödinger, Inc.) was used for virtual docking of compounds that utilized a grid-based ligand docking with energetics algorithm. Lipinski’s rule of 5 was adopted for filtering out orally inactive molecules. For maximum diversity of the chemicals, different tautomeric and ionization states were also generated. Thus the final library contains multiple states of some of the chemicals. The crystal structure of ERK2 with N, N-dimethyl-4-(4-phenyl-1H-pyrazol-3-yl)-1H-pyrrole-2-carboxamide (PDB ID 2OJG) bound in the ATP-binding site was used as a starting model for the virtual screening. After removing all the crystallographic water molecules, the protein structure was corrected by adding all the missing hydrogen atoms. Then the protein-ligand structure was subjected to energy minimization using the Optimized Potentials for Liquid Simulations- All Atom (OPLS-AA) force field. A hierarchical filtering procedure based on different levels of precision score function was used to identify potential inhibitors. Initial docking precision uses a high throughput virtual screening (HTVS) procedure, followed by a standard precision (SP) procedure, and finally the extra precision (XP) procedure. The binding affinity of the docked molecules can be considered as directly proportional to the docking score. The 25 molecules with a high XP score were selected as potential inhibitors of ERK2 with a potentially high affinity to bind with ERK2. The XP score of norathyriol was determined to be −9.6 kcal/mol.
Synthesis of norathyriol
The synthesis was performed based on a described procedure (21). Borontribromide (1 M in DCM, 100 mL, 2.5 eqv. per each OCH3 group) was added drop wise to a stirred suspension of 1, 3, 6, 7-tetramethoxyxanthone (3.16 g, 10 mmol in 25 mL dichloromethane at −78 °C) over a period of 30 min under a nitrogen atmosphere. After the addition, the reaction mixture was slowly brought to room temperature and stirred at this temperature for 48 h. After this time, the mixture was cooled to 0 °C and excess borontribromide was quenched by the slow addition of ice-water. The resulting precipitate was filtered and dried under vacuum. The crude material was purified by column chromatography (20–50 % ethyl acetate in hexane) followed by recrystallization using methanol-water to yield 2.0 g (77%) of norathyriol. The compound was confirmed by 1H NMR and comparing it with an authentic commercially available sample. 1H NMR (400 MHz, DMSO-d6): δ 13.21 (s, 1H), 10.75 (br s, 1H), 7.36 (s, 1H), 6.84 (s, 1H), 6.31 (d, J = 1.8 Hz, 1H), 6.13 (d, J = 1.8 Hz, 1H); ESI MS 259 (M-H+).
Cytotoxicity assay
To estimate cytotoxicity, JB6 P+ cells were seeded (2×104 cells/well) in 96-well plates with 5% FBS/MEM at 37°C in a 5% CO2 incubator. After 4h cells were fed with fresh medium and treated with norathyriol at the concentrations indicated (0, 1, 10, 25 or 50 µM). After culturing for the indicated times, 20 µl of Cell Titer 96 Aqueous One Solution were added to each well, and the cells were then incubated for 1 h at 37°C in a 5% CO2 incubator. Absorbance was measured at 490 and 690 nm.
UVB irradiation
A UVB irradiation system was used to stimulate cells in serum-free media. The spectral peak from the UVB source (Bio-Link crosslinker, Vilber Lourmat, Cedex 1, France) was 312 nm.
Western blotting
After cells (1×106) were cultured in a 10-cm dish overnight, they were starved in serum-free medium for another 24 h to eliminate the influence of FBS on the activation of mitogen-activated protein kinases. The cells were then treated with norathyriol (0–25 µM) for 2 h before they were exposed to UVB (4 kJ/0.5 m2) and then harvested after 30 min. The harvested cells were disrupted, and the supernatant fractions were boiled for 5 min. The protein concentration was determined using a dye-binding protein assay kit (Bio-Rad) as described in the manufacturer’s manual. Lysate proteins (30–50 µg) were subjected to 10% SDS-PAGE and electrophoretically transferred to a polyvinylidene difluoride membrane (GE Healthcare). After blotting, the membrane was incubated with a specific primary antibody at 4°C overnight. Protein bands were visualized by a chemiluminescence detection kit (GE Healthcare) after hybridization with an AP-linked secondary antibody.
Luciferase assay to determine AP-1 or NF-κB transactivation
Confluent monolayers of JB6 P+ cells stably transfected with an AP-1 or NF-κB luciferase reporter plasmid were trypsinized, and 4×104 viable cells suspended in 1 ml of 5% FBS/MEM were added to each well of a 24-well plate. Plates were incubated overnight at 37°C in a humidified atmosphere of 5% CO2. Cells were starved in serum-free medium for another 24 h. The cells were then treated for 2 h with norathyriol (0–25 µM) and then exposed to UVB (4 kJ/m2) and then harvested after 3 h. Cells were then disrupted with 100 µl of lysis buffer (0.1 M potassium phosphate pH 7.8, 1% Triton X-100, 1 mM dithiothreitol, and 2 mM EDTA), and luciferase activity was measured using a luminometer (Luminoskan Ascent, Thermo Electro, Helsinki, Finland).
In vitro ERK1 and ERK2 kinase assay
The recombinant non-phosphorylated GST-tagged RSK2 (residues 326–740; 500 ng) was used as a substrate in an in vitro kinase assay with 39.8 ng of active ERK1 or 10 ng of active ERK2 (Upstate Biotechnology). Reactions were carried out in 1× kinase buffer (25 mM Tris-HCl pH 7.5, 5 mM β-glycerophosphate, 2 mM dithiothreitol (DTT), 0.1 mM Na3VO4, 10 mM MgCl2) containing 50 µmol/L unlabeled ATP with or without 10 µCi of [γ-32P]ATP at 30°C for 30 min. Reactions were stopped and then proteins resolved by 10% SDS-PAGE and visualized by autoradiography.
In vitro pull-down binding assay
Sepharose 4B powder (0.3 g) was suspended in 1 mmol/L HCl and the coupled solution [0.1 mol/L NaHCO3 (pH 8.3) and 0.5 mol/L NaCl containing norathyriol was mixed and rotated at 4°C overnight. The medium was transferred to 0.1 mol/L Tris-HCl buffer (pH 8.0) and again rotated end over end at 4 °C overnight. The medium was washed thrice with 0.1 mol/L acetate buffer (pH 4.0) containing 0.5 mol/L NaCl followed by a wash with 0.1 mol/L Tris-HCl (pH 8.0) containing 0.5 mol/L NaCl. Active ERK1 or ERK2 proteins (0.2 µg) were individually incubated with 100 µl of the norathyriol–Sepharose 4B (or Sepharose 4B only as a control) (100 µl, 50% slurry) in a reaction buffer [50 mM Tris (pH 7.5), 5 mM EDTA, 150mM NaCl, 1 mM DTT, 0.01% Nonidet P-40, 2 lg/ml bovine serum albumin, 0.02 mM PMSF and 1× protease inhibitor mixture]. After incubation with gentle rocking overnight at 4°C, ATP (1, 10 and 100 µM) was added to a final volume of 500 µl and incubated for 30 min, the beads were washed five times with buffer comprised of 50 mM Tris (pH 7.5), 5 mM EDTA, 150 mM NaCl, 1 mM DTT, 0.01% Nonidet P-40 and 0.02 mM PMSF. The proteins bound to the beads were analyzed by western blotting using ERK1/2 antibody.
ATP and norathyriol competition assay
Active ERK2 (0.2 µg each) was incubated with 100 µl of norathyriol–Sepharose 4B or 100 µl of Sepharose 4B in a reaction buffer (see direct pull-down assay) for 12 h at 4°C, and ATP (1, 10 or 100 µM) was added to a final volume of 500 µl and incubated for 30 min. The samples were washed, and proteins were then detected by Western blotting.
Recombinant ERK2 expression, purification, crystallization
Full length (residues 1–360) human ERK2 (NM_002745) was cloned into the pET-28a vector (EMD Chemicals, Gibbstown, NJ) at the NdeI/HindIII restriction sites and expressed in BL21-CodonPlus (DE3)-RIPL E.coli (Stratagene, La Jolla, CA). The cells were induced with 0.25 mM IPTG and grown for an additional 4–5 h at 25 °C. The frozen pellet was resuspended in washing buffer containing 20 mM imidazole, 500 mM NaCl, 50 mM NaH2PO4 (pH 8.0), and 10% glycerol. The cells were lysed by French press. Soluble His-tagged ERK2 (about 90% of total protein) was loaded on Ni-NTA (Qiagen), washed thoroughly with washing buffer, eluted with 60 mM, 100 mM and 200 mM imidazole, and 150 mM NaCl, 20 mM Tris-HCl (pH 8.0). All fractions were combined and loaded onto Econo-pack 10DG desalting columns (Bio-Rad) to exchange buffer for 150 mM NaCl, 20 mM Tris-HCl (pH 8.0). To remove the His-tag, the protein was incubated with thrombin (EMD Chemicals) at 0.25 U thrombin/mg protein for 2–2.5 hours at room temperature. Untagged ERK2 was loaded on a Superdex ™ 200 10/30 GL FPLC column and concentrated to 10–15 mg/ml. Diffraction quality crystals were produced by the sitting drops vapor diffusion technique at 20 °C. The protein was diluted with 10 mM Tris-HCl (pH 8.0) to 7 mg/ml, and 10 mM β-mercapthoethanol was added prior to crystallization. The protein was mixed with an equal volume of precipitant comprised of 1.1 M – 1.3 M ammonium sulfate, 2% PEG 500 MME, 0.1 M Hepes-NaOH (pH 7.5). The crystals appeared in 2–4 days. Norathyriol powder was dissolved in 100% DMSO at 100 mM concentration. To produce the ERK2/narathyriol complex, the crystals of apo ERK2 were soaked overnight with the solution containing norathyriol. The compound from the stock solution was added to a two-fold diluted well solution (0.5 M– 0.7 M ammonium sulfate, 2% PEG 500 MME, 0.1 M Hepes-NaOH pH 7.5) at a final 2.5 mM concentration. That solution was added to the pre-existing drop with the crystals at a 1:1 (v:v) ratio. After soaking, the crystals were cryo-protected in buffer comprised of 1.6 M ammonium sulfate, 2% PEG 500 MME, 0.1 M Hepes (pH 7.5), and 20% xylitol. They were then flash-cooled in liquid nitrogen. The His-tagged ERK2 mutant, Q105A, was purified by a similar procedure.
ERK2/norathyriol co-crystal structure determination
ERK2 crystals belong to the primitive monoclinic lattice P21 with one molecule in the asymmetric unit cell. The high-resolution data sets were collected at the Advanced Photon Source (APS) beamline 24ID using 30×50 micron beam. The crystal to Quantum 315 CCD detector distance was 220 mm and the crystals were rotated around the spindle axis with images collected over 180° to a resolution of 1.6 Å. Data were integrated and scaled using the HKL2000 package (22). Integrated intensities from 3 different crystals were scaled together to increase redundancy and improve overall statistics. The real resolution of the data, used for structure refinement, was estimated taking into consideration the completeness of the last resolution shell, I/σ ratio and R-merge values. The atomic coordinates of the refined ERK2 structure (PDB code 1TVO) were used for the initial crystallographic phasing by molecular replacement. All calculations were performed using PHENIX (23). With the model given by molecular replacement, a rigid body refinement was carried out at 3.5 Å resolution. All data with a high-resolution limit of 1.6 Å were used for structure refinement with riding hydrogens. Once a satisfactory description of the protein electron density was complete, water molecules were added, followed by the modeling of norathyriol. A few cycles of slow-cooling annealing (2500–> 100K), positional, restrained isotropic temperature factor and TLS refinements were followed by visual inspection of the electron density maps, including omit maps, coupled with manual model building (when necessary) using the graphics program COOT (24). The refined electron density clearly matched the amino acid sequence of ERK2 with the exception of the N- and C-terminus (residues 3–17 and 358–361) and a weak electron density was observed for residues 23–34. Strong stereochemical restraints were imposed during the crystallographic refinement and the final ERK2 structure possessed a very good stereochemistry with a root mean square deviation (r.m.s.d.) of ~0.013 Å for bond lengths and ~1.4° for angles. The quality of the stereochemistry of the final protein structure was assessed with the PROCHECK package (25). The Ramachandran plot showed no residues in disallowed regions (data not shown). As a better guide to the quality of the structure, the values of the free R-factor were monitored during the course of the crystallographic refinement. The final value of free R-factors did not exceed the overall R-factor by more than 3%. The refined coordinates of ERK2/norathyriol complex structure have been deposited into the Protein Data Bank with PBD ID 3SA0.
Cell growth and death assays
JB6 P+ cells were seeded (8×104 cells/well) in 6-well plates with 5% FBS/MEM at 37°C in a 5% CO2 incubator overnight and then starved in a serum-free medium for 24 h. They were then fed with fresh medium and treated with different doses of norathyriol (0, 1, 10, or 25 µM). After 24 or 72 h of treatment, total cells were collected by brief trypsinization, and washed with PBS. Total cell number was determined by counting each sample in duplicate using a hemocytometer under an inverted microscope. Cell viability was determined using the trypan blue exclusion method. The data shown in this study are the mean of 3 independent experiments.
Cell cycle assay
JB6 P+ cells were seeded (2×105 cells/well) in 60 mm dishes with 5% FBS/MEM at 37°C in a 5% CO2 incubator overnight. Then cells were starved in a serum-free medium for 24 h followed by treatment with norathyriol (0, 1, 10, or 25 µM) in MEM containing 5% FBS for 24 or 72 h. The cells were trypsinized, washed twice with cold PBS, and fixed with ice-cold 70% ethanol at −20°C overnight. Cells were then washed twice with PBS, incubated with 20 mg/ml RNase A and 200 mg/ml propidium iodide in PBS at room temperature for 30 min in the dark, and subjected to flow cytometry using the FACSCalibur flow cytometer. Data were analyzed using ModFit LT (Verity Software House, Inc., Topsham, ME).
Mouse skin tumorigenesis study
Female SKH-1 hairless mice (5–13 wks of age; mean body weight, 25 g) were purchased from the National Cancer Institute (NIH) and were maintained under “specific pathogen-free” conditions according to guidelines established by Research Animal Resources, University of Minnesota. Skin carcinogenesis in mice was induced using a solar UV irradiation system. The solar UV radiation source (Q-Lab Corporation) emitted at wavelengths of 295–365 nm and the peak emission was 340 nm. SKH-1 mice were divided into 4 groups of 10 animals each. In the control group, the dorsal skin was topically treated with 200 µL acetone only. In the solar UV-treated group, the dorsal skin was topically treated with 200 µL acetone 1 h before UVB. The mice in groups 3 and 4 received topical application of norathyriol (0.5 or 1 mg, respectively) in 200 µL acetone 1 h before solar UV irradiation. At week 1, the solar UV dose was 30 kJ/m2 UVA/1.8 kJ/m2 UVB given twice/wk. The dose of solar UV was progressively increased (10% each week). At week 6, the dose was 48 kJ/m2 UVA/2.9 kJ/m2 UVB and this dose was maintained for week 6–15. The tumor was defined as an outgrowth of >1 mm in diameter that persisted for 2 weeks or more. Tumor numbers and volume were recorded every wk until the end of the experiment. One-half of the samples were immediately fixed in 10% neutral buffered formalin and processed for H&E staining and immunostaining. The other samples were frozen and used for Western blot analysis.
Immunostaining
Mouse skin and tumor samples were blocked with 5% donkey serum albumin in 600 µl 1× phosphate-buffered saline/0.03% Triton X-100, (pH 6.0) in a humidified chamber for 1 h at room temperature and then were immunostained with antibodies as follows: (i) 1:200 anti-pERK1/2 (Tyr-202/Tyr-204) raised in rabbit (Cell Signaling Technology) and 1:200 donkey anti-rabbit IgG conjugated to Cy3 (Jackson ImmunoResearch Laboratories); and (ii) 1:200 anti-ERK1/2 raised in rabbit (Cell Signaling Technology) and 1:200 donkey anti-rabbit IgG conjugated to Dylight 488 (Jackson ImmunoResearch Laboratories).
Statistical analysis
Significant differences were determined using one-way ANOVA.
Results
Norathyriol suppresses UVB-induced phosphorylation of ERKs, AP-1 and NF-κB activation in JB6 P+ cells
Hyperactivation of ERKs results in unregulated cell proliferation in several human cancers including skin cancer (26–27), suggesting that inhibition ERKs represents a potential approach for the prevention of cancer. Using in silico virtual screening, we found that norathyriol (Fig. 1A) might be a potential ERK2 inhibitor. First, we examined the cytotoxicity of this compound. The varying concentrations (0–50 µM) of norathyriol and the time (0–48 h) of exposure had minimal effect on the viability of JB6 P+ cells (Figure 1B) exposed to norathyriol. We also measured the effect of norathyriol on UVB-induced MAPKs phosphorylation in JB6 P+ cells. Results showed that norathyriol inhibited UVB induced phosphorylation of ERKs and RSK, but had no obvious effect on JNKs or p38 (Figure 2A). The transcription factor activator protein-1 (AP-1) and nuclear factor kappa B (NF-κB) are activated through the MAPKs pathway upon stimulation with UV (1, 28). To detect the transactivation of AP-1 and NF-κB, we exposed JB6 P+ cells stably transfected with an AP-1 or NF-κB luciferase reporter plasmid to norathyriol and this compound suppressed UVB-induced transactivation of AP-1 and NF-κB in a dose-dependent manner (Figure 2B).
Figure 2. Norathyriol inhibits UVB-induced AP-1 and NF-κB transactivation in JB6 cells through inhibition of the ERKs signaling pathway.
(A) Norathyriol inhibits UVB-induced phosphorylation of ERKs and p90RSK. Cells were treated with norathyriol at the indicated concentrations (0–25 µM) for 2 h, and then exposed to UVB (4 kJ/0.5 m2) and harvested after 30 min. The levels of phosphorylated and total ERKs, p90RSK, JNKs and p38 proteins were determined by Western blot analysis. (B) For the luciferase assay, JB6 cells stably transfected with an AP-1 or NF-κB luciferase reporter plasmid were cultured. Cells were starved in serum free medium for 24 h, and then treated with norathyriol (0–25 µM), or its vehicle, DMSO (negative control), in serum free medium for 2 h. Cells were then exposed to UVB (4kJ/m2) and harvested 3 h later. Luciferase activity was measured and AP-1 or NF-κB activity is expressed relative to control cells without UVB treatment. Data are represented as means ± S.E. The asterisk (*) indicates a significant difference (p < 0.05) between groups treated with UVB and norathyriol and the group treated with UVB alone.
ERKs are potential targets of norathyriol
We focused on ERKs signaling and determined the effects of norathyriol on the kinase activity of ERK1 and ERK2. Kinase assay data revealed that norathyriol (10 µM) strongly suppressed ERK1 and 2 activities in vitro (Figure 3A). A direct interaction of norathyriol with ERK1 and 2 was demonstrated by an in vitro pull-down assay (Figure 3B). To examine the means by which norathyriol binds directly with ERK2, we performed an ATP competition assay. The results indicated that norathyriol inhibits ERK2 activity competitively with ATP (Figure 3C).
Figure 3. Norathyriol inhibits ERK1 and 2 kinase activities and directly binds with ERK1 or 2 in an ATP-competitive manner.
(A) Norathyriol inhibits ERK1 or 2 kinase activity. An in vitro ERK1 or 2 kinase assay was performed as described in “Materials and Methods”. A GST-RSK2 fusion protein was used in an in vitro kinase assay with active ERK1 or 2 and results were visualized by autoradiography. Coomassie blue staining of the GST fusion protein serves as a loading control. Left panels: Lane 1 – positive control, which indicates that active ERK2 phosphorylates the GST RSK2 fusion protein; lanes 2 and 3 are positive controls and indicate that increasing amounts of CAY10561 (commercially available ERK2 inhibitor) suppresses ERK2 kinase activity; lanes 4, 5, and 6 - increasing amounts of norathyriol suppresses ERK2 kinase activity. Right panels: Lane 1 – positive control, which indicates that active ERK1 phosphorylates the GST-RSK2 fusion protein; lanes 2 and 3 - increasing amounts of norathyriol suppresses ERK1 kinase activity. (B) Norathyriol directly binds with ERK1 or 2. Binding of norathyriol with ERK1 or 2 was confirmed by immunobloting using an antibody against ERK1 or 2. Lane 1 (input control), ERK1 or ERK2 protein standard; lane 2 (control), Sepharose 4B was used to pull down ERK1 or ERK2; and lane 3, ERK1 or ERK2 was pulled down using norathyriol-conjugated Sepharsoe 4B beads as described in “Materials and Methods”. (C) Norathyriol binds to ERK2 competitively with ATP. Active ERK2 (0.2 µg) was incubated with ATP at the indicated concentrations (0, 1, 10, 100 µM) and 100 µl of norathyriol-Sepharose 4B or 100 µl of Sepharose 4B (as a negative control) beads in a reaction buffer to a final volume of 500 µl. The pulled down proteins were detected by Western blot as described in “Materials and Methods”. Lane 1 (input control), ERK2 protein standard; lane 2, negative control, indicating that ERK2 does not bind with Sepharose 4B; lane3, positive control, indicating that ERK2 binds with norathyriol-Sepharose 4B beads; lane 4, 5 and 6, increasing amounts of ATP inhibits norathyriol binding with ERK2.
To confirm further that ERKs are a molecular target for norathyriol, we initiated a crystallographic study. Human ERK2 was cloned and purified from E.coli, and successfully crystallized. To obtain the ERK2/norathyriol complex, the crystals were soaked with solution containing norathyriol. The three-dimensional structure of ERK2 in complex with norathyriol was refined to a resolution of 1.6 Å. The data collection and refinement statistics are presented in Supplemental Table 1. Norathyriol was found in the ATP-binding site, between thetwo kinase lobes (Figure 4A and B). The xanthone moiety is located below the phosphate-binding loop and occupies a position similar to that of the adenine ring of ATP. Three hydrogen bonds are formed between norathyriol and the hinge loop of ERK2 that involves the side-chain of Gln105 and the backbone chain of Asp106 and Met108 (Figure 4B and C). The side chain carboxyl of Gln105, the identified gatekeeper residue (29), hydrogen bonded with the 7-OH group of norathyriol. The backbone of Asp106 and Met108 form hydrogen bonds with the 6-OH group of norathyriol. A recombinant ERK2 mutant, Q105A, showed decreased binding to the Sepharose 4B conjugated with norathyriol (Figure 4D). The Ile31, Val39, and Ala52 residues from the N-lobe contribute to the hydrophobic interactions with the xanthone moiety of norathyriol. The carbonyl oxygen of norathyriol orients toward the conserved Lys52 from the β3-strand, but forms no contacts with that residue (2.91 A). Together, the hydrogen bonds and hydrophobic interactions stabilize norathyriol in the ATP-binding pocket.
Figure 4. The crystal structure of the ERK2/norathyriol complex.
(A) Overall ribbon presentation showing norathyriol bound to the hinge loop, a crossover connection between the N- and the C-lobes, in the ATP-binding pocket. The α–helices are shown in magenta, and the β–strands are shown in cyan. The norathyriol molecule in stick presentation is shown in green. (B) Close-up view of the active site showing the hydrogen bonding between norathyriol and the residues in the hinge loop. The Gln105, Asp106 and Met108 residues each form a hydrogen bond at 2.86 Å, 2.43 Å and 2.86 Å, respectively. (C) The 2|Fo|-|Fc| electron density map countered at 1.1σ. The hinge loop, including the residues Gln105, Asp106 and Met108 are shown in sticks presentation. (D) Comparison of norathyriol binding with a wildtype recombinant ERK2 or an ERK2 Q105A mutant. Proteins were incubated with norathyriol-conjugated Sepharose 4B beads, and analyzed by Western blot with an ERKs antibody.
Norathyriol inhibits growth by inducing cell cycle arrest in JB6 P+ cells
Early studies showing a link between ERKs signaling and cell cycle machinery included the demonstration that blockade of thrombin-induced growth of Chinese hamster lung fibroblasts correlated with suppression of ERKs activation (30). Similarly, dominant-negative forms of ERKs inhibited growth of NIH3T3 fibroblasts (31). Because norathyriol inhibited ERKs signaling, we assessed the effect of norathyriol on JB6 P+ cell growth, cell death and cell cycle. Cells were treated with different concentrations of the agent (1, 10, or 25 µM, final concentration in medium) dissolved in DMSO (vehicle) for 24 or 72 h. At the end of each treatment time, determination of total cell number as well as number of dead cells showed that norathyriol inhibits cell growth in a dose- as well as time-dependent manner but does not cause cell death (Figure 5A and B). The middle dose of norathyriol (10 µM) showed 29 and 52% decreases in total cell number after 24 and 72 h of treatment, respectively (Figure 5A). The high dose (25 µM) decreased cell number by 45% and 68% after 24 and 72 h of treatment, respectively (Figure 5A). Using the trypan blue dye exclusion method, we observed that the decrease in cell number caused by norathyriol was not accompanied by an increase in cell death (Figure 5B). These data suggested that a decrease in cell number did not contribute to the cell-death-inducing effect of norathyriol. We investigated the effect of norathyriol on cell cycle progression to determine whether the inhibitory effect on proliferation is caused by modulation of cell cycle progression. After treatment with norathyriol, cells were stained with propidium iodide and analyzed by flow cytometry. Cell cycle distribution analysis showed that the high dose of norathyriol for 24 and 72 h resulted in an increase in the number of cells in G2-M-phase (Figure 5C). The G2-M-phase of the cell cycle is reportedly controlled primarily by cyclin B1 and its associated catalytically active partner Cdk1 (32). Therefore, we tested the effect of norathyriol on cyclin B1 and Cdk1 expression and Western blot data showed increases in cyclin B1 and phosphorylated Cdk1 protein level after treatment for 72 h. These results suggested norathyriol inhibits cell growth by inducing cell cycle G2-M arrest.
Figure 5. Norathyriol inhibits JB6 cell growth by inducing G2-M arrest.
Cells were starved in serum free medium for 24 h, and then treated with norathyriol (0–25 µM), or its vehicle, DMSO (control), in 5% FBS/MEM for 24 or 72 h. At the end of each treatment time, both floating and attached cells were collected and processed for (A) determination of total cell number and (B) number of dead cells. Data are represented as means ± S.E. The asterisk (*) indicates a significant difference (p < 0.05) between groups treated with norathyriol and the group treated with DMSO. (C) Cells were starved in serum free medium for 24 h, and then treated with norathyriol (0–25 µM), or DMSO (control), for the indicated times. Cell cycle analysis was performed by flow cytometry. Data are represented as means ± S.E. The asterisk (*) indicates a significant difference (p < 0.05) between groups treated with norathyriol and the group treated with DMSO. (D) For Western-blot analysis, cells were treated with norathyriol at the indicated concentrations for 72 h.
Norathyriol inhibits solar UV-induced skin tumorigenesis by blocking ERKs in SKH-1 hairless mice
To study the antitumorigenic activity of norathyriol in vivo, we evaluated the effect of norathyriol in a solar UV-induced mouse skin tumorigenesis model. Photographic data showed that norathyriol inhibited skin cancer development in mice treated with solar UV compared with mice treated only with solar UV (Figure 6A). Topically applied norathyriol (0.5 or 1 mg) on mouse skin resulted in a significant inhibition of average tumor number per mouse (p < 0.05; Figure 6B). The volume of tumors developed in solar UV-treated mouse skin was also significantly attenuated by norathyriol treatment (p < 0.05; Fig 6C). At the end of the study, skin and tumor samples were processed for H&E staining. After treatment with solar UV, epidermal thickness increased by edema and epithelial cell proliferation, while norathyriol significantly inhibited epidermal thickness and inflammation (Figure 6D). Immunostaining throughout the skin showed that chronic exposure to solar UV strongly increased phosphorylated and total ERKs expression compared with mice exposed to solar UV but treated only with acetone. However, treatment of skin with norathyriol resulted in a downregulation of phosphorylated and total ERKs levels (Figure 6E). Overall, these results indicate that norathyriol might serve as an effective chemopreventive agent against solar UV-mediated skin cancer.
Figure 6. Norathyriol inhibits solar UV-induced skin tumorigenesis in SKH-1 hairless mice.
The treatment groups were as follows: (a) not irradiated (control); (b) solar UV (SUV) treated; or (c) treated with 0.5 or 1mg of norathyriol in 200 µL of acetone before SUV treatment. The compound was applied topically to the dorsal surface of each mouse before solar UV exposure (SUV + Norathyriol 0.5 mg or SUV + Norathyriol 1 mg) as detailed in “Materials and Methods”. (A) External appearance of tumors. (B) Average tumor number per mouse. Data are represented as means ± S.E. The asterisk (*) indicates a significant difference (p < 0.05) between the SUV groups treated with norathyriol and the group treated with acetone. (C) Tumor volume was calculated using the formula: tumor volume (mm3) = (length×width×height×0.52). Data are represented as means ± S.E. The asterisk (*) indicates a significant difference (p < 0.05) between the SUV groups treated with norathyriol and the group treated with acetone. At the end of the study, skin and tumor samples were fixed in 10% neutral buffered formalin and processed for H&E staining (D) and immunostaining with specific primary antibodies to detect phsophorylated ERKs (Tyr-202/Tyr-204) and total ERKs (E).
Discussion
Epidemiological, clinical and laboratory studies demonstrated that chronic solar UV radiation exposure-induced skin cancer is caused by the excessive induction of inflammation, oxidative stress and DNA damage (3). The use of chemopreventive agents, especially naturally occurring plant products, to inhibit these events in UV-exposed skin is gaining more and more attention. A variety of phytochemicals have been reported to possess substantial skin photoprotective effects (2–3, 5). Mangiferin is a xanthone (2-b-D-glucopyranosyl-1, 3, 6, 7-tetrahydroxy-9H-xanthen-9-one) widely distributed in mango (11). Norathyriol is a main metabolite of mangiferin in vivo, derived from a deglycosilation process (33–34). Norathyriol possesses antioxidant, anti-inflammatory and antitumor effects. Recent studies showed that norahtyriol is more active than mangigerin (11, 14). In the present study, we demonstrated a chemopreventive effect of norathyriol against UV-induced skin cancer development and have identified a molecular mechanism(s) and possible protein target(s).
Cellular homeostasis, the equilibrium between cell proliferation and cell death is controlled by cell cycle progression and apoptosis induction (35). Unchecked proliferative potential involving deregulation of cell cycle progression is generally described as a central process in the development of cancer (36). We examined the effects of norathyriol on mouse epidermal JB6 P+ cell proliferation, cell death and cell cycle. These data showed that the inhibition of proliferation does not contribute to the cell death effect, but instead is associated with a G2/M cell cycle arrest induced by norathyriol. ERK1 and ERK2 are reportly involved in regulating the cell cycle in mammalian cells (10). An early study showed that in the G1 phase and around the M phase, ERK1 and ERK2 are activated in CHO cells (37). Recent data suggested that ERK1 and ERK2 are required for epidermal G2/M progression (38). Growth inhibition caused by ERK1/2 loss is rescued by reintroducing ERK2, but not by activating ERKs effectors that promote G1 cell cycle progression (38). Some natural compounds, such as oridonin (39) and Gleditsis sinensis thorn extract, reportedly induce G2/M arrest through ERK signaling (40). Our data showed that induction of cell cycle arrest by norathyriol may be mediated through ERKs signaling because norathyriol strongly suppresses ERK1 and 2 phosphorylation and kinase activities. Animal study results revealed that treatment with norathyriol inhibits solar UV-induced carcinogenesis by blocking ERKs activation.
AP-1 (activator protein-1) is a transcription factor that is extremely important in the progresses of cell proliferation, cell differentiation, inflammation, cell survival, cell transformation, as well as playing a major role in tumorigenesis (2, 5, 41). ERKs play a critical role in the transcriptional activity of AP-1. AP-1 and ERKs are key molecules activated after UVB exposure. Our results showed that norathyriol inhibits AP-1 transcriptional activation through suppression of the ERKs pathway. Norathryiol inhibited UVB-induced phosphorylation of ERKs and p90RSK. Using in silico virtual screening, we found that the molecular target of norathyriol might be ERKs. The results showed that norathyriol strongly inhibited ERKs kinase activity and that the inhibition resulted from ATP-competitive binding of norathyriol with ERKs.
The ERK2/norathyriol co-crystal structure presented herein first showed that ERKs may bind with xanthon-based compounds. Norathyriol was found to occupy the ATP-binding site with the xanthone moiety acting as an adenine mimeric and anchoring the compound to the hinge region by hydrogen bonds. The involvement of amino acids, Gln105, Asp106, and Met 108 residing at the hinge loop, in the interaction with inhibitors was previously observed in the ERK2/ pyrazolo[3,4-c]pyridazine derivative complex structure (42) and in the ERK2/FR 180204 complex structure (26). The Met108 residue was also involved in hydrogen bonding with olomoucine (43). The hydrogen bonding between inhibitor and the backbone nitrogen of methionine in the hinge loop (Met108 in ERK2) is conserved in all ATP-competitive kinase inhibitors (44). Overall, the ERK2/norathyriol complex structure is similar to the previously reported ERK2 structures complexed with different compounds (26).
In summary, our results clearly showed that topical application of norathyriol markedly inhibits the formation of skin cancer in SKH-1 hairless mice exposed to solar UV. This inhibition occurs mainly through the suppression of proliferation by inducing G2/M arrest and the downregulation of AP-1 activity by blocking ERKs. Norathyriol might be an ideal chemopreventive agent for skin cancer.
Table 1.
X-ray data collection and refinement statisticsa)
| Data collection | |
|---|---|
| Space group | P21 |
| Cell dimensions | |
| a, b, c (Å) | 48.69, 69.41, 59.72 |
| beta (°) | 108.99 |
| Resolution b) (Å) | 30-1.6 (1.66-1.60) |
| Rmerge | 0.101 (0.80) |
| I/σ | 40.9 (3.2) |
| Completeness (%) | 99.9 (100) |
| Redundancy | 15.3 (13.5) |
| Refinement | |
| Resolution (Å) | 30 - 1.6 |
| No. reflections | 48776 |
| Rwork/ Rfree c | 0.163 / 0.199 |
| No. atoms | |
| Protein | 5878 (with hydrogens) |
| Norathyriol | 19 (no hydrogens) |
| Water | 309 |
| R.m.s. deviations | |
| Bond lengths (Å) | ~0.013 Å |
| Bond angles (°) | ~1.4 Å |
Three crystals were used for the structure determination.
Highest resolution shell is shown in parenthesis.
Rfree was calculated from a randomly chosen 5% of reflections excluded from refinement
Acknowledgments
The crystallography work is based upon research conducted at the Northeastern Collaborative Access Team beamlines 24ID of the Advanced Photon Source (APS) and was supported by award RR-15301 from the National Center for Research Resources at the National Institutes of Health. Use of the APS is supported by the US Department of Energy, Office of Basic Energy Sciences, under contract No. W-31-109-ENG-38. This work was mainly supported by The Hormel Foundation and National Institutes of Health Grants R37 CA081064, CA027502, CA120388, and ES016548.
References
- 1.Bode AM, Dong Z. Mitogen-activated protein kinase activation in UV-induced signal transduction. Sci STKE. 2003;2003:RE2. doi: 10.1126/stke.2003.167.re2. [DOI] [PubMed] [Google Scholar]
- 2.Jung SK, Lee KW, Byun S, Kang NJ, Lim SH, Heo YS, et al. Myricetin suppresses UVB-induced skin cancer by targeting Fyn. Cancer Res. 2008;68:6021–6029. doi: 10.1158/0008-5472.CAN-08-0899. [DOI] [PubMed] [Google Scholar]
- 3.Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng D, Bode AM, Zhao Q, Cho YY, Zhu F, Ma WY, et al. The cannabinoid receptors are required for ultraviolet-induced inflammation and skin cancer development. Cancer Res. 2008;68:3992–3998. doi: 10.1158/0008-5472.CAN-07-6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byun S, Lee KW, Jung SK, Lee EJ, Hwang MK, Lim SH, et al. Luteolin inhibits protein kinase C(epsilon) and c-Src activities and UVB-induced skin cancer. Cancer Res. 2010;70:2415–2423. doi: 10.1158/0008-5472.CAN-09-4093. [DOI] [PubMed] [Google Scholar]
- 6.Assefa Z, Garmyn M, Bouillon R, Merlevede W, Vandenheede JR, Agostinis P. Differential stimulation of ERK and JNK activities by ultraviolet B irradiation and epidermal growth factor in human keratinocytes. J Invest Dermatol. 1997;108:886–891. doi: 10.1111/1523-1747.ep12292595. [DOI] [PubMed] [Google Scholar]
- 7.She QB, Ma WY, Zhong S, Dong Z. Activation of JNK1, RSK2, and MSK1 is involved in serine 112 phosphorylation of Bad by ultraviolet B radiation. J Biol Chem. 2002;277:24039–24048. doi: 10.1074/jbc.M109907200. [DOI] [PubMed] [Google Scholar]
- 8.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 9.Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 11.Chieli E, Romiti N, Rodeiro I, Garrido G. In vitro effects of Mangifera indica and polyphenols derived on ABCB1/P-glycoprotein activity. Food Chem Toxicol. 2009;47:2703–2710. doi: 10.1016/j.fct.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Hsu MF, Lin CN, Lu MC, Wang JP. Inhibition of the arachidonic acid cascade by norathyriol via blockade of cyclooxygenase and lipoxygenase activity in neutrophils. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:507–515. doi: 10.1007/s00210-004-0922-9. [DOI] [PubMed] [Google Scholar]
- 13.Lee HZ, Lin WC, Yeh FT, Lin CN, Wu CH. Decreased protein kinase C activation mediates inhibitory effect of norathyriol on serotonin-mediated endothelial permeability. Eur J Pharmacol. 1998;353:303–313. doi: 10.1016/s0014-2999(98)00385-9. [DOI] [PubMed] [Google Scholar]
- 14.Wilkinson AS, Monteith GR, Shaw PN, Lin CN, Gidley MJ, Roberts-Thomson SJ. Effects of the mango components mangiferin and quercetin and the putative mangiferin metabolite norathyriol on the transactivation of peroxisome proliferator-activated receptor isoforms. J Agric Food Chem. 2008;56:3037–3042. doi: 10.1021/jf800046n. [DOI] [PubMed] [Google Scholar]
- 15.Teng CM, Ko FN, Wang JP, Lin CN, Wu TS, Chen CC, et al. Antihaemostatic and antithrombotic effect of some antiplatelet agents isolated from Chinese herbs. J Pharm Pharmacol. 1991;43:667–669. doi: 10.1111/j.2042-7158.1991.tb03561.x. [DOI] [PubMed] [Google Scholar]
- 16.Ko FN, Lin CN, Liou SS, Huang TF, Teng CM. Vasorelaxation of rat thoracic aorta caused by norathyriol isolated from Gentianaceae. Eur J Pharmacol. 1991;192:133–139. doi: 10.1016/0014-2999(91)90079-6. [DOI] [PubMed] [Google Scholar]
- 17.Wang JP, Raung SL, Lin CN, Teng CM. Inhibitory effect of norathyriol, a xanthone from Tripterospermum lanceolatum, on cutaneous plasma extravasation. Eur J Pharmacol. 1994;251:35–42. doi: 10.1016/0014-2999(94)90440-5. [DOI] [PubMed] [Google Scholar]
- 18.Kang JJ, Cheng YW, Ko FN, Kuo ML, Lin CN, Teng CM. Induction of calcium release from sarcoplasmic reticulum of skeletal muscle by xanthone and norathyriol. Br J Pharmacol. 1996;118:1736–1742. doi: 10.1111/j.1476-5381.1996.tb15599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu MF, Raung SL, Tsao LT, Lin CN, Wang JP. Examination of the inhibitory effect of norathyriol in formylmethionyl-leucyl-phenylalanine-induced respiratory burst in rat neutrophils. Free Radic Biol Med. 1997;23:1035–1045. doi: 10.1016/s0891-5849(97)00132-9. [DOI] [PubMed] [Google Scholar]
- 20.Wang JP, Raung SL, Tsao LT, Lin CN. Evidence for the involvement of protein kinase C inhibition by norathyriol in the reduction of phorbol ester-induced neutrophil superoxide anion generation and aggregation. Eur J Pharmacol. 1997;336:81–88. doi: 10.1016/s0014-2999(97)01214-4. [DOI] [PubMed] [Google Scholar]
- 21.Lin CN, Liou SS, Ko FN, Teng CM. Gamma-pyrone compounds. II: Synthesis and antiplatelet effects of tetraoxygenated xanthones. J Pharm Sci. 1992;81:1109–1112. doi: 10.1002/jps.2600811114. [DOI] [PubMed] [Google Scholar]
- 22.Otwinowski Z, Minor W. Processing of x-ray diffraction data collected in oscillation mode. Methods in Enzymology. 1997;276 doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 23.Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, et al. PHENIX: building new software for automated crystallographic structure determination. Acta crystallographica. 2002;58:1948–1954. doi: 10.1107/s0907444902016657. [DOI] [PubMed] [Google Scholar]
- 24.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta crystallographica. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 25.Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Cryst. 1993;26:283–291. [Google Scholar]
- 26.Ohori M, Kinoshita T, Okubo M, Sato K, Yamazaki A, Arakawa H, et al. Identification of a selective ERK inhibitor and structural determination of the inhibitor-ERK2 complex. Biochem Biophys Res Commun. 2005;336:357–363. doi: 10.1016/j.bbrc.2005.08.082. [DOI] [PubMed] [Google Scholar]
- 27.Russo AE, Torrisi E, Bevelacqua Y, Perrotta R, Libra M, McCubrey JA, et al. Melanoma: molecular pathogenesis and emerging target therapies (Review) Int J Oncol. 2009;34:1481–1489. doi: 10.3892/ijo_00000277. [DOI] [PubMed] [Google Scholar]
- 28.Bode HB, Zeeck A. UV mutagenesis and enzyme inhibitors as tools to elucidate the late biosynthesis of the spirobisnaphthalenes. Phytochemistry. 2000;55:311–316. doi: 10.1016/s0031-9422(00)00307-1. [DOI] [PubMed] [Google Scholar]
- 29.Aronov AM, Baker C, Bemis GW, Cao J, Chen G, Ford PJ, et al. Flipped out: structure-guided design of selective pyrazolylpyrrole ERK inhibitors. J Med Chem. 2007;50:1280–1287. doi: 10.1021/jm061381f. [DOI] [PubMed] [Google Scholar]
- 30.Meloche S, Seuwen K, Pages G, Pouyssegur J. Biphasic and synergistic activation of p44mapk (ERK1) by growth factors: correlation between late phase activation and mitogenicity. Mol Endocrinol. 1992;6:845–854. doi: 10.1210/mend.6.5.1603090. [DOI] [PubMed] [Google Scholar]
- 31.Pages G, Lenormand P, L'Allemain G, Chambard JC, Meloche S, Pouyssegur J. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc Natl Acad Sci U S A. 1993;90:8319–8323. doi: 10.1073/pnas.90.18.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plesca D, Crosby ME, Gupta D, Almasan A. E2F4 function in G2: maintaining G2-arrest to prevent mitotic entry with damaged DNA. Cell Cycle. 2007;6:1147–1152. doi: 10.4161/cc.6.10.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bock C, Waldmann KH, Ternes W. Mangiferin and hesperidin metabolites are absorbed from the gastrointestinal tract of pigs after oral ingestion of a Cyclopia genistoides (honeybush tea) extract. Nutr Res. 2008;28:879–891. doi: 10.1016/j.nutres.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Ye G, Ma CH, Tang YH, Fan MS, Li ZX, et al. Identification and determination of four metabolites of mangiferin in rat urine. J Pharm Biomed Anal. 2007;45:793–798. doi: 10.1016/j.jpba.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 35.Singh RP, Agarwal R. Natural flavonoids targeting deregulated cell cycle progression in cancer cells. Curr Drug Targets. 2006;7:345–354. doi: 10.2174/138945006776055004. [DOI] [PubMed] [Google Scholar]
- 36.Deep G, Singh RP, Agarwal C, Kroll DJ, Agarwal R. Silymarin and silibinin cause G1 and G2-M cell cycle arrest via distinct circuitries in human prostate cancer PC3 cells: a comparison of flavanone silibinin with flavanolignan mixture silymarin. Oncogene. 2006;25:1053–1069. doi: 10.1038/sj.onc.1209146. [DOI] [PubMed] [Google Scholar]
- 37.Tamemoto H, Kadowaki T, Tobe K, Ueki K, Izumi T, Chatani Y, et al. Biphasic activation of two mitogen-activated protein kinases during the cell cycle in mammalian cells. J Biol Chem. 1992;267:20293–20297. [PubMed] [Google Scholar]
- 38.Dumesic PA, Scholl FA, Barragan DI, Khavari PA. Erk1/2 MAP kinases are required for epidermal G2/M progression. J Cell Biol. 2009;185:409–422. doi: 10.1083/jcb.200804038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng Y, Qiu F, Ye YC, Tashiro S, Onodera S, Ikejima T. Oridonin induces G2/M arrest and apoptosis via activating ERK-p53 apoptotic pathway and inhibiting PTK-Ras-Raf-JNK survival pathway in murine fibrosarcoma L929 cells. Arch Biochem Biophys. 2009;490:70–75. doi: 10.1016/j.abb.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 40.Lee SJ, Park K, Ha SD, Kim WJ, Moon SK. Gleditsia sinensis thorn extract inhibits human colon cancer cells: the role of ERK1/2, G2/M-phase cell cycle arrest and p53 expression. Phytother Res. 2010;24:1870–1876. doi: 10.1002/ptr.3214. [DOI] [PubMed] [Google Scholar]
- 41.Cooper SJ, Bowden GT. Ultraviolet B regulation of transcription factor families: roles of nuclear factor-kappa B (NF-kappaB) and activator protein-1 (AP-1) in UVB-induced skin carcinogenesis. Curr Cancer Drug Targets. 2007;7:325–334. doi: 10.2174/156800907780809714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kinoshita T, Warizaya M, Ohori M, Sato K, Neya M, Fujii T. Crystal structure of human ERK2 complexed with a pyrazolo[3,4-c]pyridazine derivative. Bioorg Med Chem Lett. 2006;16:55–58. doi: 10.1016/j.bmcl.2005.09.055. [DOI] [PubMed] [Google Scholar]
- 43.Wang Z, Canagarajah BJ, Boehm JC, Kassisa S, Cobb MH, Young PR, et al. Structural basis of inhibitor selectivity in MAP kinases. Structure. 1998;6:1117–1128. doi: 10.1016/s0969-2126(98)00113-0. [DOI] [PubMed] [Google Scholar]
- 44.Rastelli G, Rosenfeld R, Reid R, Santi DV. Molecular modeling and crystal structure of ERK2-hypothemycin complexes. J Struct Biol. 2008;164:18–23. doi: 10.1016/j.jsb.2008.05.002. [DOI] [PubMed] [Google Scholar]