Abstract
The neural mechanisms supporting social bonds between adult men remain uncertain. In this double-blind, placebo-controlled study, we investigate the impact of intranasally administered oxytocin (OT) and vasopressin (AVP) on behavior and brain activity among men in the context of an iterated Prisoner’s Dilemma game, which models a real-life social situation. fMRI results show that, relative to both AVP and placebo, OT increases the caudate nucleus response to reciprocated cooperation, which may augment the reward of reciprocated cooperation and/or facilitate learning that another person can be trusted. OT also enhances left amygdala activation in response to reciprocated cooperation. Behaviorally, OT was associated with increased rates of cooperation following unreciprocated cooperation in the previous round compared with AVP. AVP strongly increased cooperation in response to a cooperative gesture by the partner compared with both placebo and OT. In response to reciprocated cooperation, AVP increased activation in a region spanning known vasopressin circuitry implicated in affiliative behaviors in other species. Finally, both OT and AVP increase amygdala functional connectivity with the anterior insula relative to placebo, which may increase the amygdala’s ability to elicit visceral somatic markers that guide decision making. These findings extend our knowledge of the neural and behavioral effects of OT and AVP to the context of genuine social interactions.
Keywords: oxytocin, vasopressin, cooperation, fMRI
Introduction
In nature, the paradigmatic social bond is that between mother and offspring. Research with animal models implicates both the oxytocin (OT) and dopamine (DA) systems in the establishment and maintenance of maternal attachment and caregiving. OT enables mothers to overcome avoidance of proximity to offspring and, together with dopamine, may render maternal caregiving rewarding (Swain et al., 2007, 2010). The OT and DA systems interact in the ventral striatum, implicating the latter in maternal motivation (Skuse and Gallagher, 2009; Strathearn et al., 2009). This mechanism also appears to mediate female attachment to adult male partners in pair-bonding voles (Liu and Wang, 2003; Young et al., 2005) and possibly humans (Bartels and Zeki, 2004; Aron et al., 2005; Fisher et al., 2005), prompting the suggestion that modification of neural systems involved in maternal care is a parsimonious mechanism for the evolution of adult female pair-bonding (Ross and Young, 2009).
Non-reproductive social bonds between unrelated adults have been less thoroughly investigated, however similar mechanisms may be involved. Reciprocated cooperation from adult strangers activates the caudate nucleus (Rilling et al., 2002; Rilling et al., 2004; Delgado et al., 2005), a region known to receive DA projections from the midbrain, and caudate activation predicts future reciprocity (King-Casas et al., 2005). Intranasal OT is associated with increased trust (Kosfeld et al., 2005; Baumgartner et al., 2008; Andari et al., 2010), and a recent study presented evidence suggesting that OT mediates bonding among men in groups (De Dreu et al., 2010). Thus, OT and DA may interact to support bonds among adult men. Here, we administered OT to men as they played an iterated Prisoner’s Dilemma (PD) game to determine if OT would augment activation in the caudate nucleus in response to reciprocated cooperation.
OT appears to reduce the salience of negative social stimuli and increase the salience of positive social stimuli (Heinrichs et al., 2003; Baumgartner et al., 2008; Guastella et al., 2008; Petrovic et al., 2008; Unkelbach et al., 2008; Heinrichs et al., 2009; Theodoridou et al., 2009; Gamer et al., 2010). Recent studies have revealed a potential neural mechanism for these effects. OT decreases the amygdala response to aversive social stimuli in men (Kirsch et al., 2005; Domes et al., 2007; Baumgartner et al., 2008; Petrovic et al., 2008; Gamer et al., 2010). Domes et al (2007) also showed that OT decreased amygdala activation to all emotional face stimuli (including happy faces) in the right amygdala, and a recent study showed that OT increased amygdala activation to happy faces in the left amygdala (Gamer et al., 2010). Collectively, these studies led us to the prediction that OT would decrease the right amygdala response to unreciprocated cooperation (an aversive social stimulus), and perhaps also reciprocated cooperation, whereas OT would increase the left amygdala response to reciprocated cooperation.
While OT is anxiolytic, AVP is anxiogenic (Heinrichs et al., 2009) and may play a role in inter-male aggressive communication (Thompson et al., 2004; Thompson et al., 2006). Polymorphisms of the V1a vasopressin receptor (AVPR1a) have been linked with differences in amygdala responses to emotional facial expressions (Meyer-Lindenberg et al., 2008). Consequently, we predicted that AVP administration would be associated with increased amygdala activation and decreased rates of cooperation in our sample of male subjects.
Finally, we hypothesized that neuropeptide effects on behavior and brain activity would be specific to interactions with assumed human partners and would not be observed for interactions with known computer partners. Importantly, this is the first study to simultaneously test the effects of both OT and AVP on behavior and brain activation in a social interactive context.
Methods
Subjects
91 men from the Emory University community between the ages of 18 and 22 (mean = 20.2) were randomized to receive intranasal OT (n=27), intranasal AVP (n=27) or intranasal placebo (n=36). 5 subjects (AVP n=2, OT n=1, and placebo n=2) were excluded from the neuroimaging analysis due to excessive motion (>1.3mm) (n=3) or to missing data (n=2). One subject was excluded from the behavioral analysis due to missing data. All potential subjects completed a full medical history questionnaire. Subjects with a history of head trauma, seizures or other neurological disorders, psychiatric illness, alcoholism or any other substance abuse, hypertension, cardiovascular disease, diabetes and other endocrine diseases or malignancy were excluded from the study. Subjects who had used medications with known psychoactive effects over the past year were also excluded. All studies were conducted between the hours of 9 AM and 6 PM across the entire year. All subjects gave written informed consent, and the study was approved by the Emory University Institutional Review Board.
Prisoner’s Dilemma Task
The iterated Prisoner’s Dilemma game is a model for relationships based on reciprocal altruism. In the game, two players choose to either cooperate or defect and receive a payoff that depends upon the interaction of their respective choices. The game version we use here is a sequential-choice PD game in which player 1 chooses and player 2 is then able to view player 1’s choice before making his own choice. Each of the four outcomes is associated with a different payoff. Player cooperation followed by partner cooperation (CC) pays $2 to both player and partner, player cooperation followed by partner defection (CD) pays $0 to the player and $3 to the partner, player defection followed by partner defection (DD) pays $1 to both player and partner, and player defection followed by partner cooperation (DC) pays $3 to the player and $0 to the partner.
Preparation of OT, AVP and placebo
Intranasal AVP
6 ml of 20 unit/ml AVP (American Reagent Laboratories, Shirley, NY) were transferred to a plastic bottle with nasal applicator.
Intranasal AVP placebo
The AVP placebo consisted of the vasopressin vehicle only, and was prepared by adding 125 mg of 0.5% chlorobutanol to 50 ml saline, followed by acetic acid until the pH fell within the range of 2.5 to 4.5 as measured with a pH meter. The solution was then sterilized using a 0.22 micron filter. 6 ml were transferred to a plastic bottle with nasal applicator.
Intranasal OT
3.6 ml of 40 IU/ml OT (Syntocinin-Spray, Novartis) was added to 2.4 ml OT placebo to make 6.0 ml of 24 IU/ml OT. 6 ml were transferred to a plastic bottle with a nasal applicator.
Intranasal OT placebo
The OT placebo consisted of the Syntocinon vehicle only. Each 5 ml of OT placebo consists of: Chlorobutanol hemihydrate 12.5 mg, Methyl-4-hydroxybenzoate 2.0 mg, Propyl-4-hydroxybenzoate 1.0 mg, 85% ethanol 125 μl, Sodium acetate 14.0 mg, purified water 4.8455 g. 6 ml were transferred to a plastic bottle with nasal applicator.
Behavioral Procedures
PD tutorial and practice trials
All subjects completed a 10 minute computer tutorial that explained the Prisoner’s Dilemma game, and were given a 4-question multiple choice quiz to evaluate their understanding. If any question was answered incorrectly, study personnel explained to participants why that answer was wrong and why another answer was correct. If necessary, subjects repeated the tutorial. Study personnel continued with the experiment only after they were convinced that the subject fully comprehended the task. Subjects then completed two practice rounds of the game using the response box they would be holding while in the scanner. The practice trials familiarized subjects with the feel of the game and the operation of the response box.
Administration of OT, AVP or placebo
Both experimenters and subjects were blind to the treatment subjects received. All solutions were administered intranasally. The OT group self-administered 24 IU oxytocin (Syntocinin-Spray, Novartis), and the AVP group self-administered 20 IU of AVP (American Reagent Laboratries, Shirley, NY). In each case, this required 10 nasal puffs to administer 1 ml of solution. The placebo group self-administered 10 nasal puffs of either OT placebo or AVP placebo. Half of the placebo subjects received OT placebo and half received AVP placebo. Subjects were instructed to place the nasal applicator in one nostril and depress the lever until they felt a mist of spray in the nostril, to then breathe in deeply through the nose, and afterwards to place the applicator in the other nostril and repeat the process.
Monitor vital signs and administer PANAS
To monitor for unintended side-effects of neuropeptide administration, subjects’ ear temperature, heart rate and blood pressure were measured prior to drug administration and again approximately 20 minutes later. Blood pressure was also measured at 5 minute intervals during scanning. To evaluate any effects of neuropeptides on mood or anxiety, subjects also completed the Positive and Negative Affect Schedule (Watson et al., 1988) at both of these time points, as well as immediately after the scan.
Blood sample
Twenty minutes after drug administration, a blood sample was drawn for measurement of plasma OT and AVP levels. Due to difficult vascular access, samples were not obtained from 12 subjects. Samples were centrifuged at 4C within 20 minutes of blood draw. Plasma was collected and frozen at −80C until assay.
Confederate introductions
Prior to entering the scanner, subjects met two male confederates, one of whom partnered the subject when the subject played as player 1 and another who partnered the subject when the subject played as player 2. Subjects were told that they would play 30 rounds of an iterated PD game with each of the two partners. The meeting between subjects and their partners consisted of a very brief introduction in which the two parties could greet one another but were not given the opportunity to engage in conversation. A total of ten different confederates were used. Although confederates were matched to subjects on sex and age (mean age = 22.9), they were not matched on race. However, behavioral analyses showed no significant difference in outcome frequency as a function of whether subjects were playing with racial ingroup or outgroup partners. Confederates were not intentionally randomized over the three treatment groups. However, a post-hoc analysis revealed that for 9 of the 10 confederates, the proportion of subjects in each treatment group that the confederate faced did not differ significantly from the proportion of subjects in each treatment group in the overall sample. These proportions were significantly different for only one subject (χ2 (2 N=5) = 6.33, p=0.04) who was over-represented in the OT group and underrepresented in the placebo group.
Anatomical image acquisition
Subjects were next positioned in the MRI scanner (Siemens Trio 3T). Subjects lay motionless in a supine position in the scanner with padded head restraint to minimize head movement during scanning. Each scanning session began with a 15 second scout, followed by a 5 minute T1-weighted MPRAGE scan (TR= 2600 ms, TE = 3.02 ms, matrix = 256×256, FOV=256 mm, slice thickness = 1.00 mm, gap = 0 mm).
Following intranasal administration of AVP, CSF concentrations begin rising within 10 minutes, continue to increase for up to 80 minutes, and remain above those of placebo-treated subjects at 100–120 minutes after administration (Born et al., 2002). Previous studies using intranasal OT in human subjects have sampled behavior or brain activity at 50 minutes post-injection (Kirsch et al., 2005; Kosfeld et al., 2005). Thompson et al (Thompson et al., 2006) tested subjects at 15 and 50 minutes after intranasal vasopressin administration. Accordingly, our goal was for subjects to be fully immersed in the task at 50 minutes post drug administration. We therefore aimed to start both the task and fMRI scan at 40 minutes after drug administration. In actuality, this time period averaged 42 minutes (s.d.= 3.62) across subjects.
Task
Prior to the start of each game, the visual display inside the scanner indicated with which partner the subject was about to play the game. While being imaged with fMRI, subjects played 30 rounds of a sequential-choice, iterated Prisoner’s Dilemma game in each of four sessions. For two sessions, subjects were told they were playing with the human partners they were introduced to. For the other two sessions, subjects were told they were playing with a computer partner. In actuality, subjects were always playing with a pre-programmed computer algorithm (described below). Subjects were compensated with two-thirds of their total earnings across all four sessions.
For both human and computer partners, in one of the two sessions subjects played in the role of first mover (player 1) and their partner played in the role of second mover (player 2). In the second session, roles were reversed. The order of human and computer sessions was counterbalanced across subjects so that half of the subjects were scanned in the order: player 1 with human partner (H1), player 2 with human partner (H2), player 1 with computer partner (C1), player 2 with computer partner (C2), and the other half were scanned in the order: C1, C2, H1, H2. Note that statistical comparisons were made between human and computer runs as player 1 or as player 2, but player 1 runs were not compared with player 2 runs so these need not be counterbalanced.
E-prime software (Psychology Software Tools, Pittsburgh) was used for stimulus presentation. Stimuli were projected onto a screen that subjects could view through a mirror mounted on the head coil. Subject responses were recorded using a response box. A timeline for a single PD trial is depicted in figure 1. At the beginning of each round, the round number and partner’s photo were displayed for 2 seconds (s). Player 1 then had 4 s to choose to cooperate or defect. Players were informed that if they did not decide within this 4 s interval, their response would default to defection. Player 1’s choice was immediately revealed to player 2 and displayed for 1 s. A variable length fixation epoch of either 2, 4 or 6 s followed. Afterwards, player 2 had 4 seconds to cooperate or defect. Once player 2 decided, the outcome of the round was displayed for 4s. Finally, the trial concluded with another variable length fixation epoch of either 2, 4 or 6 s. Trials were approximately 20 s long. Five null trials were interspersed among 30 PD trials in each session. Null trials consisted of 14 seconds of fixation. One session lasted approximately 12 minutes. All four sessions lasted about 48 minutes.
Figure 1.
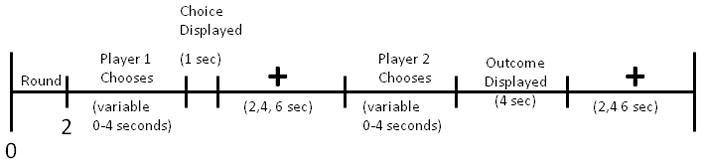
Timeline of PD task.
In all four sessions, partner choices were administered by a computer algorithm. Computer strategies were designed to mimic actual human strategies. With the subject playing as player 1, the computer reciprocated defection at 90% and cooperation at 67%. This is realistic because some people will defect in response to partner cooperation in order to earn an extra dollar, even at their partner’s expense. When the subject took the role of player 2, the computer played a “forgiving tit for tat” strategy, which cooperated in round 1 and always reciprocated partner cooperation from the previous round. Following mutual defection, it cooperated 33% of the time. Following unreciprocated cooperation (computer cooperated, player defected), it cooperated 10% of the time, but it never cooperated after two consecutive such outcomes. 100% reciprocation of cooperation is a natural human response in this role because there is no temptation to defect as player 1, given that player 2 would naturally counter with defection. Mutual cooperation is the best one can hope for in this role. On the other hand, it may be worth occasionally cooperating in response to partner defection to attempt to disrupt runs of mutual defection and establish mutual cooperation.
Behavioral analysis
For player 1 data, outcome frequencies and transition probabilities were compared across partner types (human, computer) using paired t-tests and across treatment groups (AVP, OT, placebo) using two sample t tests. For player 2 data, probabilities of reciprocating partner cooperation and defection were compared across partner types and treatment groups, using paired and two sample t tests, respectively.
Neuroimaging Procedures
fMRI image acquisition
Subjects were imaged while playing the PD game. Functional scans used an EPI sequence with the following parameters: TR = 2000 ms, TE = 28 ms, matrix = 64 × 64, FOV = 224 mm, slice thickness = 2.5 mm, 34 axial slices. TE was minimally decreased from the typical value (32 ms) in order to reduce magnetic susceptibility artifact in the orbitofrontal region. The duration of each EPI scan was about 12 minutes (30 PD rounds × ~20 seconds per round, plus 5 null trials at 14 seconds per trial). After each of the four sessions, while still in the scanner, subjects rated their emotional reaction to the four PD game outcomes (CC, CD, DC, and DD). Seven-point Likert scales were used to rate the following emotions or feelings: afraid, angry, happy, guilty, disappointed, and relieved.
fMRI image analysis
Image preprocessing was conducted with Brain Voyager QX (version 2.0.8) software (Brain Innovation, Maastricht, The Netherlands). Preprocessing involved image realignment by six-parameter 3-D motion correction, slice scan time correction using cubic spline interpolation, spatial smoothing with a 8-mm full width at half maximum (FWHM) Gaussian kernel, and temporal high-pass filtering using linear and nonlinear trend removal and a frequency cutoff of three cycles per run length. Images were subsequently normalized into Talairach space (Talairach and Tournoux, 1988). Subjects were scanned when playing both in the role of player 1 and in the role of player 2. For the player 1 runs, a separate general linear model (GLM) was defined for each subject that examined the neural response to both the epoch in which the choice to cooperate or defect was made, as well as to the epoch in which the game outcome was revealed. More specifically, the following regressors were defined for each subject in the role of player 1: 1) the beginning epoch when round number and the partner’s face or a picture of the computer were displayed, 2) the choice epoch when the subject chose to cooperate (Choice C), 3) the choice epoch when the subject chose to defect (Choice D), 4) CC outcomes, 5) CD outcomes, 6) DC outcomes, and 7) DD outcomes. These regressors were specified separately for runs with human and computer partners, resulting in a total of 14 distinct regressors per subject. For player 2 runs, a separate general linear model (GLM) was defined for each subject that examined the neural response to the epoch in which the partner’s choice was revealed, the epoch while the subject was choosing, and the epoch in which outcome was revealed. More specifically, the following regressors were defined for each subject in the role of player 2: 1) the beginning epoch when the subject saw his partner’s picture and the round number, 2) partner choice C, 3) partner choice D, 4) player choice C, 5) player choice D, 6) CC outcome, 7) CD outcome, 8) DC outcome, 9) DD outcome. These regressors were specified separately for runs with human and computer partners. Each regressor was convolved with a standard model of the hemodynamic response.
We performed a region of interest analysis based on a priori hypotheses, supplemented with whole brain analysis. The ROI analysis focused on the right and left caudate nucleus and the left amygdala response to reciprocated cooperation, as well as the right amygdala response to unreciprocated cooperation. The caudate nucleus ROI was defined as a 9 mm-side cube (729 mm3) centered on the activation maximum for the contrast between mutual cooperation and the average of the other three PD game outcomes from our previous study (Rilling et al., 2002), as well as its left hemisphere homologue. The cube volume was chosen to match that of the functional activation as closely as possible. The left amygdala ROI was a 10 mm-side cube centered on the coordinates of the activation peak reported in Gamer et al (Gamer et al., 2010), after converting from MNI to Talairach coordinates (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach), and then manually adjusting the ROI location to more closely match the anatomical features of the MNI template. The right amygdala ROI was a 10 mm-side cube centered on the peak coordinate in Baumgartner et al (2008) after converting from MNI to Talairach coordinates. For each subject, contrasts of parameter estimates for predictors were averaged across all voxels within each ROI. We also computed the difference in these contrasts between human and computer partners, i.e., the interaction between the factor specified by the contrast and the factor of partner type. In a second level, random-effects analysis, individual subject contrast values were compared across treatment groups with two sample t tests. A statistical threshold of p<0.05 was adopted.
For the whole brain analyses, contrasts of parameter estimates for various predictors were computed at every voxel within the brain. We also directly compared the values of these contrasts between human and computer partners. In a second level, random-effects analysis, individual subject contrast values were compared across treatment groups with two sample t tests. The resulting map of the t statistic image was thresholded at p<0.05 corrected for multiple comparisons based on the volume of clusters of contiguous voxels, using a 3D extension (implemented in BrainVoyager) of a 2D Monte Carlo simulation procedure (Forman et al., 1995). At this threshold, very widespread activations were observed when testing for partner effects (i.e., human vs. computer). Therefore, a more stringent threshold of p<0.01 corrected was used when reporting partner effects (human vs. computer). Individual subject contrast values were also correlated with plasma OT and AVP levels in both ROI and whole brain analyses. Within a priori ROIs, contrast values were averaged across voxels within the ROI. For the whole brain analysis, the map of the Pearson correlation statistic (r) was thresholded at p<0.05 corrected for multiple comparisons based on the volume of clusters of contiguous voxels.
Functional connectivity analysis
This analysis examined how OT and AVP modulate amygdala connectivity across all epochs of our task. A mask was drawn on the right amygdala of one subject’s T1 scan warped to Talairach space. This mask was used as a seed region in a functional connectivity analysis. The potentially confounding effect of global signal changes was removed by scaling the voxels intensities in each volume by the average of all in-brain voxels intensities at that time point. Further, a high-pass filter with a cutoff of three cycles per time course was applied to remove low frequency physiological and scanner induced noise. For each subject, the Pearson’s correlation coefficients between the amygdala time course and the time course of every other voxel of the brain were computed. In a second level random-effects analysis, individual subject parameter estimates, after being subjected to a Fischer’s z-transform to improve distributional characteristics, were compared across treatment groups with a two sample t test. The resulting t-statistic map was thresholded at p<0.05 corrected for multiple comparisons based on the volume of clusters of contiguous voxels.
Post-scanning procedures
After scanning, subjects completed the PANAS for a third time. They were also asked several questions about their experience during the PD game. Subjects were asked to guess whether they had received a drug (either OT or AVP) or a placebo. The proportion of subjects who guessed they had received a drug were: AVP (65%), OT (44%), AVP placebo (38%), OT placebo (31%). There was no significant difference in the proportion of subjects who guessed they received a drug when comparing each drug to its respective placebo (95% C.I. for the difference in proportions between AVP and its placebo was (−0.03, 0.58); for OT and its placebo (−0.18, 0.45)). Nor was there a significant difference between the two drugs (AVP vs OT; (−0.48, 0.06)) or the two placebos (AVP placebo vs. OT placebo; (−0.41, 0.28)).
Subjects were compensated with a total of approximately $120; the exact amount varied as a function of task performance and was obtained by multiplying the total earnings across all four games by 2/3.
Within two weeks of being scanned, subjects returned to complete a battery of personality measures.
Neuropeptide Assays
Vasopressin and Oxytocin Assays
Plasma was assayed for AVP and OT in the Clinical Pathology Translational Research Laboratory at Emory University.
Plasma OT was measured using a standard RIA kit from Phoenix Pharmaceuticals with a sensitivity of approximately 5 pg/ml. Quality control measures failed on one plate of samples such that reliable plasma OT values were only obtained from 32 subjects. One subject with a value more than 1.5*the interquartile range (Q3 - Q1) greater than the third quartile (Q3) for his treatment group (placebo) was considered an outlier and excluded from analyses.
Plasma AVP was assayed as follows. The procedure utilizes an antiserum supplied by Dr. TJ.B.VanWimersma Greidanus. Synthetic standard material (Arg8-Vasopressin) is obtained from Bachem (Torrance, CA). Iodinated vasopressin is purchased from PerkinElmer. Standard curves are prepared in human plasma which has been “stripped” of endogenous vasopressin. Prior to assay, plasma standards and samples are extracted on C-18 Sep Paks (Waters, Milford, MA). The extracts are then concentrated by lyophilization and reconstituted by in assay buffer. The incubation with the primary antisera is for 24 hours at 4°C. After addition of the tracer the incubation is continued for an additional 24 hours. A second antibody, goat anti-rabbit gamma globulin, is then added and the incubation continues for another 3 hours. The bound component is separated by centrifugation.
The tubes are then decanted and counted in a gamma counter (Packard Instrs., Downers Grove, IL). The data is analyzed by standard RIA log/logit procedures. The working sensitivity of the assay is 0.3 pg/mL. The assay shows only limited cross-reactivity with lys-vasopressin and no cross-reactivity with oxytocin. The interassay C.V. is 9% and the intra-assay C.V. is 5%, at a level of 10 pg/mL. The curve is linear to 250 pg/mL and the normal range in fully hydrated adults is 0.5 to 3.0 pg/mL (n=35). Samples were assayed in 57 subjects, four of whom had values more than two standard deviations beyond the mean for their respective groups (n=3 OT, n =1 placebo). These four were treated as outliers and excluded from analyses involving plasma AVP.
Results
Plasma levels of AVP and OT
Plasma AVP levels were higher in the AVP group (4.5 pg/mL, +/− 0.49 pg/mL) than the placebo group (3.1 pg/mL, +/− 0.40 pg/mL) by an average of 1.4 pg/mL (t(31)=2.15, p=0.04). However plasma AVP levels were not significantly higher in the AVP group compared with the OT group (3.5 pg/mL, +/− 0.34 pg/mL). Plasma OT levels were not significantly higher in the OT group (134.7 pg/mL, +/− 10.2 pg/mL) compared with either the placebo (129.4 pg/mL, +/− 12.2 pg/mL) or the AVP group (120.6 pg/mL, +/− 7.3 pg/mL).
PANAS
The Positive and Negative Affect Schedule (PANAS) was used to measure self-reported mood and anxiety both before and 20 minutes after drug administration. Although for all three groups combined decreases were observed in “nervous” (paired t(80)= −2.63, p=0.01), “scared” (paired t(80)= −2.02, p= 0.05) and “afraid” (paired t(80)= −2.97, p=0.004), there were no significant repeated-measure changes in any of the PANAS measures within any of the individual drug treatment groups. A third PANAS score was collected after the scan. Importantly, there were no significant differences across treatment groups for any of these measures at any of the three time points.
Psychophysiological Data
Ear temperature, heart rate and blood pressure were collected before and 20 minutes after drug administration. Although systolic blood pressure increased across this interval for AVP (paired t(25)= 2.34, p=0.03) and placebo (paired t(36)= 2.66, p=0.01) and diastolic blood pressure increased for AVP (paired t(25)= 3.02, p=0.006), there were no significant differences among treatment groups at either time. Nor was there any effect of time or treatment group on heart rate or ear temperature. Blood pressure was also collected at 5 minute intervals throughout the scan, and there were no significant differences among the three treatment groups in their average blood pressure during scanning.
Behavior
Player 1
After correction for multiple comparisons, there were no significant differences between the OT placebo and the AVP placebo on any of 16 behavioral measures for player 1. Neuroimaging analyses similarly showed comparable effects of the two placebos. Thus, the two groups were combined for subsequent analyses.
The frequency of the four possible PD outcomes (CC, CD, DC, DD) did not differ as a function of either partner (human vs. computer) or drug treatment (AVP, OT, Pl). On the other hand, transition probabilities (i.e., the probability of cooperating following specific outcomes in the previous round) were significantly modulated by both partner and drug treatment (figure 2), as outlined below.
Figure 2.
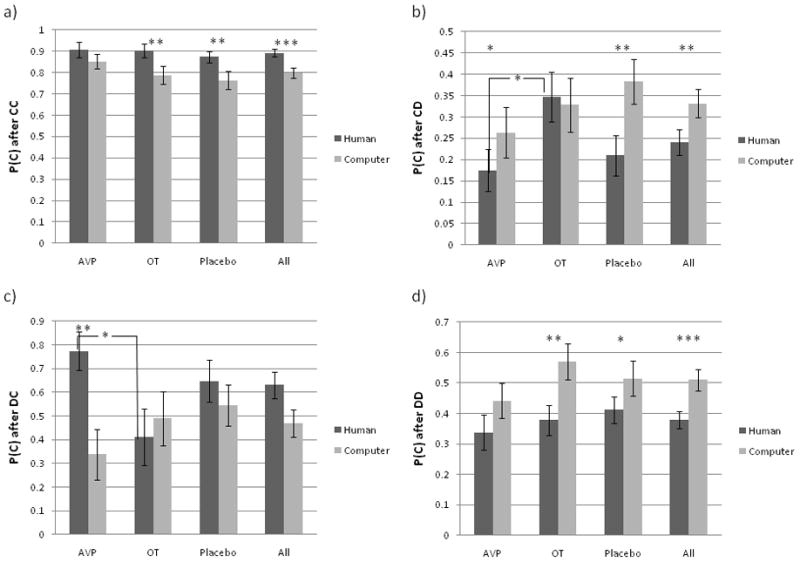
Player 1 behavioral data. Transition probabilities as a function of partner and drug treatment. Probability of cooperation after a) CC outcome, b) CD outcome, c) DC outcome, and d) DD outcome in the previous round. * = p<0.05, **=p<0.01, ***=p<0.001.
Effects of partner type
Collapsing across all drug treatment groups, subjects were 9% more likely to cooperate after a CC outcome (p C/CC) when playing with putative human (89%) compared with computer (80%) partners (p=0.0002). This relationship also held within both the OT group (9% higher for human partners; p=0.007) and the placebo group (11% higher with human partners; p=0.005), but not the AVP group. On the other hand, subjects were 9% less likely to cooperate following unreciprocated cooperation in the previous round (p C/CD) when playing with human (24%) compared with computer (33%) partners (p=0.005). This relationship also held within the AVP group (9% lower with human partners; p<0.05) and the placebo group (17% lower with human partners; (p=0.004), but not the OT group.
Collapsing across all drug treatment groups, subjects were 13% less likely to cooperate following DD outcomes (p C/DD) when playing with putative human (38%) compared with computer (51%) partners (p=0.0002). This relationships also held for both the OT (19% lower with human partners; p=0.003) and the placebo group (10% lower with human partners; p=0.05), but not the AVP group.
Collapsing across all drug treatment groups, partner type had no significant effect on p C/DC. However, within the AVP group, the p C/DC was 44% higher with human than computer partners (p=0.01).
Drug treatment effects when playing with putative human partners
There were no effects of drug treatment for either p C/CC or p C/DD when playing with putative human partners. On the other hand, following CD outcomes with putative human partners, OT treated subjects (35%) were 18% more likely to cooperate than AVP-treated subjects (17%) (p=0.03). Neither group differed significantly from the placebo group (21%). Given its link with male agonistic behavior, we expected that AVP would provoke retaliation (i.e., defection) in response to unreciprocated cooperation. In other words, we expected AVP to decrease rates of cooperation following a CD outcome. In addition, as OT is linked with trust (Baumgartner et al., 2008), we expected it would increase rates of cooperation following CD outcomes. This is the trend we observe. Although the differences from placebo group are not significant, the OT vs. placebo difference is marginally significant (p=0.07). We suspect that this is a statistical power issue and that a larger sample size would yield a statistically significant difference. Following DC outcomes (player defection followed by partner cooperation) with putative human partners, OT-treated subjects (41%) were 36% less likely to cooperate than AVP-treated subjects (77%) (p=0.02). Again, neither group differed significantly from placebo (65%).
Drug treatment effects when playing with computer partners
Consistent with our hypothesis, there were no significant effects of drug treatment when playing with computer partners for any of the transition probabilities.
Interaction between partner type and drug treatment
The effect of partner type did not differ across treatment groups for either p C/CC or p C/DD. However, for p C/CD, the difference between human and computer partners was larger in the placebo than the OT group by an average probability of 0.17 (p=0.04). Although trending in the same direction, partner effects for the AVP group were not significantly greater than those in the OT group. For p C/DC, the difference between human and computer partners was greater in the AVP compared with the OT group by an average probability of 0.54 (p=0.03).
Player 2
The probability of cooperation, conditional on the partner’s choice, was significantly modulated by both partner and drug treatment (figure 3), as outlined below.
Figure 3.
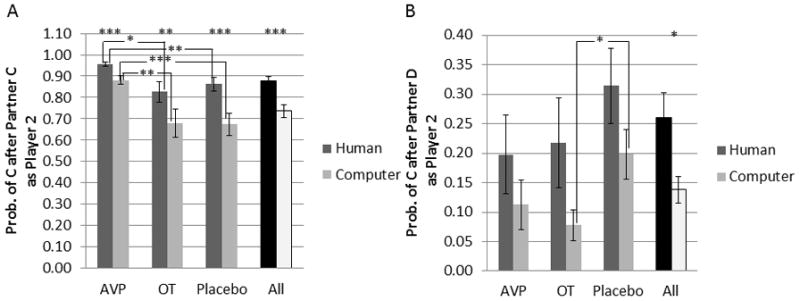
Player 2 behavioral data. Probability of cooperation conditional on player 1 choice. Probability of cooperation after a) partner cooperation, b) partner defection. * = p<0.05, **=p<0.01, ***=p<0.001.
Main effects of partner type
Collapsing across all drug treatment groups, subjects were 14% more likely to reciprocate cooperation from human (88%) than computer (74%) partners (p=3×10−7). Subjects were also 11% more likely to cooperate following partner defection when playing with human (26%) compared with computer (14%) partners (p=0.02).
Drug treatment effects when playing with putative human partners
AVP treated subjects reciprocated cooperation at 96% with human partners, which was 10% greater than for placebo (86%) (p=0.008) and 13% greater than for OT treated subjects (83%) (p=0.01). There were no effects of drug treatment on the probability of cooperating after partner defection.
Drug treatment effects when playing with computer partners
AVP treated subjects reciprocated cooperation at 88% with computer partners, which was 21% greater than for placebo (67%; p=0.0006) and 20% greater than for OT treated subjects (68%; p=0.007). OT subjects cooperated after partner defection only 8% of the time, which was 12% less than for the placebo group (20%; p=0.02).
Interaction between partner type and drug treatment
For the probability of reciprocating cooperation, the human-computer difference for AVP (7%) was less than that for placebo (19%) by 12% (p=0.04). Neither group significantly differed from OT (15%). The effect of partner type did not differ across treatment groups for the probability of cooperating after a partner defection.
Neuroimaging
Player 1
Neuropeptide modulation of the response to reciprocated cooperation
In an earlier fMRI study in which subjects played an iterated PD game with human and computer partners, the contrast between mutual cooperation and the average of the other three PD game outcomes yielded activation in the head of the right caudate nucleus that was specific to interactions with human partners (Rilling et al., 2002; Rilling et al., 2004). Within this region of interest (ROI) and its left hemisphere equivalent, we tested for drug and partner effects on the response to reciprocated cooperation in the current study. Collapsing across all treatment groups, the left caudate nucleus activated more strongly to reciprocated compared with unreciprocated cooperation from a human partner (paired t(80)=2.41, p=0.02). It also activated more strongly to reciprocated cooperation from putative human compared with computer partners (paired t(73)=2.56, p=0.01). This difference in activation between human and computer partners was greater for the OT group compared with either the placebo (two sample t(47)=2.17, p=0.03) or AVP groups (two sample t(41)=2.05, p<0.05) (figure 4b). Examination of the time-course of the BOLD response to CC outcomes within this ROI (figure 4c) shows that the maximum percent signal change is significantly larger for the OT group during interactions with human partners compared with each of the other five conditions (OT human > AVP human t(48)=3.20, p=0.003; OT human > Placebo human t(57)=2.95, p=0.005; OT human > AVP computer t(44)=2.36, p=0.02; OT human > Placebo computer t(55)=3.13, p=0.003; OT human > OT computer t(47)=2.14, p=0.04). In the right caudate ROI, although all effects trended in the same direction, only the contrast between reciprocated and unreciprocated cooperation from human subjects reached significance (t(80)=2.24, p=0.03).
Figure 4.
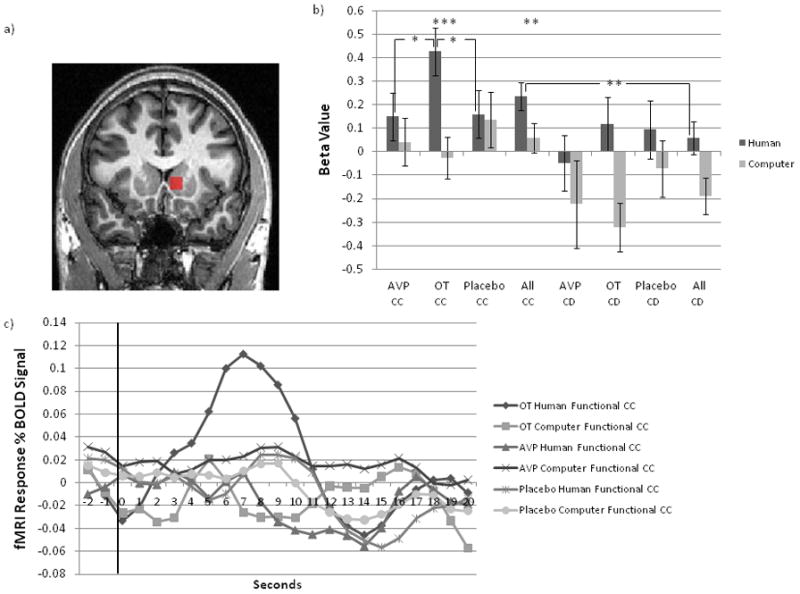
Left caudate nucleus response to reciprocated and unreciprocated cooperation, as a function of partner type and treatment group. a) functional ROI in left caudate, b) left caudate beta values as a function of outcome, partner type and drug treatment, c) time course of left caudate response as a function of partner type and drug treatment. Outcome is revealed at t=0 seconds and displayed for 4 seconds.
Within the left amygdala ROI from Gamer et al (2010), OT treatment was not associated with significantly stronger activation in response to reciprocated cooperation from human partners, compared with either placebo (two sample t(54)=1.77, p=0.08) or AVP (two sample t(46)=1.05 p=0.30). Despite this, both the left caudate nucleus and the left amygdala were activated in a whole brain analysis that examined the contrast [(CC human – CC computer) OT – (CC human – CC computer) placebo)]. To ensure that results were not driven by OT-mediated suppression of activation for computer partners, we further masked out activations from the contrast (CCcomputer) placebo – (CCcomputer) OT, and the activations in the left caudate and left amygdala remained (supplementary figure 1, supplementary table 1). Thus, for both the left caudate nucleus and the left amygdala, the response to CC outcomes was augmented by OT when interacting with human partners. Furthermore, activation in these two areas was highly correlated across subjects (r=0.50, n=81, p<0.001).
For the subgroup of subjects from whom we obtained plasma OT measures (n=29), plasma OT was significantly positively correlated with activation in response to CC outcomes in the left caudate ROI, both for the overall sample (n=29, r=0.48, p=0.01), and also within the placebo (n=10, r=0.69, p=0.03), but not the OT (n=10, r=0.62, p=0.06;) or AVP groups (n=9) (figure 5a). Within the left amygdala ROI, activation in response to CC outcomes was not significantly correlated with plasma OT for the overall sample (n=29, r=0.21, p=0.28), the AVP group (n=9, r=0.10, p=0.80), the OT group (n=10, r=−0.24, p=0.50), or the placebo group (n=10, r=0.49, p=0.15).
Figure 5.

Correlation between plasma OT levels and response to reciprocated cooperation in a) left caudate and b) the basal forebrain. Image is thresholded at p<0.05 corrected for multiple comparisons based on the volume of clusters of contiguous voxels.
Entering plasma OT as a covariate in an exploratory, whole brain, voxel-wise analysis revealed a positive and highly specific correlation between plasma OT and the response to reciprocated cooperation within the basal forebrain (figure 5b, supplementary table 2), a region that includes areas known to have the highest densities of OT receptors in the human brain, such as the preoptic area of the hypothalamus, the ventral pallidum, the basal nucleus of Meynert and the nucleus of the vertical limb of the diagonal band of Broca (Loup et al., 1991).
AVP treatment was associated with increased activation in response to CC outcomes, relative to placebo, in several areas, most notably a region spanning known vasopressin circuitry, including the BNST, lateral septum and stria terminalis (supplementary figure 2, supplementary table 3).
Neuropeptide modulation of the response to unreciprocated cooperation
We also investigated the effect of both OT and AVP on the response to unreciprocated cooperation. Within an a priori functional ROI centered on the right amygdala coordinates reported in Baumgartner et al (2008), OT was not associated with decreased activation relative to placebo when playing with human partners only, although there was a trend in that direction (two sample t(50)= −1.80, p=0.08). Nor was there a difference between the AVP and placebo group in this ROI. In whole brain analyses, both OT and AVP were associated with increased activation to CD outcomes in both ventromedial and ventrolateral prefrontal cortex, compared with placebo (supplementary figure 3, supplementary table 4).
Neuropeptide modulation of amygdala functional connectivity
In rats, central amygdala (CeA) neurons that trigger autonomic fear responses through projections to the hypothalamus and brainstem are modulated by OT and AVP in opposite ways, with OT decreasing and AVP increasing firing rates (Huber et al., 2005). We therefore compared functional connectivity from the amygdala between the OT and placebo groups and between the AVP and placebo groups. OT and, unexpectedly, AVP were both associated with decreased amygdala connectivity with the brainstem compared with placebo. Additionally, compared with the placebo group, the AVP group had stronger amygdala connectivity with several regions including bilateral ventral anterior insula, subgenual anterior cingulate cortex and inferior lateral temporal cortex (supplementary table 5, figure 6). Like the AVP group, the OT group also had stronger amygdala connectivity with right ventral anterior insula and right inferior lateral temporal cortex compared with placebo. However, several regions showing increased amygdala connectivity for AVP did not show increased connectivity for OT, and AVP-related increases were more widespread in general (supplementary table 5, figure 6).
Figure 6.
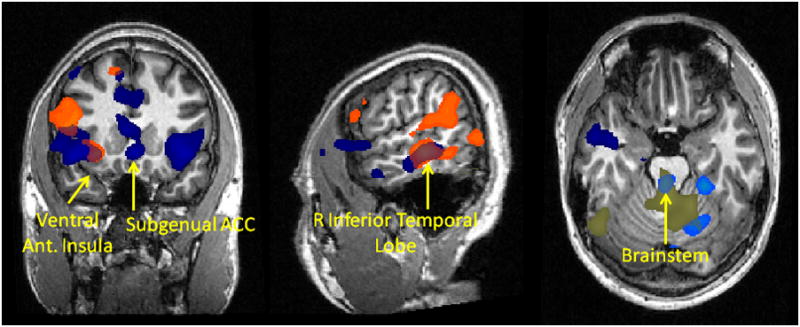
Functional connectivity with right amygdala as a function of drug treatment. Orange = stronger connectivity with right amygdala for OT vs. placebo group, light blue = weaker connectivity with right amygdala for OT vs. placebo group, dark blue = stronger connectivity with right amygdala for AVP vs. placebo group, beige = weaker connectivity with right amygdala for AVP vs. placebo group. T statistic map is thresholded at p<0.05 corrected for multiple comparisons based on the volume of clusters of contiguous voxels.
Human vs. computer differences
As mentioned above, the transition probabilities of cooperation differed when playing with putative human vs. computer partners. Subjects were more likely to cooperate with human than computer partners after a CC outcome in the previous round. On the other hand, subjects were less likely to cooperate with human than computer partners after partner defection in the previous round. To identify potential neural correlates of these behavioral effects, we contrasted the response to reciprocated cooperation (CC) by human vs. computer partners. We also contrasted the response to defection (CD+DD) by human vs. computer partners. Both of these contrasts revealed stronger activation in medial prefrontal cortex for human compared with computer partners (supplementary figure 4a). Partner defection from human partners was additionally associated with stronger activation in rostral ACC, anterior insula and hypothalamus compared with defection by computer partners (supplementary table 6, supplementary figure 4b).
Player 2
Neuropeptide modulation of the response to cooperation
Within the right caudate nucleus ROI used for player 1 analyses, the response to cooperation from human partners was stronger for the OT than for the AVP group (two sample t(46) = 2.26, p=0.03), however neither group differed significantly from placebo. In the left caudate, the difference between OT and AVP was in the same direction but not significant (two sample t(46) = 1.69, p=0.10), and again, neither differed from placebo. In response to cooperation from computer partners, AVP treatment was associated with less activation in both the right and left caudate nucleus compared with placebo (left caudate two-sample t(54)= −2.06, p=0.04; right caudate two-sample t(53)= −2.35, p=0.02), but did not differ from OT. As expected given the results of these ROI analyses, neither OT nor AVP significantly modulated activation in the caudate nucleus in whole brain analyses of the contrasts (C human – C computer) OT – (C human – C computer) placebo) and (C human – C computer) AVP – (C human – C computer) placebo) (supplementary table 7).
Neuropeptide modulation of the response to defection
Within the right amygdala ROI from the player 1 analysis, the response to defection did not differ among the three treatment groups for either human or computer partners. In whole brain analyses, OT attenuated activation in the subgenual ACC for the contrast (partner D human – partner D computer), relative to placebo. For the same contrast, AVP treatment was associated with decreased activation in medial prefrontal cortex and left insula (supplementary table 8).
Human vs. computer differences
As mentioned above, subjects were significantly more likely to reciprocate cooperation from human than computer partners when playing in the role of player 2. To identify a potential neural correlate of this effect, we compared the response to partner cooperation from human vs. computer partners. This contrast revealed several regions that responded more strongly to cooperation from human partners, including dorsomedial and ventromedial prefrontal cortex, as reported previously by us and others using similar paradigms (McCabe et al., 2001; Rilling et al., 2004). Several regions also responded more strongly to defection from human vs. computer partners, including dorsomedial prefrontal cortex and hypothalamus (supplementary table 9, figure 7).
Figure 7.
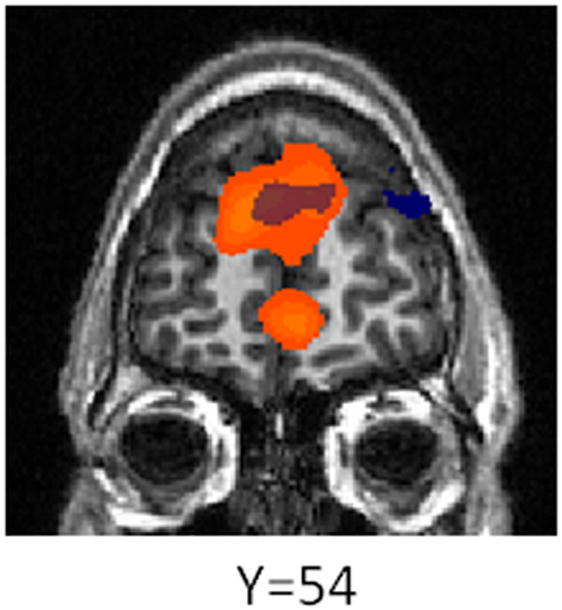
Effect of partner type on brain activation as player 2. Areas in orange activate more strongly to cooperation from human vs. computer partners. Areas in blue activate more strongly to defection from human vs. computer partners.
Discussion
OT effects on brain and behavior
In the iterated PD game, players must learn whether they can trust their partners. The human caudate nucleus tracks a social partner’s decision to reciprocate or not reciprocate cooperation in trust or PD games (Rilling et al., 2002; Rilling et al., 2004; Delgado et al., 2005; King-Casas et al., 2005). Specifically, reciprocated cooperation activates the caudate nucleus, and caudate activation is associated with increased future reciprocity (Rilling et al., 2002; King-Casas et al., 2005). Thus, the caudate may register social prediction errors that guide decisions about reciprocity. For player 1, we found that OT augmented the caudate response to reciprocated trust (i.e., CC outcomes), suggesting that OT may enhance the reward from reciprocated cooperation and/or facilitate learning that another person can be trusted to reciprocate cooperation. Indeed, a recent study with autistic patients found OT to facilitate learning that another person can be trusted in a virtual ball tossing game (Andari et al., 2010). Interestingly, another study has shown that the caudate nucleus response to reciprocated cooperation is enhanced for partners with trustworthy reputations (Phan et al., 2010). Thus, given the relationship between OT and trust (Kosfeld et al., 2005; Baumgartner et al., 2008), OT could be augmenting caudate nucleus activation in our paradigm by increasing trust. For player 2, OT was similarly associated with a stronger caudate response to cooperation compared with the AVP group. Dopamine and oxytocin interact in the nucleus accumbens to facilitate pair-bonding in female prairie voles (Liu and Wang, 2003). However, midbrain dopamine cells project to both ventral and dorsal striatum (Lynd-Balta and Haber, 1994), including the caudate nucleus. Our results therefore suggest that a similar mechanism involving the interaction of DA and OT may mediate cooperation among men.
Plasma OT levels were positively correlated with the response to mutual cooperation with human partners in the left caudate nucleus, as well as the basal forebrain, which includes several areas with known high densities of OT receptors in the human brain (Loup et al., 1991). These correlations suggest that plasma OT levels were correlated with central OT levels in specific brain areas in our experiment. However, plasma and central OT levels are not always correlated (Landgraf and Neumann, 2004). In our study, plasma OT levels are expected to be the sum of endogenous peripheral secretion plus intranasally administered OT that leaks back to the periphery from the brain. Our observed correlations could emerge from coordinated endogenous release of OT into the brain and the periphery. Indeed, recent evidence suggests that the same neuronal populations that project to the posterior pituitary and release OT into the plasma also project to the striatum (Ross et al., 2009). Alternatively, the putative correlation between plasma and central OT levels we observed could result from across subject variability in the amount of OT that is absorbed through the nasal epithelium and then enters the brain and periphery. Given that plasma OT levels were not significantly higher in the OT compared with the placebo group, we favor the former possibility. Two previous studies did report increases in plasma OT from baseline following intranasal administration (Andari et al., 2010; Domes et al., 2010), which begs the question of why our intranasal OT group did not have elevated plasma OT compared with placebo (though it did trend in that direction). Unlike AVP (Born et al., 2002), the kinetics of intranasal OT administration are not well-known. We measured plasma OT at 20 minutes post-administration. Domes et al (2010) reported a 34.2 pg/ml increase at 45 minutes post-administration, whereas Andari et al (2010) reported a 1.6 pg/ml increase 10 minutes post-administration. Thus, we may have been sampling prior to the time when marked increases in plasma OT are observed. In addition, the lack of a baseline sample may have precluded our ability to detect a small increase. That is, our OT group may have increased from a lower baseline than the placebo group such that no significant difference was observed after OT administration. In sum, although these results should be interpreted with caution, we speculate that subjects with higher levels of OT in the caudate nucleus and basal forebrain have a stronger response to reciprocated cooperation in these areas, further supporting the above conclusion that OT augments this response.
Whole brain, but not ROI analyses, also showed that OT augmented the left amygdala response to reciprocated cooperation from human partners. Although the amygdala is classically known for its role in fear conditioning (Davis, 1997; Ledoux, 1998), a recent study implicated it in OT-mediated enhancement of socially reinforced learning (Hurlemann et al., 2010). Thus, the left amygdala may be involved in OT-mediated enhancement of learning whether a partner can be trusted to reciprocate cooperation. The left amygdala response to CC outcomes was strongly positively correlated with the left caudate nucleus response to CC outcomes. Both regions are targets of the mesolimbic dopamine system (De Keyser et al., 1988), so the correlation may reflect simultaneous release of DA in these two regions. Several studies have reported that OT decreases amygdala activation in response to negative social stimuli (Kirsch et al., 2005; Domes et al., 2007; Baumgartner et al., 2008; Petrovic et al., 2008; Gamer et al., 2010). However, OT did not significantly attenuate the right amygdala response to a negative social stimulus, namely unreciprocated cooperation from human partners. Behaviorally, OT was associated with an increased probability, relative to AVP, of disregarding the “betrayal” and choosing to cooperate rather than retaliate in the subsequent round, consistent with previous findings (Baumgartner et al., 2008).
AVP effects on brain and behavior
We did not observe any significant behavioral effects of AVP for player 1. On the other hand, robust behavioral effects of AVP were found for player 2. AVP-treated subjects were more likely to reciprocate cooperation from both human and computer partners compared with either OT or placebo treated subjects. This finding runs contrary to our hypothesis that AVP would increase male-male agonism and decrease cooperation. However, if AVP is anxiogenic, as has been suggested (Heinrichs et al., 2009), then reciprocating cooperation might minimize anxiety by avoiding conflict. Indeed, girls with anxiety disorders reciprocate cooperation in the PD game more reliably than do their healthy peers (McClure et al., 2007). Moreover, AVP has been linked with affiliative behavior in some species (reviewed in (Goodson and Thompson, 2010)).
It is therefore of interest that AVP increased activation in response to reciprocated cooperation (i.e., CC outcomes) in a region spanning known AVP circuitry that is implicated in affiliation in many species, namely the BNST, lateral septum and stria terminalis. Interestingly, the vasopressin/vasotocin projections from the bed nucleus of the stria terminalis to the lateral septum typically show increased immediate early gene expression in response to affiliation-related, but not socially aversive stimuli (Goodson and Thompson, 2010).
OT and AVP effects on amygdala functional connectivity
Consistent with a previous study, we found that OT decreased amygdala connectivity with brainstem effector sites of the autonomic nervous system (Kirsch et al., 2005). Contrary to our expectation, however, we also found AVP to have a similar effect. AVP can bind the OT receptor (Audigier and Barberis, 1985; Derick et al., 2002), so AVP could be working through the OT receptor to have this effect. This might also explain the overlap between the two peptides in regions showing increased amygdala connectivity, such as the ventral anterior insula and ventral lateral temporal cortex. The ventral anterior insula is involved in awareness of visceral feedback from the body thought to support subjective feeling states (Craig, 2002, 2004; Critchley et al., 2004). Notably, this region consistently coactivates with the amygdala in response to social-emotional stimuli (Mutschler et al., 2009; Kurth et al., 2010). Stronger functional connectivity from the amygdala to the insula may enhance the amygdala’s ability to elicit subjective feeling states in response to salient social stimuli. The ventral lateral temporal regions that show increased functional connectivity with amygdala are part of the ventral visual stream, and can be modulated by back-projections from the amygdala to increase visual attention to emotionally salient stimuli (Amaral et al., 2003). Our data suggest that OT and AVP may augment this function. AVP was associated with more widespread increases in amygdala functional connectivity than was OT. One region where AVP showed stronger amygdala connectivity is the subgenual ACC. The only published neuroimaging study of vasopressin effects on human brain function found significantly greater subgenual cingulate cortex activation after vasopressin administration compared with placebo during face emotion processing, however AVP did not significantly impact functional connectivity between amygdala and subgenual ACC as we found here (Zink et al., 2010). AVP’s effects on patterns of functional connectivity may therefore differ according to the behavioral demands of the task. Both elevated AVP levels and increased subgenual ACC activity have been linked with depression and anxiety disorders (Mayberg et al., 2005; Drevets et al., 2008; Surget and Belzung, 2008). Thus, elevated vasopressin levels could potentially increase the risk of these illnesses by enhancing the amygdala’s ability to drive activity in the subgenual ACC.
Effects of partner type on brain and behavior
We also observed effects of partner type (human vs. computer) on both behavior and brain activation. As player 1, subjects were less likely to cooperate following partner defection (either CD or DD) from human compared with computer partners. This is likely attributable to betrayal aversion (Bohnet and Zeckhauser, 2004; Baumgartner et al., 2008), in which subjects find the prospect of unreciprocated cooperation from a human partner to be more aversive than the same from a computer, and are therefore less likely to risk cooperation with the former. For player 1, defection by human partners was associated with stronger activation in bilateral ventral anterior insula, rostral ACC and hypothalamus compared with defection by computer partners. The hypothalamus was also activated more strongly by defection from human compared with computer partners as player 2. All three structures are involved in autonomic arousal (Critchley et al., 2000), which may motivate subsequent defection (Rilling et al., 2008).
The reluctance to cooperate following partner defection (player 1) is somewhat balanced by an increased tendency to cooperate following CC outcomes with human compared with computer partners. Subjects were also more likely to reciprocate cooperation from human vs. computer partners in the role of player 2. In each case, cooperative feedback from human partners was associated with activation in ventromedial prefrontal cortex (VMPFC), consistent with previous studies (McCabe et al., 2001; Rilling et al., 2004). VMPFC activation could represent the abstract nature of this social reward or the value placed on future benefits from sustained cooperation (Rilling and Sanfey, 2011).
In sum, both OT and AVP augmented the BOLD response to reciprocated cooperation within regions that encompass their respective circuitry. OT treatment was associated with an increased response to reciprocated cooperation in the caudate nucleus and in the left amygdala (for whole brain but not ROI analyses), and plasma OT levels were correlated with the response to CC outcomes in basal forebrain regions rich in OT receptors. On the other hand, AVP increased activation in a region spanning known AVP circuitry including BNST, lateral septum and stria terminalis, regions implicated in affiliative behavior. Both AVP and OT were associated with increased amygdala functional connectivity with ventral anterior insula, suggesting that both may increase the amygdala’s ability to elicit visceral somatic markers that guide decision making.
Behaviorally, OT treatment was associated with increased cooperation following unreciprocated cooperation in the previous round when compared with AVP treatment. AVP strongly increased cooperation in response to a cooperative gesture by the partner (as player 2). In addition to these drug effects, robust effects of partner type were also observed. Subjects were more likely to reciprocate cooperation when interacting with human vs. computer partners, and this was paralleled by greater VMPFC activation in response to cooperation by human partners. Finally, subjects were less likely to cooperate following partner defection (either CD or DD outcome) from human compared with computer partners, and this behavior was paralleled by stronger activation in several areas involved in autonomic arousal. These findings extend our knowledge of the neural and behavioral effects of OT and AVP to the context of genuine social interactions.
Supplementary Material
Acknowledgments
We thank Paula Kincheloe, Karl Fernstrom, James Ritchie, Susan Rogers, Jianguo Xu and Larry Young for assistance with various aspects of this experiment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaral DG, Behniea H, Kelly JL. Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience. 2003;118:1099–1120. doi: 10.1016/s0306-4522(02)01001-1. [DOI] [PubMed] [Google Scholar]
- Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci U S A. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL. Reward, motivation, and emotion systems associated with early-stage intense romantic love. J Neurophysiol. 2005;94:327–337. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- Audigier S, Barberis C. Pharmacological characterization of two specific binding sites for neurohypophyseal hormones in hippocampal synaptic plasma membranes of the rat. EMBO J. 1985;4:1407–1412. doi: 10.1002/j.1460-2075.1985.tb03794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Bohnet I, Zeckhauser R. Trust, risk and betrayal. J Econ Behav Organ. 2004;55:467–484. [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Human feelings: why are some more aware than others? Trends Cogn Sci. 2004;8:239–241. doi: 10.1016/j.tics.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Critchley H, Elliot R, Mathias C, Dolan R. Neural activity relating to the generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging study. J Neurosci. 2000;20:3033–3040. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Davis M. Neurobiology of fear responses: the role of the amygdala. J Neuropsych Clin N. 1997;9:382–402. doi: 10.1176/jnp.9.3.382. [DOI] [PubMed] [Google Scholar]
- De Dreu CK, Greer LL, Handgraaf MJ, Shalvi S, Van Kleef GA, Baas M, Ten Velden FS, Van Dijk E, Feith SW. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science. 2010;328:1408–1411. doi: 10.1126/science.1189047. [DOI] [PubMed] [Google Scholar]
- De Keyser J, Claeys A, De Backer JP, Ebinger G, Roels F, Vauquelin G. Autoradiographic localization of D1 and D2 dopamine receptors in the human brain. Neurosci Lett. 1988;91:142–147. doi: 10.1016/0304-3940(88)90758-6. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Frank RH, Phelps EA. Perceptions of moral character modulate the neural systems of reward during the trust game. Nature Neuroscience. 2005;8:1611–1618. doi: 10.1038/nn1575. [DOI] [PubMed] [Google Scholar]
- Derick S, Cheng LL, Voirol MJ, Stoev S, Giacomini M, Wo NC, Szeto HH, Ben Mimoun M, Andres M, Gaillard RC, Guillon G, Manning M. [1-deamino-4-cyclohexylalanine] arginine vasopressin: a potent and specific agonist for vasopressin V1b receptors. Endocrinology. 2002;143:4655–4664. doi: 10.1210/en.2002-220363. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35:83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher H, Aron A, Brown LL. Romantic love: an fMRI study of a neural mechanism for mate choice. J Comp Neurol. 2005;493:58–62. doi: 10.1002/cne.20772. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Gamer M, Zurowski B, Buchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proc Natl Acad Sci U S A. 2010;107:9400–9405. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Thompson RR. Nonapeptide mechanisms of social cognition, behavior and species-specific social systems. Curr Opin Neurobiol. 2010 doi: 10.1016/j.conb.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Mathews F. Oxytocin enhances the encoding of positive social memories in humans. Biol Psychiatry. 2008;64:256–258. doi: 10.1016/j.biopsych.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30:548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, Dziobek I, Gallinat J, Wagner M, Maier W, Kendrick KM. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci. 2010;30:4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65:768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Casas B, Tomlin D, Anen C, Camerer CF, Quartz SR, Montague PR. Getting to know you: reputation and trust in a two-person economic exchange. Science. 2005;308:78–83. doi: 10.1126/science.1108062. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214:519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Ledoux J. Fear and the Brain: Where have we been, and where are we going? Biological Psychiatry. 1998;44:1229–1238. doi: 10.1016/s0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Res. 1991;555:220–232. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- Lynd-Balta E, Haber SN. The organization of midbrain projections to the ventral striatum in the primate. Neuroscience. 1994;59:609–623. doi: 10.1016/0306-4522(94)90181-3. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McCabe K, Houser D, Ryan L, Smith V, Trouard T. A functional imaging study of cooperation in two-person reciprocal exchange. Proc Natl Acad Sci U S A. 2001;98:11832–11835. doi: 10.1073/pnas.211415698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EB, Parrish JM, Nelson EE, Easter J, Thorne JF, Rilling JK, Ernst M, Pine DS. Responses to Conflict and Cooperation in Adolescents with Anxiety and Mood Disorders. J Abnorm Child Psychol. 2007 doi: 10.1007/s10802-007-9113-8. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kolachana B, Gold B, Olsh A, Nicodemus KK, Mattay V, Dean M, Weinberger DR. Genetic variants in AVPR1A linked to autism predict amygdala activation and personality traits in healthy humans. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutschler I, Wieckhorst B, Kowalevski S, Derix J, Wentlandt J, Schulze-Bonhage A, Ball T. Functional organization of the human anterior insular cortex. Neurosci Lett. 2009;457:66–70. doi: 10.1016/j.neulet.2009.03.101. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J Neurosci. 2008;28:6607–6615. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Sripada CS, Angstadt M, McCabe K. Reputation for reciprocity engages the brain reward center. Proc Natl Acad Sci U S A. 2010;107:13099–13104. doi: 10.1073/pnas.1008137107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling J, Gutman D, Zeh T, Pagnoni G, Berns G, Kilts C. A neural basis for social cooperation. Neuron. 2002;35:395–405. doi: 10.1016/s0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Sanfey AG. The neuroscience of social decision-making. Annu Rev Psychol. 2011;62:23–48. doi: 10.1146/annurev.psych.121208.131647. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Sanfey AG, Aronson JA, Nystrom LE, Cohen JD. Opposing BOLD responses to reciprocated and unreciprocted altruism in putative reward pathways. NeuroReport. 2004;15:2539–2543. doi: 10.1097/00001756-200411150-00022. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Dagenais JE, Goldsmith DR, Glenn AL, Pagnoni G. Social cognitive neural networks during in-group and out-group interactions. Neuroimage. 2008;41:1447–1461. doi: 10.1016/j.neuroimage.2008.03.044. [DOI] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162:892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuse DH, Gallagher L. Dopaminergic-neuropeptide interactions in the social brain. Trends Cogn Sci. 2009;13:27–35. doi: 10.1016/j.tics.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34:2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surget A, Belzung C. Involvement of vasopressin in affective disorders. Eur J Pharmacol. 2008;583:340–349. doi: 10.1016/j.ejphar.2007.11.065. [DOI] [PubMed] [Google Scholar]
- Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent-infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. J Child Psychol Psychiatry. 2007;48:262–287. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers; New York: 1988. p. 122. [Google Scholar]
- Theodoridou A, Rowe AC, Penton-Voak IS, Rogers PJ. Oxytocin and social perception: oxytocin increases perceived facial trustworthiness and attractiveness. Horm Behav. 2009;56:128–132. doi: 10.1016/j.yhbeh.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Thompson R, Gupta S, Miller K, Mills S, Orr S. The effects of vasopressin on human facial responses related to social communication. Psychoneuroendocrinology. 2004;29:35–48. doi: 10.1016/s0306-4530(02)00133-6. [DOI] [PubMed] [Google Scholar]
- Thompson RR, George K, Walton JC, Orr SP, Benson J. Sex-specific influences of vasopressin on human social communication. Proc Natl Acad Sci U S A. 2006;103:7889–7894. doi: 10.1073/pnas.0600406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkelbach C, Guastella AJ, Forgas JP. Oxytocin selectively facilitates recognition of positive sex and relationship words. Psychol Sci. 2008;19:1092–1094. doi: 10.1111/j.1467-9280.2008.02206.x. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality & Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Young LJ, Murphy Young AZ, Hammock EA. Anatomy and neurochemistry of the pair bond. J Comp Neurol. 2005;493:51–57. doi: 10.1002/cne.20771. [DOI] [PubMed] [Google Scholar]
- Zink CF, Stein JL, Kempf L, Hakimi S, Meyer-Lindenberg A. Vasopressin modulates medial prefrontal cortex-amygdala circuitry during emotion processing in humans. J Neurosci. 2010;30:7017–7022. doi: 10.1523/JNEUROSCI.4899-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


