Abstract
Bone marrow is thought to be a primary hematopoietic organ. However, accumulated evidences demonstrate that active function and trafficking of immune cells, including regulatory T cells, conventional T cells, B cells, dendritic cells, natural killer T (NKT) cells, neutrophils, myeloid-derived suppressor cells and mesenchymal stem cells, are observed in the bone marrow. Furthermore, bone marrow is a predetermined metastatic location for multiple human tumors. In this review, we discuss the immune network in the bone marrow. We suggest that bone marrow is an immune regulatory organ capable of fine tuning immunity and may be a potential therapeutic target for immunotherapy and immune vaccination.
Keywords: bone marrow, immunity, memory T cell, regulatory T cell, tumor
Introduction
Bone marrow is the tissue comprising the center and the epiphysis of bones, which is the place where new blood cells are produced. Bone marrow has been long thought to be a hematopoietic organ. However, it is well known that B cells are produced and matured in the bone marrow. Antigen-specific antibody producing, long-term lived plasma cells are largely found in the bone marrow. Thus, bone marrow contributes to humoral immune responses. Although normal bone marrow lacks the organized T- and B-cell areas, bone marrow is a nest for function, migration and selective retainment of innate and adaptive immune cells. In this review, we discuss the immune networks in the bone marrow. We suggest that bone marrow is an immune regulatory organ capable of fine tuning immunity and may be a potential therapeutic target for immunotherapy and immune vaccination.
Bone marrow structure
Bone is an organ composed of cortical and trabecular bone, cartilage, hemopoetic and connective tissues. Spongy or trabecular bone is composed of a lattice of fine bone plates filled with hematopoietic marrow, fat containing marrow or blood vessels. Arterial vessels enter the marrow through foramina nutricia and then divide into several arterioles. Small arterioles and capillaries from these vessels span throughout the bone marrow and supply sinusoids, which are interconnected by intersinusoidal capillaries (Figures 1 and 2).1 The bone marrow cavity in trabecular bone is subdivided into four regions: endosteal, subendosteal, central and perisinusoidal. Bone marrow consists of a hematopoietic component (parenchyma) and a vascular component (stroma) (Figure 1). The parenchyma includes hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (Figure 1), which are not randomly distributed in the bone marrow but rather are localized close to the endosteum of the bone and more around blood vessels (Figure 2).
Figure 1.
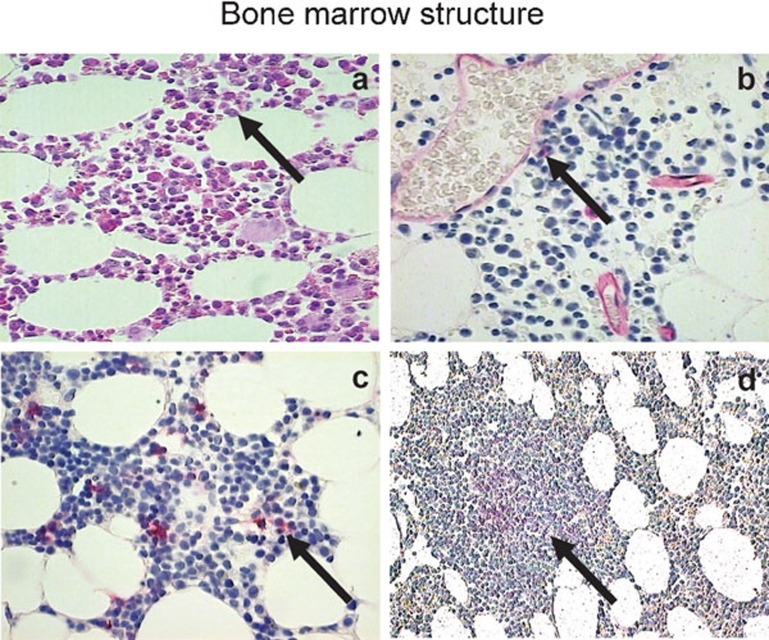
Bone marrow morphology and key cellular components: bone marrow core biopsy sections were subjected to HE staining (a), anti-CD34 staining (b), anti-CD38 staining (c) and anti-CD20 staining (d). The stained sections were revealed with fast red, analyzed by conventional microscope and images were shown with ×40 (a–c) and ×10 (d) magnification. The positive cells were judged by positive staining (red, b–d) as well as the morphology. The control mAb staining reveals no positive cells (not shown). HE, hematoxylin and eosin; mAb, monoclonal antibody.
Figure 2.
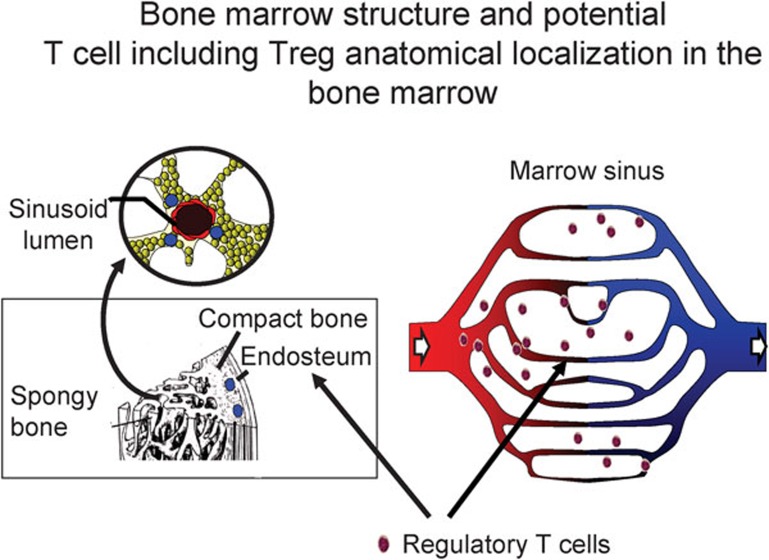
Bone marrow structure. The bone marrow is encased by cortical bone and traversed by trabecular bone. Bone marrow consists of a highly organized meshwork of thin-walled capillary-venous with extracellaur matrix that fills the space between the bony trabeculae. The artery and the periosteal capillary network are the two sources of arterial blood for the bone marrow. By successive bifurations, small branches of the artery ultimately form the capillary-venous sinus network. Murine and human bone marrow harbor immune cells including Treg cells. T cells including Treg cells may reside in the marrow sinus. Treg, regulatory T.
Bone marrow stroma contains multipotential non-hematopoietic progenitor cells (Figure 1c) capable of differentiating into various tissues of mesenchymal origin, including osteoblasts, endothelial cells, reticular cells, fibroblasts and adipocytes. The bone marrow's microvasculature with a single layer endothelium forms sinusoid (Figure 1b), which radically distributes around the draining central sinus. The vasculature provides the barrier between the bone marrow compartment as a functional and spatial entity from extralymphoid organ and the peripheral circulation.1
The stromal cells including endothelial cells provide signals for migration of individual leukocytes into and out of the bone marrow, involving in rolling/extravasations along the vascular endothelium. Compared with other organ-specific endothelial cells, bone marrow-derived endothelial cells constitutively express certain cytokines and adhesion molecules like vascular cell adhesion molecule 1 (VCAM-1 or CD106) and E-selectin (Figure 3). Additionally, organ regeneration relies on the presence of endothelial precursors among the grafted cell population, which improves vascularization of damaged tissues or by secretion of pro-angiopoietic factors by the infused cells.2 Therefore, it not only implies a role of bone marrow microvasculature system in stem cell mobilization and development, but also indicates that they play key roles in migration and maintenance of leukocyte function in bone marrow environment.
Figure 3.
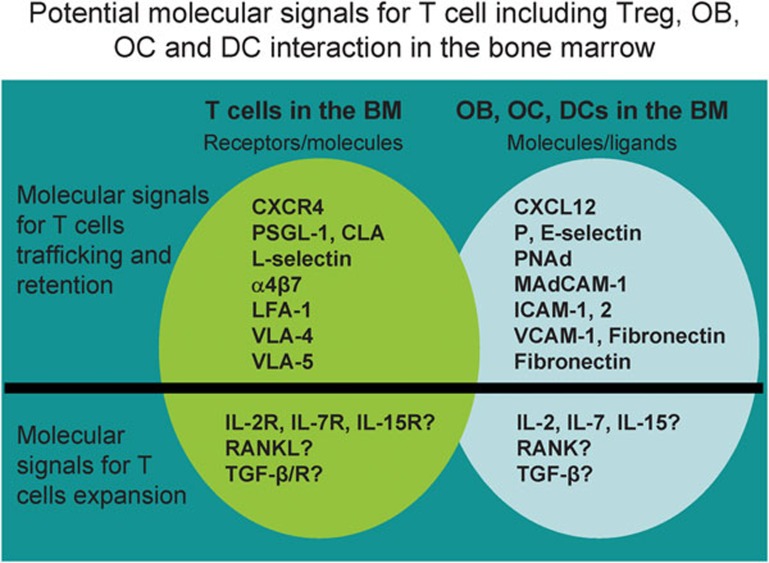
Molecular and cellular interaction between T cells and marrow sinus: T cells including Treg cells might be in the sinus areas or/and endosteum areas. The organization of molecular and cellular niches is known to have a key role in regulating T-cell immunity. Bone marrow contains a large amount of chemokines, adhesion and integrin molecules. These molecular signals may be important for immune cell bone marrow trafficking, retention and expansion. These structural and molecular niches may play a role in bone pathology in cancer patients with bone metastasis including prostate cancer and breast cancer. BM, bone marrow; CLA, conjugated linoleic acid; DC, dendritic cell; ICAM, intercellular adhesion molecule; LFA, leucocyte function antigen; OB, osteoblast; OC, osteoclast; PSGL, P-selectin glycoprotein ligand; RANKL, receptor activator of NF-kappaB ligand; TGF, transforming growth factor; Treg, regulatory T; VCAM, vascular cell adhesion molecule; VLA, very late antigen.
Immune cells in the bone marrow
Immune system is functionally compartmentalized into primary lymphoid organs and secondary lymphoid tissues where immune responses are initiated and maintained. T-cell areas of secondary lymphoid organs have a unique architecture and cellular composition which is thought to be a prerequisite for primary T-cell responses.3 Bone marrow displays structural and functional features resembling a secondary lymphoid organ, and contains follicle-like structures similar to lymph nodes or spleen, although it lacks the organized T- and B-cell areas (Figure 1d). Bone marrow microenvironment provides appropriate support for T cells to develop in the absence of the thymus.4 Lymphoid follicles in the bone marrow are increased during infections, inflammation and autoimmunity.
Bone marrow is vascularized by blood (Figure 1b), not by lymphatic vessels, and could represent a major part of the lymphocyte recirculation network, with billions of lymphocytes recirculating through it per day. It has been shown that bone marrow contains various immune cells (Table 1). Approximately 8%–20% of bone marrow mononuclear cells are lymphocytes, with a T cell/B cell ratio of 5∶1.5, 6 Bone marrow lymphocytes are distributed throughout stroma and parenchyma, and condensed in lymphoid follicles. Approximately 1% of the bone marrow mononuclear population represents plasma cells, which can produce antibodies. Mouse bone marrow contains 1%–5% CD3+ T cells in mononuclear cells.6, 7 Among T cells, there are about 1.5% CD4+ T cells and 2.0%–2.5% CD8+ T cells.8, 9, 10, 11 Interestingly, approximately one-third of CD4+ T cells are CD4+CD25+ regulatory T (Treg) cells,12 and the CD4/CD8 ratio in the bone marrow is 1∶2, which is inverted as compared to both peripheral lymph nodes and the blood.8, 10 Two-thirds of bone marrow T cells express surface markers indicative of antigen experience, such as CD44hi and CD122+, whereas most T cells in spleen and peripheral lymph nodes exhibit naive phenotypes.8 In addition to T cells, there are 1%–2% CD11c+ dendritic cells6, 7, 13 and 0.4%–4% natural killer T (NKT) cells in bone marrow.14, 15, 16 Therefore, bone marrow contains substantial amount of immune cells. Altogether, the evidences suggest that bone marrow is a lymphoid organ which may play a key role in immunity.
Table 1. Immune cells in the bone marrow.
| Immune cells | Percent | Reference |
|---|---|---|
| CD4+ T cells | ∼1.5% | 10, 11 |
| CD8+ T cells | 2–2.5% | 8, 9 |
| Regulatory T cells (Treg) | ∼0.5% | 10–12 |
| CD11c+ dendritic cells (DCs) | 1–2% | 6, 7, 13 |
| B cells | ∼1% | 63 |
| Plasma cell | ∼0.5% | 5, 66 |
| Natural killer T (NKT) cells | 0.4–4% | 10, 14–16 |
| Mesenchymal stem cells (MSCs) | 0.01–0.1% | 109, 117 |
| Myeloid-derived suppressor cells (MDSCs) | 20–30% | 97, 100 |
CD4+ T cell
Bone marrow contains a high proportion of CD4+ T cells displaying a memory phenotype, which express high levels of CD44 in mice and low levels of CD45RA in humans (Figures 2 and 3).8, 11 Similar to the CD8+ T cells, basal homeostatic proliferation and survival of CD4+ memory T cells are regulated by IL-7, identified as the dominant cytokine, and IL-15, an accessory cytokine.17 Although human CD4+ memory T cells proliferate in vitro in response to IL-7 and IL-15,18 studies in mice showed that acute homeostatic proliferation of ‘memory-phenotype' CD4+ T cells is independent of IL-7 and IL-15.19 Both cytokines were also ruled out to participate in CD4+ memory T-cell survival, because CD4+ memory T cells deficient for CD132 (γc-chain, jointly used by IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 receptors) are effectively maintained in vivo, but other work demonstrated that IL-7 is actually required for survival of both memory phenotype and T-cell receptor transgenic CD4+ memory T cells.20
Treg cell
Naturally occurring CD4+CD25+ Treg cells represent 5%–10% of the CD4+ T cells. However, our group has reported that 30% of CD4+ T cells are functional Treg cells in bone marrow.12 In patients with prostate cancer bone marrow metastasis, bone marrow Treg cells are further increased (Zou et al., unpubl. data). It suggests that bone marrow is a preferential site for migration, or selective retainment and function of Treg cells.12, 21, 22 Furthermore, we have demonstrated that CXCR4/CXCL12 (CXC chemokine ligand 12, stromal cell-derived factor-1) signaling mediates Treg cell trafficking to bone marrow (Figures 2 and 3).12 Mouse Treg cells are known to reduce the severity of graft-versus-host disease (GVHD).6, 23 Granulocyte colony-stimulating factor (G-CSF) decreases bone marrow CXCL12, and in turn mobilizes bone marrow Treg cells. These findings may help explain why G-CSF administration reduces the severity and mortality in acute GVHD.13, 24
Given that high levels of Treg cells are found in the bone marrow, bone marrow transplantation may result in increased numbers of Treg cells and in turn lead to a reduction of autoantibody production and protection from lethality caused by severe GVHD.25 Additionally, antigen presentation by bone marrow dendritic cells can induce the expansion of CD4+CD25+ T cells while simultaneously activating their ability to suppress cytokine secretion by effector T cells. Therefore, agents that mobilize Treg cells from bone marrow would be therapeutically beneficial in some clinical settings.
It is well known that Treg cells are implicated in the pathogenesis of autoimmune diseases, tumors and organ transplantation.26, 27, 28, 29 Our unpublished data indicate that Treg cells may regulate bone biology. For example, Treg cells can suppress osteoclastogenesis and ameliorate osteolytic bone resorption and destruction. The levels of bone marrow Treg cells are much higher in patients with prostate cancer. Patients with prostate cancer often have bone metastases with bone precipitation as a pathological characteristic. It is assumed that bone marrow Treg cells may contribute to bone pathology in patients with prostate cancer. The studies in our laboratory will address this possibility. Nonetheless, bone marrow Treg cells might be a novel therapeutic strategy for clinical diseases and transplantation.30, 31
IL-17+CD4+ T (Th17) cell
IL-17 (originally termed CTLA8, also known as IL-17A) belongs to a family of six members and has been of great interest recently owing to the discovery that the production of IL-17 characterizes a subset of CD4+ helper T cells (Th17 cells).32 The development of Th17 cells is coupled to signal transducer and activator of transcription 3 and the transcription factor RORγt and depends on IL-6 and transforming growth factor (TGF)-β, which is shared by regulatory T cells for development and function.33 Th17 cells are characterized by the production of a distinct profile of effector cytokines, including IL-17 (or IL-17A), IL-17F, IL-21 and IL-22.34, 35, 36 Th17 cells play an important role in inflammation, autoimmune disease and tumor.37, 38, 39
Th17 cells are induced and elevated in multiple myeloma by the elevated cytokine production of IL-6, IL-1β and TGF-β in bone marrow microenvironment.33 IL-17 in turn promotes myeloma cell growth and suppresses immune function in multiple myeloma.33 It is also reported that elevated Th17 cells mediate bone resorption and destruction by inducing osteoclast formation in multiple myeloma patients.40 Bone marrow mesenchymal stem cells (MSCs) inhibit the differentiation and function of Th17 cells and ameliorate multiple sclerosis in an experimental autoimmune encephalomyelitis model.41, 42 Administration of bone marrow stromal cells ameliorates experimental autoimmune myasthenia gravis by reducing Th17 cells.43 Several groups have shown that Th17 cells contribute to GVHD after bone marrow transplantation.44, 45 There is evidence showing increased Th17 cells in low risk myelodysplastic syndrome.46 Thus, Th17 cells play an important role in bone marrow-mediated immunity and may serve as a therapeutic target for bone marrow-related diseases.
CD8+ T cell
Bone marrow is the preferred site for proliferation of memory CD8+ T cells.47 Antigen specific memory CD8+ T cells receive proliferative signals by IL-7 and/or IL-15 in the bone marrow. It suggests that the bone marrow is a ‘niche' for the antigen-independent proliferation of memory CD8+ T cells. Memory CD8+ T lymphocyte populations in bone marrow display a CD44-positive phenotype with a higher percentage of HLA-DR molecules, which suggests that CD8+ T cells in bone marrow are in an activated state. High numbers of tumor-associated antigen-specific CD8+ T cells were shown to persist in the bone marrow for several months after acute infection or tumor development.48, 49 Adoptive transfer of bone marrow cells from lymphochoriomeningitis virus-immunized mice to immunodeficient recipients provides antiviral protection.9 Thus, memory CD8+ T cells in the bone marrow are able to mount an effective secondary response. A long time after priming, memory CD8+ T cells proliferate more extensively in the bone marrow than they do in either secondary lymphoid or extra-lymphoid organs and undergo basal proliferation in the bone marrow.47, 50 Indeed, bone marrow-resident T cells are functionally distinct from those in other compartments.10 Compared to their blood counterparts, bone marrow-derived CD8+ T cells induce milder GVHD and possess higher anti-tumor activity.6
Bone marrow also functions as a site of recruitment and retention for central memory T cells by providing specific recruitment signals that mediate the recruitment of central memory T cells from the blood.8 Central memory T cells constitute the largest endogenous subset of CD8+ T cells in murine bone marrow and are also prominent in human bone marrow. There are also naive CD8+ T cells and effector memory CD8+ T cells in bone marrow, but these populations are smaller (at least in mice) than naive T cells in secondary lymphoid organs or effector memory T cells in spleen, liver and lung.51 Accordingly, adoptively transferred central memory T cells from immunized mice accumulated and colonized in recipient bone marrow more effectively than effector T cells and naive T cells. Overall, the bone marrow may be an important organ for CD8+ T cell-mediated immunity.
NKT cell
NKT cells are defined as T cells bearing the common natural killer cell marker such as NK1.1 (NKR-P1C) in mice or CD161 (NKR-P1A) in humans, expressing IL-2Rβ (CD122), Ly49 family receptors and T-cell receptor.52, 53 In normal adult mice, NKT cells are found in thymus, bone marrow, liver and spleen at a level of 0.5–1.5 million cells per organ. NKT cells are rare in lymph nodes and virtually absent from gut intraepithelial lymphocytes. Besides thymus and liver, NKT cells can be generated in bone marrow of nude mice.14, 54 Reconstitution of adult thymectomized irradiated mice with syngeneic bone marrow cells gives rise to NKT cells in the recipient organs,55, 56 suggesting that NKT cells can develop extrathymically from the bone marrow. Peripheral NKT cells are rapidly deleted upon activation and replaced by NKT cells that have been generated by de novo proliferation in bone marrow. Thus, bone marrow plays a major role in restoring NKT cell homeostasis.
Bone marrow NKT cells may play immune stimulatory and inhibitory roles in the regulation of bone marrow transplantation immunity15, 57, 58 and tumor immunity.59, 60 Adoptive transfer of donor NKT cells significantly ameliorates GVHD in a murine model of bone marrow transplantation.58 The relative protection against GVHD was contributed to, to some extent, the population of NKT cells in bone marrow.58, 61 NKT cells are also believed to be among the most important anti-cancer cell populations in the mouse, causing rejection of malignant cells in vivo.59, 60 Thus, bone marrow NKT cells could differentially regulate immune responses in different settings.
B cell
Bone marrow is major organ for the development and maturation of B cells. B cells are generated from HSCs and developed in bone marrow before they egress into peripheral blood to reach peripheral lymphoid organs. Specific cellular niches for B-cell development include CXCL12-expressing cells and IL-7-expressing cells.62 Immature B cells, like pro-B and pre-B stage cells, are regulated by extrinsic signals from the bone marrow during their development.63 B-cell precursors and plasma cells reside in the specific niches and move between the niches as development proceeds.62
The majority of serum antibody is produced by terminally differentiated plasma cells. These non-dividing cells differ from memory B cells in typical B-cell markers, including major histocompatibility (MHC) class II and surface immunoglobulin. The main function of plasma cells is to continuously secrete large quantities of specific antibody. In contrast, memory B cells do not spontaneously secrete antibody. These cells proliferate and differentiate into antibody-secreting cells following appropriate stimulation. Interestingly, some plasma cells are long-lived. Most importantly, the long-lived plasma cells are found in the bone marrow. The bone marrow is a reservoir for long-lived plasma cells and is involved in the maintenance of long immunity.64, 65 Antigen-specific bone marrow plasma cells have been detected for more than 300 days post-viral infection.64 The persistence of plasma cells within the bone marrow might be supported by soluble factors and/or cell–cell contact in the bone marrow microenvironment, in which one critical element is the bone marrow reticular stromal cells. Stromal cells provide growth factors as well as cell contact-dependent signals, such as IL-6 and cell contact-mediated signals (e.g., very late antigen-4) (Figure 2).66 In addition to stromal elements, plasma cells could interact with various other bone marrow resident cells, including developing lymphoid and myeloid lineage cells. Therefore, bone marrow plasma cells are not intrinsically long-lived. Bone marrow stromal cells provide survival factors to them. The plasmablasts can migrate to the bone marrow67, 68, 69 and differentiate into memory plasma cells after docking with CXCL12-expressing stromal cells.62, 69 The overall impact of these interactions supports the longevity of the plasma cells in bone marrow. Therefore, bone marrow contributes to humoral immune responses.
Neutrophil
Neutrophils are an essential component of innate immune system and may represent a critical link between the innate and adaptive immune system.70 They are differentiated from stem cells in the bone marrow by a process termed granulopoiesis.71 Neutrophils are generated at a rate of 1 to 2×1011 cells per day in a normal adult human under normal condition.72 Several myeloid transcription factors are essential for granulopoiesis, including CCAAT-enhanced binding protein α PU.1 and growth factor independent-1 (GFI-1).73 PU.1 is an ETS family transcription factor encoded by spleen focus forming virus proviral integration oncogene in human74 and absolutely required for myeloid lineage commitment.75, 76 The balance between PU.1 and CCAAT-enhanced binding protein α determines the commitment of granulocytes and monocytes.77, 78, 79 High expression of PU.1 drives monocytic differentiation and CCAAT-enhanced binding protein α promotes granulocytopoiesis.72, 80 GFI-1 is also necessary for neutrophil differentiation and it is upregulated during granulocytic lineage commitment.81, 82 G-CSF is critical in regulating granulopoiesis at several stages. G-CSF directs the commitment of multipotent progenitor cells down to the myeloid lineage, stimulates proliferation of granulocytic precursors and reduces the transit time of neutrophils through granulocytic compartment.83, 84
Bone marrow is a large pool for the mature neutrophils.85 There are 1%–2% of mature neutrophils in the circulation in mice.86 The majority of neutrophils are reserved in the bone marrow. A large amount of neutrophils can be mobilized rapidly in response to infection and stress, which suggests that the bone marrow reserve is critical for host defense. CXCR4/CXCL12 signaling pathway plays a crucial role for maintaining neutrophils in bone marrow (Figure 3).87, 88 It is reported that administration of G-CSF reduces the expression of CXCR4 on bone marrow neutrophils and Treg cells, and the levels of bone marrow CXCL12.12 This explains why G-CSF administration mobilizes neutrophils from bone marrow to peripheral circulation.89, 90
Once released from the bone marrow, neutrophils circulate in the peripheral blood and have a relatively short half-life (about 6–8 h).71, 91 Bone marrow serves as an important site for neutrophil clearance under homeostatic conditions. Senescent neutrophils home back to bone marrow depending on Gαi subunit of the heterotrimeric G-protein.92 Senescent neutrophils highly express CXCR493 and may home back to bone marrow via the CXCR4/CXCL12 chemokine axis.88 Once senescent neutrophils return to bone marrow, they are phagocytosed and destroyed by resident stromal macrophages in bone marrow, which in turn stimulates the production of G-CSF by bone marrow macrophages after uptake of apoptotic neutrophils and subsequently G-CSF acts as a positive feedback to promote granulopoiesis and regulate neutrophils release.91, 92 Thus, bone marrow plays an important role in the homeostasis of neutrophils.
Dendritic cell
Dendritic cells (DCs) play a key role in linking innate and adaptive immune responses.13, 94 Circulating DCs migrate to the bone marrow where they are retained better than in spleen, liver and lung tissues.95 Homing of DCs to the bone marrow depends on constitutively expressed VCAM-1 and endothelial selectins in bone marrow microvessels (Figure 3). The migration of DCs to non-lymphoid organs might be advantageous for ‘boosting' memory responses to previously antigen-experienced T cells.96 Bone marrow DCs are able to trigger central memory T cells-mediated responses with antigen-dependent contacts. Bone marrow-derived dendritic cells are distinct from counterpart DCs in extralymphoid tissue, and are essential for innate immunity to intracellular infection. Bone marrow-derived dendritic cells are able to uptake the blood-derived cell-associated tumor-associated antigen, process them and induce antigen-specific systemic protective T cells-mediated immunity.5 Thus, bone marrow DCs are functionally important in adaptive immunity.
Myeloid-derived cell
Myeloid-derived cells are a heterogeneous population of cells consisting of myeloid progenitors, immature myeloid cells and macrophages.97 Immature myeloid cells that generated in bone marrow differentiate into mature myeloid cells under normal condition. But under pathological conditions (e.g., tumor), immature myeloid cells are expanded and activated, and become myeloid-derived suppressor cells (MDSCs), and acquire immunosuppressive activity by producing immune suppressive factors such as arginase I or inducible nitric oxidase synthase or TGF-β.97, 98 MDSCs express Gr-1 and CD11b, the surface marker for myeloid cell lineage in mice.99 In healthy mice, 20%–30% of whole bone marrow cells express Gr-1+CD11b+ phenotype.100 Based on the two different epitopes of Gr-1 antibodies, Ly6G and Ly6C, mouse MDSCs are subdivided into granulocytic MDSCs (CD11b+Ly6G+Ly6Clow) and monocytic MDSCs (CD11b+Ly6G-Ly6Chi).101, 102 MDSCs may be defined as cells with CD14−CD11b+HLA-DRdim phenotype in human.103, 104 MDSCs are shown to have the ability to induce the development and differentiation of Treg cells through cytokine production or cell–cell interaction,105, 106 which indicates that MDSCs and Treg cells might cooperate to regulate immune response.97 Adoptive transfer of MDSCs inhibits T-cell alloresponses, prevents GVHD and prolongs the survival of mice.107, 108 MDSC–T cell interactions play an important role in protective anti-tumor immunity, infection, inflammation and GVHD.107 Bone marrow MDSCs may be an important regulator of immune response and may serve as a potential therapeutic target for several clinical diseases.
MSC
In adult life, stem cells of mesenchymal lineage (MSCs), which compromise 0.01%–0.1% of total adult bone marrow cells, are mainly confined to the bone marrow,109 where they are multipotential non-hematopoietic progenitor cells capable of differentiating into various tissues of mesenchymal origin. Human MSCs express human leukocyte antigen (HLA) class I but not HLA class II110 or costimulatory molecules CD80 (B7.1), CD86 (B7.2) or CD40.111 MSCs produce cytokines and growth factors for hematopoiesis and may attract infused HSCs to the bone marrow by inducing expression of homing receptors.112, 113
In the bone marrow, MSCs display a great potential role in immunoregulatory activity, which comes from the observation that MSCs from various species can exert profound immunosuppression by inhibiting T-cell proliferation in responses to polyclonal stimuli and to their cognate peptide in a MHC-independent manner.24, 114, 115, 116 Although the conflicting results about MSC-mediated immunosuppression have been produced with different methods and species, overall data suggest that some soluble factors and mechanisms of cell–cell interaction might contribute to the inhibition of MSCs.24, 115, 116 It is reported that MSCs could inhibit naive and memory T-cell responses to their cognate antigens, but it does not appear to be antigen-specific.24 MSCs also induce T-cell anergy117 or T-cell apoptosis,118 suppress T-cell IFN-γ production and increase IL-4 secretion, and enhance Treg cell compartment.119 MSCs selectively targets antigen-experienced T-cell responses in the contact with antigen-presenting cells in a non-cognate fashion, sparing those that have not been activated by T-cell receptor engagement.116 Moreover, MSCs appear to discriminate between cellular responses to alloantigens and recall antigens.120 The expression of early activation markers such as CD25 and CD69 on T cell is unaffected in the presence of MSCs, but IFN-γ production is reduced. The inhibitory effect of MSCs is directed mainly at the level of cell proliferation without interfering with early T-cell activation. MSCs do not preferentially target any T-cell subset. MSCs have a role in inhibiting proliferation and affecting differentiation, antibody production and chemotactic behavior of B cells.117, 121 MSCs may inhibit differentiation of hematopoietic progenitors into DCs and promote anti-inflammatory cytokine production of monocyte-derived DCs.122, 123 MSCs suppress cytokine-induced proliferation and prevent cytokine production and cytotoxic activity of natural killer cells.124, 125 In the context of limited understanding about MHC expression on MSCs, it is difficult to ascribe specific T-cell receptor/MHC/peptide interactions to their mechanism of immunoregulation. Notably, the inhibitory effect of MSCs does not require the presence of antigen-presenting cells and is not mediated through Treg cells.116 MSCs have been reported to be used in clinical trials for autologous or allogeneic engraftment of bone marrow transplants,126 osteogenesis imperfect,127 stroke,128 myocardial infarction129 and GVHD.130 Therefore, bone marrow MSCs have an important role in immune regulation and are potential candidates for clinical treatment.
Cytokines and chemokines
Bone marrow-derived cells including leukocytes, HSCs and stromal cells could secret lots of cytokines. Stroma cells and cells of hemopoietic lineage in the bone marrow produce both IL-7 and IL-15. IL-7 is a ‘stromal cytokine' produced by a variety of stromal tissues including those in bone marrow. The production of IL-7 by bone marrow stromal cells is thought to be essential for early B lymphocyte development in mouse (but not in human),131, 132, 133 and secreted IL-7 in bone marrow is postulated to play a critical role in post-thymic T-cell homeostasis.54, 134 IL-15 promotes basal homeostatic proliferation and survival of memory T cells in different experimental systems, whereas IL-7 performs an overlapping and complementary role during acute homeostatic proliferation in lymphopenic environments.135, 136 Recently, exogenous IL-15 was shown to replace exogenous IL-2 therapy during the treatment of established, non-manipulated poorly immunogenic tumors.137 IL-21 plays a role in the proliferation and maturation of natural killer cell populations from bone marrow, and contributes to the proliferation of T/B cell populations and anti-tumor effect.138, 139
Chemokines could also be involved in the generation and regulation of bone marrow immune cells. Mobilization and interactions of stromal cell/hematopoietic precursors are thought to be controlled by cytokines, particularly, chemokines. Among chemokines, CXCL12 is particularly intriguing. CXCL12-expressing cells are a small population of bone marrow stromal cells scattered throughout bone marrow, such as osteoblasts, marrow fibroblasts and endothelial cells.140 CXCL12-mediated interaction of progenitors with the bone marrow vascular niche allows the progenitors to relocate to a microenvironment that is permissive and instructive for megakaryocyte maturation and thrombopoiesis.23 It has been recently determined that chemokine stimulation of HSCs and bone marrow-derived endothelial cells by CXCL12 leads to an enhancement in transendothelial and stromal migration via activation of adhesion molecules, in addition to its well-known ability to stimulate motility.141, 142 Signals for the translocation of HSCs from the fetal liver to bone marrow are provided by CXCL12. In response to CXCL12, HSCs or lymphocytes that express its specific seven transmembrane-span G protein-coupled CXCR4 receptor leave the fetal liver and colonize in the bone marrow, where they finally establish hematopoiesis.143, 144, 145 As we discussed above, CXCR4/CXCL12 signaling also regulates the bone marrow trafficking of memory T cells, Treg cells and neutrophils.12, 67, 68, 87, 88, 93
In addition to the role of chemokines in bone marrow, the adhesion molecules also regulate leukocytes migration to the bone marrow (Figure 3). Normal bone marrow sinusoids express VCAM-1 and E-selectin. The migration of B cells, CD4+ and CD8+ T cells to bone marrow is impaired in conditional VCAM-1-deficient mice, resulting in reduced B cells and T cells in bone marrow and mild leukocytosis in peripheral blood.146 E-selectin and VCAM-1 are necessary for recruitment of hematopoietic progenitor cells to bone marrow.147 Neutralizing antibody to E-selectin attenuates CD8+ central memory T-cell rolling in bone marrow.8 Thus, bone marrow plays an important role in immune cells homeostasis via the expression of cytokines, chemokines and adhesion molecules.
Concluding remarks
Bone marrow is well-known as a primary hematopoietic organ. However, bone marrow contains high levels of multiple immune cell subsets with important and unique functionalities. It is evident that bone marrow can supplant the secondary lymphoid tissue either as a site of primary immune response or memory response. Immune regulation occurs in the bone marrow microenvironment in cell–cell contact manners or/and through soluble factors including cytokines. Thus, bone marrow is an immune regulatory organ, which may importantly affect systemic immunity and therapeutic efficacy of conventional and immune therapy/vaccination. Given that multiple human cancers including breast and prostate cancer preferentially metastasize to bone marrow, specific cellular and molecular niches in the bone marrow including high levels of Treg cells and MDSCs may impact tumor bone metastasis and contribute to bone pathology in cancer patients with bone marrow metastasis. Therefore, understanding the immune regulatory mechanisms in the bone marrow microenvironment will generate significant insight into human bone biology and immunology. Furthermore, given the unique functionalities of bone marrow memory T cells, one may expect that bone marrow serves as an excellent source of immune cells for adoptive immunotherapy for both malignancies and infectious diseases.
Acknowledgments
This research is supported (in part) by NIH/NCI R01 grants (R01CA133620) (WZ) and the NIH through the University of Michigan's Cancer Center Support Grant (5 P30 CA46592).
References
- Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda) 2005;20:349–356. doi: 10.1152/physiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- Tripp RA, Topham DJ, Watson SR, Doherty PC. Bone marrow can function as a lymphoid organ during a primary immune response under conditions of disrupted lymphocyte trafficking. J Immunol. 1997;158:3716–3720. [PubMed] [Google Scholar]
- Dejbakhsh-Jones S, Jerabek L, Weissman IL, Strober S. Extrathymic maturation of alpha beta T cells from hemopoietic stem cells. J Immunol. 1995;155:3338–3344. [PubMed] [Google Scholar]
- Schirrmacher V, Feuerer M, Fournier P, Ahlert T, Umansky V, Beckhove P. T-cell priming in bone marrow: the potential for long-lasting protective anti-tumor immunity. Trends Mol Med. 2003;9:526–534. doi: 10.1016/j.molmed.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Feuerer M, Beckhove P, Mahnke Y, Hommel M, Kyewski B, Hamann A, et al. Bone marrow microenvironment facilitating dendritic cell: CD4 T cell interactions and maintenance of CD4 memory. Int J Oncol. 2004;25:867–876. [PubMed] [Google Scholar]
- Feuerer M, Beckhove P, Garbi N, Mahnke Y, Limmer A, Hommel M, et al. Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat Med. 2003;9:1151–1157. doi: 10.1038/nm914. [DOI] [PubMed] [Google Scholar]
- Mazo IB, Honczarenko M, Leung H, Cavanagh LL, Bonasio R, Weninger W, et al. Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity. 2005;22:259–270. doi: 10.1016/j.immuni.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Slifka MK, Whitmire JK, Ahmed R. Bone marrow contains virus-specific cytotoxic T lymphocytes. Blood. 1997;90:2103–2108. [PubMed] [Google Scholar]
- Zeng D, Hoffmann P, Lan F, Huie P, Higgins J, Strober S. Unique patterns of surface receptors, cytokine secretion, and immune functions distinguish T cells in the bone marrow from those in the periphery: impact on allogeneic bone marrow transplantation. Blood. 2002;99:1449–1457. doi: 10.1182/blood.v99.4.1449. [DOI] [PubMed] [Google Scholar]
- Price PW, Cerny J. Characterization of CD4+ T cells in mouse bone marrow. I. Increased activated/memory phenotype and altered TCR Vbeta repertoire. Eur J Immunol. 1999;29:1051–1056. doi: 10.1002/(SICI)1521-4141(199903)29:03<1051::AID-IMMU1051>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Zou L, Barnett B, Safah H, Larussa VF, Evdemon-Hogan M, Mottram P, et al. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. 2004;64:8451–8455. doi: 10.1158/0008-5472.CAN-04-1987. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Sykes M. Unusual T cell populations in adult murine bone marrow. Prevalence of CD3+CD4−CD8− and alpha beta TCR+NK1.1+ cells. J Immunol. 1990;145:3209–3215. [PubMed] [Google Scholar]
- Higuchi M, Zeng D, Shizuru J, Gworek J, Dejbakhsh-Jones S, Taniguchi M, et al. Immune tolerance to combined organ and bone marrow transplants after fractionated lymphoid irradiation involves regulatory NK T cells and clonal deletion. J Immunol. 2002;169:5564–5570. doi: 10.4049/jimmunol.169.10.5564. [DOI] [PubMed] [Google Scholar]
- Zeng D, Gazit G, Dejbakhsh-Jones S, Balk SP, Snapper S, Taniguchi M, et al. Heterogeneity of NK1.1+ T cells in the bone marrow: divergence from the thymus. J Immunol. 1999;163:5338–5345. [PubMed] [Google Scholar]
- Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174:6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4+ T cells. J Exp Med. 2001;194:1711–1719. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz O, Grandjean I, Matzinger P, Di Santo JP. Gamma chain required for naive CD4+ T cell survival but not for antigen proliferation. Nat Immunol. 2000;1:54–58. doi: 10.1038/76917. [DOI] [PubMed] [Google Scholar]
- Wei S, Kryczek I, Edwards RP, Zou L, Szeliga W, Banerjee M, et al. Interleukin-2 administration alters the CD4+FOXP3+ T-cell pool and tumor trafficking in patients with ovarian carcinoma. Cancer Res. 2007;67:7487–7494. doi: 10.1158/0008-5472.CAN-07-0565. [DOI] [PubMed] [Google Scholar]
- Wei S, Kryczek I, Zou W. Regulatory T-cell compartmentalization and trafficking. Blood. 2006;108:426–431. doi: 10.1182/blood-2006-01-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. . Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- Herrmann MM, Gaertner S, Stadelmann C, van den Brandt J, Boscke R, Budach W, et al. Tolerance induction by bone marrow transplantation in a multiple sclerosis model. Blood. 2005;106:1875–1883. doi: 10.1182/blood-2004-12-4607. [DOI] [PubMed] [Google Scholar]
- Kryczek I, Liu R, Wang G, Wu K, Shu X, Szeliga W, et al. FOXP3 defines regulatory T cells in human tumor and autoimmune disease. Cancer Res. 2009;69:3995–4000. doi: 10.1158/0008-5472.CAN-08-3804. [DOI] [PubMed] [Google Scholar]
- Wilke CM, Wu K, Zhao E, Wang G, Zou W. Prognostic significance of regulatory T cells in tumor. Int J Cancer. 2010;127:748–758. doi: 10.1002/ijc.25464. [DOI] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- Issa F, Wood KJ. CD4+ regulatory T cells in solid organ transplantation. Curr Opin Organ Transplant. 2010;15:757–764. doi: 10.1097/MOT.0b013e32834017ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2011;10:248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhala RH, Pelluru D, Fulciniti M, Prabhala HK, Nanjappa P, Song W, et al. Elevated IL-17 produced by TH17 cells promotes myeloma cell growth and inhibits immune function in multiple myeloma. Blood. 2010;115:5385–5392. doi: 10.1182/blood-2009-10-246660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Wei S, Vatan L, Escara-Wilke J, Szeliga W, Keller ET, et al. Cutting edge: opposite effects of IL-1 and IL-2 on the regulation of IL-17+ T cell pool IL-1 subverts IL-2-mediated suppression. J Immunol. 2007;179:1423–1426. doi: 10.4049/jimmunol.179.3.1423. [DOI] [PubMed] [Google Scholar]
- Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114:357–359. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Bruce AT, Gudjonsson JE, Johnston A, Aphale A, Vatan L, et al. Induction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J Immunol. 2008;181:4733–4741. doi: 10.4049/jimmunol.181.7.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, et al. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- Noonan K, Marchionni L, Anderson J, Pardoll D, Roodman GD, Borrello I. A novel role of IL-17-producing lymphocytes in mediating lytic bone disease in multiple myeloma. Blood. 2010;116:3554–3563. doi: 10.1182/blood-2010-05-283895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannam S, Pene J, Torcy-Moquet G, Jorgensen C, Yssel H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol. 2010;185:302–312. doi: 10.4049/jimmunol.0902007. [DOI] [PubMed] [Google Scholar]
- Bai L, Lennon DP, Eaton V, Maier K, Caplan AI, Miller SD, et al. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009;57:1192–1203. doi: 10.1002/glia.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong QF, Sun B, Wang GY, Zhai DX, Mu LL, Wang DD, et al. BM stromal cells ameliorate experimental autoimmune myasthenia gravis by altering the balance of Th cells through the secretion of IDO. Eur J Immunol. 2009;39:800–809. doi: 10.1002/eji.200838729. [DOI] [PubMed] [Google Scholar]
- Kappel LW, Goldberg GL, King CG, Suh DY, Smith OM, Ligh C, et al. IL-17 contributes to CD4-mediated graft-versus-host disease. Blood. 2009;113:945–52. doi: 10.1182/blood-2008-08-172155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Vodanovic-Jankovic S, Johnson B, Keller M, Komorowski R, Drobyski WR. Absence of regulatory T-cell control of TH1 and TH17 cells is responsible for the autoimmune-mediated pathology in chronic graft-versus-host disease. Blood. 2007;110:3804–3813. doi: 10.1182/blood-2007-05-091074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordasti SY, Afzali B, Lim Z, Ingram W, Hayden J, Barber L, et al. IL-17-producing CD4+ T cells, pro-inflammatory cytokines and apoptosis are increased in low risk myelodysplastic syndrome. Br J Haematol. 2009;145:64–72. doi: 10.1111/j.1365-2141.2009.07593.x. [DOI] [PubMed] [Google Scholar]
- Becker TC, Coley SM, Wherry EJ, Ahmed R. Bone marrow is a preferred site for homeostatic proliferation of memory CD8 T cells. J Immunol. 2005;174:1269–1273. doi: 10.4049/jimmunol.174.3.1269. [DOI] [PubMed] [Google Scholar]
- Marshall DR, Turner SJ, Belz GT, Wingo S, Andreansky S, Sangster MY, et al. Measuring the diaspora for virus-specific CD8+ T cells. Proc Natl Acad Sci USA. 2001;98:6313–6318. doi: 10.1073/pnas.101132698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- Parretta E, Cassese G, Barba P, Santoni A, Guardiola J, Di Rosa F. CD8 cell division maintaining cytotoxic memory occurs predominantly in the bone marrow. J Immunol. 2005;174:7654–7664. doi: 10.4049/jimmunol.174.12.7654. [DOI] [PubMed] [Google Scholar]
- Weninger W, Crowley MA, Manjunath N, von Andrian UH. Migratory properties of naive, effector, and memory CD8+ T cells. J Exp Med. 2001;194:953–966. doi: 10.1084/jem.194.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- MacDonald HR. NK1.1+ T cell receptor-alpha/beta+ cells: new clues to their origin, specificity, and function. J Exp Med. 1995;182:633–638. doi: 10.1084/jem.182.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci USA. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikly K, Dennert G. Evidence for extrathymic development of TNK cells. NK1+ CD3+ cells responsible for acute marrow graft rejection are present in thymus-deficient mice. J Immunol. 1992;149:403–412. [PubMed] [Google Scholar]
- Sato K, Ohtsuka K, Hasegawa K, Yamagiwa S, Watanabe H, Asakura H, et al. Evidence for extrathymic generation of intermediate T cell receptor cells in the liver revealed in thymectomized, irradiated mice subjected to bone marrow transplantation. J Exp Med. 1995;182:759–767. doi: 10.1084/jem.182.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi K, Takahashi T, Hiruma K, Kanda Y, Tanaka Y, Ogawa S, et al. Recovery of Valpha24+ NKT cells after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2004;34:595–602. doi: 10.1038/sj.bmt.1704582. [DOI] [PubMed] [Google Scholar]
- Margalit M, Ilan Y, Ohana M, Safadi R, Alper R, Sherman Y, et al. Adoptive transfer of small numbers of DX5+ cells alleviates graft-versus-host disease in a murine model of semiallogeneic bone marrow transplantation: a potential role for NKT lymphocytes. Bone Marrow Transplant. 2005;35:191–197. doi: 10.1038/sj.bmt.1704719. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Crowe NY, Hayakawa Y, Takeda K, Yagita H, Godfrey DI. NKT cells - conductors of tumor immunity. Curr Opin Immunol. 2002;14:165–171. doi: 10.1016/s0952-7915(02)00316-3. [DOI] [PubMed] [Google Scholar]
- Dean J, McCarthy D, Lawler M, Doherty DG, O'Farrelly C, Golden-Mason L. Characterization of NKR+ T-cell subsets in human bone marrow: implications for immunosurveillance of neoplasia. Clin Immunol. 2005;114:42–51. doi: 10.1016/j.clim.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Zeng D, Lewis D, Dejbakhsh-Jones S, Lan F, Garcia-Ojeda M, Sibley R, et al. Bone marrow NK1.1− and NK1.1+ T cells reciprocally regulate acute graft versus host disease. J Exp Med. 1999;189:1073–1081. doi: 10.1084/jem.189.7.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20:707–718. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Sandel PC, Gendelman M, Kelsoe G, Monroe JG. Definition of a novel cellular constituent of the bone marrow that regulates the response of immature B cells to B cell antigen receptor engagement. J Immunol. 2001;166:5935–5944. doi: 10.4049/jimmunol.166.10.5935. [DOI] [PubMed] [Google Scholar]
- Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997;388:133–134. doi: 10.1038/40540. [DOI] [PubMed] [Google Scholar]
- Minges Wols HA, Underhill GH, Kansas GS, Witte PL. The role of bone marrow-derived stromal cells in the maintenance of plasma cell longevity. J Immunol. 2002;169:4213–4221. doi: 10.4049/jimmunol.169.8.4213. [DOI] [PubMed] [Google Scholar]
- Hargreaves DC, Hyman PL, Lu TT, Ngo VN, Bidgol A, Suzuki G, et al. A coordinated change in chemokine responsiveness guides plasma cell movements. J Exp Med. 2001;194:45–56. doi: 10.1084/jem.194.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser AE, Debes GF, Arce S, Cassese G, Hamann A, Radbruch A, et al. Chemotactic responsiveness toward ligands for CXCR3 and CXCR4 is regulated on plasma blasts during the time course of a memory immune response. J Immunol. 2002;169:1277–1282. doi: 10.4049/jimmunol.169.3.1277. [DOI] [PubMed] [Google Scholar]
- Tokoyoda K, Hauser AE, Nakayama T, Radbruch A. Organization of immunological memory by bone marrow stroma. Nat Rev Immunol. 2010;10:193–200. doi: 10.1038/nri2727. [DOI] [PubMed] [Google Scholar]
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- Christopher MJ, Link DC. Regulation of neutrophil homeostasis. Curr Opin Hematol. 2007;14:3–8. doi: 10.1097/00062752-200701000-00003. [DOI] [PubMed] [Google Scholar]
- Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–670. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Rosmarin AG, Yang Z, Resendes KK. Transcriptional regulation in myelopoiesis: hematopoietic fate choice, myeloid differentiation, and leukemogenesis. Exp Hematol. 2005;33:131–143. doi: 10.1016/j.exphem.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Ray D, Culine S, Tavitain A, Moreau-Gachelin F. The human homologue of the putative proto-oncogene Spi-1: characterization and expression in tumors. Oncogene. 1990;5:663–668. [PubMed] [Google Scholar]
- Nerlov C, Graf T. PU.1 induces myeloid lineage commitment in multipotent hematopoietic progenitors. Genes Dev. 1998;12:2403–2412. doi: 10.1101/gad.12.15.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki H, Somoza C, Shigematsu H, Duprez EA, Iwasaki-Arai J, Mizuno S, et al. Distinctive and indispensable roles of PU.1 in maintenance of hematopoietic stem cells and their differentiation. Blood. 2005;106:1590–1600. doi: 10.1182/blood-2005-03-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy VA, Iwama A, Iotzova G, Schulz M, Elsasser A, Vangala RK, et al. Granulocyte inducer C/EBPalpha inactivates the myeloid master regulator PU.1: possible role in lineage commitment decisions. Blood. 2002;100:483–490. doi: 10.1182/blood.v100.2.483. [DOI] [PubMed] [Google Scholar]
- Dahl R, Walsh JC, Lancki D, Laslo P, Iyer SR, Singh H, et al. Regulation of macrophage and neutrophil cell fates by the PU.1:C/EBPalpha ratio and granulocyte colony-stimulating factor. Nat Immunol. 2003;4:1029–1036. doi: 10.1038/ni973. [DOI] [PubMed] [Google Scholar]
- Laslo P, Spooner CJ, Warmflash A, Lancki DW, Lee HJ, Sciammas R, et al. Multilineage transcriptional priming and determination of alternate hematopoietic cell fates. Cell. 2006;126:755–766. doi: 10.1016/j.cell.2006.06.052. [DOI] [PubMed] [Google Scholar]
- Zhang DE, Zhang P, Wang ND, Hetherington CJ, Darlington GJ, Tenen DG. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein alpha-deficient mice. Proc Natl Acad Sci USA. 1997;94:569–574. doi: 10.1073/pnas.94.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsunky H, Zeng H, Schmidt T, Zevnik B, Kluge R, Schmid KW, et al. Inflammatory reactions and severe neutropenia in mice lacking the transcriptional repressor Gfi1. Nat Genet. 2002;30:295–300. doi: 10.1038/ng831. [DOI] [PubMed] [Google Scholar]
- Hock H, Hamblen MJ, Rooke HM, Traver D, Bronson RT, Cameron S, et al. Intrinsic requirement for zinc finger transcription factor Gfi-1 in neutrophil differentiation. Immunity. 2003;18:109–120. doi: 10.1016/s1074-7613(02)00501-0. [DOI] [PubMed] [Google Scholar]
- Lord BI, Bronchud MH, Owens S, Chang J, Howell A, Souza L, et al. The kinetics of human granulopoiesis following treatment with granulocyte colony-stimulating factor in vivo. Proc Natl Acad Sci USA. 1989;86:9499–9503. doi: 10.1073/pnas.86.23.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards MK, Liu F, Iwasaki H, Akashi K, Link DC. Pivotal role of granulocyte colony-stimulating factor in the development of progenitors in the common myeloid pathway. Blood. 2003;102:3562–3568. doi: 10.1182/blood-2003-02-0593. [DOI] [PubMed] [Google Scholar]
- Cartwright GE, Athens JW, Wintrobe MM. The kinetics of granulopoiesis in normal man. Blood. 1964;24:780–803. [PubMed] [Google Scholar]
- Semerad CL, Liu F, Gregory AD, Stumpf K, Link DC. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity. 2002;17:413–423. doi: 10.1016/s1074-7613(02)00424-7. [DOI] [PubMed] [Google Scholar]
- Eash KJ, Means JM, White DW, Link DC. CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood. 2009;113:4711–4719. doi: 10.1182/blood-2008-09-177287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19:583–593. doi: 10.1016/s1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
- Semerad CL, Christopher MJ, Liu F, Short B, Simmons PJ, Winkler I, et al. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 2005;106:3020–3027. doi: 10.1182/blood-2004-01-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher MJ, Liu F, Hilton MJ, Long F, Link DC. Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization. Blood. 2009;114:1331–1339. doi: 10.1182/blood-2008-10-184754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin SM. The bone marrow: a site of neutrophil clearance. J Leukoc Biol. 2010;88:241–251. doi: 10.1189/jlb.0210112. [DOI] [PubMed] [Google Scholar]
- Furze RC, Rankin SM. The role of the bone marrow in neutrophil clearance under homeostatic conditions in the mouse. FASEB J. 2008;22:3111–3119. doi: 10.1096/fj.08-109876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H, Miyamasu M, Yamaguchi M, Imanishi M, Tsuno NH, Matsushima K, et al. Cytokine-mediated regulation of CXCR4 expression in human neutrophils. J Leukoc Biol. 2002;71:711–717. [PubMed] [Google Scholar]
- Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- Cavanagh LL, Bonasio R, Mazo IB, Halin C, Cheng G, van der Velden AW, et al. Activation of bone marrow-resident memory T cells by circulating, antigen-bearing dendritic cells. Nat Immunol. 2005;6:1029–1037. doi: 10.1038/ni1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–999. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- Kusmartsev S, Gabrilovich DI. Role of immature myeloid cells in mechanisms of immune evasion in cancer. Cancer Immunol Immunother. 2006;55:237–245. doi: 10.1007/s00262-005-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movahedi K, Guilliams M, van den Bossche J, van den Bergh R, Gysemans C, Beschin A, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa AC, Zea AH, Hernandez C, Rodriguez PC. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res. 2007;13:721s–726s. doi: 10.1158/1078-0432.CCR-06-2197. [DOI] [PubMed] [Google Scholar]
- Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- Yang R, Cai Z, Zhang Y, Yutzy WH 4 th, Roby KF, Roden RB. CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer Res. 2006;66:6807–6815. doi: 10.1158/0008-5472.CAN-05-3755. [DOI] [PubMed] [Google Scholar]
- Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- Ribechini E, Greifenberg V, Sandwick S, Lutz MB. Subsets, expansion and activation of myeloid-derived suppressor cells. Med Microbiol Immunol. 2010;199:273–281. doi: 10.1007/s00430-010-0151-4. [DOI] [PubMed] [Google Scholar]
- Highfill SL, Rodriguez PC, Zhou Q, Goetz CA, Koehn BH, Veenstra R, et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. 2010;116:5738–5747. doi: 10.1182/blood-2010-06-287839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW, et al. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12:47–57. doi: 10.1007/s11373-004-8183-7. [DOI] [PubMed] [Google Scholar]
- Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP, Gerson SL. Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res. 2000;9:841–848. doi: 10.1089/152581600750062264. [DOI] [PubMed] [Google Scholar]
- Cheng L, Hammond H, Ye Z, Zhan X, Dravid G. Human adult marrow cells support prolonged expansion of human embryonic stem cells in culture. Stem Cells. 2003;21:131–142. doi: 10.1634/stemcells.21-2-131. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- Plumas J, Chaperot L, Richard MJ, Molens JP, Bensa JC, Favrot MC. Mesenchymal stem cells induce apoptosis of activated T cells. Leukemia. 2005;19:1597–1604. doi: 10.1038/sj.leu.2403871. [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003;171:3426–3434. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080–2087. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16:557–564. [PubMed] [Google Scholar]
- Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- Miller JP, Izon D, DeMuth W, Gerstein R, Bhandoola A, Allman D. The earliest step in B lineage differentiation from common lymphoid progenitors is critically dependent upon interleukin 7. J Exp Med. 2002;196:705–711. doi: 10.1084/jem.20020784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roifman CM, Zhang J, Chitayat D, Sharfe N. A partial deficiency of interleukin-7R alpha is sufficient to abrogate T-cell development and cause severe combined immunodeficiency. Blood. 2000;96:2803–2807. [PubMed] [Google Scholar]
- Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. . Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieper WC, Tan JT, Bondi-Boyd B, Gapin L, Sprent J, Ceredig R, et al. Overexpression of interleukin (IL)-7 leads to IL-15-independent generation of memory phenotype CD8+ T cells. J Exp Med. 2002;195:1533–1539. doi: 10.1084/jem.20020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci USA. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Carlo E, Comes A, Orengo AM, Rosso O, Meazza R, Musiani P, et al. IL-21 induces tumor rejection by specific CTL and IFN-gamma-dependent CXC chemokines in syngeneic mice. J Immunol. 2004;172:1540–1547. doi: 10.4049/jimmunol.172.3.1540. [DOI] [PubMed] [Google Scholar]
- Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- Peled A, Grabovsky V, Habler L, Sandbank J, Arenzana-Seisdedos F, Petit I, et al. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34+ cells on vascular endothelium under shear flow. J Clin Invest. 1999;104:1199–1211. doi: 10.1172/JCI7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34+ cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95:3289–3296. [PubMed] [Google Scholar]
- Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia. 2002;16:1992–2003. doi: 10.1038/sj.leu.2402684. [DOI] [PubMed] [Google Scholar]
- Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10:463–471. doi: 10.1016/s1074-7613(00)80046-1. [DOI] [PubMed] [Google Scholar]
- Avigdor A, Goichberg P, Shivtiel S, Dar A, Peled A, Samira S, et al. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103:2981–2989. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- Koni PA, Joshi SK, Temann UA, Olson D, Burkly L, Flavell RA. Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J Exp Med. 2001;193:741–754. doi: 10.1084/jem.193.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenette PS, Subbarao S, Mazo IB, von Andrian UH, Wagner DD. Endothelial selectins and vascular cell adhesion molecule-1 promote hematopoietic progenitor homing to bone marrow. Proc Natl Acad Sci USA. 1998;95:14423–14428. doi: 10.1073/pnas.95.24.14423. [DOI] [PMC free article] [PubMed] [Google Scholar]