Abstract
Purpose
To investigate the reproducibility of choroidal thickness measurements in normal subjects on three Spectral Domain Optical Coherence Tomography (SDOCT) instruments, Zeiss Cirrus HD-OCT (Carl Zeiss Meditec Inc, Dublin, California, USA), Heidelberg Spectralis (Heidelberg Engineering, Heidelberg, Germany) and Optovue RTVue (Optovue Inc., Fremont, CA).
Design
Cross-sectional non-interventional study
Participants
Images were obtained in 28 eyes of 28 healthy undilated volunteers without ocular pathology in a clinical setting.
Methods
All subjects were imaged on the fovea using Cirrus HD 1-line raster, Spectralis enhanced depth imaging and RTVue retina-cross.
Main Outcome Measures
The choroid was measured subfoveally, 750 μm temporal and 750 μm nasal to the fovea. All measurements were performed by 2 independent observers. Two way analysis of variance (ANOVA) with Bonferroni's post-test, Pearson correlation and the Bland-Altman analysis were used to compare measurements.
Results
The group of 28 subjects consisted of 7 men and 21 women, with an average age of 35.2 years (range, 23 to 64 years). A two way ANOVA with Bonferroni's post-test revealed no significant difference in the average subfoveal choroidal thickness (P >0.05) between systems for any location: subfoveally, 750μm temporal and 750 μm nasal to the fovea. The measurements of choroidal thickness from any pair of three instruments (Cirrus vs. Spectralis, Cirrus vs. RTVue, Spectralis vs. RTVue) were also strongly correlated. The Pearson correlation between all two system pairs of the three systems was greater than 0.9 p <0.0001. The 95% limits of agreement between four choroidal thickness measurements between Cirrus and RTVue were +11.21% to -13.57% (Bias -1.17), between Spectralis and RTVue +10.85% to -12.45% (Bias -0.80) and between Cirrus and Spectralis +12.81% to -13.33% (Bias -0.25).
Conclusions
In our population of young healthy adults with normal vision, there was good reproducibility between choroidal thickness measurements of images acquired with Cirrus, Spectralis and RTVue.
Introduction
The choroid plays a vital role in the pathophysiology of many conditions such as central serous chorioretinopathy, 1 age-related macular degeneration, 2 choroidal melanoma, 3 Vogt-Koyanagi-Harada 4 and others. However, adequate morphological examination of the choroid using spectral domain optical coherence tomography (SDOCT) has not been possible until recently, owing to its posterior location, the presence of pigmented cells that attenuate the incident light and the limited depth of penetration inherent to the design of SDOCT instruments which is independent of these factors.
The recent development of enhanced depth imaging (EDI) has made choroidal examination with SDOCT possible. 5 In the EDI technique, scan acquisition of the choroid-scleral interface is set up adjacent to the zero delay, where the sensitivity in SDOCT images is highest. This allows better visualization of the choroid. Another technique used to visualize the choroid, image averaging, uses multiple B-scans from the same retinal location, which are subsequently averaged together. Between scans, the signal remains constant and the noise is variable. Consequently, when the images are averaged, there is an increase in signal to noise ratio and reduction in “speckle.” Recent reports showed successful examination and measurement of choroidal thickness in normal and pathologic states using the Heidelberg Spectralis (Heidelberg Engineering, Heidelberg, Germany) and Cirrus HD-OCT (Carl Zeiss Meditec Inc, Dublin, California, USA) SDOCT instruments. 5-8
The ability to reliably image the choroid with different SDOCT instruments makes this an emerging area of study. At present a number of commercially available SDOCT systems are able to image the choroid. Consequently, it is important to validate the reproducibility of the choroidal measurements by different instruments in order to understand how precise and comparable the measurements are. The aim of this study is to investigate the reproducibility of the choroidal measurements in normal subjects performed by three different SDOCT instruments Cirrus HD OCT, Heidelberg Spectralis and Optovue RTVue (Optovue Inc., Fremont, CA).
Methods
A cross-sectional analysis was performed on 28 eyes of 28 healthy undilated volunteers, who underwent OCT examination at the New England Eye Center, Tufts Medical Center, Boston, Massachusetts, between January 13, 2011 and January 28, 2011. This study was approved by the institutional review board of the Tufts Medical Center and is adherent to the tenets of the Declaration of Helsinki. All volunteers had best corrected visual acuity of 20/20 or better in both eyes and were examined clinically. Any subject with retinal or choroidal pathology was excluded.
Scan patterns
Subjects were scanned on Zeiss Cirrus HD-OCT instrument software 5.0, Optovue RTVue software version 3.5, and Heidelberg Spectralis HRA+ OCT software version 5.1 within 10 minutes on the same eye.
The scan pattern used on Zeiss Cirrus, HD 1-line raster, is a 6-mm line consisting of 4096 A-scans. Images were taken with the vitreoretinal interface adjacent to the zero-delay and were not inverted to bring the choroid adjacent to zero-delay as image inversion using the Cirrus software results in a low-quality image. The HD 1-line raster has 20 B-scans averaged together without tracking. This method was described previously. 9
The scan pattern used on Optovue RTVue was the retina cross line which consists of two orthogonally oriented 6mm lines consisting of 1024 A-scans. Only the nasal-temporally oriented line was used for measurement. The image is automatically inverted so that the chorioretinal interface is adjacent to the zero delay. The retina cross line scan has 32 frames averaged, 16 per direction, without tracking.
The scan pattern used on Spectralis was a line scan of 30 degrees consisting of 768 A-scans per frame. The EDI option was used which places the chorioretinal interface adjacent to the zero delay. One hundred frames were averaged together with the aid of eye tracking. This method has been described previously. 5
Scan analysis
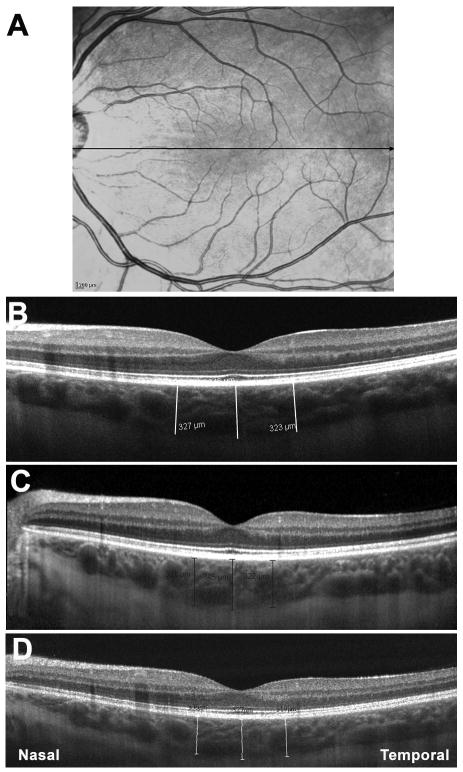
All images were taken as close to the fovea as possible in order to ensure, to the best extent possible, that the same retinal area was being scanned across the three machines. One eye per patient was selected for measurements. If images from both eyes met inclusion criteria, then the eye in which the choroid/sclera border could be most clearly visualized was selected. Of the 28 eyes, 15 right eyes and 13 left eyes were measured in this study. Using Cirrus, RTVue and Spectralis linear measurement tools, 2 independent observers measured choroidal thickness perpendicularly from the outer edge of the hyper-reflective retinal pigment epithelium (RPE) to the inner sclera at the fovea, 750 μm temporal to the fovea and 750 μm nasal to the fovea (Figure 1). The measurements from the 2 observers were then averaged together for analysis.
Figure 1.
Optical coherence tomography (OCT) scans showing choroidal thicknesses of the same subject on three different systems. A: Scanning Laser Ophthalmoscopy fundus image used by Spectralis for eye tracking. Black line indicates location and direction of scan pattern. B: OCT image of subject on Cirrus. Image averaging is used for choroidal visualization. White lines indicate choroidal thickness measurements taken perpendicularly from the outer edge of the hyper-reflective retinal pigment epithelium (RPE) to the inner sclera at the fovea, 750 μm temporal to the fovea and 750 μm nasal to the fovea. C: OCT image of subject on Spectralis. Image averaging with the aid of eye tracking and enhanced depth imaging are used for choroidal visualization. Black lines indicate choroidal thickness measurements at the fovea, 750 μm temporal to the fovea and 750 μm nasal to the fovea. D: OCT image of subject on RTVue. Image averaging and enhanced depth imaging are used for choroidal visualization. White lines indicate choroidal thickness measurements at the fovea, 750 μm temporal to the fovea and 750 μm nasal to the fovea.
Statistical analysis
Interobserver Pearson correlations were calculated for all choroidal thickness measurements. Pearson correlations between choroidal thickness measurements and age, gender and ethnicity were calculated.
Two-way analysis of variance (ANOVA) with Bonferroni's post-test was used to compare choroidal thickness at the different locations examined (subfoveal, nasal and temporal). Pearson correlations between choroidal thickness of 2 instrument pairs (RTVue vs Cirrus; RTVue vs Spectralis; Cirrus vs Spectralis) were also calculated.
The methods of Bland and Altman were then used to calculate the mean difference between 2 instrument pairs (RTVue vs Cirrus; RTVue vs Spectralis; Cirrus vs Spectralis). The reproducibility of subfoveal choroidal thickness was quantified using the bias (mean difference) and analyzed by the concordance coefficient with a 95% confidence interval.
Graph Pad Prism 5.0 software for Macintosh was used to perform the statistical analysis. A 95% confidence interval and a 5% level of significance were adopted. P values ≤0.05 were considered to be significant.
Results
The group of 28 subjects consisted of 7 men and 21 women, with an average age of 35.2 years (range, 23 to 64 years). Eighteen subjects were caucasian, 5 subjects were asian, 3 subjects were hispanic and 2 subjects were african american. No significant correlation was found between gender, age and ethnicity respectively and choroidal thickness measurements on any system. All eyes had normal foveal contour with no retinal pathology and no abnormalities of the choroid. The delineation between choroid and sclera could be easily visualized on all systems to permit reliable thickness measurements in 27 out of 28 (96.4%) of the images evaluated in this study.
The mean subfoveal choroidal thickness derived from Cirrus was 347.51 μm ± 94.37μm (mean ± deviation), from Spectralis, 347.46 μm ± 97.92 μm and from RTVue 337.67 μm ± 89.01μm. A two way ANOVA with Bonferroni's post-test revealed no significant difference in the average subfoveal choroidal thickness (P >0.05) between systems for any location, subfoveally, 750 μm temporal and 750 μm nasal to the fovea (Table 1, Figure 1). The measurements of choroidal thickness from any pair of three instruments (Cirrus vs. Spectralis, Cirrus vs. RTVue, Spectralis vs. RTVue) were also strongly correlated. The Pearson correlation between all two system pairs of the three systems (Cirrus vs. Spectralis, Cirrus vs. RTVue, Spectralis vs. RTVue) was greater than 0.9 <0.0001 (Table 2). Further, Interobserver correlations for all choroidal thickness measurements were equal to or greater than 0.93 (p<0.0001) (Table 3).
Table 1.
Choroidal thickness measurements for Cirrus, Spectralis and RTVue at the fovea, 750 μm temporal to the fovea and 750 μm nasal to the fovea (mean ± standard deviation).
| Choroidal thickness measurements | ||||
|---|---|---|---|---|
| Location | Cirrus | Spectralis | RTVue | P |
| Nasal | 320.48 ± 87.84 | 319.80 ± 90.18 | 313.35 ± 84.00 | > 0.05 |
| Subfoveal | 347.51 ± 94.37 | 347.46 ± 97.92 | 337.67 ± 89.01 | > 0.05 |
| Temporal | 339.39 ± 85.80 | 346.87 ± 86.88 | 335.94 ± 75.54 | > 0.05 |
Bonferroni's post-test revealed no significant difference in the average subfoveal choroidal thickness (P >0.05) between systems for any location.
Table 2.
Pearson Correlations of choroidal thickness measurements from any pair of three instruments.
| Correlations of choroidal thickness between instruments | ||
|---|---|---|
| Instruments | Pearson Correlation | P |
| Cirrus vs Spectralis | 0.976 | < 0.0001 |
| Cirrus vs RTVue | 0.965 | < 0.0001 |
| Spectralis vs RTVue | 0.964 | < 0.0001 |
All systems are strongly correlated.
Table 3.
Interobserver Pearson Correlations of choroidal thickness measurements.
| Interobserver correlation of choroidal thickness measurements | ||||
|---|---|---|---|---|
| Cirrus | Spectralis | RTVue | P | |
| Nasal | 0.94 | 0.96 | 0.95 | <0.0001 |
| Subfoveal | 0.95 | 0.96 | 0.93 | <0.0001 |
| Temporal | 0.96 | 0.93 | 0.94 | <0.0001 |
All measurements are strongly correlated.
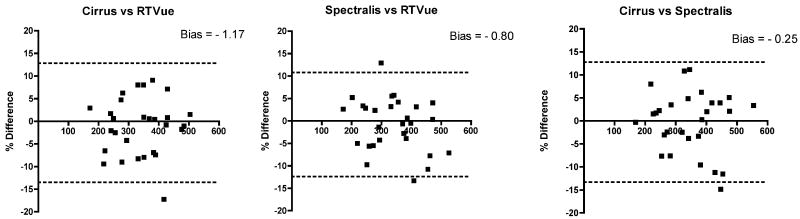
The difference between two of the three instruments (Cirrus vs. RTVue, Spectralis vs. RTVue, Cirrus vs. Spectralis) against the mean of the same two of the three instruments measurements of choroidal thickness was analyzed using Bland Altman plots (Figure 2). It is evident from Figure 2 that there is no substantial bias between any two of the three instruments and that close agreement was found for the majority of subjects. The 95% limits of agreement between four choroidal thickness measurements between Cirrus and RTVue were +11.21% to -13.57% (Bias -1.17), between Spectralis and RTVue +10.85% to -12.45% (Bias -0.80) and between Cirrus and Spectralis +12.81% to -13.33% (Bias -0.25). There is a trend toward increased % difference (bias) with increasing choroidal thickness.
Figure 2.
Bland-Altman plots for Cirrus vs. RTVue, Spectralis vs. RTVue, and Cirrus vs. Spectralis. A: Bland-Altman plot of Cirrus vs. RTVue. There is no substantial bias between these two systems. The 95% limits of agreement for choroidal thickness measurements between Cirrus and RTVue are +11.21% to -13.57%. B: Bland-Altman plot of Spectralis vs. RTVue. There is no substantial bias between these two systems. The 95% limits of agreement between Spectralis and RTVue are +10.85% to -12.45%. C: Bland-Altman plot of Cirrus vs. Spectralis. There is no substantial bias between these two systems. The 95% limits of agreement for choroidal thickness measurements between Cirrus and Spectralis are +12.81% to -13.33%.
Discussion
Previous studies have used OCT to measure choroidal thickness in both normal patients and those with ocular disease using both Cirrus and Spectralis. 2, 4-12 To our knowledge choroidal thickness measurements using RTVue have not been reported. This is the first study to directly compare reproducibility of choroidal thickness measurements across three different OCT instruments. In our population of young healthy adults with normal vision, there was good reproducibility between Cirrus, Spectralis and RTVue. There was also good interobserver correlation for all choroidal thickness measurements. There is a nonsignificant trend toward RTVue measurements being smaller than the other systems by 10 μm. This could be due to a difference in scale factor during image processing.13, 14 There is a minimal bias between the three systems when comparing the measurements using Bland-Altman plots (Figure 2). Additionally, there is a trend toward a larger bias when the choroidal thickness is larger. This may be due to the fact that visualization of the choroid sclera junction is more difficult in images where the choroid is thicker.
Because the choroid-sclera interface is not routinely apparent on standard SDOCT, imaging the choroid using SDOCT requires specific imaging protocols. One technique to visualize the choroid uses multiple scans from the same retinal location which are subsequently averaged together which reduces noise, thereby increasing the signal to noise ratio in the resulting image. This technique results in images with improved continuity of retinal features and the ability in most cases to identify the choroid-sclera junction.
The three systems tested in this examination, Cirrus HD-OCT, Spectralis and RTVue all use image averaging. Cirrus HD-OCT averages 20 B-scans to create the HD 1-line raster. The retina cross-line scan used on the RTVue is a cross shaped acquisition pattern where 16 B-scans are averaged in the superior inferior orientation and 16 B-scans are averaged in the nasal temporal orientation. Only the images taken in the nasal temporal orientation were used for measurement. The reason that this scan pattern was used in comparison to the retina line scan which averages 32 B-scans only in the nasal temporal orientation, was to be more confident that the scan was directly through the fovea. Spectralis uses eye tracking to ensure that all of the images to be oversampled are taken from the same retinal location. This allows for a greater number of images that can be averaged. In this study, 100 B-scans were averaged.
Another imaging protocol that is used to visualize the choroid is enhanced depth imaging (EDI) which takes advantage of the variation in sensitivity with delay and the mirror image behavior of SDOCT. Spectral / Fourier domain detection cannot distinguish between positive and negative echo delays because the interference spectra are identical for an echo at + or − given delay. Therefore, if a retinal structure crosses zero delay, it will appear reflected about the zero delay position, as a mirror like image.
Both Spectralis and RTvue support EDI imaging. Spectralis has an EDI function, and RTVue uses “chorioretinal imaging mode.” By adjusting the zero delay position, or the position of the instrument relative to the patient's eye, it is possible to image with the zero delay adjacent to the choroidal-scleral interface, such that sensitivity to the choroid is maximized (sensitivity to structures at the vitreoretinal interface are reduced). By setting the scan acquisition so that the choroid-scleral interface is adjacent to the zero delay, sensitivity roll-off is minimized. Cirrus does not have this functionality. Attempting to place the choroid adjacent to the zero delay on Cirrus results in a low quality image because the image processing is optimized for the case where the vitreo-retinal boundary is placed near the zero delay.
Two of the three systems in this investigation employ EDI to image the choroid. Our findings demonstrate comparable choroidal thickness measurements across the three machines tested. This suggests that EDI though helpful, may not be necessary in the measurement of choroidal thickness in normal subjects. By contrast, all of the systems tested in this investigation use image averaging to visualize the choroid. The number of B-scans averaged varies by system. The lowest number of averaged images was 16 images on RTVue where the highest was 100 images on Spectralis. The sensitivity of Spectralis for single images is lower than for other instruments. Therefore it is difficult to determine a minimum number of averaged images needed to visualize the choroid sclera junction which would apply to all instruments. The number of averaged images necessary to visualize the choroid-sclera junction may also vary with age, the presence of ocular pathology, amount of RPE pigment and image quality.
Normal choroidal thickness has been described by Margolis and Spaide using Spectralis and by Manjunath et. al. using Cirrus. The reported subfoveal choroidal thickness was 287 ± 76 μm (mean ± standard deviation) on the Spectralis with a sample size of 30 patients (54 eyes) and a mean age of 50.4 years (range, 19 to 85 years). Additionally, they reported regression analysis demonstrating a decrease of 1.56 μm in subfoveal choroidal thickness per year. 6 This is consistent with the subfoveal choroidal thickness found on Spectralis in this investigation when corrected for age.
Manjunath et. al. reported a subfoveal choroidal thickness of 272 ± 81 μm on Cirrus with a sample size of 34 subjects (34 eyes), a mean age of 51.1 years (range, 22 to 78 years) and a negative correlation between age and average choroidal thickness beneath the fovea (r = −0.61, P < 0.0001). 9 This data is consistent with the subfoveal choroidal thickness found on Cirrus in this investigation when corrected for age. Where Manjunath reported the ability to reliably visualize the choroid sclera junction in 74% of eyes on Cirrus, to our knowledge, the percentage of eyes where the choroid sclera junction is visible has not been reported on Spectralis or RTVue.
One limitation of this investigation is that due to the nature of our subject selection, there was minimal motion during image acquisition. This is because our subjects were relatively young with an average age of 35.2 years (range, 23 to 64 years) with good vision. This population was chosen because the aim of this study was to compare the three systems. Further investigation is needed in order to compare the ability to measure the choroid on these three systems in patients with low vision and older age.
In summary, in our population of young healthy adults with normal vision, there was good reproducibility between choroidal thickness measurements of images acquired with Cirrus, Spectralis and RTVue. Additionally choroidal thickness can be measured using RTVue. Our measurements were similar with those described previously using Cirrus HD and Spectralis. Knowing that choroidal thickness measurements are reproducible across three different OCT systems potentially adds to the usefulness of choroidal imaging in clinical practice. Further investigation of reproducibility in subjects with disease is necessary to confirm.
Acknowledgments
Financial Support: This work was supported in part by a Research to Prevent Blindness Challenge grant to the New England Eye Center/Department of Ophthalmology -Tufts University School of Medicine, NIH contracts RO1-EY11289-25, R01-EY13178-10, R01-EY013516-07, R01-EY019029-02, Air Force Office of Scientific Research FA9550-10-1-0551 and FA9550-10-1-0063.
Footnotes
Conflict of Interest: James G. Fujimoto, P, receives royalties from intellectual property owned by M.I.T. and licensed to Carl ZeissMeditech, Inc.; O, has stock options in Optovue, Inc..
Jay S. Duker, S, receives research support from Carl ZeissMeditech, Inc., Optovue, Inc., and Topcon Medical Systems, Inc..
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gemenetzi M, De Salvo G, Lotery AJ. Central serous chorioretinopathy: an update on pathogenesis and treatment. Eye (Lond) 2010;24:1743–56. doi: 10.1038/eye.2010.130. [DOI] [PubMed] [Google Scholar]
- 2.Spaide RF. Age-related choroidal atrophy. Am J Ophthalmol. 2009;147:801–10. doi: 10.1016/j.ajo.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Torres VL, Brugnoni N, Kaiser PK, Singh AD. Optical coherence tomography enhanced depth imaging of choroidal tumors. Am J Ophthalmol. 2011;151:586–93. doi: 10.1016/j.ajo.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 4.Maruko I, Iida T, Sugano Y, et al. Subfoveal choroidal thickness after treatment of Vogt-Koyanagi-Harada disease. Retina. 2011;31:510–7. doi: 10.1097/IAE.0b013e3181eef053. [DOI] [PubMed] [Google Scholar]
- 5.Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496–500. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 6.Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147:811–5. doi: 10.1016/j.ajo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29:1469–73. doi: 10.1097/IAE.0b013e3181be0a83. [DOI] [PubMed] [Google Scholar]
- 8.Manjunath V, Fujimoto JG, Duker JS. Cirrus HD-OCT high definition imaging is another tool available for visualization of the choroid and provides agreement with the finding that the choroidal thickness is increased in central serous chorioretinopathy in comparison to normal eyes [letter] Retina. 2010;30:1320–1. doi: 10.1097/IAE.0b013e3181e798b1. author reply 1321–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manjunath V, Taha M, Fujimoto JG, Duker JS. Choroidal thickness in normal eyes measured using Cirrus HD optical coherence tomography. Am J Ophthalmol. 2010;150:325–9. doi: 10.1016/j.ajo.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spaide RF. Enhanced depth imaging optical coherence tomography of retinal pigment epithelial detachment in age–related macular degeneration. Am J Ophthalmol. 2009;147:644–52. doi: 10.1016/j.ajo.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Maruko I, Iida T, Sugano Y, et al. Subfoveal choroidal thickness after treatment of central serous chorioretinopathy. Ophthalmology. 2010;117:1792–9. doi: 10.1016/j.ophtha.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Fujiwara T, Imamura Y, Margolis R, et al. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol. 2009;148:445–50. doi: 10.1016/j.ajo.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 13.Wolf-Schnurrbusch UE, Ceklic L, Brinkmann CK, et al. Macular thickness measurements in healthy eyes using six different optical coherence tomography instruments. Invest Ophthalmol Vis Sci. 2009;50:3432–7. doi: 10.1167/iovs.08-2970. [DOI] [PubMed] [Google Scholar]
- 14.Pierro L, Giatsidis SM, Mantovani E, Gagliardi M. Macular thickness interoperator and intraoperator reproducibility in healthy eyes using 7 optical coherence tomography instruments. Am J Ophthalmol. 2010;150:199–204. doi: 10.1016/j.ajo.2010.03.015. [DOI] [PubMed] [Google Scholar]