Abstract
Recent work has implicated dopaminergic mechanisms in overeating and obesity with some researchers suggesting parallels between the dopamine dysregulation associated with addiction and an analogous dysregulation in obesity. The precise role of dopamine in mediating reward and reinforcement, however, remains controversial. In contrast to drugs of abuse, pursuit of a natural reward, such as food, is regulated by homeostatic processes that putatively maintain a stable energy balance keeping unrestrained consumption and reward pursuit in check. Understanding how the reward system is constrained by or escapes homeostatic regulation is a critical question. The widespread use of food restriction to motivate animal subjects in behavior paradigms precludes investigation of this relationship as the homeostatic system is locked into deficit mode. In the present study, we examine the role of dopamine in modulating adaptive feeding behavior in semi-naturalistic home cage paradigms where mice earn all their food from lever pressing. We compared consumption and meal patterning between hyperdopaminergic dopamine transporter knock-down mice (DATkd) with wild-type (WT) in two paradigms that introduce escalating costs for procuring food. We found that hyperdopaminergic mice exhibited similar demand elasticity, weight loss and energy balance in response to cost. However, the DATkd show clear differences in meal patterning. Consistent with expectations of enhanced motivation, elevated dopamine increased meal size and reduced intrameal cost sensitivity. Nonetheless, this did not alter overall energy balance. We conclude that elevated dopamine enhances incentive or willingness to work locally within meals without shifting energy balance, enhancing global food-seeking or generating an energy surplus.
Keywords: mouse, dopamine, feeding, homeostatic regulation, semi-naturalistic homecage operant, dopamine transporter knockdown
INTRODUCTION
The cause of the dramatic rise in obesity in recent years (Wang & Beydoun, 2007; Prevention, 2009) is not fully understood and no clear program for reversing this trend has yet emerged. Historically viewed primarily from the perspective of biologically determined homeostatic mechanisms, energy balance is increasingly seen as arising from complex interactions between genetic and environmental factors (Hill, 2006; Neel, 1999 #4}. The last decade has seen a growing appreciation for the role of non-homeostatic systems and processes, particularly the reward and incentive system and reinforcement learning (Kelley et al., 2005a; Berthoud, 2007; Rowland et al., 2008; Berridge et al., 2010; Kenny, 2010).
Recent work has implicated dopaminergic mechanisms in overeating and obesity, though the nature of its role is unclear. Both increased and decreased reward sensitivity, in overweight and obese subjects, respectively, have been observed (Kenny, 2010), making simple attributions about the role of dopamine difficult. An idea with growing currency is to liken overeating and obesity to addiction, invoking dopaminergic dysregulation in the reward system as a key mechanisms underlying excessive caloric intake, lack of executive control and the resulting net positive energy balance and obesity (Volkow & Wise, 2005; Volkow et al., 2010). Notably, however, the role of dopamine in both addiction and natural reward remain controversial (Dayan & Balleine, 2002; Redish, 2004; Wise, 2004; Balleine, 2005; Everitt & Robbins, 2005; Berridge, 2007; Di Chiara & Bassareo, 2007; Salamone, 2007; Schultz, 2007). Viewing food as an addiction--broadly and widely construed as the ‘hijacking’ of the natural reward system—adds an additional layer of complexity to the already controversial role of dopamine in addiction and reward. Specifically, it suggests that natural rewards can ‘hijack’ the natural reward system, begging the question what are the presumably homeostatic controls on this natural reward system and what causes them to fail? It is precisely the absence of such regulatory systems that is believed to underlie vulnerability of the reward system to drugs of abuse (Di Chiara, 2005). Though fairly recent work has begun to highlight the interactions between reward/incentive and homeostatic mechanisms, much of this work is in its early stages and continues to evolve (Kelley et al., 2005b; Fulton et al., 2006; Hommel et al., 2006; Figlewicz et al., 2007; Palmiter, 2007; Berthoud & Morrison, 2008; Lutter & Nestler, 2009; Davis et al.; Figlewicz & Sipols).
Behavioral studies of incentive and reward processes traditionally use food (or water) restriction to motivate subjects to perform the experimental task. Doing so artificially locks homeostatic systems into a deficit state, effectively driving motivation but precluding investigation of how homeostatic and incentive processes jointly contribute to self-regulated energy balance behaviors. Such investigation remains an important challenge. Consequently, we have adopted a semi-naturalistic homecage operant paradigm in which mice earn all of their food through lever pressing with no explicit food restriction, allowing both homeostatic and incentive processes to determine on-going behavior.
In the present study, we examine the role of dopamine in adaptively modulating appetitive behavior in response to environmental cost contingencies. Using dopamine transporter knock-down mice (DATkd) that exhibit elevated extracellular dopamine and increased tonic firing of dopamine neurons (Zhuang et al., 2001), we ask how hyperdopaminergia alters subjects’ consummatory behavior in response to increasing costs. Associated with enhanced incentive (Berridge, 2007)and decreased sensitivity to cost (Salamone & Correa, 2002), we hypothesized that increased tonic dopamine would enhance adaptation to high costs associated with acquiring food. Increased dopamine did not substantially increase resistance to cost nor improve survival. However, it did alter meal patterning in favor of longer but fewer meals. This finding is consistent with a role for dopamine in increasing incentive and/or decreasing cost sensitivity locally within individual meals while overall energy balance remains under homeostatic regulation. We propose that dopamine mediated enhancement of incentive does not act globally to increase episodes of goal pursuit or food-seeking but is restricted locally to augmenting pursuit of a goal once initiated.
METHODS
Subjects
Mice for the demand study were all male between 6–8 weeks of age. Mice in the homecage studies were all male (progressive ratio) or mixed sex (progressive interval) between 12–16 weeks of age. All mice were housed under standard 12:12 light cycles with free access to water and access to food as described below. Wild-type C57BL/6 mice were obtained from Jackson Laboratories. All animal procedures were approved by the Institutional Animal Care and Use Committee at The University of Chicago.
Dopamine transporter knock-down mice
The DATkd were from an established colony backcrossed with C57BL/6 more than ten generations. The DATkd have been described and extensively characterized (Zhuang et al., 2001; Pecina et al., 2003; Cagniard et al., 2006a; Cagniard et al., 2006b; Yin et al., 2006; Beeler et al., 2010). These mice exhibit 85% reduction in dopamine transporter expression (DAT) resulting in elevated extracellular dopamine and increased tonic dopamine cell activity (Zhuang et al., 2001; Cagniard et al., 2006b). Phasic dopamine cell activity is unaltered with this mutation (Cagniard et al., 2006b); however, there is a 25% reduction in the amplitude of dopamine release arising from phasic activation (Zhuang et al., 2001). Unlike the DAT knock out mice (Bosse et al., 1997), these mice show no developmental abnormalities and multiple studies have demonstrated no deficits in learning (Cagniard et al., 2006b; Yin et al., 2006; Beeler et al., 2010). High performance liquid chromotagraphy (HPLC) analysis of tissue dopamine comparing the DATkd to wild-type shows that intracellular dopamine is diminished and dopamine turnover, as reflected by DOPAC/DA and HVA/DA ratios, is increased in the dorsal and ventral striatum as well as the hypothalamus, the greatest effect observed in the dorsal striatum (Table 1). Within the prefrontal cortex, dopamine reuptake is mediated primarily by norepinephrine transporters (NE) and available evidence suggests that diminished DAT does not significantly alter the pharmacokinetics of dopamine reuptake in the PFC (Sesack et al., 1998; Mundorf et al., 2001; Moron et al., 2002).
Table 1.
HPLC analysis of intracellular dopamine and dopamine turnover comparing wild-type (WT) and DATkd. All genotype differences significant, p < .01.
| Dopamine | DOPAC/DA | HVA/DA | ||||
|---|---|---|---|---|---|---|
| WT | DATkd | WT | DATkd | WT | DATkd | |
| Dorsal Striatum | 86.83 ± 6.8 | 40.16 ± 7.4 | .064 ± .003 | .135 ± .017 | .109 ± .008 | .491 ± .006 |
| Ventral Striatum | 37.68 ± 1.1 | 26.45 ± 3.4 | .131 ± .008 | .146 ± .009 | .128 ± .014 | .278 ± .040 |
| Hypothalamus | 1.84 ± .087 | 1.25 ± .134 | .259 ± .034 | .305 ± .021 | .572 ± .072 | .905 ± .098 |
Behavior setup and housing
Mice were singly housed in standard cages equipped with two levers placed on one side of the cage approximately 15 cm a part with a food hopper between the levers (Med-Associates, St. Albans, VT). A pellet dispenser delivered 20 mg grain-based precision pellets (Bio-Serv, Frenchtown, NJ) contingent on lever presses according to a programmed schedule. No other food was available. Water was available ad libitum. Upon initial placement in the operant homecages, three pellets were placed in the food hopper and the first 50 lever presses on the active lever yielded a pellet (continuous reinforcement), after which the experimental design was initiated. All mice acquired the lever pressing response overnight. One lever was active and yielded reward, the other was inactive and had no programmed consequences.
Behavioral paradigms
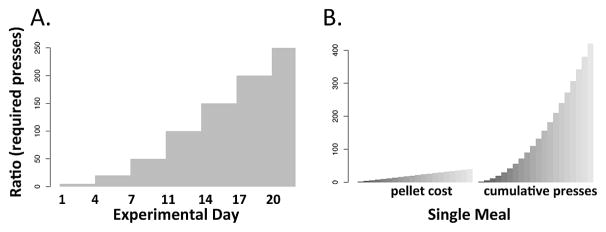
In the demand study, the active lever operates on a fixed ratio schedule incremented across the experiment (5,20,50,100,150,200,250,300). Each ratio is in effect for three days (Fig 1A). Data is collected using Med-PCIV software (Med-Associates, St. Albans, VT) and flushed daily at 9 a.m. The program tracks total daily consumption, active and inactive lever presses, the number of meals in a 24 hour period, the average size and duration of meals, the average intermeal interval, and the average rate of pressing across meals (ie., # of presses/ meal duration). A meal is qualitatively defined as a sustained period of eating and effort. We operationally defined a meal as follows: the first pellet earned signaled the ‘start’ of a meal. A meal was considered terminated after 30 minutes without earning a reward, at which time the meal termination was recorded as having occurred 30 minutes prior (Chaney & Rowland, 2008). Single pellet ‘meals’ were discarded as mice tend to almost continuously sample the levers at a low rate unless asleep. Such random pressing occasionally results in an earned pellet that does not reflect sustained effort or eating. An event recorder codes and time-stamps every lever press, pellet delivery, meal start and meal end. This data is used to construct a meal dataset (ie., rather than daily averages across meals) for more detailed analysis.
Figure 1.
Schematic of cost schedule for (A) homecage demand paradigm and (B) the homecage progressive ratio paradigm.
In the homecage progressive ratio, mice are give 24 hours of training in which the first 50 pellets are earned on an FR1 schedule, followed by FR5 until training is over. Following this, the progressive ratio (PR2) is initiated. After each reward during a meal, the lever press requirement for the next pellet is increased by 2. Though this results in only a moderate increase in the ratio requirement for each pellet, the cumulative number of presses required to continue a meal grows dramatically (Fig 1B). The ratio resets back to 2 after 30 minutes of inactivity. A 1 second cue light signals each pellet delivery. When the ratio resets, the cue light is set to constant illumination until the next active lever press initiating a new meal. Breakpoint is defined as the last successfully completed ratio. The program tracks the number, size and duration of meals (defined as a PR reset) as well as the average breakpoint, total consumption and total lever presses, both active and inactive. The progressive interval is identical to (ie., training, cue light) to the progressive ratio except a delay interval rather than required ratio increments. In the progressive interval paradigm only one press is required to dispense a pellet; however, after each pellet delivery, there is a delay before another pellet becomes available. This delay progressively increments analogous to the progressive ratio above and also resets after 30 minutes of inactivity.
Analyses
All statistical analyses were performed using R statistical software (R version 2.12.1 (2010-12-16); The R Foundation for Statistical Computing, http://www.r-project.org). To analyze the mixed within and between groups design of the demand study (Figs 3/5), unbalanced due to mice dropping out at different points, we used a linear mixed effects model (nlme package) with mouse as a random, within-subjects variable as follows:
Two sample comparisons used t-tests and survival analysis were performed using the R survival package. Where data is balanced (eg., supplemental Fig 3), traditional repeated measures ANOVA were used. The meal data (Figs 7/8; Table 1) was constructed from the same dataset only the unit of observation was individual meals rather than daily averages. Within the meal data, we removed meals consisting of a single pellet considering these as arising from accumulating, random pressing rather than occurring within a bout of focused effort. There were no differences between genotypes in terms of the number of single pellet meals overall (t = .5808, p = .571) nor during either the active (t = .6935, p = .499) or inactive cycle (t = .3959, p = .698). This dataset was also unbalanced as mice varied in their daily number of meals. In addition, meal size and duration were periodic with a bimodal distribution corresponding to the active and inactive circadian phases. To model these, we used linear regression incorporating both fixed effects and covariance structures, specifically matrices for covariance by mouse (M), start time of meal (T) and mouse x time (M*T). These analyses were performed using the R package Regress. To assess the contribution of individual factors, we removed each individually starting with interaction terms and compared each subsequent log-likelihood of the reduced model to the log-likelihood of the full model using chi-square distribution. When a term did not contribute to a significantly better model, it was removed (the least significant being removed first) and was then considered the full model. Only two terms were removed, both from the intermeal duration model. Once the models with the fewest parameters that best fit the data were determined, p values within that model were determined using z-scores (ie., pnorm(coefficient/standard error of coefficient)).
To evaluate elasticity, we used an established model developed by Hursh and colleagues (Hursh & Silberberg, 2008) as follows:
where Q is consumption/demand, Q0 baseline demand (we used FR5 as Q0), k determines the range of the demand, P is price (ratio) and alpha represents the elasticity coefficient.
In Figure 9, energy balance dynamics was evaluated as follows: Peak energy stores occur at termination of a meal and trough energy stores at initiation. To approximate the energy balance thresholds for initiating and terminating meals, we defined initiation threshold as the degree to which a prior energy peak is depleted prior to initiating a new meal:
where kdepletion is a constant that represents the average rate of basal energy expenditure which we set equal to one. This value represents the percentage of energy stores remaining since the previous peak such that larger numbers reflect less depletion while smaller numbers reflect greater depletion. To make this more intuitive, we plot (1-initiation threshold) which reflects the percentage to which energy previously ingested is depleted.
We assessed termination thresholds as follows:
again setting the constant kdepletion equal to one. This reflects the degree to which the current meal restores energy lost since the last energy peak. Larger numbers reflect greater energy restoration. These data were analyzed using a linear mixed effects model (nlme package).
RESULTS
DATkd exhibit no differences in consumption or bodyweight at baseline low cost conditions
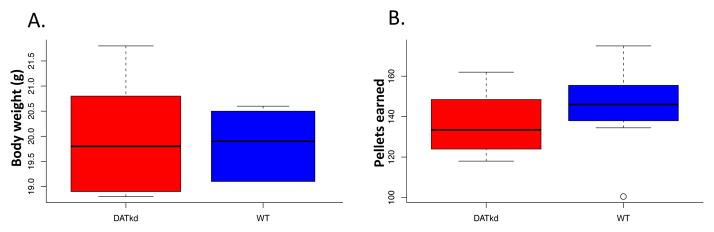
There were no initial differences between genotype in body weight (Fig 2A; t = 1.55, p = .124, N=15). Consistent with previous reports (Beeler et al., 2010), during the low cost, FR5 portion of the demand experiment, there was no significant difference between genotypes in either consumption (Fig 2B; genotype main, F(1,15) =.6857, p = .4215) or body weight (genotype main, F(1,15) =.1377, p = .7161, data not shown). These data indicate that the DATkd and wild-type exhibit similar energy balance under baseline conditions prior to environmental challenge.
Figure 2.
Baseline comparison of genotypes. (A) Initial body weight and (b) daily consumption at low cost FR5 schedule. N=8.
Increased dopamine does not improve adaptation to escalating costs or survival
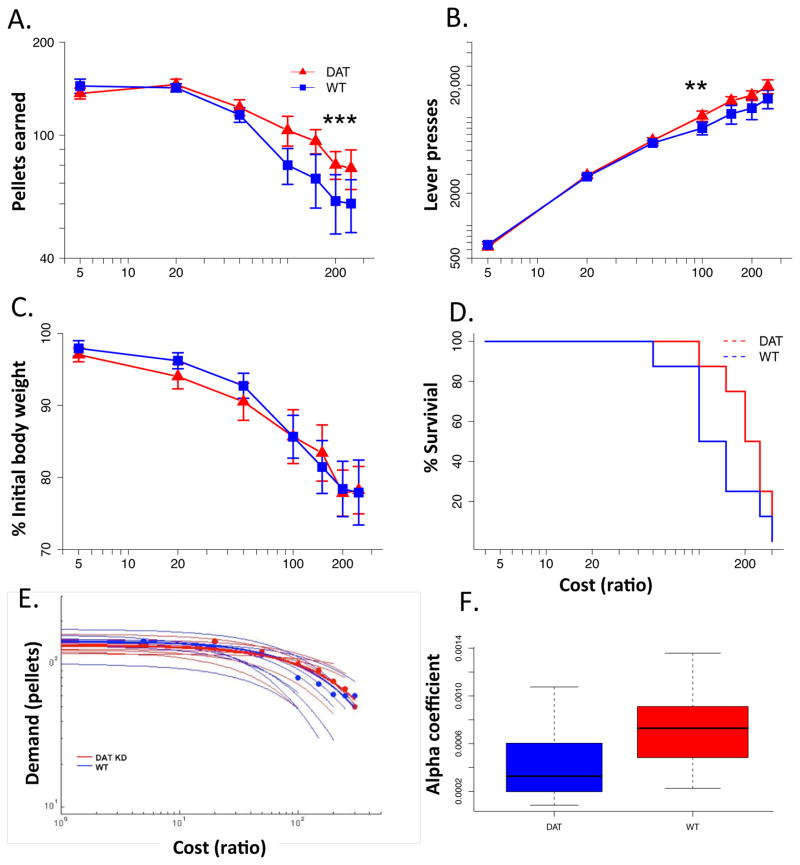
In the first experiment, mice were housed in homecages equipped with operant levers through which they were required to earn their entire food intake through lever pressing. The ‘cost’ of a 20mg grain pellet changed on an incrementing schedule, starting at low cost (FR5) and increasing to high cost (FR250), allowing 3 days on each cost schedule. The change in consumption as cost increased is linear (see supplemental Fig 1) and we used a linear mixed effects model to evaluate statistical significance (see methods). As cost increases, both genotypes significantly decrease their daily consumption (Fig 3A; cost main effect, t = 10.03, p < .0001), though increasing overall responding (Fig 3B; cost main effect, t = 15.7, p < .0001). A significant genotype by cost interaction can be observed, indicating that at higher costs the DATkd press and consume more than the wild-type (Fig 3A/B; genotype X cost, pellets, t = 3.49, p = .0008; genotype X cost, lever presses, t = 3.05, p = .0031), consistent with expectations that hyperdopaminergic mice would be more willing to work (Salamone et al., 1994; Salamone et al., 1997). However, the magnitude of this effect, compared to the cost-related decline in responding, is surprisingly small. Presses on the inactive lever as percentage of total presses were not significantly different by genotype (Supplemental Fig 2A; geno main effect, t = .689, p = .5026, geno X cost, t = .239, p = .8112) though these declined dramatically for both groups as cost increased (cost main effect, t = 5.04, p < .0001). The rate of lever pressing between genotypes was not significantly different (Supplemental Fig 2B; geno main effect, t = .380, p = .7098, geno X cost, t = 1.13, p = .2597). Nor were there any significant differences in inter-response times or post-reinforcement pauses (Supplemental Fig 2C/D; IRTs, geno main t = .001, p = .9991; PRPs, geno main, t = .9234, p = .3714).
Figure 3.
Demand, survival and elasticity. (A) Average daily consumption of 20 mg pellets at each ratio/cost (cost x genotype, *** p < .001), (B) average daily lever presses (cost x genotype, ** p < .01), (C) average daily body weight as percentage of initial weight, (D) survival curve (defined as asymptotic lever pressing), (E) demand curve (see methods) of individual mice (light traces) with average for each genotype represented as points with bold trace, (F) boxplot of elasticity coefficient for each genotype. N = 8.
The minimal impact of this genotype difference is observed in adaptive outcomes. Despite slightly more consumption at higher costs, there is no resulting significant difference in body weight between the groups (Fig 3C; geno main effect, t = .377, p = .7122) nor in survival, defined here as the breakpoint at which individual responding asymptotes regardless of increasing cost, generally necessitating removal from the experiment (Fig 3D; survival difference chi-square, p = .179).
In (neuro)economics, the degree to which consumption or ‘demand’ adjusts in response to associated costs is referred to as elasticity. The present data suggests that although the hyperdopaminergic mice exhibit a slightly greater resistance to cost (ie., slightly greater high cost responding, Fig 3A/B), overall they remain subject to the same processes that induce food and energy related elasticity in response to escalating costs. To look at the impact of hyperdopaminergia specifically on elasticity, we fit the data to a well established model developed by Hursh and colleagues (Hursh & Silberberg, 2008), see methods). We observe no difference between genotype in this model fit (Fig 3E) and no statistically significant difference in alpha values, the parameter that measures elasticity (Fig 3F; t = 1.509, p = .1599).
These results suggest that although dopamine does alter an animal’s response to cost (Fig 3A/B), it is not the only, nor most important, factor mediating cost sensitivity. In particular, increased cost in this paradigm induces environmental scarcity: that is, food is harder to come by as the experiment progresses. Consequently, scarcity may induce energy conservation, possibly through homeostatic mechanisms, that are either not significantly altered by dopaminergic function or actually modulate dopamine (Hommel et al., 2006; Lutter & Nestler, 2009; Figlewicz & Sipols, 2010). This would be consistent with the observed similarity in demand and survival between the genotypes.
It is possible that at higher costs, the energy required to press the lever enough to earn a pellet is greater than the energy obtained, artificially forcing an energy deficit. This problem, however, would only apply to those mice that sustained pressing at higher costs and not explain those that decreased demand at lower costs. It is difficult to assess the caloric costs of lever pressing; however, the results presented in the remainder of the paper argue that the observed decrease in demand reflects a regulatory process rather than an artifact arising from forced energetic deficits. This issue will be revisited in the discussion.
Increased dopamine alters meal patterning
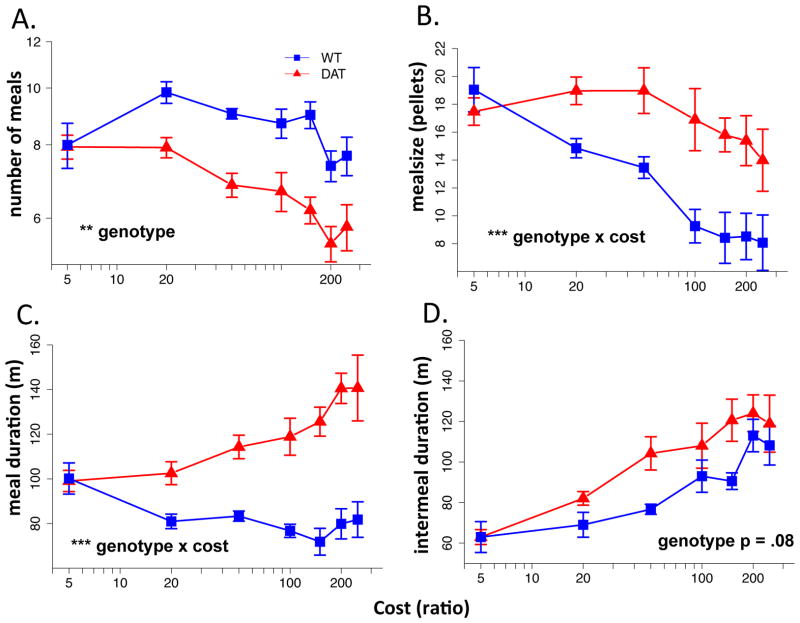
To further investigate the impact of hyperdopaminergia on behavior in this paradigm, we analyzed meal patterning. Meals were defined as starting with the first pellet earned and terminating after 30 minutes having not received a pellet (subtracting the 30 minutes the meal duration). Figure 4 shows two example raster plots of meals across the course of the experiment. We analyzed number of meals (Fig 5A), meal size(Fig 5B), meal duration (Fig 5C) and length of intermeal intervals (Fig 5D). Again, at low cost baseline (FR5), no differences were observed between genotypes (Fig 5A–D). However, with escalating costs, meal patterning diverged. Overall the DATkd mice ate fewer (Fig 5A; geno main effect, t = 3.14, p = .0078) but larger meals with meal size, in contrast to wild-type, showing relative insensitivity to cost (Fig 5B; geno x cost, t = 3.19, p = .0020). Larger meals are compensated by marginally significant greater intermeal intervals (Fig 5D; geno main effect, t = 1.85, p = .0861). These data suggest that increased dopamine, though having only limited impact on overall energy balance in response to increasing cost and scarcity, nonetheless have a clear and highly significant effect on meal patterns. To determine more precisely what aspect of meal patterning increased dopamine alters (ie., what component of behavior), we developed a model of the meal data.
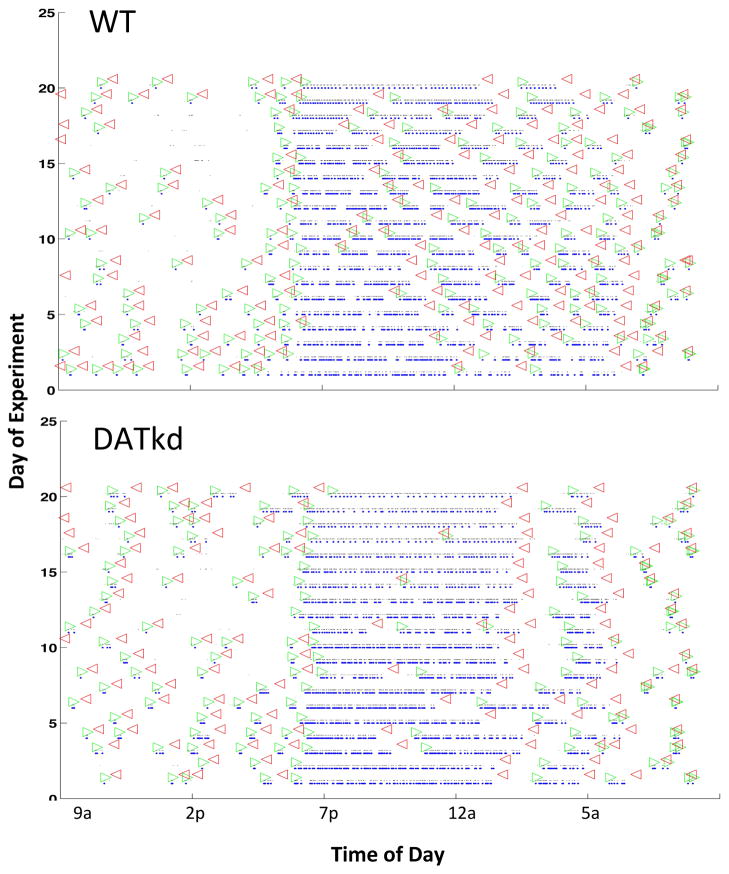
Figure 4.
Example raster plot of pellets and meals. Pellets earned (blue dots) were plotted against time of day horizontally with each line representing a single experimental day (vertical axis). Meal initiation as identified by the program (30 minute without a pellet delivery) is marked with open green arrows and termination with open red arrows. One wild-type and one DATkd are shown.
Figure 5.
Meal patterning. (A) average number of meals per day across ratio/costs, (B) average meal size, (C) average duration of meals and (D) average intermeal duration. ** p < .01, *** p < .001. N = 8.
Meal pattern model: incorporating time
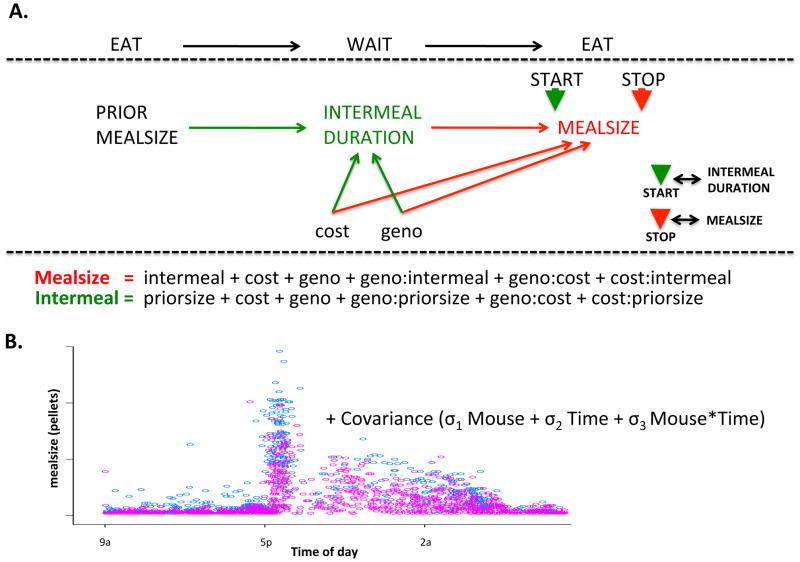
We started with the basic premise that meal patterning is the composite of two processes: initiation and termination of meals. We examined these separately, viewing meal size as in index of termination and intermeal interval as an index of meal initiation. The repeated cycles of eating, terminated by a particular meal size, not eating, and initiating a new meal (indexed by intermeal interval) formed the backbone of our model. We assume that meal size and meal termination is dependent upon how hungry an animal is at the time of the meal, which will be determined by how long it has been since they last ate a meal. Conversely, how long an animal waits to eat will be determined by how large their last meal was. Both cost and genotype may directly impact meal size and intermeal interval as well as exhibit interactive effects. Our model is illustrated in figure 6A and the intent is to discern where genotype exhibits its greatest effects. Because there is a periodicity to meals, resulting in a bimodal distribution of short (during inactive period) and long (during active period) meals (Fig 6B), time had to be incorporated into the model. To accomplish this, we included covariance structures in the model (see methods), accounting for covariance arising from (a) individual mouse subjects (M matrix), (b) meal start time (T matrix) and (c) the interaction between mouse and start time (M*T).
Figure 6.
Model schematic. (A) starting and stopping meals were modeled separately with meal start (green arrow) indexed by intermeal duration and meal end (red arrow) indexed by meal size. Factors hypothesized to contribute to the start and stop models are indicated in green and red, respectively. Fixed effects are identified under the graphic. (B) a dot plot showing distribution of meal size for all mice across the experiment as a function of time of day. To capture this time-dependent distribution of meal size, covariance was explicitly modeled as indicated.
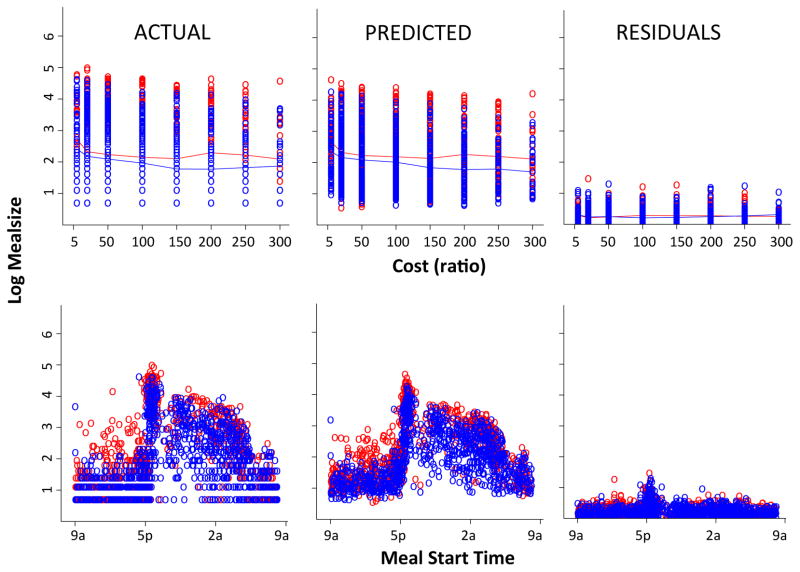
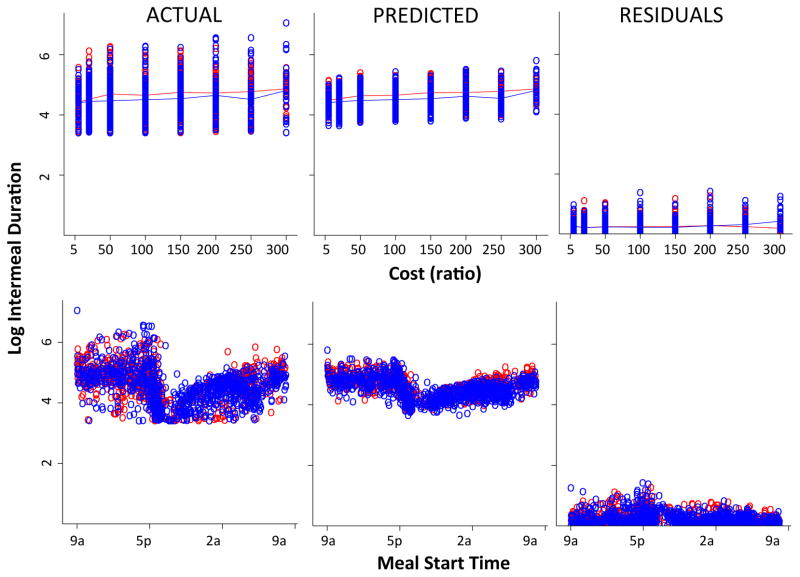
Figures 7 and 8 show the model fits for meal size and intermeal interval, respectively, with columns showing actual data, predicted values and residuals, respectively, plotted against cost (top) and time of day (bottom). On visual inspection, the model captures the distribution of the data quite well. Moreover, the model recapitulates the both the temporal and cost related patterns of the data. Examination of residuals against several variables (eg., genotype, cost, time of day) indicate that the variance between actual and predicted values are evenly distributed (data not shown). To assess the goodness of fit of the total model and the necessity of each factor included, we evaluated a series of models in which parameters were successively subtracted and compared each of these partial models with the full model using a chi square test of significance (see methods). In the meal size model, all factors were significant. In the intermeal model, the geno:cost and cost:priorsize interactions did not contribute significantly to the model. Consequently, they were removed (resulting in no significant difference between the full and the modified model with two less parameters) and statistics reported below are derived from the modified model.
Figure 7.
Performance of meal termination (meal size) model. Actual data, predicted values and residuals presented left to right. Each point represents a meal within the entire dataset (all subjects, all days). Top row plotted by cost, bottom row plotted by meal start time. Wild-type (red) and DATkd (blue). N = 8.
Figure 8.
Performance of meal initiation (intermeal duration) model. Actual data, predicted values and residuals presented left to right. Each point represents a meal within the entire dataset (all subjects, all days). Top row plotted by cost, bottom row plotted by meal start time. Wild-type (red) and DATkd (blue). N = 8.
Increased dopamine delays termination of meals
The regressor coefficients and p-values associated with the different factors and their interactions are presented in Table 2. Examining first the model of meal size (termination), the preceding intermeal duration is a significant predictor of subsequent meal size; however, in the opposite direction from what was predicted. That is, longer intermeal durations are associated with shorter meals. This inverse relationship between intermeal duration and meal size may reflect an underlying energy balance regulation (see below) such that as costs increase, the subject reduces consumption and associated energy expenditure, observed in the cost main effect and delays initiating new meals. The positive coefficient for the interaction between intermeal duration and cost suggest that as both cost and time since last meal increases, the degree to which these two factors predict a smaller meal is diminished, perhaps reflecting accumulating energetic urgency. Genotype exerts a significant main effect on meal size, with wild-type exhibiting reduced meal size, consistent with the observations above. In addition, the significant cost-genotype interaction indicates that wild-type show greater declines in meal sizein response to cost, consistent with greater cost sensitivity observed in Fig 5B. Finally, wild-type exhibit a significant positive offset to the negative main effect of intermeal (ie., intermeal:geno(WT)). The interpretation of this is unclear.
Table 2.
Regressor coefficients and p value for models of meal size (meal termination) and intermeal duration (meal initiation).
| model | factor | coefficient | p value |
|---|---|---|---|
| y = mealsize | intermeal duration | − .0050 | < .0001 |
| cost | − .0020 | < .0001 | |
| geno(wt) | − .2987 | .0043 | |
| cost:geno (wt) | −.0021 | < .0001 | |
| intermeal:geno (wt) | .0011 | .0169 | |
| intermeal:cost | .000015 | < .0001 | |
| y = Intermeal duration | prior mealsize | .0004 | .2873 |
| cost | .0011 | < .0001 | |
| geno (wt) | −.0175 | .3940 | |
| prior mealsize: geno (wt) | −.0045 | .0379 |
In contrast to the meal size/termination model, in the model of intermeal duration (meal initiation), cost is clearly the primary factor controlling initiation, consistent with an underlying energy conservation process. The lack of a significant genotype:cost interaction is consistent with the hypothesis that both genotypes are equally subject to this conservation process. Surprisingly, prior meal size is not a significant predictor of when a subject will initiate a new meal although we observe a significant interaction term between prior meal size and genotype. We interpret this interaction as the indirect inclusion in this model of genotype main effect observed in the meal size model.
A clear pattern emerges where cost exerts strong, highly significant main effects on both initiation (intermeal duration) and termination (meal size) of meals, consistent with increased energy conservation as cost escalates. Genotype, however, appears to exert its influence only on meal size/termination, including a main effect and interactions with other factors. This divergence in factors controlling the initiation and termination of meals suggests two potentially independent processes, with the termination process being dopamine dependent while the initiation process appears dopamine independent. One possibility is that the dopamine dependent process reflects engagement of the incentive system in the pursuit of a goal, that is, once a meal is initiated. Hyperdopaminergia, then, may facilitate goal pursuit resulting in larger meals. However, the present data suggest this does not significantly shift energy balance, including conservation in the face of scarcity. Episodes of goal pursuit, ie., meals, occur within an overarching homeostatic system that compensates for larger meals with delayed initiation of the next meal. To assess this notion, we compare the two genotypes on energy balance dynamics.
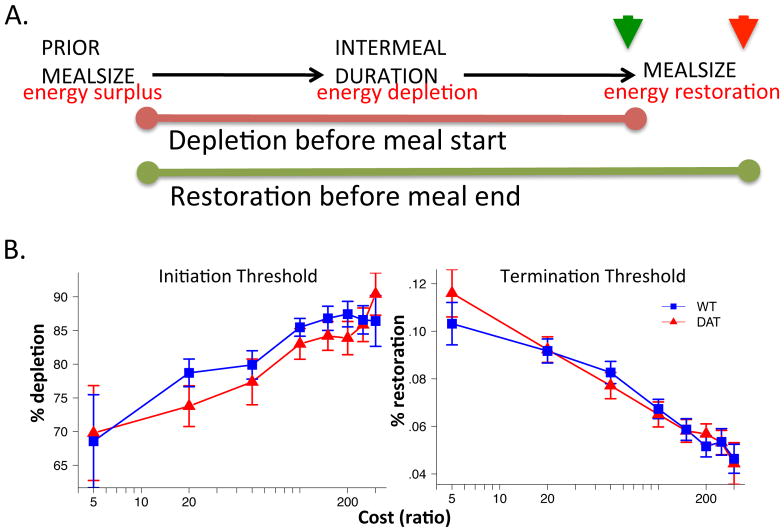
Increased dopamine and altered meal patterning does not alter gross rate of energy depletion or restoration
To assess the rate of energy depletion and restoration (Fig 9A), we used two indexes. First, energy depletion was calculated for the beginning of each meal as the size of the prior meal divided by the intermeal interval, approximating the degree to which an energy surplus is depleted before initiating a new meal. Second, energy restoration was calculated at the end of each meal as the size of the current meal divided by the total time since the end of the last meal (ie., sum of preceding intermeal interval and current meal duration); that is, the degree to which new consumption compensates for accumulating energy depletion since the last meal and restores a previous surplus.
Figure 9.
Energy balance dynamics. (A) Schematic of indices used to characterize energy balance dynamics (see Methods). Meal initiation indicated by green arrow, meal termination by red arrow. (B) percent depletion of prior energy stores before initiating a new meal (left) and percent restoration of energy stores prior to terminating a meal (right). No significant differences between genotypes. N=8.
As cost increases, both groups delayed meal initiation until greater levels of energy depletion were reached (Fig 9B, left; cost main effect, t = 4.66, p < .0001) and terminated meals at lower levels of restoration (Fig 9B, right; cost main effect, t = 8.19, p < .0001), reflecting a clear shift toward greater energy conservation and a net decrease in energy balance, as observed in Fig 3. No genotype differences were observed in either of these measures (Fig 9B, geno main effect, depletion, t = 1.18, p = .2571; restoration, t = .111, p = .9126). Viewing the meal data from the perspective of decreasing and increasing energy stores, the DATkd show identical patterns of energy depletion before initiating a meal as well as identical patterns of energy restoration before terminating a meal. These data suggest a level of homeostatic regulation of energy balance that is unaffected by elevated dopamine. Though increased dopamine delays meal termination (prolongs pursuit) and changes meal patterning, energy balance dynamics are identical between the groups. This explains why, despite increased within meal motivation/effort, increased dopamine does not substantially impact energy balance and survival in response to escalating cost; both the DATkd and wild-type are equally subject to homeostatic conservation mechanisms induced by increasing caloric restriction and food scarcity in the demand paradigm used here.
Increased dopamine enhances effort in pursuit of individual meals without altering overall consumption
To address the role of dopamine in meal patterning without the confound of increasing scarcity and putative homeostatic conservation mechanisms, we used an alternative homecage progressive ratio paradigm in which increasing costs are incorporated into individual meals. In this way, each meal indicates a subject’s willingness to continue working for food. However, after stopping a meal, the cost resets, allowing the animal access once again to lower cost food. In this way, mice can maintain their energy balance by eating more frequent, smaller meals, eliminating caloric restriction and implicit food scarcity. In the course of the study, both groups reduced their initial body weight by 7–8% with no differences between genotypes (t = .427, p = .6801, data not shown). There was, however, a significant difference between the groups in initial weight (means, DAT = 25.7, WT =21.4, p = .008). Consequently, we used two-way ANOVA with initial body weight and genotype as factors, except for number of meals where only genotype was included. No significant interaction effects were observed. P values in Fig 10 indicate genotype main effect.
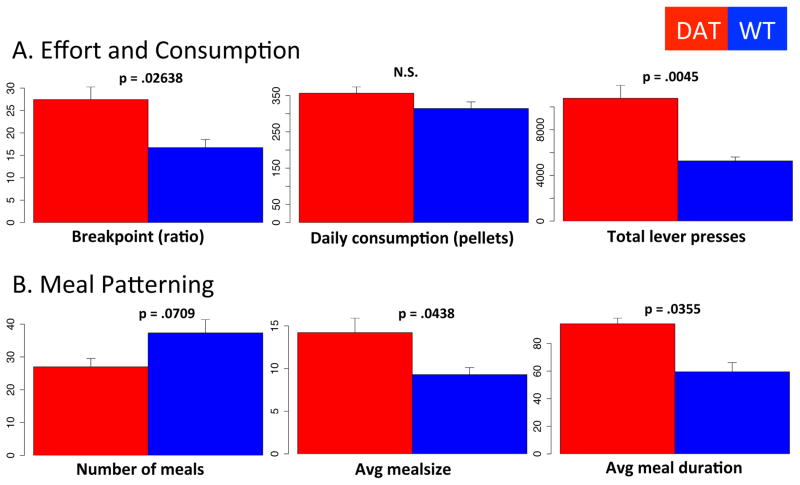
Figure 10.
Homecage progressive ratio. (A) Comparison of genotypes on breakpoint (left, defined as last ratio completed before pressing stopped for 30 minutes and incrementing ratio reset), total daily consumption (middle) and total daily lever presses (right). (B) Comparison of genotype on average number of meals (left), average meal size (middle) and average meal duration (right). N = 5.
In this paradigm, the cost of a 20mg pellet increments by two after each pellet earned (see methods). The incrementing schedule resets after 30 minutes of inactivity. Highly motivated mice may eat large meals. Less motivated mice may eat smaller meals; however, by eating a greater number of small meals, mice can easily maintain their desired intake. Consistent with meal patterning results above, the DATkd show a statistically significant increase in their breakpoint (Fig 10A; F (8,1) = 7.86, p = .0263), meal size and meal duration (Fig 10B; meal size, F (8,1) = 6.02, p = .0438; duration, F (8,1) = 6.74, p = .0355), with a corresponding marginally significant decrease in their total number of meals (Fig 10B, . F (8,1) = 4.19, p = .0709). However, their overall consumption is essentially equivalent (Fig 10A; F (8,1) = .988, p = .3533), although they work considerably more to maintain the same energy balance (Fig 10A; F (8,1) = 16.9, p = .0045). These data are consistent with the above results where increased dopamine affects motivation and willingness to work within individual meals (episodes of goal pursuit) without altering presumably homeostatically regulated overall energy balance.
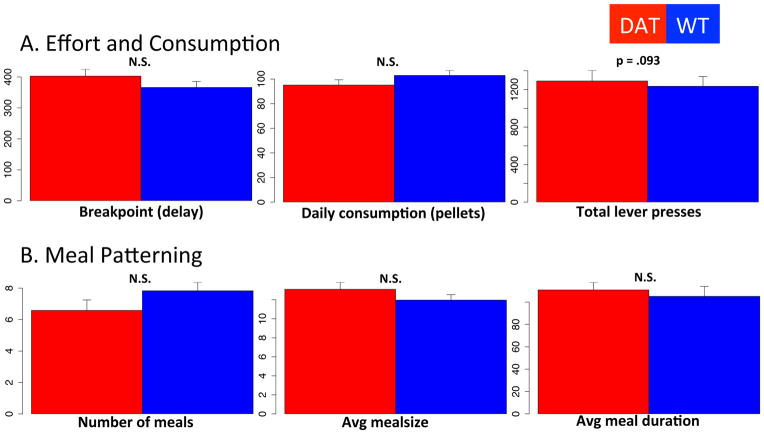
Finally, to discern whether the ‘cost’ associated with pressing reflects the effort required or the delay associated with obtaining a reward as the number of required lever presses increase, we tested a homecage progressive interval paradigm where a single press always resulted in a pellet, but an incrementing delay was introduced between pellets (incrementing by 30s). In this paradigm, no differences were observed between genotypes (Fig 11), indicating that within these homecage studies it is effort rather than delay that comprises cost (Floresco et al., 2008). In this study, initial weights were identical between groups (t = .0511, p = .9603).
Figure 11.
Homecage progressive interval. (A) Comparison of genotypes on breakpoint (left, defined as last interval before pressing stopped for 30 minutes and incrementing interval reset), total daily consumption (middle) and total daily lever presses (right). (B) Comparison of genotype on average number of meals (left), average meal size (middle) and average meal duration (right). N = 5.
DISCUSSION
Dopamine is thought by many to enhance incentive motivation and willingness to work toward a goal, an idea that has linked it to addiction and compulsive drug-seeking (Berridge, 2007; Di Chiara & Bassareo, 2007)and, more recently, the regulation of food intake and obesity (Volkow &Wise, 2005; Kenny, 2010; Volkow et al., 2010). In the DATkd mice with elevated tonic dopamine, we did observe an increase in responding at higher costs, but this increase over wild-type responding was small compared to the decline observed in both genotypes as costs escalate. Critically, this difference did not alter adaptive survival and comparison of elasticity between groups using an established model of demand elasticity showed no significant genotype difference. These data, together with those showing no difference between genotypes at baseline, low cost conditions, suggest that dopaminergic modulation of incentive and willingness to work is subject to other processes regulating food intake, presumably homeostatic mechanisms. In the demand study, we suggest that increasing caloric restriction resulting from escalating work requirement induces food scarcity and engages mechanisms of homeostatic energy conservation, though elaborating such mechanisms is beyond the scope of the present study.
Although we cannot conclusively rule out in the demand study that the energy required to obtain pellets at higher ratios exceeds the energy obtained, this is unlikely for several reasons. First, those mice that persevered at higher ratios were able to maintain a reduced body weight, suggesting that those that dropped out at lower costs were not simply responding to an energetic deficit. Second, the homecage progressive ratio shows the same pattern of results—different meal patterning between the genotypes with comparable overall energy balance—without the problem of a potential energetic ceiling. Finally, even if the demand curve partially reflects diminishing caloric gain from each pellet earned, the mice still show a differential response to this diminishing return that reflects their motivation to persevere in the face of those costs and the implicit scarcity they induce.
In contrast to overall energy balance, in which the two genotypes are more similar than different across costs, hyperdopaminergia has a clear effect on meal patterning with DATkd eating larger but fewer meals. This effect is more pronounced as the cost of food increases, with the DATkd showing relative insensitivity to cost in terms of meal size, consistent with views that dopamine increases incentive and willingness to work. However, this effect does not appear to significantly alter energy balance as prolonged intermeal intervals compensate for larger meals resulting in similar net caloric intake. Modeling of the meal data indicates that significant genotype effects center upon delaying the termination of a meal (ie., increasing meal size) with no direct effects on meal initiation (ie., intermeal interval). Whether dopamine is acting to enhance a positive incentive value or diminish the impact of increasing satiety was not specifically addressed by the present study. However, the lack of difference between genotypes in the homecage progressive interval suggests that elevated dopamine is not acting to diminish satiety signals. If this were the case, we might expect to see prolonged meals in the progressive interval study as well.
Taken as a whole, these data suggest that increased dopamine can clearly and significantly increase incentive and willingness to work but that this effect is constrained to temporally local episodes of goal pursuit, in this case a meal (for related study of localization of sensitivity to costs in RL learning see (Desrochers et al.). Put another way, dopamine does not appear to globally enhance incentive value and propensity to work but rather modulates local effort once goal pursuit is initiated ‘in the heat of the meal’ leaving overall homeostatic control of energy balance intact. As a consequence, hyperdopaminergia prolongs meals increasing meal size. Net energy balance is maintained, however, with a decrease in meal frequency, suggesting dopamine is not playing a significant role in initiating meals. Interestingly, Zorrilla et al (Zorrilla et al., 2005) found the opposite pattern where administration of acute leptin primarily reduced the frequency but not size of meals. Importantly, these conclusions may not apply across the entire range of dopamine concentrations. For example, a complete lack of dopamine in the dorsal striatum eliminates feeding behavior altogether (Palmiter, 2008). At lower dopamine concentrations, meal initiation may be altered, though whether frequency would be decreased, as suggested by the work of both Palmiter (2008) and Salamone (Salamone et al., 1990; Salamone et al., 1991), or increased, as proposed by the ‘reward deficiency’ hypothesis (Kenny, 2010) will require further investigation. At lower dopamine concentrations, dopamine’s role in motor control may impact initiation of meals as well, i.e., ‘meal initiating akinesia’ as it were.
Several limitations need to be noted. First, though the present findings are consistent with the role ascribed to the striatum in appetitive motivation, we cannot attribute the present observations to a discrete striatal region. Both the dorsal and ventral striatum have been implicated in control of feeding (Kelley, 2004; Balleine et al., 2007; Palmiter, 2008) and both are affected in the DATkd. In addition, though the effects are less striking than in the striatum, the DATkd show increased dopamine turnover in the hypothalamus (see methods), leaving open the possibility that hypothalamic dopamine contributes to the present observations. Interestingly, if so this would suggest that hypothalamic dopamine is not contributing to overall homeostatic regulation of energy balance. A more likely interpretation is that the alterations in hypothalamic dopamine are marginal and have little impact on behavior and the dramatic changes in striatal dopamine account for the observed changes in appetitive and instrumental responding, consistent with decades of literature on the role of striatum in mediating these behaviors. Importantly, a critical component of the addiction perspective is the loss of prefrontal cortex executive control inhibiting compulsive responding (George & Koob; Koob & Volkow). In the DATkd, altered dopamine function in the PFC is unlikely to contribute to the present observations as dopamine reuptake in the PFC is mediated primarily by NET and alterations in DAT have little impact on PFC dopamine reuptake kinetics (Sesack et al., 1998; Mundorf et al., 2001; Moron et al., 2002). Thus, increased mealsize observed here is unlikely to be attributable to a loss of inhibitory cortical control but rather reflects enhanced incentive via striatal signals or, possibly, hypothalamic signals.
Second, we examine hyper-but not hypo-dopaminergia and cannot assume a simple inverse of results. Though the work of Salamone and colleagues demonstrates clearly that decreased dopamine can diminish effort (Salamone et al., 1990; Salamone et al., 1991), the effect of decreased dopamine on meal patterns in a semi-naturalistic environment remains to be tested empirically. The present data, nonetheless, are difficult to reconcile with the ‘dopamine deficiency’ hypothesis currently gaining prominence (Kenny, 2010). In this theory, reduced dopamine function causes a deficiency in reward signaling leading to increased consumption in order to compensate for this reduction in reward. If dopamine signaling provided direct reward experience as suggested by this hypothesis, we would expect that hyperdopaminergic mice would decrease their consumption due to an analogous ‘dopamine excess,’ which they do not.
Third, we used a standard grain diet. It is widely proposed that palatable, energy rich foods are highly rewarding and promote consumption beyond homeostatically regulated energy needs (Saper et al., 2002; Zheng et al., 2009; Kenny, 2010; Oswald et al., 2010; Volkow et al., 2010). Under these circumstances, it may be that hyperdopaminergia would not only increase meal size but also food-seeking and meal initiation, significantly contributing to positive energy balance and homeostatic dysregulation. Finally, in the mice tested here there is no known pathophysiology in neuroendocrine, homeostatic systems, such as deficits in leptin signaling. It is possible that in the context of dysregulated homeostatic mechanisms, dopamine may exert different, unexpected effects on both energy balance and meal patterning. Addressing these issues require further study.
We propose the following simplified model. As energy stores are depleted, hunger signals increase until reaching a threshold that initiates food seeking and consumption. As energy is ingested, metabolic signals indicate rising energy levels, moving the organism back below the meal initiation threshold. If only a single threshold controlled consumption, then shortly after initiating a meal, a signalled rise in energy (eg., blood sugar, insulin) would drop below the ‘initiating threshold’ and the animal would discontinue eating after only a short period of ingestion. A single energy threshold for eating/not-eating would result in distributed, constant grazing, precluding the ability to store energy, decreasing the animal’s ability to exploit found food sources and increasing the amount of time spent foraging, notably increasing risk of predation. However, establishing two energy thresholds, one for initiating and one for terminating a meal would alleviate this problem. Extracellular dopamine increases after initiation of a meal and remains elevated for 20–60 minutes (Hernandez & Hoebel, 1988; Hoebel et al., 1992; Yoshida et al., 1992; Wilson et al., 1995; Martel & Fantino, 1996; Taber & Fibiger, 1997; Sokolowski et al., 1998; Cousins et al., 1999; Ostlund et al., 2010). We suggest that this increase in dopaminergic tone after initiating a meal enhances the incentive associated with pursuit and consumption, effectively generating a different ‘termination’ threshold, prolonging motivation despite signals indicating increasing energy stores, enabling the animal to consume a larger meal. This would result in larger but fewer meals. The resulting intermeal interval could be used for other activities, including further exploration. The lack of a genotype difference in the homecage progressive interval study suggests that this dopaminergic setting of a ‘termination’ threshold may arise through enhancing willingness to expend energy in pursuit of a goal rather than through directly diminishing the effects of satiety.
Though making inferences from a mouse study to processes contributing to human behavior and obesity requires caution, the present study suggests a couple of potentially important points.
The present data suggests that increased reward/dopaminergic function would prolong individual episodes of eating (meals/snacks), increasing caloric intake, without necessarily increasing frequency of eating. Human eating, however, is not entirely homeostatic (Strubbe & Woods, 2004;Levitsky, 2005; Lowe & Levine, 2005; Woods & D’Alessio, 2008; Grill). That is, we do not eat only when hungry, but at scheduled meal times, in social settings when food is provided (eg., donuts in the office, celebrations) and for a variety of other reason, including stress and anxiety (Dallman, 2010). Moreover, caloric consumption is not limited to meals but often occurs almost continuously throughout the day in the form of calorie rich drinks such as soft drinks and lattes. Under these circumstances, enhanced dopamine function may prolong ingestion during any single episode of consumption—the ‘one more potato chip’ phenomenon—without a corresponding increase in wait time prior to the next meal or snack, resulting in a net positive energy balance. Individual differences in dopamine function and reward sensitivity may contribute to vulnerability to overeating and obesity (Campbell & Eisenberg, 2007)by enhancing prolongation of ingestion. In addition, as frequently suggested, the prevalence of and easy access to highly palatable, energy rich foods may itself contribute to enhanced dopaminergic responses to food related stimuli (Hajnal & Norgren, 2002; Small et al., 2003; Avena et al., 2008; Davis & Carter, 2009) generating dopaminergic dysregulation. The present data do not, however, suggest that elevated dopamine would increase food-seeking, though as noted earlier, whether this holds true with high sugar and high fat food remains to be tested.
In contrast, as weight accumulates and overweight transitions into obesity, an opposite mechanism may be engaged. In this case, elevated leptin may result in two physiological adaptations: (1) leptin receptor insensitivity (Considine et al., 1996; Maffei et al., 1996; Munzberg et al., 2004; Farooqi & O’Rahilly, 2005; Enriori et al., 2007; Myers et al., 2008; Opland et al., 2010)and (2)diminished dopamine function (Fulton et al., 2006; Roseberry et al., 2007). Under these conditions, prolongation of consumption during individual episodes of ingestion might be replaced by greater food seeking and increased frequency of consumption as the homeostatic system behaves as if it were in energy deficit (ie., reduced leptin signaling) keeping the goal of energy acquisition consistently active. Diminished dopaminergic function may result in diminished motivation to overcome costs in these individuals; however, cost is rarely an issue in our modern culture where energy dense foods are cheap, ubiquitous and plentiful. This view would suggest that these two populations—overweight versus obese—may benefit from different interventions, both behaviorally and pharmacologically. To our knowledge, there have been no human studies that investigate meal patterning across the natural course of obesity, ie., from overweight to obese. The present study suggests that dopaminergic effects on meal patterning could play a role in the transition from overweight to obese and suggest a specific way in which dopaminergic, incentive mechanisms and environmental factors may interact to promote net positive energy balance, weight gain and obesity.
Supplementary Material
Supplemental Figure 1 Plots of consumption by cost for individual mice.
Supplemental Figure 2 Activity patterns. (A) Average daily inactive lever presses at each ratio/cost, (B) average daily within meal lever pressing rate, (C) histogram of interresponse times(IRTs) as percentage of total IRTs in 1 second bins, (D) histogram of post-reinforcement pauses (PRPs) in 10 second bins from 1 to 600s (5 min). No significant genotype main effect or interactions observed. N=8.
Acknowledgments
This work was supported by NIDA, DA25875 (JB), NIDDK, R56DK088515(XZ), NARSAD (XZ) and NIDA, F31DA026802 (CF).
References
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neuroscience and biobehavioral reviews. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW. Neural bases of food-seeking: affect, arousal and reward in corticostriatolimbic circuits. Physiology & behavior. 2005;86:717–730. doi: 10.1016/j.physbeh.2005.08.061. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler JA, Daw N, Frazier CR, Zhuang X. Tonic dopamine modulates exploitation of reward learning. Front Behav Neurosci. 2010;4:170. doi: 10.3389/fnbeh.2010.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR. Interactions between the “cognitive” and “metabolic” brain in the control of food intake. Physiology & behavior. 2007;91:486–498. doi: 10.1016/j.physbeh.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- Bosse R, Fumagalli F, Jaber M, Giros B, Gainetdinov RR, Wetsel WC, Missale C, Caron MG. Anterior pituitary hypoplasia and dwarfism in mice lacking the dopamine transporter. Neuron. 1997;19:127–138. doi: 10.1016/s0896-6273(00)80353-0. [DOI] [PubMed] [Google Scholar]
- Cagniard B, Balsam PD, Brunner D, Zhuang X. Mice with chronically elevated dopamine exhibit enhanced motivation, but not learning, for a food reward. Neuropsychopharmacology. 2006a;31:1362–1370. doi: 10.1038/sj.npp.1300966. [DOI] [PubMed] [Google Scholar]
- Cagniard B, Beeler JA, Britt JP, McGehee DS, Marinelli M, Zhuang X. Dopamine scales performance in the absence of new learning. Neuron. 2006b;51:541–547. doi: 10.1016/j.neuron.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Campbell BC, Eisenberg D. Obesity, attention deficit-hyperactivity disorder and the dopaminergic reward system. Coll Antropol. 2007;31:33–38. [PubMed] [Google Scholar]
- Chaney MA, Rowland NE. Food demand functions in mice. Appetite. 2008;51:669–675. doi: 10.1016/j.appet.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Trevitt J, Atherton A, Salamone JD. Different behavioral functions of dopamine in the nucleus accumbens and ventrolateral striatum: a microdialysis and behavioral investigation. Neuroscience. 1999;91:925–934. doi: 10.1016/s0306-4522(98)00617-4. [DOI] [PubMed] [Google Scholar]
- Dallman MF. Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab. 2010;21:159–165. doi: 10.1016/j.tem.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, Carter JC. Compulsive overeating as an addiction disorder. A review of theory and evidence. Appetite. 2009;53:1–8. doi: 10.1016/j.appet.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Davis JF, Choi DL, Schurdak JD, Fitzgerald MF, Clegg DJ, Lipton JW, Figlewicz DP, Benoit SC. Leptin regulates energy balance and motivation through action at distinct neural circuits. Biol Psychiatry. 2010;69:668–674. doi: 10.1016/j.biopsych.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan P, Balleine BW. Reward, motivation, and reinforcement learning. Neuron. 2002;36:285–298. doi: 10.1016/s0896-6273(02)00963-7. [DOI] [PubMed] [Google Scholar]
- Desrochers TM, Jin DZ, Goodman ND, Graybiel AM. Optimal habits can develop spontaneously through sensitivity to local cost. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:20512–20517. doi: 10.1073/pnas.1013470107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. Dopamine in disturbances of food and drug motivated behavior: a case of homology? Physiology & behavior. 2005;86:9–10. doi: 10.1016/j.physbeh.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn’t do. Curr Opin Pharmacol. 2007;7:69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5:181–194. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, O’Rahilly S. Monogenic obesity in humans. Annu Rev Med. 2005;56:443–458. doi: 10.1146/annurev.med.56.062904.144924. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, MacDonald Naleid A, Sipols AJ. Modulation of food reward by adiposity signals. Physiology & behavior. 2007;91:473–478. doi: 10.1016/j.physbeh.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP, Sipols AJ. Energy regulatory signals and food reward. Pharmacol Biochem Behav. 2010;97:15–24. doi: 10.1016/j.pbb.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Tse MT, Ghods-Sharifi S. Dopaminergic and glutamatergic regulation of effort-and delay-based decision making. Neuropsychopharmacology. 2008;33:1966–1979. doi: 10.1038/sj.npp.1301565. [DOI] [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neuroscience and biobehavioral reviews. 2010;35:232–247. doi: 10.1016/j.neubiorev.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ. Leptin and the systems neuroscience of meal size control. Front Neuroendocrinol. 2009;31:61–78. doi: 10.1016/j.yfrne.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnal A, Norgren R. Repeated access to sucrose augments dopamine turnover in the nucleus accumbens. Neuroreport. 2002;13:2213–2216. doi: 10.1097/00001756-200212030-00010. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Feeding and hypothalamic stimulation increase dopamine turnover in the accumbens. Physiology & behavior. 1988;44:599–606. doi: 10.1016/0031-9384(88)90324-1. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Mark GP, West HL. Conditioned release of neurotransmitters as measured by microdialysis. Clin Neuropharmacol. 1992;15(Suppl 1):Pt A, 704A–705A. doi: 10.1097/00002826-199201001-00364. [DOI] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neuroscience and biobehavioral reviews. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol. 2005a;493:72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiology & behavior. 2005b;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron. 2010;69:664–679. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitsky DA. The non-regulation of food intake in humans: hope for reversing the epidemic of obesity. Physiology & behavior. 2005;86:623–632. doi: 10.1016/j.physbeh.2005.08.053. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Levine AS. Eating motives and the controversy over dieting: eating less than needed versus less than wanted. Obes Res. 2005;13:797–806. doi: 10.1038/oby.2005.90. [DOI] [PubMed] [Google Scholar]
- Lutter M, Nestler EJ. Homeostatic and hedonic signals interact in the regulation of food intake. J Nutr. 2009;139:629–632. doi: 10.3945/jn.108.097618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei M, Stoffel M, Barone M, Moon B, Dammerman M, Ravussin E, Bogardus C, Ludwig DS, Flier JS, Talley M, et al. Absence of mutations in the human OB gene in obese/diabetic subjects. Diabetes. 1996;45:679–682. doi: 10.2337/diab.45.5.679. [DOI] [PubMed] [Google Scholar]
- Martel P, Fantino M. Mesolimbic dopaminergic system activity as a function of food reward: a microdialysis study. Pharmacol Biochem Behav. 1996;53:221–226. doi: 10.1016/0091-3057(95)00187-5. [DOI] [PubMed] [Google Scholar]
- Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci. 2002;22:389–395. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundorf ML, Joseph JD, Austin CM, Caron MG, Wightman RM. Catecholamine release and uptake in the mouse prefrontal cortex. J Neurochem. 2001;79:130–142. doi: 10.1046/j.1471-4159.2001.00554.x. [DOI] [PubMed] [Google Scholar]
- Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- Opland DM, Leinninger GM, Myers MG., Jr Modulation of the mesolimbic dopamine system by leptin. Brain Res. 2010;1350:65–70. doi: 10.1016/j.brainres.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Wassum KM, Murphy NP, Balleine BW, Maidment NT. Extracellular dopamine levels in striatal subregions track shifts in motivation and response cost during instrumental conditioning. J Neurosci. 2010;31:200–207. doi: 10.1523/JNEUROSCI.4759-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald KD, Murdaugh DL, King VL, Boggiano MM. Motivation for palatable food despite consequences in an animal model of binge eating. Int J Eat Disord. 2010;44:203–211. doi: 10.1002/eat.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007;30:375–381. doi: 10.1016/j.tins.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Palmiter RD. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: lessons from dopamine-deficient mice. Ann N Y Acad Sci. 2008;1129:35–46. doi: 10.1196/annals.1417.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher “wanting” but not “liking” for sweet rewards. J Neurosci. 2003;23:9395–9402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevention, C.F.D.C.a; Government, U.S. US Obesity Trends: Trends by State 1985–2008. 2009. [Google Scholar]
- Redish AD. Addiction as a computational process gone awry. Science. 2004;306:1944–1947. doi: 10.1126/science.1102384. [DOI] [PubMed] [Google Scholar]
- Roseberry AG, Painter T, Mark GP, Williams JT. Decreased vesicular somatodendritic dopamine stores in leptin-deficient mice. J Neurosci. 2007;27:7021–7027. doi: 10.1523/JNEUROSCI.1235-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland NE, Vaughan CH, Mathes CM, Mitra A. Feeding behavior, obesity, and neuroeconomics. Physiology & behavior. 2008;93:97–109. doi: 10.1016/j.physbeh.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD. Functions of mesolimbic dopamine: changing concepts and shifting paradigms. Psychopharmacology (Berl) 2007;191:389. doi: 10.1007/s00213-006-0623-9. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137:3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, McCullough LD, Carriero DL, Berkowitz RJ. Nucleus accumbens dopamine release increases during instrumental lever pressing for food but not free food consumption. Pharmacol Biochem Behav. 1994;49:25–31. doi: 10.1016/0091-3057(94)90452-9. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Snyder BJ. Behavioral functions of nucleus accumbens dopamine: empirical and conceptual problems with the anhedonia hypothesis. Neuroscience and biobehavioral reviews. 1997;21:341–359. doi: 10.1016/s0149-7634(96)00017-6. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology (Berl) 1991;104:515–521. doi: 10.1007/BF02245659. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Zigmond MJ, Stricker EM. Characterization of the impaired feeding behavior in rats given haloperidol or dopamine-depleting brain lesions. Neuroscience. 1990;39:17–24. doi: 10.1016/0306-4522(90)90218-s. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annual review of neuroscience. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Guido MA, Levey AI. Cellular and subcellular localization of the dopamine transporter in rat cortex. Adv Pharmacol. 1998;42:171–174. doi: 10.1016/s1054-3589(08)60720-6. [DOI] [PubMed] [Google Scholar]
- Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- Sokolowski JD, Conlan AN, Salamone JD. A microdialysis study of nucleus accumbens core and shell dopamine during operant responding in the rat. Neuroscience. 1998;86:1001–1009. doi: 10.1016/s0306-4522(98)00066-9. [DOI] [PubMed] [Google Scholar]
- Strubbe JH, Woods SC. The timing of meals. Psychol Rev. 2004;111:128–141. doi: 10.1037/0033-295X.111.1.128. [DOI] [PubMed] [Google Scholar]
- Taber MT, Fibiger HC. Feeding-evoked dopamine release in the nucleus, accumbens: regulation by glutamatergic mechanisms. Neuroscience. 1997;76:1105–1112. doi: 10.1016/s0306-4522(96)00450-2. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2010;15:37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Wang Y, Beydoun MA. The obesity epidemic in the United States--gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- Wilson C, Nomikos GG, Collu M, Fibiger HC. Dopaminergic correlates of motivated behavior: importance of drive. J Neurosci. 1995;15:5169–5178. doi: 10.1523/JNEUROSCI.15-07-05169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Woods SC, D’Alessio DA. Central control of body weight and appetite. J Clin Endocrinol Metab. 2008;93:S37–50. doi: 10.1210/jc.2008-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Zhuang X, Balleine BW. Instrumental learning in hyperdopaminergic mice. Neurobiology of learning and memory. 2006;85:283–288. doi: 10.1016/j.nlm.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Yokoo H, Mizoguchi K, Kawahara H, Tsuda A, Nishikawa T, Tanaka M. Eating and drinking cause increased dopamine release in the nucleus accumbens and ventral tegmental area in the rat: measurement by in vivo microdialysis. Neurosci Lett. 1992;139:73–76. doi: 10.1016/0304-3940(92)90861-z. [DOI] [PubMed] [Google Scholar]
- Zheng H, Lenard NR, Shin AC, Berthoud HR. Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. Int J Obes (Lond) 2009;33(Suppl 2):S8–13. doi: 10.1038/ijo.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, Hen R. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:1982–1987. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Inoue K, Valdez GR, Tabarin A, Koob GF. Leptin and post-prandial satiety: acute central leptin more potently reduces meal frequency than meal size in the rat. Psychopharmacology (Berl) 2005;177:324–335. doi: 10.1007/s00213-004-1952-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 Plots of consumption by cost for individual mice.
Supplemental Figure 2 Activity patterns. (A) Average daily inactive lever presses at each ratio/cost, (B) average daily within meal lever pressing rate, (C) histogram of interresponse times(IRTs) as percentage of total IRTs in 1 second bins, (D) histogram of post-reinforcement pauses (PRPs) in 10 second bins from 1 to 600s (5 min). No significant genotype main effect or interactions observed. N=8.