Abstract
Actigraphic measures of physical activity do not rely on participant self-report and may be of particular importance for efforts to examine the health benefits of physical activity across the full spectrum of older individuals especially those with dementia, a group in which loss of motor function is particularly salient. We tested whether actigraphy could be employed to examine the relationship between total daily physical activity and motor function in community-dwelling older persons both with (n=70) and without clinical dementia (n=624). Total daily activity was measured with actigraphy for a median of 9 (range 2–16) days. All participants also underwent a structured examination including 9 muscle strength and 9 motor performance measures summarized as a composite measure. In linear regression models controlling for age, sex, and education, total daily activity was associated with global motor scores (β=0.13, SD=0.01, p<0.001). This association remained significant after adjusting for body composition, cognition, depressive symptoms, disability, vascular risk factors and diseases (β=0.07, SD=0.01, p < 0.001). The association did not vary by dementia status (interaction p=0.53). In persons without dementia, the association was independent of self-reported physical activity. Total daily activity was associated with both muscle strength (β=0.10, SD=0.02, p<0.001) and motor performance (β=0.16, SD=0.02, p<0.001). Actigraphy can be employed in the community-setting to provide objective measures of total daily activity that are associated with a broad range of motor performances and these associations did not vary by dementia status. Actigraphy may provide a means to more fully explicate the nature and course of motor impairment in old age.
Keywords: actigraphy, physical activity, motor function
INTRODUCTION
Motor impairments are common in old age 1 and associated with a wide range of adverse health consequences including mortality,2 disability,3 and loss of independence.4 This association between motor impairments and function in old age may be especially important for older persons who have dementia, as motor impairments are common in dementia and may be an early sign of Alzheimer’s disease (AD).5–6 Gaps in our knowledge about the relationship between physical activity and motor function in older persons and especially those with dementia, who cannot provide self-reports, impede the formulation of evidence-based recommendations for optimal physical activity interventions to decrease the burden of motor impairments in old age.7 Effective methods to measure both physical activity and motor function in the community of older persons both with and without dementia will help to alleviate this impediment.
There is now mounting evidence that health benefits may accrue from the total energy expenditure achieved through both structured and non-structured physical activity accumulated throughout the day.8 However, supporting data are lacking from community-based studies of the general older population, since many studies rely on self-reported physical activity questionnaires which limit the type of activities that are sampled and may be affected by recall bias due to age-related cognitive impairments such as dementia. Prior efforts to objectively quantify physical activity have been limited to the laboratory setting. However, rapid advances in technology have led to the development of portable devices that store large amounts of data making it possible to collect quantitative measures of physical activity for prolonged periods of time in the community setting.9, 10 These devices are minimally intrusive or burdensome to participants and have the distinct advantage of recording total daily physical activity and do not rely on participant recall.
In the current study, we tested the hypothesis that objective measures of total daily physical activity are associated with the level of motor function in community-dwelling older persons with and without dementia. We used data from approximately 700 participants of the Rush Memory and Aging Project who underwent structured motor exam including strength from 9 muscle groups and 9 motor performance measures which were summarized as a composite global motor score and 2 subcomponents (muscle strength and motor performance) as previously described.11, 12 Objective measures of total daily physical activity were obtained using an actigraph, a portable device which was worn on the non-dominant wrist 24 hours a day for up to 10 days as previously described.13 In secondary analyses, we tested whether the association of total daily activity with motor function was independent of level of self-reported physical activities in persons without dementia. We also tested whether the association differed for persons with dementia, a group in which motor function impairment is particularly salient and whose cognitive impairments prevent accurate self-reporting of physical activities.
METHODS
Participants
Participants were individuals from the Rush Memory and Aging Project, an ongoing longitudinal, community study of common chronic conditions of old age.12 Participants were recruited from retirement facilities and subsidized housing facilities from around the Chicago metropolitan area. All participants signed an informed consent agreeing to annual clinical evaluation (see below), and organ donation at the time of death. The study was in accordance with the latest version of the Declaration of Helsinki and was approved by the institutional review board of Rush University Medical Center.
Between 1997 and January, 2010, 1,322 older persons had enrolled in the Memory and Aging Project and completed a baseline evaluation. Of the 1,144 participants enrolled after actigraphy was added in 2005, 156 refused actigraphy. Of the 988 who agreed to actigraphy testing, 792 (80.2%) had actigraphs placed, 63 (6.4%) died before actigraphy could be obtained, and 133 (13.5%) are still being recruited for actigraphy. Of the 792 who had actigraphy testing, there were 23 (2.9%) instances in which the device failed or the participant removed the device, leaving 769 with actigraphy data for these analyses. Another 47 were excluded for being non-ambulatory, and 28 did not have complete motor testing or cognitive assessment data, leaving a total of 694 participants for these analyses. Participants with actigraphy data were older (82.2 vs. 78.8 years, t = −6.75, p<0.001), more educated (14.7 vs. 13.7 years, t = −4.70, p<0.001), had better global cognitive function (0.06 vs. −0.25, t = −6.27, p<0.001), less depressive symptoms (1.15 vs. 1.60 on CES-D, t = 3.37, p<0.001), and less disabilities (0.22 vs. 0.95 on KATZ, t = 7.76, p<0.001) than eligible participants without actigraphy.
Assessment of Total Daily Activity
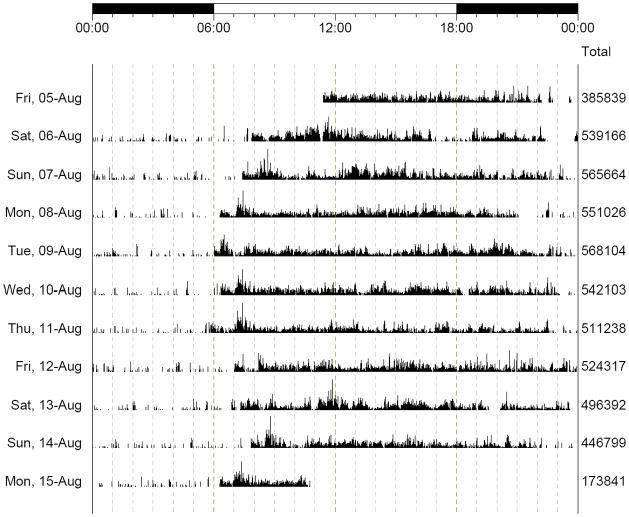
Objective measures of physical activity were obtained using a portable actigraph (Actical®; Mini Mitter, Bend, Oregon). Actical is a compact, battery-operated activity monitor similar in size to a wristwatch and worn on the wrist. We chose a device for the wrist rather than the waist or leg in order to minimize participant burden and missing data, based on pilot testing, Upper extremity movement is highly correlated with movement measured at the waist and leg.14 The device was worn 24 hours per day as it is completely waterproof (i.e., subjects can bathe or shower with the device). Actical utilizes a piezoeletric triaxial accelerometer to monitor the occurrence and degree of motion. This type of sensor and associated digital integration signal processing considers both the degree and intensity of motion to produce an electrical current that varies in magnitude. An increased degree of speed and motion produces an increase in voltage which is stored by the device as Activity Counts. The area under the curve for each 1-second sample is integrated and stored as “activity counts”, which are summed over user-specified intervals of time (epoch) and valued at 0 if there is no activity during an epoch. In the current study, the Actical device was placed on the non-dominant wrist for up to 10 days. We employed an epoch duration of 15 seconds which yields 5760 epochs/day. Figure 1 shows a time-series for a single participant and illustrates the changing levels and distribution of physical activity during 10 days.
Figure 1. Distribution and Magnitude of Total Daily Activity Over Nine Consecutive Days.
A graphical summary of Total Daily Activity from a single Memory and Aging participant. In this recording, the device recorded and averaged physical activity every 15 seconds for nine complete days (August 6th–14th) and portions of two additional days (August 5th and 15th). The bar on the top of the figure displays the time of day for 24 hours (0.00 to 0.00). Each line with a date on the far left, represents a full 24 hours of activity. Activity can be seen to vary during the day, with low or absent activity usually noted between 23:00 and 6:00 when the participant was likely sleeping. The column on the right of the figure, displays the total daily activity counts used to calculate Total Daily Activity/day for all 9 days with complete data.
We developed three measures to summarize the actigraphy data as previously reported.13 Total daily activity, which is the sum of all activity counts recorded during a 24 hour period was used as our primary objective measure of physical activity in these analyses. A second measure, intensity of daily activity, was obtained by dividing the total daily activity counts by the time of all nonzero epochs during each day (24 hours) to yield the mean activity/active hours/day. Since epochs without activity had a value of 0, we also calculated a third measure, the percentage of the day during which there was no activity, by dividing the sum of epochs with zero activity during the 24 hours by 5760, the total number of 15 second epochs in a 24 hour period. Since there were more than 300,000 activity counts/day on average, raw counts were divided by 100,000 (about 1 Standard deviation [SD]) to facilitate presentation and interpretation of the results, as previously reported.13
Assessment of Motor Function
Muscle strength was measured in both arms and legs including: arm abduction, arm flexion, arm extension, hip flexion, knee extension, plantar flexion, and ankle dorsiflexion using Hand-held dynamometers (Lafayette Manual Muscle Test System, Model 01163, Lafayette, Indiana). The Jamar hydraulic dynamometers (Lafayette Instruments, Lafayette, Indiana) were used to measure grip and pinch strength bilaterally.
Motor performance measures included the time and number of steps it took to walk 8 feet and turn 360°; how long participants could stand on each leg and then on their toes for up to 10 seconds; and the number of steps off line when walking an 8 foot line in a heel to toe manner. We also measured: the number of pegs that could be placed (Purdue pegboard) in 30 seconds, and the maximum number of finger taps in 10 seconds using an electronic tapper (Western Psychological Services, Los Angeles, California).
Motor measures were summarized as a global motor score as well as subcomponents for muscle strength and motor performance, by averaging the z scores for each of the individual tests, as previously reported.11 Z scores for grip strength and timed walk were sex-adjusted before creating the composite scores, due to differences in performance on these tests between men and women.
Assessment of Cognitive Function and Diagnosis of Dementia
Cognitive function was assessed using a battery of 19 tests used to create a composite measure of global cognition as described elsewhere.12, 15 Dementia was diagnosed in a three-step process. Cognitive testing was scored by computer and reviewed by a neuropsychologist to diagnose cognitive impairment. Participants were then evaluated by a clinician who used all cognitive and clinical data to diagnose dementia.12
Other Covariates
Sex and years of education were recorded at the study entry. Age in years at the time of the motor function testing was computed from self-reported date of birth. Self-report physical activity was assessed using questions adapted from the 1985 National Health Interview Survey.16 Time engaged in each of five activities (walking for exercise, gardening or yardwork, calisthenics or general exercise, bicycle riding, and swimming or water exercise) was summed and expressed as hours of activity per week, as previously described.17 Body-mass index (BMI) was calculated from measured weight and height. Because of the inverted J-shaped relationship between BMI and health, terms for both BMI and BMI squared were used in analyses. Percent body fat was derived from whole body bioimpedance measures using the portable Body Comp Scale (American Weights & Measure, California).18 Depressive symptoms were assessed with the 10-item version of the Center for Epidemiologic Studies Depression Scale.12 Disabilities in activities of daily living (ADLs) were assessed using a modified version of the Katz index.19 The sum of three vascular risk factors and four vascular diseases were used as previously described (listed in Table 1).20
Table 1.
Characteristics of the Cohort (n=694).
| Variable* | Mean (SD) or n (%) | Range | Correlation with total daily activity† | P Value | Correlation with global motor score† | P Value |
|---|---|---|---|---|---|---|
| Age | 82.2 (7.0) | 56, 98 | −0.23 | <0.001 | −0.45 | <0.001 |
| Years of education | 14.7 (3.0) | 5, 28 | −0.003 | 0.93 | 0.21 | <0.001 |
| Dementia diagnosis | 70 (10.1%) | 5.8 | <0.001 | 6.6 | <0.001 | |
| MMSE | 27.3 (3.4) | 0, 30 | 0.25 | <0.001 | 0.32 | <0.001 |
| Global cognition | 0.1 (0.7) | −2.6, 1.4 | 0.28 | <0.001 | 0.44 | <0.001 |
| BMI | 26.8 (5.1) | 12.8, 56.9 | −0.10 | 0.015 | −0.11 | 0.006 |
| Percent body fat | 37.5 (7.0) | 10.4, 53.5 | −0.07 | 0.066 | −0.28 | <0.001 |
| Depressive Symptoms | 1.2 (1.7) | 0, 9 | −0.12 | 0.001 | −0.24 | <0.001 |
| ADL disability | 0.2 (0.7) | 0, 6 | −0.22 | <0.001 | −0.37 | <0.001 |
| Vascular risk factors | 1.2 (0.8) | 0, 3 | −0.11 | 0.003 | −0.11 | 0.006 |
| Hypertension | 454 (65.4%) | 4.78 | <0.001 | 4.99 | <0.001 | |
| Diabetes mellitus | 115 (16.6%) | 2.69 | 0.007 | 2.65 | 0.008 | |
| Smoking | 278 (40.1%) | −1.62 | 0.106 | −2.29 | 0.022 | |
| Vascular diseases | 0.5 (0.8) | 0, 4 | −0.19 | <0.001 | −0.22 | <0.001 |
| Myocardial infarction | 92 (13.3%) | 3.11 | 0.002 | 1.72 | 0.086 | |
| Congestive heart failure | 41 (6.5%) | 2.41 | 0.016 | 1.90 | 0.058 | |
| Claudication | 103 (14.8%) | 2.55 | 0.011 | 7.02 | <0.001 | |
| Stroke | 82 (12.3%) | 3.84 | <0.001 | 3.71 | <0.001 |
Abbreviations: SD, standard deviation; MMSE, Mini-Mental State Examination; BMI, body mass index; IADL, instrumental activities of daily living.
Mini-mental state exam (MMSE) score: range, 18 to 30 (a higher score indicates a higher level of cognition). Global cognition: composite measure of cognition based on performances on 19 cognitive tests (a higher score indicates a higher level of cognition). Body Mass Index (BMI): calculated as weight in kilograms divided by height in meters squared. Percent body fat: from whole body bioimpedance measures using the portable Body Comp Scale (American Weights & Measure, Calif.). Depressive symptoms: a modified 10-item Center for Epidemiologic Studies Depression scale (a higher score indicates greater depressive symptomatology). Katz activities of daily living (ADL) disability: a 6-item measure of basic activities of daily living (a higher score indicates greater disability). Vascular risk factors: sum of smoking, diabetes, and hypertension (self-reported). Vascular diseases: sum of myocardial infarction, congestive heart failure, claudication, and stroke (self-reported).
Pearson correlations used for continuous variables; t-tests used for categorical variables.
Statistical Analysis
Since actigraphy measures were positively skewed, Spearman correlations were used to assess the relationship between measures of total daily activity and demographic variables and other covariates at baseline. t Tests were used to compare men and women. A linear regression model that adjusted for age, sex, and education was then used to examine the association of total daily activity with motor function (core model). A term for total daily activity squared was significant, but was not retained in subsequent analyses because it accounted for very little additional variance in motor function and did not significantly improve model fit. To test for interactions with demographics, the core model was run with interaction terms for total daily activity and each demographic variable. We repeated the core model with mean intensity of daily activity and percentage of the day without activity. We then ran the core model controlling for covariates that might confound the relationship between total daily activity and motor function. To examine whether the relationship between total daily activity and motor function differed by dementia status, we first stratified the cohort by dementia status and reran the core model. Then we repeated the core model and added interaction terms for dementia status. Next we examined whether self-reported physical activity attenuated the association of total daily activity and motor function in non-demented participants. Finally we examined whether total daily activity was related to muscle strength and motor performances Model validation was performed graphically and analytically. Programming was done in SAS 9.2 (SAS Institute Inc., Cary, North Carolina).
RESULTS
Descriptive Properties of Actigraphy Measures
There were 694 participants whose mean age was 82.2 years (SD=7.0); 75.9% were women, 93.7% were white, non-Hispanic, and 10.1% had clinical dementia. Additional characteristics of these participants are included in Table 1. Participants wore the Actical for an average of 9.3 days (SD=1.1 days, median= 9 days, range=2–16 days). Total daily activity ranged from 0.40 × 105 counts/day to 7.18 × 105 counts/day (mean: 2.98 × 105 counts/day; SD = 1.35 × 105 counts/day). Mean daily activity/active hours was 0.29 × 105 counts/hour (SD = 0.09 × 105 counts/hour, range: 0.07 × 105 counts/hour, 0.56 × 105 counts/hour). The average participant showed no activity for almost 60% of each day (mean 59% [SD = 9%0, range: 28%, 85%]). Total daily activity was inversely related to age and women had higher levels of total daily activity (Table 1). A higher level of total daily activity was related to lower BMI, higher level of cognition, less depressive symptoms, less disability and fewer chronic conditions (Table 1).
Actigraphy Measures and Motor Function
Global motor score was approximately normally distributed and ranged from -1.83 to 2.44 (mean = 0.06, SD = 0.63) with higher scores indicating better motor function. Global motor score was inversely related to age and was correlated with all of the covariates we examined (Table 1). Men had higher global motor scores than women (0.25 vs. 0.00, p < 0.001).
In a linear regression model adjusting for age, sex, and education, a higher level of total daily activity was associated with a higher global motor score (β = 0.13, S.D. = 0.015, p < 0.001, adjusted r2 = 0.34). To contextualize the magnitude of this association, we compared its magnitude to the association of age with global motor score in the same model (β =−0.034, S.D. = 0.0029). Thus, a 1 standard deviation increase in total daily activity was equivalent to a participant being more than 5 years younger ([βtotal daily activity =0.13/S.D.total daily activity distribution =1.35]/βage =−0.034). In secondary analyses, mean daily activity/active hours (i.e. intensity of activity) (β = 2.15, S.D. = 0.23, p < 0.001, adjusted r2 = 0.34) and the percentage of the day without activity (β = −1.50, S.D. = 0.22, p < 0.001, adjusted r2 = 0.31) were also related to global motor score.
The association of total daily activity and motor function did not vary by sex when the cohort was stratified (females: β = 0.13, S.D. = 0.02, p < 0.001; males: β = 0.15, S.D. = 0.03, p < 0.001). When we included interaction terms for demographic variables, the association of total daily activity and global motor score did not vary by age, sex or education (results not shown).
Since a number of variables may affect both the level of physical activity or motor function, we repeated the core model described above including terms for potential confounding variables, including body composition (BMI and percent body fat), global cognition, depressive symptoms, ADL disability, vascular risk factors and vascular diseases. The association of total daily activity and motor function remained unchanged when adjusting for each of these covariates separately (data not shown). In a single model with all the above covariates, total daily activity remained associated with motor function, though the magnitude of the association was attenuated (β = 0.07, S.D. = 0.01, p < 0.001). Of the variables adjusted for, global cognition and disability contributed the most to the attenuation, and could potentially reflect overadjustment.
Objective and Self-Reported Measures of Physical Activity, and Motor Function
These analyses were restricted to persons without dementia to avoid recall bias. Mean hours per week of physical activity as assessed by self-report was 3.31 (SD = 3.61). Total daily activity and self-reported physical activity were modestly related (Pearson correlation coefficient=0.20, p<0.001). In separate linear regression models adjusted for age, sex, and education, both total daily activity and self-reported physical activity were associated with global motor score, though the model with total daily activity accounted for a larger percentage of the total variance in global motor function (Table 2, models 2 versus 3). Next we examined whether self-reported physical activity attenuated the association of total daily activity and motor function when both were included in a single model. In this model, both total daily activity and self-report physical activity were independently associated with global motor score (Table 2, model 4).
Table 2.
Association of Total Daily Activity and Self-Report Physical Activity with Global Motor Score in Participants Without Dementia (n = 624)
| Model 1 β (SE) | Model 2 β (SE) | Model 3 β (SE) | Model 4 β (SE) | Model 5 β (SE) | |
|---|---|---|---|---|---|
| Age (years) | −0.038 (0.003)*** | −0.034 (0.003)*** | −0.037 (0.003)*** | −0.034 (0.003)*** | −0.026 (0.003)*** |
| Sex (male vs. female) | 0.255 (0.051)*** | 0.296 (0.049)*** | 0.241 (0.050)*** | 0.284 (0.049)*** | 0.186 (0.061)** |
| Education (years) | 0.032 (0.007)*** | 0.032 (0.007)*** | 0.029 (0.007)*** | 0.030 (0.007)*** | 0.005 (0.008) |
| Total daily activity1 | 0.124 (0.016)*** | 0.116 (0.016)*** | 0.068 (0.016)*** | ||
| Self-report physical activity2 | 0.024 (0.006)*** | 0.015 (0.006)*** | 0.006 (0.006) | ||
| BMI | 0.030 (0.025) | ||||
| BMI2 | −0.001 (0.001) | ||||
| % body fat | 0.016 (0.021) | ||||
| % body fat squared | −0.001 (0.001) | ||||
| Global cognition | 0.291 (0.046)*** | ||||
| Depression (CESD) | −0.025 (0.013) | ||||
| Disability (KATZ) | −0.019 (0.033)*** | ||||
| Vascular risk factors (#) | −0.019 (0.025) | ||||
| Vascular diseases (#) | −0.036 (0.027) | ||||
| Adjusted r2 | 0.248 | 0.317 | 0.266 | 0.323 | 0.439 |
Abbreviations: β = estimated coefficient for association of variable with global motor score; SE = standard error.
p<0.05
p<0.01
p<0.001
Estimates are for every 105 activity counts/day as measured by actigraphy
Estimates are for every hour of activity per week as measured by self-report [Figure 1]
Total Daily Activity and Motor Function in Participants with and without Dementia
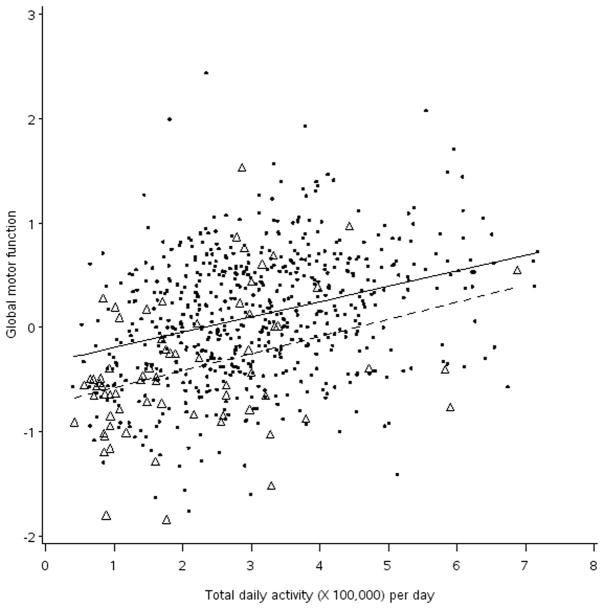
Participants with dementia (10.1%, 70/694) had significantly lower levels of both total daily activity (2.11 vs. 3.07, t test = 5.77 [df = 692], p < 0.001) and global motor score (−0.40 vs. 0.11, t test = 6.61 [df = 692], p < 0.001) as compared to participants without dementia. In separate regression models stratified by dementia status, total daily activity was associated with global motor score for both nondemented (β = 0.12, S.D. = 0.02, p < 0.001) and demented (β = 0.11, S.D. = 0.05, p = 0.029) participants. Next, we added an interaction term for dementia status to the core model. The association of total daily activity and global motor score did not vary by dementia status (interaction β = −0.030, S.D. = 0.047, p = 0.53). This finding is illustrated in Figure 2, which shows that while those with dementia (triangles) had lower levels of both global motor function and lower total daily activity than in persons without dementia (circles), the estimated relationship of total daily activity and motor function was similar in participants with and without dementia. Finally, in a secondary fully adjusted model with all of the covariates described above, the association of total daily activity and global motor score did not vary by dementia status (interaction β = 0.008, S.D. = 0.047, p = 0.87).
Figure 2. The Association of Total Daily Activity Measured With Actigraphy and Global Motor Scores In Participant’s With and Without Dementia.
Circle = observed data, nondemented participants
Triangle = observed data, demented participants
Solid line = regression line for estimated global motor score against total daily activity in nondemented participants, adjusted for age, sex, and education.
Dashed line = regression line for estimated global motor score against total daily activity in demented participants, adjusted for age, sex, and education.
Total Daily Activity, Muscle Strength and Motor Performance
Motor function is not a unitary process so we examined the association between total daily activity and the subcomponents of global motor score, muscle strength and motor performance in the total cohort. Muscle strength ranged from −2.36 to 4.67 (mean = 0.23, SD = 0.77). Motor performance ranged from −1.70 to 1.57 (mean = −0.03, SD = 0.68). Total daily activity was associated with both muscle strength (β = 0.10, SD = 0.02, p < 0.001, adjusted r2 = 0.22) and motor performance (β = 0.16, SD = 0.02, p < 0.001, adjusted r2 = 0.34).
DISCUSSION
In a group of nearly 700 community-dwelling older persons, higher levels of total daily activity, as measured by actigraphy, were associated with higher levels of motor function. The association between total daily activity and motor function persisted after adjustment for possible confounders, including body composition, cognition, depression, disability, and chronic conditions and did not vary in participants with and without dementia. In secondary analyses restricted to participants without dementia, higher levels of physical activity as measured both by actigraphy and self-report were independently associated with better motor function, though actigraphy measures accounted for more of the variance in motor function than self-report physical activities. Finally, total daily activity was associated with subcomponents of motor function, measures of motor performance and muscle strength. These results suggest that using currently available technology, objective measures of total daily activity can be obtained in the community setting and that these measures are associated with a range of motor performance measures in older persons with and without dementia.
Our work extends the growing body of research using objective measures of activity to study the relationship of physical activity, health, and function in older adults.10, 21, 22 The use of objective measures of activity such as actigraphy may help to elucidate this relationship in several important ways. Recent work suggests that the total daily energy expenditure from both structured and non-structured physical activity may be more relevant to the health and functioning of older persons.8 Actigraphy can be used to capture total energy expenditure over the course of the day since it does not differentiate between structured and non-structured activities, which individuals at the lower ends of motor functioning may not engage in. In the current study, both total daily activity as measured by actigraphy and self-reported exercise activities were independently associated with motor function, supporting the notion that non-structured physical activity may be associated with health benefits above and beyond what is captured in self-reports of structured activity. Further work is needed to clarify the independent contributions of structured and non-structured activity for the maintenance of motor function in older persons
Actigraphy measures of physical activity do not rely on participant self-report. Thus we were able to examine the relationship of physical activity and motor function in persons with dementia, a group in which loss of motor function is particularly salient and for whom it has been difficult to obtain objective measures of physical activity in the community setting. Impaired motor function is commonly observed in persons with AD 23–26, associated with worse outcomes for AD patients,27, 28 and is hypothesized to be an early sign of AD in that it predicts the clinical onset of dementia.29–32 Our findings show that while demented persons on average are less physically active and have lower motor function, the relationship between physical activity as measured by actigraphy and motor function in demented persons was essentially the same as it was for persons without dementia. Further work using actigraphy in the community setting is vital to determine whether physical activity even after the diagnosis of dementia might serve as an intervention to modify the trajectory of further declines.
This work may help to elucidate the mechanisms linking activity to motor function. In the current study objective measures of total daily activity were associated with a broad range of motor measures. Physical activity can affect muscle strength,8 but there is also evidence that physical activity may have direct effects on the spinal motor neurons,33, 34 and it may also influence neuronal plasticity and neurogenesis throughout the entire central nervous system.35 In the current study, total daily activity was associated with both muscle strength and motor performance, indicating that it may have effects on both central brain pathways which regulate performance as well as peripheral musculoskeletal components which are integral to effective movements. In addition to providing objective measures of total daily activity, actigraphy provides data about the patterns of physical activity over the course of single and multiple days which may also underlie the relationship between physical activity and health. Both the intensity of activity (the amount of activity during only active hours) as well as the percent of the day spent in inactivity were related to motor function. This supports a body of literature indicating that sleep disturbances and prolonged periods of inactivity as measured by actigraphy are related to physical and functional limitations in older persons.36–38 More research is needed to characterize whether the overall patterns of activity and non-activity over the course of a single day or several days might serve an important indicators of health, in addition to total daily activity.39
There are limitations to this study including the use of cross-sectional observational data so that causal inferences cannot be established between physical activity and motor function. Impaired motor function may of course limit physical activity. Also, MAP participants are a volunteer cohort, and thus their level of activity may not be representative of the general population of older adults. There are limitations to the use of actigraphy to measure physical activity, such as certain types of activity may not be captured depending on where the device is located on the body, and removal of the device cannot always be distinguished from periods of no activity. Furthermore, it can be expensive to implement sensor-based measurement of activity in community-based studies, though it is cheaper than the gold standard measure of total daily energy expenditure using doubly labeled water.40
The main strength of this study is the use of actigraphy as an objective measure of total daily activity. Further, this study had the ability to compare objective to self-report physical activity in a relatively large number of older persons who may be more representative of the cognitive spectrum than previous studies of the health benefits of physical activity relying on self-report only. An additional strength is robust measurement of motor function, evaluated as part of a uniform clinical evaluation that incorporated a number of accepted and reliable strength and motor performance measures. The aggregation of these measures into composite scores yielded a more stable measure of motor function as well as motor performance and muscle strength. The modes of assessment for both activity and motor function in this study can be used in people with dementia, and motor impairments are common in dementia and may be an early sign of AD. Establishing this connection would provide the means to fill the gaps in our knowledge, particularly with respect to individuals with dementia, about the relationship between physical activity and the development motor impairment and to what degree physical activity might modify its trajectory. In addition, especially given the recent re-conceptualization of AD and the increased recognition of the motor impairment as an early sign of AD, the use of objective quantitative measures of motor function may be important for identifying subtle motor findings that might be used to detect individuals at risk for AD.
Acknowledgments
This work received funding from the National Institutes of Health (See Acknowledgements).
We are indebted to the participants and the staff of the Rush Memory and Aging Project and the Rush Alzheimer’s Disease Center for this work, and to Sue Leurgans, PhD for biostatistical consultation and Woojeong Bang, MS for help with statistical programming. This work was supported by the Illinois Department of Public Health (James), the National Institute on Aging grants R01AG17917 (Bennett), R01AG24480 (Buchman), R01AG34374 (Boyle), R01AG33678 (Boyle), and the Robert C. Borwell Endowment Fund (Bennett).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to report.
References
- 1.Vandervoort AA. Aging of the human neuromuscular system. Muscle & nerve. 2002 Jan;25(1):17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- 2.Ostir GV, Kuo YF, Berges IM, Markides KS, Ottenbacher KJ. Measures of lower body function and risk of mortality over 7 years of follow-up. Am J Epidemiol. 2007 Sep 1;166(5):599–605. doi: 10.1093/aje/kwm121. [DOI] [PubMed] [Google Scholar]
- 3.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995 Mar 2;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994 Mar;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 5.Hebert LE, Scherr PA, McCann JJ, Bienias JL, Evans DA. Change in direct measures of physical performance among persons with Alzheimer’s disease. Aging & mental health. 2008 Nov;12(6):729–734. doi: 10.1080/13607860802154390. [DOI] [PubMed] [Google Scholar]
- 6.Hebert LE, Bienias JL, McCann JJ, Scherr PA, Wilson RS, Evans DA. Upper and lower extremity motor performance and functional impairment in Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2010 Aug;25(5):425–431. doi: 10.1177/1533317510367636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keysor JJ. Does late-life physical activity or exercise prevent or minimize disablement? A critical review of the scientific evidence. Am J Prev Med. 2003 Oct;25(3 Suppl 2):129–136. doi: 10.1016/s0749-3797(03)00176-4. [DOI] [PubMed] [Google Scholar]
- 8.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006 Mar 14;174(6):801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis MG, Fox KR. Physical activity patterns assessed by accelerometry in older people. Eur J Appl Physiol. 2007 Jul;100(5):581–589. doi: 10.1007/s00421-006-0320-8. [DOI] [PubMed] [Google Scholar]
- 10.Pruitt LA, Glynn NW, King AC, et al. Use of accelerometry to measure physical activity in older adults at risk for mobility disability. J Aging Phys Act. 2008 Oct;16(4):416–434. doi: 10.1123/japa.16.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchman AS, Boyle PA, Wilson RS, Bienias JL, Bennett DA. Physical activity and motor decline in older persons. Muscle & nerve. 2007 Mar;35(3):354–362. doi: 10.1002/mus.20702. [DOI] [PubMed] [Google Scholar]
- 12.Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25(4):163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 13.Buchman AS, Wilson RS, Bennett DA. Total daily activity is associated with cognition in older persons. Am J Geriatr Psychiatry. 2008 Aug;16(8):697–701. doi: 10.1097/JGP.0b013e31817945f6. [DOI] [PubMed] [Google Scholar]
- 14.Heil DP, Bennett GG, Bond KS, Webster MD, Wolin KY. Influence of activity monitor location and bout duration on free-living physical activity. Res Q Exerc Sport. 2009 Sep;80(3):424–433. doi: 10.1080/02701367.2009.10599580. [DOI] [PubMed] [Google Scholar]
- 15.Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc. 2005 Jul;11(4):400–407. [PubMed] [Google Scholar]
- 16.McPhillips JB, Pellettera KM, Barrett-Connor E, Wingard DL, Criqui MH. Exercise patterns in a population of older adults. Am J Prev Med. 1989 Mar-Apr;5(2):65–72. [PubMed] [Google Scholar]
- 17.Wilson RS, Mendes De Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002 Feb 13;287(6):742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 18.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002 May;50(5):889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 19.Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv. 1976;6(3):493–508. doi: 10.2190/UURL-2RYU-WRYD-EY3K. [DOI] [PubMed] [Google Scholar]
- 20.Boyle PA, Wilson RS, Aggarwal NT, et al. Parkinsonian signs in subjects with mild cognitive impairment. Neurology. 2005 Dec 27;65(12):1901–1906. doi: 10.1212/01.wnl.0000188878.81385.73. [DOI] [PubMed] [Google Scholar]
- 21.Brach JS, FitzGerald S, Newman AB, et al. Physical activity and functional status in community-dwelling older women: a 14-year prospective study. Arch Intern Med. 2003 Nov 24;163(21):2565–2571. doi: 10.1001/archinte.163.21.2565. [DOI] [PubMed] [Google Scholar]
- 22.Barnes DE, Blackwell T, Stone KL, Goldman SE, Hillier T, Yaffe K. Cognition in older women: the importance of daytime movement. J Am Geriatr Soc. 2008 Sep;56(9):1658–1664. doi: 10.1111/j.1532-5415.2008.01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson RS, Bennett DA, Gilley DW, Beckett LA, Schneider JA, Evans DA. Progression of parkinsonian signs in Alzheimer’s disease. Neurology. 2000 Mar 28;54(6):1284–1289. doi: 10.1212/wnl.54.6.1284. [DOI] [PubMed] [Google Scholar]
- 24.Goldman WP, Baty JD, Buckles VD, Sahrmann S, Morris JC. Motor dysfunction in mildly demented AD individuals without extrapyramidal signs. Neurology. 1999 Sep 22;53(5):956–962. doi: 10.1212/wnl.53.5.956. [DOI] [PubMed] [Google Scholar]
- 25.Pettersson AF, Olsson E, Wahlund LO. Motor function in subjects with mild cognitive impairment and early Alzheimer’s disease. Dement Geriatr Cogn Disord. 2005;19(5–6):299–304. doi: 10.1159/000084555. [DOI] [PubMed] [Google Scholar]
- 26.Scarmeas N, Hadjigeorgiou GM, Papadimitriou A, et al. Motor signs during the course of Alzheimer disease. Neurology. 2004 Sep 28;63(6):975–982. doi: 10.1212/01.wnl.0000138440.39918.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scarmeas N, Albert M, Brandt J, et al. Motor signs predict poor outcomes in Alzheimer disease. Neurology. 2005 May 24;64(10):1696–1703. doi: 10.1212/01.WNL.0000162054.15428.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson RS, Bennett DA, Gilley DW, Beckett LA, Schneider JA, Evans DA. Progression of parkinsonism and loss of cognitive function in Alzheimer disease. Arch Neurol. 2000 Jun;57(6):855–860. doi: 10.1001/archneur.57.6.855. [DOI] [PubMed] [Google Scholar]
- 29.Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch Neurol. 2009 Nov;66(11):1339–1344. doi: 10.1001/archneurol.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buchman AS, Wilson RS, Boyle PA, Bienias JL, Bennett DA. Grip strength and the risk of incident Alzheimer’s disease. Neuroepidemiology. 2007;29(1–2):66–73. doi: 10.1159/000109498. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Larson EB, Bowen JD, van Belle G. Performance-based physical function and future dementia in older people. Arch Intern Med. 2006 May 22;166(10):1115–1120. doi: 10.1001/archinte.166.10.1115. [DOI] [PubMed] [Google Scholar]
- 32.Wilson RS, Schneider JA, Bienias JL, Evans DA, Bennett DA. Parkinsonianlike signs and risk of incident Alzheimer disease in older persons. Arch Neurol. 2003 Apr;60(4):539–544. doi: 10.1001/archneur.60.4.539. [DOI] [PubMed] [Google Scholar]
- 33.Kanda K, Hashizume K. Effects of long-term physical exercise on age-related changes of spinal motoneurons and peripheral nerves in rats. Neurosci Res. 1998 May;31(1):69–75. doi: 10.1016/s0168-0102(98)00026-1. [DOI] [PubMed] [Google Scholar]
- 34.Adkins DL, Boychuk J, Remple MS, Kleim JA. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J Appl Physiol. 2006 Dec;101(6):1776–1782. doi: 10.1152/japplphysiol.00515.2006. [DOI] [PubMed] [Google Scholar]
- 35.Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. J Appl Physiol. 2006 Oct;101(4):1237–1242. doi: 10.1152/japplphysiol.00500.2006. [DOI] [PubMed] [Google Scholar]
- 36.Ensrud KE, Blackwell TL, Redline S, et al. Sleep disturbances and frailty status in older community-dwelling men. J Am Geriatr Soc. 2009 Nov;57(11):2085–2093. doi: 10.1111/j.1532-5415.2009.02490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldman SE, Stone KL, Ancoli-Israel S, et al. Poor sleep is associated with poorer physical performance and greater functional limitations in older women. Sleep. 2007 Oct 1;30(10):1317–1324. doi: 10.1093/sleep/30.10.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Healy GN, Dunstan DW, Salmon J, et al. Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes care. 2008 Apr;31(4):661–666. doi: 10.2337/dc07-2046. [DOI] [PubMed] [Google Scholar]
- 39.Ueda T, Mukai T, Higashi M, Kirime E, Hitomi K. Evaluation of depression with actigraphy. Sleep and Biological Rhythms. 2005;3(1):22–26. [Google Scholar]
- 40.Manini TM, Everhart JE, Patel KV, et al. Daily activity energy expenditure and mortality among older adults. JAMA. 2006 Jul 12;296(2):171–179. doi: 10.1001/jama.296.2.171. [DOI] [PubMed] [Google Scholar]