Abstract
In recent years, we have witnessed a considerable advance in the understanding of the processes involved in pubertal development. This is partially due to the discovery of the kisspeptin system and its fundamental role in the control of reproductive physiology. In addition, the suspected relationship between increasing rates of childhood obesity and the apparent advance in the age of puberty onset in girls have generated a crescent interest in identifying the mechanisms by which nutrition may influence reproductive maturation. This review will focus on recent data unveiling the sites of leptin’s action in pubertal development that was generated using novel molecular techniques and genetically engineered mouse models. It will also emphasize areas of contention and the many relevant questions that remain unanswered.
Introduction
The role of leptin in pubertal development is a recurrent theme in the specialized literature and several excellent reviews on this issue have been published in the last decade (1–3). Our understanding of the processes and physiological contribution of metabolic cues involved in pubertal development has grown significantly in recent years. This is partially due to the discovery of the kisspeptin system and its fundamental role in the control of the reproductive neuroendocrine axis, and also to the availability of innovative molecular tools and animal models in which genes can be manipulated. In addition, increased interest in identifying the mechanisms linking metabolism and reproduction have been brought about by several recent publications suggesting the existence of a relative correspondence between the increasing rate of childhood obesity and the apparent advance in the age of puberty onset in girls. Puberty is a complex physiological process, and many factors (e.g. circulating signals and neuroendocrine pathways) have been found to influence the normal development of pubertal maturation. This review will highlight recent advances in our understanding of the role of the adipocyte-derived hormone leptin as a permissive factor for the onset of puberty. Different interpretations of the effects of leptin have been suggested, and the data collected in a variety of species and animal models have generated some debate in the field. Controversial findings, alternative interpretations and discussions on leptin’s actions can be accessed by examining a series of important articles (3–6). Recent data generated using novel molecular techniques and genetically engineered mouse models support a permissive role for leptin in the normal progression of pubertal development, but areas of contention still exist and many relevant questions remain unanswered.
Puberty
Puberty is a complex physiological process that can be described as the continuous transition to adulthood that occurs within a defined period of time. It is discernible by the development of genital organs, of secondary sex characteristics and the resultant ability to reproduce. In humans and higher primates, puberty culminates with the first menstrual cycle (menarche) in females (5, 7). Importantly, the pubertal maturation is unique from species to species and distinct for each sex. Within a species, some aspects of pubertal development may vary as a function of modifier genes (i.e. genetic background) and are highly dependent on the physiological condition of the individual. In particular, nutritional state, stage of development and growth are the key factors.
In many species, puberty is initiated with increase in the activity of the hypothalamic-pituitary-gonadal (HPG) axis, primarily induced by the re-awakening of the gonadotropin releasing hormone (GnRH) neurons (5, 7). At the onset of puberty, GnRH release becomes amplified consisting of secretory pulses at higher frequencies. The sustained high pulsatile levels of GnRH induce gonadotropin synthesis and secretion from the pituitary gland which in turn drives the full development of the gonads, the synthesis and secretion of sex steroids and the maturation of the gametes. Thus, the tight control of GnRH neuronal activity determines the timely onset of puberty.
During pubertal maturation, the increase in circulating levels of sex steroids triggers the development of secondary sex characteristic (5, 7). Higher estrogen production in girls promotes breast development and contributes to body fat redistribution, and androgen production in boys induces changes in the musculoskeletal system, spermatogenesis and testicular growth. As a result, the process of pubertal development is readily detectable through the identification of these physical cues, which are the clinical signs of pubertal maturation. However, the factors or physiological changes that induce the increased frequency of GnRH pulses and resultant pubertal maturation remain uncertain. For instance, it is unknown whether prepubertal GnRH neurons are under high inhibitory restraint, lack excitatory influence or both (8, 9). Several studies have indicated that an increase in excitatory inputs and/or in the sensitivity of glutamate or kisspeptin receptors on GnRH neurons is a key event at the onset of puberty (8, 10–12). On the other hand, removal of inhibitory restraint or decreased sensitivity to the negative feedback actions, also seems to play a role (8, 9, 13–15). But yet, what drives these physiological changes – neuronal inputs, sensitivity and remodeling - remains to be determined. Thus far, a series of signals have been identified as permissive for the onset of puberty (8, 16, 17). Nutrition and sufficient amount of energy storage are critical factors in this process.
Metabolism and puberty
Individual nutritional status has long been recognized as a determining factor for the onset of puberty (18, 19). It has been documented that the age of puberty onset in girls – defined by first clinical signs of breast development, Tanner stage 2 – has declined since the 19th and early 20th centuries (20–24). This is thought to be due to improved overall public health and nutrition. Variations in nutritional conditions, diet or energy expenditure may all influence the onset and progression of puberty in many species, including rodents and primates. This is in fact very intuitive as many aspects of reproductive physiology are energetically demanding for both sexes. For example, territoriality and mate acquisition/courtship for males or pregnancy and lactation for females represent significant energetic costs.
Experimental studies by Kennedy and Mitra (18) were seminal in describing the effects of nutrition and body size in the onset of puberty. They observed that the time of puberty onset in rats is correlated with body size, not chronological age. Soon after, epidemiological studies in humans reported a similar finding (19); and subsequently, a series of observations gave rise to the hypothesis that a critical amount of body fat is required for proper pubertal development (25, 26). These studies emphasized that conditions of extreme leanness may delay the initiation and progression of pubertal maturation.
In the past two decades, however, we have been facing a different situation. Studies have consistently reported a crescent incidence of earlier pubertal onset in girls (27–29). In these reports, Tanner stage 2 of breast development was considered the clinical sign of puberty onset. A cross-sectional study in girls published in 1997 reported what was at the time the youngest age of pubertal onset ever recorded in a population (9.96 ± 1.82 years) (28). Recent evidence, however, suggests that this phenomenon has been aggravated in the last decade. Compared with the previous study (28), a 5–8% increase in the number of girls at 7 to 8 years of ages showing the initial stages of breast development, was reported (27). Of note, earlier appearance of clinical signs of puberty onset was more prevalent in obese than in non-obese girls. While in the general population the onset of puberty appears to be occurring at normal ages, it is apparently advancing in obese girls (20, 30, 31). These observations have generated a passionate debate and many different interpretations have been offered. Is the timing of puberty onset really changing or we are just measuring it differently? Some methodological inconsistencies are evident. For instance, Tanner staging is determined by either visual assessment or palpation. In case of visual assessment, could the early stages of breast development be mistaken as fat depots (adipomastia)? Environmental factors including stress and exposure to endocrine disruptors have not been included in the analysis and most of these studies have not assessed gonadotropin and sex steroids levels. This is particularly relevant as the initial stages of breast development (thelarche) normally indicate gonadotropin-induced estrogen production from the ovaries. However, estrogen may also be generated from aromatization of adrenal androgens, a process facilitated by increased aromatase production in the adipocytes (32). Thus, it is theoretically possible that earlier breast development in obese girls is the consequence of an increase in estrogen production from expanded adipocytes not from the activation of the HPG axis, and therefore not representing a genuine puberty onset. The lack of conclusive data showing parallel advances of the age at menarche in obese and non-obese girls favors this argument, suggesting a temporal dissociation between the initial signs of thelarche and the completion of pubertal maturation with the first menstrual cycle (menarche) (29, 33, 34). In agreement, recent studies have shown that overweight girls with slowly progressive precocious breast development and premature adrenarche exhibit decreased LH pulsatiliy suggesting that, in this condition, adiposity may promote an apparent early onset but slow progression of puberty (35). Notably, although available data in boys show several inconsistencies, pubertal maturation in obese boys appears to be delayed rather than advanced (36–39). Additional and more conclusive data on this issue is yet to be offered and its cause, if confirmed, remains to be determined. The increased aromatization of androgens to estrogens driven by high adiposity might also partially explain this phenomenon (29).
Although the effects of childhood obesity upon the apparent increasing rates of advanced pubertal onset is debatable, it is now well-defined that the balance and interplay between metabolic cues and developmental stage are instrumental for the coordinated control of the onset of puberty and proper sexual maturation (6, 17, 40). Therefore, the existence of highly redundant neural pathways and metabolic cues is predictable. The adipocyte derived hormone leptin is one of the cues that have been studied extensively as a key permissive factor for pubertal development (1–3).
Reproductive deficits in leptin-deficient mice and humans
Leptin is primarily synthesized and secreted by adipocytes, and in normal conditions circulating levels are correlated with the amount of body fat an individual has. Mice deficient in leptin (ob/ob, in a C57BL/6 genetic background) are morbidly obese mainly due to hyperphagia and decreased energy expenditure, and display a series of neuroendocrine abnormalities (41, 42). The increased adiposity in these mice is observed early during postnatal development, and is readily detectable at weaning. The embryonic and postnatal development of the reproductive organs is apparently normal, but male and female remain in a prepubertal stage. Food restriction decreases their body weights, which can be maintained at levels similar to age-matched wild-type control mice, but does not restore fertility. Pubertal development and fertility is only attained if leptin is provided (1–3).
Gonadotropin levels are decreased in both male and female ob/ob mice but gonadotrops respond adequately to GnRH challenges (43). Despite their massive amount of body fat, which supports steroidogenesis, the circulating levels of sex steroids are reduced. Testes and ovaries of leptin-deficient mice are small with several morphological and biochemical abnormalities, compared to age-matched wild-type control mice. The lumen of the seminiferous tubules is hollow and contains fewer sperm than wild-type littermates, and the steroidogenic Leydig cells are reduced in size due to decreased cytoplasmic content (44). The ovaries of ob/ob mice have similar numbers of primordial, primary and secondary follicles compared to wild type mice, but virtually no Graafian follicles or corpora lutea (45). When transplanted to wild-type females, ovaries of ob/ob mice produce sex steroids and viable eggs. The distribution pattern of GnRH neurons and fibers as well as GnRH gene expression in ob/ob mice is comparable to wild-type littermates, suggesting normal GnRH neuronal migration during development (43, 46, 47). Thus, a close evaluation of the HPG axis of leptin-deficient ob/ob mice suggests normal postnatal development and potentially adequate functioning of gonadotropes and gonads but lack of increased GnRH pulsatility at the expected time of puberty onset.
Humans with monogenic forms of loss-of-function mutation in the leptin gene (LEP) exhibit a reproductive phenotype strikingly similar to the mutant ob/ob mice (2, 48). These individuals display severe early onset obesity and fail to undergo puberty. Leptin treatment corrects their metabolic phenotype and increases the frequency of gonadotropin secretion suggestive of pubertal onset (2, 49). Importantly, leptin replacement therapy in leptin-deficient children conducted in an early developmental stage did not induce precocious puberty. This indicates that the role of leptin in the onset of puberty depends on the individual development, strengthening the role of leptin as a permissive rather than definitive trigger of puberty. In a recent report, a novel missense variant in the leptin gene was identified in one individual from a cohort of children (78 subjects) with constitutional delay in growth and puberty (CDGP) (50). The sequence variant was not detected in control subjects (112 individuals), but was identified in his mother that also exhibited a lean constitution and delayed pubertal maturation. It is intriguing however that this sequence variant has been associated with delayed puberty in the context of decreased body mass index rather than obesity. Thus, whether pubertal delay is caused by deficient leptin signaling needs further investigation.
Sites of leptin action
The biological effects of leptin are achieved by its action in cognate receptors located in many organs and tissue. Most of leptin’s effects, including those in reproductive physiology, are mediated by the long- (signaling) form of the leptin receptor, expressed in the brain (51, 52). Of note, loss-of-function mutations of the long-form of leptin receptor, in humans and mice, produced a metabolic and reproductive phenotype similar to leptin-deficient subjects (42, 53). It is well established that in different species and in both sexes, leptin acts in the brain by increasing the pulsatile rate of GnRH secretion (54–57). But whether leptin receptor is expressed in GnRH neurons has been a matter of intense debate (58). More recently, with advances in techniques of conditional knockout, it was demonstrated that leptin action in GnRH neurons is not required for pubertal development. Mice engineered to lack leptin receptor selectively in GnRH neurons show a normal progression through puberty and exhibit normal sexual maturation and fertility (59). In fact, with the development of mouse models expressing leptin receptor reporter genes it became clear that the mouse GnRH neurons express virtually no leptin receptor (58, 60). Therefore, leptin action to stimulate GnRH secretion is attained via interneurons that converge on GnRH neurons. These neuronal populations were identified only recently through the use of an innovative molecular tracing technique (60). It was reported that, although neurons expressing leptin receptor are widespread throughout the hypothalamus, only two populations of leptin receptor-expressing neurons directly project to GnRH neurons: those in the ventral premammillary nucleus and those in the striohypothalamic nucleus, the latter of which is a newly identified neuronal population responsive to leptin. Alternatively, leptin’s effect on the reproductive neuroendocrine axis may be attained via the kisspeptin system which in turn controls GnRH neuronal activity (47, 60, 61).
Kisspeptins (the products of Kiss1/KISS1 gene) and their receptor (Gpr54/GPR54) are fundamental players in the control of the neuroendocrine reproductive axis (62–64). Loss-of-function mutations in Kiss1 or GPR54/Gpr54 cause a lack of pubertal development, resulting in hypogonadotropic hypogonadism, in humans and mice (65, 66). Because of the similarities in the reproductive phenotypes of individuals with loss-of-function mutations in leptin and kisspeptin genes and their cognate receptors, the Kiss1-GPR54 system has become the main candidate for mediating the effects of leptin on puberty. In fact, several research groups have now shown that kisspeptin neurons in the arcuate nucleus co-express leptin receptors. However, some inconsistencies are yet to be resolved. While some laboratories have reported a moderate to high degree of colocalization (67, 68), others have found very small colocalization rate (60, 61). Despite these apparent inconsistencies, a series of studies have indicated that conditions of low leptin levels – as in mutant mice or in states of negative energy balance – result in decreased Kiss1 mRNA expression or blunted kisspeptin production (69–71). Interestingly, mice with selective deletion of leptin receptors from Kiss1 neurons show normal pubertal development, sexual maturation and fertility (47). This indicates that direct leptin action in Kiss1 neurons is not required for normal puberty onset in mice. Following the same line, a recent report has demonstrated that female mice with ablation of kisspeptin neurons exhibited no deficits in pubertal development (72). Whether these findings reflect systems redundancy and/or developmental adaptations still need to be determined. Collectively, these studies have clarified the following relevant issues: leptin receptor is expressed in Kiss1 neurons of the arcuate nucleus but not in Kiss1 neurons of the preoptic area (60, 61, 71); Kiss1 neurons which express leptin receptors in the arcuate nucleus do not directly innervate GnRH neurons (60); and direct action of leptin on Kiss1 neurons is not required for pubertal maturation and fertility (47). These studies also indicate that the changes in Kiss1 mRNA expression/kisspeptin production in states of low leptin levels are mediated by indirect neuronal pathways or by the direct action of alternative (still unidentified) metabolic signals.
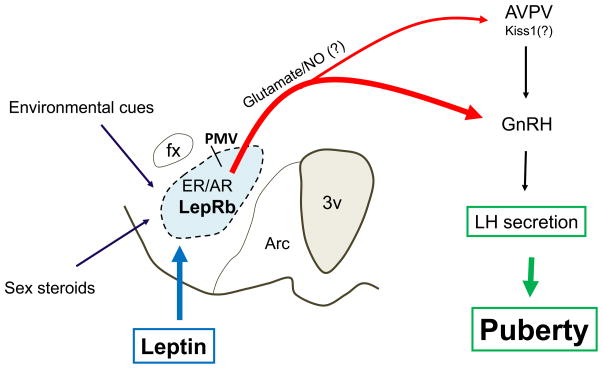
A series of studies published in recent years have demonstrated that the ventral premammillary nucleus (PMV) plays a key role in leptin’s effects on puberty initiation. The PMV is well positioned to integrate signs regarding nutritional condition (metabolic signals), reproductive status (sex steroids) and environmental cues (odor and probably daylight in seasonal breeders) (58, 73–76). These are all regulatory components of the reproductive physiology, in a variety of species. The projection pattern of PMV neurons target brain sites related to reproductive control, including those directly implicated in gonadotropin secretion (e.g. the anteroventral periventricular nucleus/AVPV and GnRH neurons), sexually dimorphic sites (e.g., the medial preoptic nucleus and the bed nucleus of stria terminalis) and the vomeronasal circuitry (e.g., medial nucleus of the amygdala) (77, 78), which integrates sensory (odorant) pathways relevant to social communication/responses and sexual behavior. The PMV houses a high density of neurons that express leptin receptors and directly projects to GnRH neurons (60, 75). High proportions of these neurons coexpress glutamate and nitric oxide, and are depolarized by leptin (47, 75, 79, 80). Collectively, these data indicated that the PMV neurons are potentially stimulated by changing levels of circulating leptin, which in turn may activate their terminal targets (e.g. AVPV and GnRH neurons) via release of excitatory neurotransmitters (glutamate/nitric oxide). In order to test this model, a series of genetic manipulation and excitotoxic lesions were performed. Endogenous re-expression of leptin receptor selectively in PMV neurons of leptin receptor null mice induced pubertal development and improved fertility in females. Male mice, however, did not exhibit any improvement of their reproductive physiology (47). In addition, when PMV neurons of ob/ob female mice were lesioned, a significant delay in leptin-induced pubertal development was observed (47). These findings suggest that leptin action in PMV neurons is sufficient and required for normal pubertal development of the female mouse (Figure 1). But they also generated a series of new questions that will be discussed in following sections (Box 1).
Figure 1.
Proposed neural pathway mediating leptin’s permissive effect on pubertal development. The ventral premammillary nucleus (PMV) expresses a dense collection of leptin receptor long-form (LepRb), sex steroids receptors (ER/estrogen receptor and AR/androgen receptor) and is responsive to environmental cues (odor and probably daylight, in seasonal breeders). Leptin stimulates PMV neurons which express glutamate and nitric oxide (NO). These PMV neurons innervate gonadotropin releasing hormone (GnRH) neurons and neurons in the anteroventral perivetricular nucleus (AVPV, supposedly those expressing Kiss1). This projection facilitates the increase in frequency of GnRH pulses and LH secretion at the onset of puberty.
Box 1. Unresolved questions.
The effect of leptin on pubertal development is believed to be mediated via leptin receptors expressed in the brain. However, both the short and long forms of leptin receptors are found in several peripheral organs, including the gonads and the pituitary gland. What is the physiological relevance of leptin receptors outside of the brain? Most of the data on leptin’s permissive effects on the onset of puberty were generated using models of leptin- or leptin receptor-deficiency, but the role of leptin at normal physiological levels is still unclear. Nocturnal peak of leptin secretion has been described in pubertal rats (87). Is there a mechanism controlling the expression and/or availability of circulating leptin during pubertal development? Is there a role for the short (circulating) form of leptin receptor in this process?
While leptin exerts an inhibitory effect in the limiting step of steroidogenesis (88–90), it increases aromatization and may favor estrogen production in the presence of higher androgen availability (91, 92). Is this mechanism related to the increase rate of the apparent earlier puberty onset in obese girls? Is the earlier puberty onset a genuine activation of the HPG axis? Could these peripheral effects also explain delayed sexual maturation in obese boys?
Studies in mice showed that leptin’s action in Kiss1 neurons in not required for normal pubertal development and fertility. However, the reproductive system is highly redundant, and therefore parallel compensatory mechanisms in the absence of kisspeptin neurons may exist. Is leptin’s action in kisspeptin neurons sufficient to induce puberty in the absence of parallel/redundant pathways?
Selective leptin action on PMV neurons is sufficient to induce puberty in leptin receptor null female, but not male mice. Is there a sexually dimorphic circuitry on which leptin acts in pubertal onset? Is the lack of improvement in the infertility phenotype of leptin receptor null male mice after reactivation of leptin receptors in the PMV a consequence of (or secondary to) the obese or diabetic phenotype? Does the PMV act directly on GnRH neurons, or does it activate kisspeptin neurons and/or any other indirect pathway? Is this effect attained by glutamatergic/nitrergic neurotransmission? Is it caused by changes in GnRH neuronal terminals (neuronal plasticity) at the median eminence? What is the role of striohypothalamic neurons?
What are the mechanisms (signaling pathways, changes in gene expression and/or cell activity) by which leptin exerts its permissive effect on pubertal development?
Mechanisms of leptin action
The leptin receptor protein is a member of the class I cytokine receptor family and activates the Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway (81). Multiple splice variants of leptin receptors have been identified, but only the leptin receptor long form contains a Box 3 motif for STAT3 activation in its cytosolic domain, and therefore it is recognized as the physiologically relevant signaling isoform (81). Consistent with this, the db/db mice - which lack only the leptin receptor long form - develop obesity, diabetes and infertility, which is virtually identical to obese leptin-deficient ob/ob mice. Neural specific deletion of STAT3 (STAT3N−/−) or deletion of leptin receptor-mediated STAT3 signaling (LRbS1138 s/s) recapitulate the db/db metabolic phenotype, producing hyperphagic obesity and diabetes (82, 83). However, notably, whereas STAT3N−/− mice are infertile similar, high proportion of LRbS1138 s/s mice are fertile. These apparent conflicting results may indicate that STAT3 expression in the brain outside of the neuronal populations expressing leptin receptor is required for normal fertility. These findings also suggest that leptin effects on reproduction may be attained by a STAT3-independent alternative signaling pathway.
In recent years, a series of important findings have suggested a role for cAMP responsive element-binding protein regulated transcription coactivator-1 (Crtc1) and mTOR as potential signaling pathways linking energy balance and reproduction (17, 84, 85). Leptin induces an increase in the dephosphorylated form of nuclear Crtc1 which stimulates Kiss1 expression by acting on the Kiss1 promoter (85). This data obtained in vitro suggests that leptin induces Kiss1 expression via a Crtc1-dependent mechanism. Of note, mice with deletion of Crtc1 gene are hyperphagic, obese and infertile (85). Subsequently, it was demonstrated that blockade of mTOR signaling in the brain, disrupts the HPG axis at puberty and blunts the stimulatory effects of leptin on pubertal development, in food-restricted female rats (84). However, the reproductive deficits reported in both studies may be secondary to a central metabolic imbalance or to the activation/inhibition of redundant signaling pathways not necessarily related to leptin signaling. Excellent reviews that focus specifically on the advances is this area, may be consulted (17). In addition, it was recently reported that the acute effects of leptin on PMV neurons require the phosphatidylinositol 3-kinase (PI3K) signaling pathway (80, 86). Whether PI3K is required for leptin’s effect on pubertal development has yet to be determined.
Concluding remarks
In the last 5 years or so we have witnessed a significant advance in the understanding of the role of leptin in pubertal development. Many of the new data were generated with the use of innovative technological tools and genetically engineered mouse models. These technologies allowed the identification of key players in the intricate circuitry involved in the onset of pubertal maturation. But, the physiological process of pubertal development is rather complex and no single player or simple explanation is expected to emerge. While some important aspects have been unveiled, several questions remain unanswered and many others have become apparent. It is hoped that an integrative perspective and the use of these novel tools will impel the field opening new fronts, answering some of the unsolved questions and advancing our understanding of the interplay between nutrition and the establishment of the reproductive competence.
Acknowledgments
I would like to thank members of my laboratory (Jose Donato Jr, Roberta Cravo and Renata Frazao) at the Department of Internal Medicine, Division of Hypothalamic Research, University of Texas Southwestern Medical Center, Dallas, TX for the active participation in the data discussed in this review. I’m also indebted with Dr. Joel Elmquist and Dr. Jeffrey Zigman for the mouse models used in our studies. The research in my laboratory has been funded by grants from NIH (R01HD061539), Foundation for Prader-Willi Research, the Regents Scholar Award and Young Investigator Research Award from UTSW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Apter DAN. The Role of Leptin in Female Adolescence. Annals of the New York Academy of Sciences. 2003;997:64–76. doi: 10.1196/annals.1290.008. [DOI] [PubMed] [Google Scholar]
- 2.Farooqi IS. Leptin and the onset of puberty: insights from rodent and human genetics. Semin Reprod Med. 2002;20:139–144. doi: 10.1055/s-2002-32505. [DOI] [PubMed] [Google Scholar]
- 3.Gueorguiev M, Goth ML, Korbonits M. Leptin and puberty: a review. Pituitary. 2001;4:79–86. doi: 10.1023/a:1012943029127. [DOI] [PubMed] [Google Scholar]
- 4.Castracane VD, Henson MC. When did leptin become a reproductive hormone? Semin Reprod Med. 2002;20:89–92. doi: 10.1055/s-2002-32499. [DOI] [PubMed] [Google Scholar]
- 5.Plant TM, Barker G, Mandi L. Neurobiological mechanisms of puberty in higher primates. Human Reproduction Update. 2004;10:67–77. doi: 10.1093/humupd/dmh001. [DOI] [PubMed] [Google Scholar]
- 6.Schneider JE. Energy balance and reproduction. Physiol Behav. 2004;81:289–317. doi: 10.1016/j.physbeh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- 8.Ojeda SR, Lomniczi A, Mastronardi C, Heger S, Roth C, Parent AS, Matagne V, Mungenast AE. Minireview: The Neuroendocrine Regulation of Puberty: Is the Time Ripe for a Systems Biology Approach? Endocrinology. 2006;147:1166–1174. doi: 10.1210/en.2005-1136. [DOI] [PubMed] [Google Scholar]
- 9.Clarkson J, Herbison AE. Development of GABA and glutamate signaling at the GnRH neuron in relation to puberty. Mol Cell Endocrinol. 2006;254–255:32–38. doi: 10.1016/j.mce.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 10.Choi J, Ha CM, Choi EJ, Jeong CS, Park JW, Baik JH, Park JY, Costa ME, Ojeda SR, Lee BJ. Kinesin Superfamily-Associated Protein 3 Is Preferentially Expressed in Glutamatergic Neurons and Contributes to the Excitatory Control of Female Puberty. Endocrinology. 2008;149:6146–6156. doi: 10.1210/en.2008-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brann DW, Mahesh VB. Excitatory amino acids: function and significance in reproduction and neuroendocrine regulation. Front Neuroendocrinol. 1994;15:3–49. doi: 10.1006/frne.1994.1002. [DOI] [PubMed] [Google Scholar]
- 12.Han S-K, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of Gonadotropin-Releasing Hormone Neurons by Kisspeptin as a Neuroendocrine Switch for the Onset of Puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev. 2001;22:111–151. doi: 10.1210/edrv.22.1.0418. [DOI] [PubMed] [Google Scholar]
- 14.Plant TM, Shahab M. Neuroendocrine mechanisms that delay and initiate puberty in higher primates. Physiol Behav. 2002;77:717–722. doi: 10.1016/s0031-9384(02)00924-1. [DOI] [PubMed] [Google Scholar]
- 15.Mayer C, Acosta-Martinez M, Dubois SL, Wolfe A, Radovick S, Boehm U, Levine JE. Timing and completion of puberty in female mice depend on estrogen receptor alpha-signaling in kisspeptin neurons. Proc Natl Acad Sci U S A. 2010;107:22693–22698. doi: 10.1073/pnas.1012406108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prevot V, Hanchate NK, Bellefontaine N, Sharif A, Parkash J, Estrella C, Allet C, de Seranno S, Campagne C, d’Anglemont de Tassigny X, et al. Function-related structural plasticity of the GnRH system: A role for neuronal-glial-endothelial interactions. Frontiers in Neuroendocrinology. 2010;31:241–258. doi: 10.1016/j.yfrne.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Roa J, Tena-Sempere M. Energy balance and puberty onset: emerging role of central mTOR signaling. Trends Endocrinol Metab. 2010;21:519–528. doi: 10.1016/j.tem.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy GC, Mitra J. Body weight and food intake as initiating factors for puberty in the rat. J Physiol. 1963;166:408–418. doi: 10.1113/jphysiol.1963.sp007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frisch RE, Revelle R. Height and weight at menarche and a hypothesis of critical body weights and adolescent events. Science. 1970;169:397–399. doi: 10.1126/science.169.3943.397. [DOI] [PubMed] [Google Scholar]
- 20.Walvoord EC. The Timing of Puberty: Is It Changing? Does It Matter? Journal of Adolescent Health. 2010;47:433–439. doi: 10.1016/j.jadohealth.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 21.Wyshak G, Frisch RE. Evidence for a secular trend in age of menarche. N Engl J Med. 1982;306:1033–1035. doi: 10.1056/NEJM198204293061707. [DOI] [PubMed] [Google Scholar]
- 22.Ong KK, Ahmed ML, Dunger DB. Lessons from large population studies on timing and tempo of puberty (secular trends and relation to body size): the European trend. Mol Cell Endocrinol. 2006;254–255:8–12. doi: 10.1016/j.mce.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 23.Kaplowitz P. Pubertal development in girls: secular trends. Current Opinion in Obstetrics and Gynecology. 2006;18:487–491. doi: 10.1097/01.gco.0000242949.02373.09. [DOI] [PubMed] [Google Scholar]
- 24.Euling SY, Herman-Giddens ME, Lee PA, Selevan SG, Juul A, Sorensen TIA, Dunkel L, Himes JH, Teilmann G, Swan SH. Examination of US Puberty-Timing Data from 1940 to 1994 for Secular Trends: Panel Findings. Pediatrics. 2008;121:S172–191. doi: 10.1542/peds.2007-1813D. [DOI] [PubMed] [Google Scholar]
- 25.Frisch RE. Fatness, menarche, and female fertility. Perspect Biol Med. 1985;28:611–633. doi: 10.1353/pbm.1985.0010. [DOI] [PubMed] [Google Scholar]
- 26.Frisch RE. The right weight: body fat, menarche and fertility. Proc Nutr Soc. 1994;53:113–129. doi: 10.1079/pns19940015. [DOI] [PubMed] [Google Scholar]
- 27.Biro FM, Galvez MP, Greenspan LC, Succop PA, Vangeepuram N, Pinney SM, Teitelbaum S, Windham GC, Kushi LH, Wolff MS. Pubertal Assessment Method and Baseline Characteristics in a Mixed Longitudinal Study of Girls. Pediatrics. 2010;126:e583–e590. doi: 10.1542/peds.2009-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herman-Giddens ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, Koch GG, Hasemeier CM. Secondary Sexual Characteristics and Menses in Young Girls Seen in Office Practice: A Study from the Pediatric Research in Office Settings Network. Pediatrics. 1997;99:505–512. doi: 10.1542/peds.99.4.505. [DOI] [PubMed] [Google Scholar]
- 29.Burt Solorzano CM, McCartney CR. Obesity and the pubertal transition in girls and boys. Reproduction. 2010;140:399–410. doi: 10.1530/REP-10-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ong KK, Emmett P, Northstone K, Golding J, Rogers I, Ness AR, Wells JC, Dunger DB. Infancy weight gain predicts childhood body fat and age at menarche in girls. J Clin Endocrinol Metab. 2009;94:1527–1532. doi: 10.1210/jc.2008-2489. [DOI] [PubMed] [Google Scholar]
- 31.Rosenfield RL, Lipton RB, Drum ML. Thelarche, pubarche, and menarche attainment in children with normal and elevated body mass index. Pediatrics. 2009;123:84–88. doi: 10.1542/peds.2008-0146. [DOI] [PubMed] [Google Scholar]
- 32.Simpson ER. Aromatase: Biologic Relevance of Tissue-Specific Expression. Semin Reprod Med. 2004;22:11, 23. doi: 10.1055/s-2004-823023. [DOI] [PubMed] [Google Scholar]
- 33.Dunger DB, Ahmed ML, Ong KK. Effects of obesity on growth and puberty. Best Pract Res Clin Endocrinol Metab. 2005;19:375–390. doi: 10.1016/j.beem.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Jasik CB, Lustig RH. Adolescent Obesity and Puberty: The “Perfect Storm”. Annals of the New York Academy of Sciences. 2008;1135:265–279. doi: 10.1196/annals.1429.009. [DOI] [PubMed] [Google Scholar]
- 35.Bordini B, Littlejohn E, Rosenfield RL. LH Dynamics in Overweight Girls with Premature Adrenarche and Slowly Progressive Sexual Precocity. Int J Pediatr Endocrinol. 2010:2010. doi: 10.1155/2010/724696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmed ML, Ong KK, Dunger DB. Childhood obesity and the timing of puberty. Trends in Endocrinology & Metabolism. 2009;20:237–242. doi: 10.1016/j.tem.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Hammoud AO, Gibson M, Peterson CM, Hamilton BD, Carrell DT. Obesity and Male Reproductive Potential. J Androl. 2006;27:619–626. doi: 10.2164/jandrol.106.000125. [DOI] [PubMed] [Google Scholar]
- 38.Fu J-f, Dong G-p, Liang L, Jiang YJ, Chen L-q, Dayan C. Early activation of the inhibin B/FSH axis in obese Tanner stage G1PH1 boys. Clinical Endocrinology. 2006;65:327–332. doi: 10.1111/j.1365-2265.2006.02597.x. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y. Is Obesity Associated With Early Sexual Maturation? A Comparison of the Association in American Boys Versus Girls. Pediatrics. 2002;110:903–910. doi: 10.1542/peds.110.5.903. [DOI] [PubMed] [Google Scholar]
- 40.Foster DL, Nagatani S. Physiological perspectives on leptin as a regulator of reproduction: role in timing puberty. Biol Reprod. 1999;60:205–215. doi: 10.1095/biolreprod60.2.205. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue [published erratum appears in Nature 1995 Mar 30;374(6521):479] [see comments] Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 42.Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14:141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- 43.Batt RAL, Everard DM, Gillies G, Wilkinson M, Wilson CA, Yeo TA. Investigation into the hypogonadism of the obese mouse (genotype ob/ob) J Reprod Fertil. 1982;64:363–371. doi: 10.1530/jrf.0.0640363. [DOI] [PubMed] [Google Scholar]
- 44.Mounzih K, Lu R, Chehab FF. Leptin treatment rescues the sterility of genetically obese ob/ob males. Endocrinology. 1997;138:1190–1193. doi: 10.1210/endo.138.3.5024. [DOI] [PubMed] [Google Scholar]
- 45.Barash IA, Cheung CC, Weigle DS, Ren H, Kabigting EB, Kuijper JL, Clifton DK, Steiner RA. Leptin is a metabolic signal to the reproductive system. Endocrinology. 1996;137:3144–3147. doi: 10.1210/endo.137.7.8770941. [DOI] [PubMed] [Google Scholar]
- 46.Johnson LM, Sidman RL. A Reproductive Endocrine Profile in the Diabetes (db) Mutant Mouse. Biol Reprod. 1979;20:552–559. doi: 10.1095/biolreprod20.3.552. [DOI] [PubMed] [Google Scholar]
- 47.Donato J, Jr, Cravo RM, Frazao R, Gautron L, Scott MM, Lachey J, Castro IA, Margatho LO, Lee S, Lee C, et al. Leptin’s effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest. 2011;121:355–368. doi: 10.1172/JCI45106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ozata M, Ozdemir IC, Licinio J. Human Leptin Deficiency Caused by a Missense Mutation: Multiple Endocrine Defects, Decreased Sympathetic Tone, and Immune System Dysfunction Indicate New Targets for Leptin Action, Greater Central than Peripheral Resistance to the Effects of Leptin, and Spontaneous Correction of Leptin-Mediated Defects. J Clin Endocrinol Metab. 1999;84:3686–3695. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 49.Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, Sanna V, Jebb SA, Perna F, Fontana S, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murray PG, Read A, Banerjee I, Whatmore AJ, Pritchard LE, Davies RA, Brennand J, White A, Ross RJ, Clayton PE. Reduced appetite and body mass index with delayed puberty in a mother and son: association with a rare novel sequence variant in the leptin gene. Eur J Endocrinol. 2011;164:521–527. doi: 10.1530/EJE-10-0656. [DOI] [PubMed] [Google Scholar]
- 51.de Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, Ludwig T, Liu SM, Chua SC., Jr Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest. 2005;115:3484–3493. doi: 10.1172/JCI24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108:1113–1121. doi: 10.1172/JCI13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction [see comments] Nature. 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 54.Wójcik-Gladysz A, Wankowska M, Misztal T, Romanowicz K, Polkowska J. Effect of intracerebroventricular infusion of leptin on the secretory activity of the GnRH/LH axis in fasted prepubertal lambs. Animal Reproduction Science. 2009;114:370–383. doi: 10.1016/j.anireprosci.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 55.Yu WH, Walczewska A, Karanth S, McCann SM. Nitric oxide mediates leptin-induced luteinizing hormone-releasing hormone (LHRH) and LHRH and leptin-induced LH release from the pituitary gland. Endocrinology. 1997;138:5055–5058. doi: 10.1210/endo.138.11.5649. [DOI] [PubMed] [Google Scholar]
- 56.Parent AS, Lebrethon MC, Gerard A, Vandersmissen E, Bourguignon JP. Leptin effects on pulsatile gonadotropin releasing hormone secretion from the adult rat hypothalamus and interaction with cocaine and amphetamine regulated transcript peptide and neuropeptide Y. Regul Pept. 2000;92:17–24. doi: 10.1016/s0167-0115(00)00144-0. [DOI] [PubMed] [Google Scholar]
- 57.Lebrethon MC, Vandersmissen E, Gerard A, Parent AS, Junien JL, Bourguignon JP. In vitro stimulation of the prepubertal rat gonadotropin-releasing hormone pulse generator by leptin and neuropeptide Y through distinct mechanisms. Endocrinology. 2000;141:1464–1469. doi: 10.1210/endo.141.4.7432. [DOI] [PubMed] [Google Scholar]
- 58.Donato J, Jr, Cravo RM, Frazao R, Elias CF. Hypothalamic sites of leptin action linking metabolism and reproduction. Neuroendocrinology. 2011;93:9–18. doi: 10.1159/000322472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quennell JH, Mulligan AC, Tups A, Liu X, Phipps SJ, Kemp CJ, Herbison AE, Grattan DR, Anderson GM. Leptin Indirectly Regulates Gonadotropin-Releasing Hormone Neuronal Function. Endocrinology. 2009;150:2805–2812. doi: 10.1210/en.2008-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Louis GW, Greenwald-Yarnell M, Phillips R, Coolen LM, Lehman MN, Myers MG. Molecular Mapping of the Neural Pathways Linking Leptin to the Neuroendocrine Reproductive Axis. Endocrinology. 2011;152:2302–2310. doi: 10.1210/en.2011-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J, Jr, Atkin S, Bookout AL, Rovinsky S, Frazao R, Lee CE, Gautron L, et al. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. 2011;173:37–56. doi: 10.1016/j.neuroscience.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Colledge WH. Kisspeptins and GnRH neuronal signalling. Trends Endocrinol Metab. 2009;20:115–121. doi: 10.1016/j.tem.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 63.Popa SM, Clifton DK, Steiner RA. The Role of Kisspeptins and GPR54 in the Neuroendocrine Regulation of Reproduction. Annual Review of Physiology. 2008;70:213. doi: 10.1146/annurev.physiol.70.113006.100540. [DOI] [PubMed] [Google Scholar]
- 64.Tena-Sempere M. Kisspeptin signaling in the brain: recent developments and future challenges. Mol Cell Endocrinol. 2010;314:164–169. doi: 10.1016/j.mce.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 65.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 67.Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18:298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- 68.Qiu J, Fang Y, Bosch MA, Ronnekleiv OK, Kelly MJ. Guinea pig kisspeptin neurons are depolarized by leptin via activation of TRPC channels. Endocrinology. 2011;152:1503–1514. doi: 10.1210/en.2010-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Castellano JM, Navarro VM, Fernandez-Fernandez R, Nogueiras R, Tovar S, Roa J, Vazquez MJ, Vigo E, Casanueva FF, Aguilar E, et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146:3917–3925. doi: 10.1210/en.2005-0337. [DOI] [PubMed] [Google Scholar]
- 70.Kalamatianos T, Grimshaw SE, Poorun R, Hahn JD, Coen CW. Fasting reduces KiSS-1 expression in the anteroventral periventricular nucleus (AVPV): effects of fasting on the expression of KiSS-1 and neuropeptide Y in the AVPV or arcuate nucleus of female rats. J Neuroendocrinol. 2008;20:1089–1097. doi: 10.1111/j.1365-2826.2008.01757.x. [DOI] [PubMed] [Google Scholar]
- 71.Quennell JH, Howell CS, Roa J, Augustine RA, Grattan DR, Anderson GM. Leptin Deficiency and Diet-Induced Obesity Reduce Hypothalamic Kisspeptin Expression in Mice. Endocrinology. 2011 doi: 10.1210/en.2010-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mayer C, Boehm U. Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nat Neurosci. 2011;14:704–710. doi: 10.1038/nn.2818. [DOI] [PubMed] [Google Scholar]
- 73.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 74.Sliwowska JH, Billings HJ, Goodman RL, Coolen LM, Lehman MN. The Premammillary Hypothalamic Area of the Ewe: Anatomical Characterization of a Melatonin Target Area Mediating Seasonal Reproduction. Biol Reprod. 2004;70:1768–1775. doi: 10.1095/biolreprod.103.024182. [DOI] [PubMed] [Google Scholar]
- 75.Leshan RL, Louis GW, Jo Y-H, Rhodes CJ, Munzberg H, Myers MG., Jr Direct Innervation of GnRH Neurons by Metabolic- and Sexual Odorant-Sensing Leptin Receptor Neurons in the Hypothalamic Ventral Premammillary Nucleus. J Neurosci. 2009;29:3138–3147. doi: 10.1523/JNEUROSCI.0155-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Donato J, Jr, Cavalcante JC, Silva RJ, Teixeira AS, Bittencourt JC, Elias CF. Male and female odors induce Fos expression in chemically defined neuronal population. Physiol Behav. 2010;99:67–77. doi: 10.1016/j.physbeh.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 77.Rondini TA, Baddini SP, Sousa LF, Bittencourt JC, Elias CF. Hypothalamic cocaine- and amphetamine-regulated transcript neurons project to areas expressing gonadotropin releasing hormone immunoreactivity and to the anteroventral periventricular nucleus in male and female rats. Neuroscience. 2004;125:735–748. doi: 10.1016/j.neuroscience.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 78.Canteras NS, Simerly RB, Swanson LW. Projections of the ventral premammillary nucleus. J Comp Neurol. 1992;324:195–212. doi: 10.1002/cne.903240205. [DOI] [PubMed] [Google Scholar]
- 79.Donato J, Jr, Frazao R, Fukuda M, Vianna CR, Elias CF. Leptin induces phosphorylation of neuronal nitric oxide synthase in defined hypothalamic neurons. Endocrinology. 2010;151:5415–5427. doi: 10.1210/en.2010-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Williams KW, Sohn J-W, Donato J, Jr, Lee CE, Zhao JJ, Elmquist JK, Elias CF. The acute effects of leptin require PI3K signaling in the hypothalamic ventral premammillary nucleus. J Neurosci. 2011 doi: 10.1523/JNEUROSCI.2602-11.2011. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Myers MG., Jr Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res. 2004;59:287–304. doi: 10.1210/rp.59.1.287. [DOI] [PubMed] [Google Scholar]
- 82.Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 83.Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI, Fu XY. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci U S A. 2004;101:4661–4666. doi: 10.1073/pnas.0303992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roa J, Garcia-Galiano D, Varela L, Sanchez-Garrido MA, Pineda R, Castellano JM, Ruiz-Pino F, Romero M, Aguilar E, Lopez M, et al. The Mammalian Target of Rapamycin as Novel Central Regulator of Puberty Onset via Modulation of Hypothalamic Kiss1 System. Endocrinology. 2009;150:5016–5026. doi: 10.1210/en.2009-0096. [DOI] [PubMed] [Google Scholar]
- 85.Altarejos JY, Goebel N, Conkright MD, Inoue H, Xie J, Arias CM, Sawchenko PE, Montminy M. The Creb1 coactivator Crtc1 is required for energy balance and fertility. Nat Med. 2008;14:1112–1117. doi: 10.1038/nm.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao AZ, Huan JN, Gupta S, Pal R, Sahu A. A phosphatidylinositol 3-kinase phosphodiesterase 3B-cyclic AMP pathway in hypothalamic action of leptin on feeding. Nature Neuroscience. 2002;5:727–728. doi: 10.1038/nn885. [DOI] [PubMed] [Google Scholar]
- 87.Nagatani S, Guthikonda P, Foster DL. Appearance of a Nocturnal Peak of Leptin Secretion in the Pubertal Rat. Hormones and Behavior. 2000;37:345–352. doi: 10.1006/hbeh.2000.1582. [DOI] [PubMed] [Google Scholar]
- 88.Lin Q, Poon S, Chen J, Cheng L, HoYuen B, Leung P. Leptin interferes with 3′,5′-Cyclic Adenosine Monophosphate (cAMP) signaling to inhibit steroidogenesis in human granulosa cells. Reproductive Biology and Endocrinology. 2009;7:115. doi: 10.1186/1477-7827-7-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tena-Sempere M, Manna PR, Zhang FP, Pinilla L, Gonzalez LC, Dieguez C, Huhtaniemi I, Aguilar E. Molecular mechanisms of leptin action in adult rat testis: potential targets for leptin-induced inhibition of steroidogenesis and pattern of leptin receptor messenger ribonucleic acid expression. J Endocrinol. 2001;170:413–423. doi: 10.1677/joe.0.1700413. [DOI] [PubMed] [Google Scholar]
- 90.Hsu HT, Chang YC, Chiu YN, Liu CL, Chang KJ, Guo IC. Leptin Interferes with Adrenocorticotropin/3′,5′-Cyclic Adenosine Monophosphate (cAMP) Signaling, Possibly through a Janus Kinase 2-Phosphatidylinositol 3-Kinase/Akt-Phosphodiesterase 3-cAMP Pathway, to Down-Regulate Cholesterol Side-Chain Cleavage Cytochrome P450 Enzyme in Human Adrenocortical NCI-H295 Cell Line. Journal of Clinical Endocrinology & Metabolism. 2006;91:2761–2769. doi: 10.1210/jc.2005-2383. [DOI] [PubMed] [Google Scholar]
- 91.Kitawaki J, Kusuki I, Koshiba H, Tsukamoto K, Honjo H. Leptin directly stimulates aromatase activity in human luteinized granulosa cells. Molecular Human Reproduction. 1999;5:708–713. doi: 10.1093/molehr/5.8.708. [DOI] [PubMed] [Google Scholar]
- 92.Geisler J, Haynes B, Ekse D, Dowsett M, Lønning PE. Total body aromatization in postmenopausal breast cancer patients is strongly correlated to plasma leptin levels. The Journal of Steroid Biochemistry and Molecular Biology. 2007;104:27–34. doi: 10.1016/j.jsbmb.2006.09.040. [DOI] [PubMed] [Google Scholar]