Abstract
Telomeres serve the dual function of protecting chromosomes from genomic instability as well as protecting the ends of chromosomes from DNA damage machinery. The enzyme responsible for telomere maintenance is telomerase, an enzyme capable of reverse transcription. Telomerase activity is typically limited to specific cell types. However, telomerase activation in somatic cells serves as a key step toward cell immortalization and cancer. Targeting telomerase serves as a potential cancer treatment with significant therapeutic benefits. Beyond targeting cancers by inhibiting telomerase, manipulating the regulation of telomerase may also provide therapeutic benefit to other ailments, such as those related to aging. This review will introduce human telomeres and telomerase and discuss pharmacological regulation of telomerase, including telomerase inhibitors and activators, and their use in human diseases.
Keywords: telomerase, pharmacological activators, inhibitors, natural compounds
Introduction
Telomeres are the DNA-protein complexes of linear chromosomes that protect the ends from genomic instability caused by the loss of small portions of the chromosomes during DNA replication [1]. In humans, telomeres are comprised of a repetitive TTAGGG sequence [1, 2]. This sequence length varies greatly across species, from approximately 300 base pairs to greater than 20 kb in length, and contains a 3′ G-rich single strand overhang [2–4]. Regulation of telomere length through replication is essential to overcoming the limitations of normal cellular division [5].
Part of the role of the telomere in generating chromosome stability is in protection of chromosomes from recognition by DNA repair machinery [6, 7]. In addition to the potential loss of genetic material associated with replicating linear chromosomes, the ends of these chromosomes resemble DNA breaks [6]. Repair machinery has the potential to see these ends and attempt to repair them [8]. This can result in both end-to-end fusions and additional loss of genetic material during additional cycles of replication. The 3′ overhang is essential to the protection of the telomere from these end-to-end fusions as well as unregulated nuclease digestion. It accomplishes this by folding upon itself forming a higher order structure known as the t-loop [9, 10]. The ability of the telomere to form higher order DNA structures is due to its GC rich component and may present a significant obstacle for DNA replication machinery. Although the inherent nature of the sequence allows for higher order structures, formation of these structures including the t-loop requires additional proteins known as telomere-associated proteins [11].
Telomere-associated proteins are essential to telomere function. Telomere-associated proteins recognize and bind to the repeat sequence stabilizing it. For example, the shelterin complex binds to the telomere organizing and defining it. Shelterin is comprised of six individual proteins: TRF1 (telomere repeat binding factor 1), TRF2 (telomere repeat binding factor 2), RAP1 (repressor/ activator protein 1), POT1 (protection of telomeres 1), TIN2 (TRF1 interacting nuclear factor 2) and TPP1 (also known as adrenocortical dysplasia homolog-mouse; TINT1, ‘TIN2 interacting protein 1’/PIP1, ‘POT1-Interacting Protein 1’/PTOP 1, ‘POT1 and TIN2 Organizing Protein’) [7, 12–14]. This simple complex has a role in many activities including regulating telomere length and forming the t-loop.
Replication of the telomere requires a specialized enzyme capable of reverse transcriptase activity called telomerase [15]. In normal, healthy cells telomerase activity is mostly limited to embryonic cells, adult male germline cells and stem cells, but is virtually absent in somatic cells [16]. In stem cells, telomerase activity serves the function of elongating telomeres thus protecting these cells from typical cellular aging and senescence [17]. The human telomerase is comprised of two major subunits, the RNA template and the catalytic enzyme [15, 18, 19]. The telomerase RNA template (hTR or hTERC) contains a complementary sequence to the human telomere that serves as the base for replication of the telomere repeat sequence [20, 21]. The extension of telomeres is completed through the catalytic enzyme, telomerase reverse transcriptase (hTERT) [19, 22]. Together this complex catalyses the addition of the six nucleotide repeat to the ends of chromosomes. Along with these two main components are additional telomere/telomerase-associated proteins. Formation of the functional holoenzyme complex requires associated proteins including the box H/ACA small nucleolar RNA proteins: dyskerin, NOP10 (NucleOlar Protein 10), NHP2 (Non-Histone Protein 2) and GAR1 (Glycine-Arginine Rich 1) [23, 24].
Telomerase activity and hTERT expression regulation is complex. Transcriptional, post-transcriptional, post-translational, localization, subunit assembly and epigenetic regulation as well as telomeric proteins and RNAs all contribute to telomerase regulation [25, 26]. Inability to properly regulate telomerase, such as in cases of genetic dysfunction of telomerase, can lead to a variety of diseases including cancer and bone marrow disorders [27–29] (for more specific reviews on telomerase regulation and disease, the reader is invited to see references 28 and 29). For example, components of the telomerase complex are up-regulated in over 90% of human malignancies and contribute to the increased proliferation and limitless replicative potential of cancer cells [30–33]. This differential expression between normal and malignant cells makes telomerase an ideal target in cancer therapeutics [28]. Artificially regulating telomerase may be useful as a treatment not only for cancer, but also for genetic and immunodeficiency disorders involving dysregulated telomerase or telomere length. It is important to note that there is a potential for additional effects of telomerase regulation due to additional activities of telomerase related to DNA repair, cell survival and death, stem cell maintenance and the regulation of gene expression [34, 35].
The development of telomerase inhibitors for cancer treatment is a major field of study. By inhibiting telomerase, it is possible to kill cancerous cells while limiting toxicity to neighbouring normal cells. Several mechanisms of telomerase inhibition have been explored for use as therapeutic agents. For example, there have been inquiries into regulating telomerase by immunotherapy vaccines. These vaccines target the active site of telomerase, which elicits an immune response against cancer cells (see Liu et al. review for more detailed discussion of this topic) [36]. In addition, adenoviruses, such as telomelysin, are being developed that can selectively replicate in cancer cells by using the TERT promoter as a molecular switch; this replication causes viral toxicity that selectively kills the cancer cells (see Nemunaitis et al. for a review on immunotherapy) [37]. While telomerase inhibition stands as a promising neoadjuvant therapy, it is important to note that activation of telomerase in some cells may prove beneficial. Telomerase activation is currently being studied for use in immunodeficient patients to stimulate proliferation of T cells as well as in regenerative medicine and a treatment to combat the signs and symptoms of aging. This review will focus on telomerase activity and the use of pharmacological intervention to alter this activity as a treatment for diseases such as cancer.
Telomerase inhibitors
Though several synthetic compounds with telomerase inhibition properties have been developed in recent years, the majority of these compounds are highly toxic [38]. In addition, it can be difficult to determine whether these inhibitors have a direct or indirect effect on telomerase (see Fig. 1 for targets of telomerase). The compounds may themselves cause telomerase inhibition (direct effect) or it may be that the compounds cause cell death and due to apoptosis telomerase activity slows or stops (indirect effect). Various targets, such as the RNA template, TERT protein and associated proteins, are all being investigated to develop telomerase inhibitors. One clinically relevant compound, imetelstat, has been developed to date as a specific oligonucleotide competitive inhibitor of telomerase activity. Imetelstat, or GRN163L, was developed by the Geron Corporation (Menlo Park, CA, USA) to target the RNA template for TERT by binding to the catalytic site of telomerase preventing action [39]. This inhibitor has been applied to breast cancers [40, 41], prostate cancers [42], glioblastoma [43], myeloma [44] and leukaemia [39]. It has been shown to augment the effects of paclitaxel in breast cancer cells [41]. Four phase I and I/II trials were completed in 2009, and the company is planning phase II studies and combinations studies for breast and lung cancers. Throughout each of these studies, few long-term side-effects of telomerase inhibition have been reported. The lack of significant detrimental side-effects enhances the potential of telomerase inhibition to continue to be used clinically to augment current treatment protocols.
Fig 1.
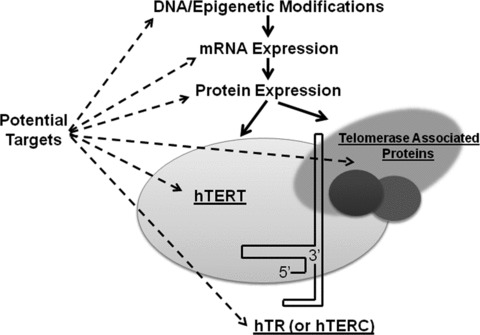
Targets of telomerase activity. Targets of telomerase and its activity are represented [29]. The pharmaceutical agents and phytochemicals discussed in this paper have been suggested to affect expression or epigenetic regulation of telomerase. More investigation into the specific anti-telomerase activities of these chemicals is necessary to define their mechanisms.
In addition to synthetic compounds, various chemical compounds that occur naturally in plants, or phytochemicals, have been suggested to inhibit telomerase activity in various cancers (Table 1). Allicin, an organophosphate derived from garlic, was shown to decrease telomerase activity and increase apoptosis in gastric adenocarcinoma cells though the mechanism is undetermined [47]. Curcumin, a phenol present in turmeric, has been shown to decrease telomerase activity in several cancer types [38, 48–53]. This inhibition has been shown to be due to the inhibition of translocation of TERT to the nucleus [38] by dissociating Hsp-90 co-chaperone p23 from TERT [50] as well as the reduction of TERT expression [48, 49]. A flavonolignan found in milk thistle, silbinin, has been shown to decrease TERT expression as well as telomerase activity [54]. The organosulfur derived from cruciferous vegetables, sulforaphane, has been shown to cause epigenetic regulation resulting in a decrease of TERT expression as well as the phosphorylation of TERT which prevents translocation to the nucleus. Epigallocatechin gallate (EGCG), a catechin in green tea, has shown to inhibit TERT expression [55], which may be largely due to epigenetic regulation of the TERT promoter [56]. Furthermore, EGCG showed inhibition of telomerase in several cervical cancer cell lines only with concurrent retinoic acid treatment. This effect was associated with a decrease in hTERT expression [45]. Several of these chemicals have been tested on normal, non-malignant, as well as cancerous cells. Curcumin [48], genistein [57], EGCG [26] and sulforaphane [58] were all tested on breast cancer cells and the non-malignant breast cell line MCF10A; curcumin [48] and sulforaphane [58] had no effect on normal cells whereas genistein was shown to inhibit telomerase in these MCF10A cells as well as the cancer cells [57]. It is important to note, not all studies on these chemicals are in agreement. Several compounds have been shown to act as both inhibitors and activators of telomerase though this may be due to treatment concentration or cell type differences. For example, resveratrol has been shown to inhibit telomerase activity in cancer cells [59] and activate telomerase in epithelial [60] and endothelial progenitor cells [61]. In addition to this, a study about genistein (soybean) suggests it may activate telomerase activity at low concentrations and inhibit telomerase activity at higher treatment concentrations (see ‘Telomerase Activators’ and Table 1) [46]. These studies suggest that more research needs to be done on these phytochemicals to ascertain their specificity for their potential development as effective telomerase inhibitors that could be utilized for clinical applications.
Table 1.
Phytochemicals shown to have telomerase regulation properties
| Phytochemical | Cancer type | Cell lines | Mechanism of regulation | |
|---|---|---|---|---|
| Allicin (Garlic) | Gastric | SGC-7901 [47] | ND | |
| Curcumin (Turmeric) | Breast | MCF-7 [48] | • Transcriptional [48] | |
| Cervical | HeLa, SiHa, Ca Ski [51] | • Translational [49] | ||
| • Post-translational – Nuclear Localization [38, 50] | ||||
| Gastric | SGC-7901 [52] | |||
| Leukaemia | HL60 [52, 53], K-562 [38] | |||
| Liver | Bel7402 [52] | |||
| Lung | H1299 [50], A549 [49] | |||
| Epigallocatechin Gallate (Green Tea) | Brain | U87-MG, 1321N1 [65] | • Transcriptional – Epigenetics [56] | |
| Breast | MCF-7 [26, 55, 56], MDA-MB-231 [26] | • Translational [55] | ||
| Cervical | OMC-4, TMCC-1 [66] | |||
| Inhibitor | Head and Neck | Hep-2 [67] | ||
| Leukaemia | HL60 [56] | |||
| Lung | H69, H69VP [68] | |||
| Genistein (Soybean) | Breast | MCF-7 [57] | • Transcriptional [69, 70] | |
| Ovarian | SKOV-3 [46] | • Post-translational – Nuclear Localization [70] | ||
| Prostate | LNCaP [69], PC-3 [66], DU-145 [70] | |||
| Resveratrol (Red Grape) | Breast | MCF-7 [59] | • Post-translational – Nuclear Localization [59] | |
| Colon | HT-29, WiDr [71] | |||
| Silibinin (Milk Thistle) | Prostate | LNCaP [54] | ND | |
| Sulforaphane | Breast | MCF-7, MDA-MB-231 [72] | • Transcriptional [58]– Epigenetics [72] | |
| (Cruciferous Vegetables) | Liver | Hep3B [58] | • Post-translational [58] | |
| Resveratrol (Red Grapes) | - | Epithelial cells [60], Endothelial progenitor cells [61] | • Post-translational [60] | |
| Activator | Genistein (Soybean) | Breast | MCF-7 [46] | • Transcriptional [46] |
| Ovarian | SKOV-3 [46] | |||
| Prostate | DU-145, LNCaP [46] |
ND: not determined.
Telomerase activators
As telomere length is associated with cellular aging, there have been interesting inquiries into the development of telomerase activators to reverse normal cellular aging and treat symptoms of aging. Geron Corp. and TA Therapeutics developed a single molecule telomerase activator, TAT2 (cycloastragenol). This small molecule has been shown to transiently activate telomerase in T lymphocytes that were no longer proliferating [62]. With continuous treatment, this agent could be useful for patients with HIV/AIDS and immunodeficiency. In addition, this molecule is being used to develop nutraceuticals and cosmeceuticals to enhance immune function and skin condition. However, more work needs to be done to determine mechanism of action and safety of these products.
Also, certain phytochemicals have been shown to activate telomerase (Table 1). Resveratrol has been shown to activate telomerase in human mammary epithelial [60] and endothelial progenitor cells [61]. It has been suggested that this may be due to the up-regulation of SIRT1 [63]. As discussed above, one study showed that the phytochemical genistein caused an activation of telomerase in DU-145 and LNCaP prostate cancer cells as well as MCF-7 breast cancer cells and SKOV-3 ovarian cancer cells at low (0.5–μM) treatment concentrations. No activation was seen in normal human prostate, PrEC, cells which are telomerase-negative. However, telomerase inhibition was seen at higher (50 μM) concentrations with all lines and this result suggests that genistein has a bilateral effect on telomerase activity in cancer cells [46]. Further screening of phytochemicals should be conducted to determine other telomerase activators, and more studies are needed to determine the effectiveness of these chemicals as telomerase activators.
Furthermore, additional studies need to be conducted on the safety of activating telomerase. There is little information to address the possible over-activation of telomerase due to pharmaceuticals that could lead to uncontrolled cell growth. Examples of this can be seen among non-pharmaceutical chemicals. For instance, a major component of cigarettes, cotinine, has been shown to activate telomerase causing abnormal proliferation [64]. In addition, it is possible that activation of telomerase could reactivate the proliferative capability of benign tumours.
Conclusions and perspectives
The potential benefits of regulating telomerase activity are clear. Pharmaceutically inhibiting telomerase may prove an important option in cancer therapy in conjunction with traditional chemotherapeutics. Conversely, the activation of telomerase could be useful to treat age-related diseases and HIV/AIDS patients where lymphocytes have stopped proliferating. However, the long-term effects of regulating telomerase either positively or negatively are unclear. It is possible that inhibition of telomerase could have adverse side effects on normal stem cell function and immune response as stem and immune cells have increased telomerase activity to accommodate frequent proliferation. Understanding of telomerase regulation in normal cells is crucial for the development of telomerase inhibitors and activators. The regulation of telomerase is complex. This complexity may make pharmaceutical regulation difficult due to compensation by other regulatory pathways. However, phytochemicals that seem to regulate telomerase provide a starting place. These chemicals can be used as lead compounds to develop drugs that may be able to be used in the clinic.
Acknowledgments
We would like to thank the anonymous reviewers for their helpful comments and suggestions. We thank R. Sloan and the Herbert laboratory for providing feedback on the manuscript. This work was supported in part by the IU Simon Cancer Center (IUSCC), by the NIH grant number T32 HL007910 (to CES; its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH), and the Indiana Genomics Initiative (INGEN), supported in part by the Lilly Endowment, Inc.
Conflict of interest
B.-S. Herbert receives imetelstat (telomerase inhibitor) and research support from Geron Corporation. The other authors confirm that there are no conflicts of interest.
References
- 1.Blackburn EH. Structure and function of telomeres. Nature. 1991;350:569–73. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 2.Moyzis RK, Buckingham JM, Cram LS, et al. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci USA. 1988;85:6622–6. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verdun RE, Karlseder J. Replication and protection of telomeres. Nature. 2007;447:924–31. doi: 10.1038/nature05976. [DOI] [PubMed] [Google Scholar]
- 4.Gomes NM, Ryder OA, Houck ML, et al. Comparative biology of mammalian telomeres: hypotheses on ancestral states and the roles of telomeres in longevity determination. Aging Cell. 2011;10:761–8. doi: 10.1111/j.1474-9726.2011.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shore D, Bianchi A. Telomere length regulation: coupling DNA end processing to feedback regulation of telomerase. EMBO J. 2009;28:2309–22. doi: 10.1038/emboj.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murnane JP. Telomere loss as a mechanism for chromosome instability in human cancer. Cancer Res. 2010;70:4255–9. doi: 10.1158/0008-5472.CAN-09-4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Sullivan RJ, Karlseder J. Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol. 2010;11:171–81. doi: 10.1038/nrm2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longhese MP, Bonetti D, Manfrini N, et al. Mechanisms and regulation of DNA end resection. EMBO J. 2010;29:2864–74. doi: 10.1038/emboj.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffith JD, Comeau L, Rosenfield S, et al. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–14. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- 10.Wei C, Price M. Protecting the terminus: t-loops and telomere end-binding proteins. Cell Mol Life Sci. 2003;60:2283–94. doi: 10.1007/s00018-003-3244-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoeftner S, Blasco MA. A ‘higher order’ of telomere regulation: telomere heterochromatin and telomeric RNAs. EMBO J. 2009;28:2323–36. doi: 10.1038/emboj.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–34. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 13.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–10. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 14.Neidle S. Human telomeric G-quadruplex: the current status of telomeric G-quadruplexes as therapeutic targets in human cancer. FEBS J. 2010;277:1118–25. doi: 10.1111/j.1742-4658.2009.07463.x. [DOI] [PubMed] [Google Scholar]
- 15.Blackburn EH. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005;579:859–62. doi: 10.1016/j.febslet.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 16.Broccoli D, Young JW, de Lange T. Telomerase activity in normal and malignant hematopoietic cells. Proc Natl Acad Sci USA. 1995;92:9082–6. doi: 10.1073/pnas.92.20.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flores I, Blasco MA. The role of telomeres and telomerase in stem cell aging. FEBS Lett. 2010;584:3826–30. doi: 10.1016/j.febslet.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 18.Wyatt HD, West SC, Beattie TL. InTERTpreting telomerase structure and function. Nucleic Acids Res. 2010;38:5609–22. doi: 10.1093/nar/gkq370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mason M, Schuller A, Skordalakes E. Telomerase structure function. Curr Opin Struct Biol. 2011;21:92–100. doi: 10.1016/j.sbi.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Shippen-Lentz D, Blackburn EH. Functional evidence for an RNA template in telomerase. Science. 1990;247:546–52. doi: 10.1126/science.1689074. [DOI] [PubMed] [Google Scholar]
- 21.Feng J, Funk WD, Wang SS, et al. The RNA component of human telomerase. Science. 1995;269:1236–41. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- 22.Morin GB. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989;59:521–9. doi: 10.1016/0092-8674(89)90035-4. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell JR, Cheng J, Collins K. A box H/ACA small nucleolar RNA-like domain at the human telomerase RNA 3′ end. Mol Cell Biol. 1999;19:567–76. doi: 10.1128/mcb.19.1.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ly HD. Genetic and environmental factors influencing human diseases with telomere dysfunction. Int J Clin Exp Med. 2009;2:114–30. [PMC free article] [PubMed] [Google Scholar]
- 25.Gustina AS, Trudeau MC. hERG potassium channel gating is mediated by N- and C-terminal region interactions. J Gen Physiol. 2011;137:315–25. doi: 10.1085/jgp.201010582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meeran SM, Patel SN, Chan TH, et al. A novel prodrug of epigallocatechin-3-gallate: differential epigenetic hTERT repression in human breast cancer cells. Cancer Prev Res (Phila) 2011;4:1243–54. doi: 10.1158/1940-6207.CAPR-11-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trudeau MA, Wong JM. Genetic variations in telomere maintenance, with implications on tissue renewal capacity and chronic disease pathologies. Curr Pharmacogenomics Person Med. 2010;8:7–24. doi: 10.2174/1875692111008010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koziel JE, Fox MJ, Steding CE, et al. Medical genetics and epigenetics of telomerase. J Cell Mol Med. 2011;15:457–67. doi: 10.1111/j.1582-4934.2011.01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia CK, Wright WE, Shay JW. Human diseases of telomerase dysfunction: insights into tissue aging. Nucleic Acids Res. 2007;35:7406–16. doi: 10.1093/nar/gkm644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shay JW, Wright WE. Telomerase therapeutics for cancer: challenges and new directions. Nat Rev Drug Discov. 2006;5:577–84. doi: 10.1038/nrd2081. [DOI] [PubMed] [Google Scholar]
- 31.Shay JW, Wright WE. Telomeres and telomerase in normal and cancer stem cells. FEBS Lett. 2010;584:3819–25. doi: 10.1016/j.febslet.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herbert BS, Wright WE, Shay JW. Telomerase and breast cancer. Breast Cancer Res. 2001;3:146–9. doi: 10.1186/bcr288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Artandi SE, DePinho RA. Telomeres and telomerase in cancer. Carcinogenesis. 2010;31:9–18. doi: 10.1093/carcin/bgp268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cong Y, Shay JW. Actions of human telomerase beyond telomeres. Cell Res. 2008;18:725–32. doi: 10.1038/cr.2008.74. [DOI] [PubMed] [Google Scholar]
- 35.Chang S, DePinho RA. Telomerase extracurricular activities. Proc Natl Acad Sci USA. 2002;99:12520–2. doi: 10.1073/pnas.212514699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu JP, Chen W, Schwarer AP, et al. Telomerase in cancer immunotherapy. Biochim Biophys Acta. 2010;1805:35–42. doi: 10.1016/j.bbcan.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Nemunaitis J, Tong AW, Nemunaitis M, et al. A phase I study of telomerase-specific replication competent oncolytic adenovirus (telomelysin) for various solid tumours. Mol Ther. 2010;18:429–34. doi: 10.1038/mt.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chakraborty S, Ghosh U, Bhattacharyya NP, et al. Inhibition of telomerase activity and induction of apoptosis by curcumin in K-562 cells. Mutat Res. 2006;596:81–90. doi: 10.1016/j.mrfmmm.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Harley CB. Telomerase and cancer therapeutics. Nat Rev Cancer. 2008;8:167–79. doi: 10.1038/nrc2275. [DOI] [PubMed] [Google Scholar]
- 40.Hochreiter AE, Xiao H, Goldblatt EM, et al. Telomerase template antagonist GRN163L disrupts telomere maintenance, tumour growth, and metastasis of breast cancer. Clin Cancer Res. 2006;12:3184–92. doi: 10.1158/1078-0432.CCR-05-2760. [DOI] [PubMed] [Google Scholar]
- 41.Goldblatt EM, Gentry ER, Fox MJ, et al. The telomerase template antagonist GRN163L alters MDA-MB-231 breast cancer cell morphology, inhibits growth, and augments the effects of paclitaxel. Mol Cancer Ther. 2009;8:2027–35. doi: 10.1158/1535-7163.MCT-08-1188. [DOI] [PubMed] [Google Scholar]
- 42.Marian CO, Wright WE, Shay JW. The effects of telomerase inhibition on prostate tumour-initiating cells. Int J Cancer. 2010;127:321–31. doi: 10.1002/ijc.25043. [DOI] [PubMed] [Google Scholar]
- 43.Marian CO, Cho SK, McEllin BM, et al. The telomerase antagonist, imetelstat, efficiently targets glioblastoma tumour-initiating cells leading to decreased proliferation and tumour growth. Clin Cancer Res. 2010;16:154–63. doi: 10.1158/1078-0432.CCR-09-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shammas MA, Koley H, Bertheau RC, et al. Telomerase inhibitor GRN163L inhibits myeloma cell growth in vitro and in vivo. Leukemia. 2008;22:1410–8. doi: 10.1038/leu.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yokoyama M, Noguchi M, Nakao Y, et al. Antiproliferative effects of the major tea polyphenol, (-)-epigallocatechin gallate and retinoic acid in cervical adenocarcinoma. Gynecol Oncol. 2008;108:326–31. doi: 10.1016/j.ygyno.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 46.Chau MN, El Touny LH, Jagadeesh S, et al. Physiologically achievable concentrations of genistein enhance telomerase activity in prostate cancer cells via the activation of STAT3. Carcinogenesis. 2007;28:2282–90. doi: 10.1093/carcin/bgm148. [DOI] [PubMed] [Google Scholar]
- 47.Sun L, Wang X. Effects of allicin on both telomerase activity and apoptosis in gastric cancer SGC-7901 cells. World J Gastroenterol. 2003;9:1930–4. doi: 10.3748/wjg.v9.i9.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramachandran C, Fonseca HB, Jhabvala P, et al. Curcumin inhibits telomerase activity through human telomerase reverse transcritpase in MCF-7 breast cancer cell line. Cancer Lett. 2002;184:1–6. doi: 10.1016/s0304-3835(02)00192-1. [DOI] [PubMed] [Google Scholar]
- 49.Hsin IL, Sheu GT, Chen HH, et al. N-acetyl cysteine mitigates curcumin-mediated telomerase inhibition through rescuing of Sp1 reduction in A549 cells. Mutat Res. 2010;688:72–7. doi: 10.1016/j.mrfmmm.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 50.Lee JH, Chung IK. Curcumin inhibits nuclear localization of telomerase by dissociating the Hsp90 co-chaperone p23 from hTERT. Lett. 2010;290:76–86. doi: 10.1016/j.canlet.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 51.Singh M, Singh N. Molecular mechanism of curcumin induced cytotoxicity in human cervical carcinoma cells. Mol Cell Biochem. 2009;325:107–19. doi: 10.1007/s11010-009-0025-5. [DOI] [PubMed] [Google Scholar]
- 52.Cui SX, Qu XJ, Xie YY, et al. Curcumin inhibits telomerase activity in human cancer cell lines. Int J Mol Med. 2006;18:227–31. [PubMed] [Google Scholar]
- 53.Mukherjee Nee Chakraborty S, Ghosh U, Bhattacharyya NP, et al. Curcumin-induced apoptosis in human leukemia cell HL-60 is associated with inhibition of telomerase activity. Mol Cell Biochem. 2007;297:31–9. doi: 10.1007/s11010-006-9319-z. [DOI] [PubMed] [Google Scholar]
- 54.Thelen P, Wuttke W, Jarry H, et al. Inhibition of telomerase activity and secretion of prostate specific antigen by silibinin in prostate cancer cells. J Urol. 2004;171:1934–8. doi: 10.1097/01.ju.0000121329.37206.1b. [DOI] [PubMed] [Google Scholar]
- 55.Mittal A, Pate MS, Wylie RC, et al. EGCG down-regulates telomerase in human breast carcinoma MCF-7 cells, leading to suppression of cell viability and induction of apoptosis. Int J Oncol. 2004;24:703–10. [PubMed] [Google Scholar]
- 56.Berletch JB, Liu C, Love WK, et al. Epigenetic and genetic mechanisms contribute to telomerase inhibition by EGCG. J Cell Biochem. 2008;103:509–19. doi: 10.1002/jcb.21417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y, Liu L, Andrews LG, et al. Genistein depletes telomerase activity through cross-talk between genetic and epigenetic mechanisms. Int J Cancer. 2009;125:286–96. doi: 10.1002/ijc.24398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moon DO, Kang SH, Kim KC, et al. Sulforaphane decreases viability and telomerase activity in hepatocellular carcinoma Hep3B cells through the reactive oxygen species-dependent pathway. Cancer Lett. 2010;295:260–6. doi: 10.1016/j.canlet.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 59.Lanzilli G, Fuggetta MP, Tricarico M, et al. Resveratrol down-regulates the growth and telomerase activity of breast cancer cells in vitro. Int J Oncol. 2006;28:641–8. [PubMed] [Google Scholar]
- 60.Pearce VP, Sherrell J, Lou Z, et al. Immortalization of epithelial progenitor cells mediated by resveratrol. Oncogene. 2008;27:2365–74. doi: 10.1038/sj.onc.1210886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xia L, Wang XX, Hu XS, et al. Resveratrol reduces endothelial progenitor cells senescence through augmentation of telomerase activity by Akt-dependent mechanisms. Br J Pharmacol. 2008;155:387–94. doi: 10.1038/bjp.2008.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fauce SR, Jamieson BD, Chin AC, et al. Telomerase-based pharmacologic enhancement of antiviral function of human CD8 +T lymphocytes. J Immunol. 2008;181:7400–6. doi: 10.4049/jimmunol.181.10.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jacob T, Clouden N, Hingorani A, et al. The effect of cotinine on telomerase activity in human vascular smooth muscle cells. J Cardiovasc Surg (Torino) 2009;50:345–9. [PubMed] [Google Scholar]
- 65.Shervington A, Pawar V, Menon S, et al. The sensitization of glioma cells to cisplatin and tamoxifen by the use of catechin. Mol Biol Rep. 2009;36:1181–6. doi: 10.1007/s11033-008-9295-3. [DOI] [PubMed] [Google Scholar]
- 66.Noguchi M, Yokoyama M, Watanabe S, et al. Inhibitory effect of the tea polyphenol, (-)-epigallocatechin gallate, on growth of cervical adenocarcinoma cell lines. Cancer Lett. 2006;234:135–42. doi: 10.1016/j.canlet.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 67.Wang X, Hao MW, Dong K, et al. Apoptosis induction effects of EGCG in laryngeal squamous cell carcinoma cells through telomerase repression. Arch Pharm Res. 2009;32:1263–9. doi: 10.1007/s12272-009-1912-8. [DOI] [PubMed] [Google Scholar]
- 68.Sadava D, Whitlock E, Kane SE. The green tea polyphenol, epigallocatechin-3-gallate inhibits telomerase and induces apoptosis in drug-resistant lung cancer cells. Biochem Biophys Res Commun. 2007;360:233–7. doi: 10.1016/j.bbrc.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 69.Ouchi H, Ishiguro H, Ikeda N, et al. Genistein induces cell growth inhibition in prostate cancer through the suppression of telomerase activity. Int J Urol. 2005;12:73–80. doi: 10.1111/j.1442-2042.2004.00973.x. [DOI] [PubMed] [Google Scholar]
- 70.Jagadeesh S, Kyo S, Banerjee PP. Genistein represses telomerase activity via both transcriptional and posttranslational mechanisms in human prostate cancer cells. Cancer Res. 2006;66:2107–15. doi: 10.1158/0008-5472.CAN-05-2494. [DOI] [PubMed] [Google Scholar]
- 71.Fuggetta MP, Lanzilli G, Tricarico M, et al. Effect of resveratrol on proliferation and telomerase activity of human colon cancer cells in vitro. J Exp Clin Cancer Res. 2006;25:189–93. [PubMed] [Google Scholar]
- 72.Meeran SM, Patel SN, Tollefsbol TO. Sulforaphane causes epigenetic repression of hTERT expression in human breast cancer cell lines. PLoS One. 2010;5:e11457. doi: 10.1371/journal.pone.0011457. [DOI] [PMC free article] [PubMed] [Google Scholar]


