Abstract
Desmoid tumors (DTs) are nonmalignant neoplasms of mesenchymal origin that mainly contain fibroblast lineage cells. These tumors often occur in Familial Adenomatous Polyposis Coli (FAP) patients who have germline mutations in the APC gene. Given emerging data that has implicated multipotent mesencyhmal stromal cells (MSCs) in the origin of mesenchymal tumors, we hypothesized that DTs may arise in FAP patients after MSCs acquire somatic mutations during the proliferative phase of wound healing. To test this idea, we examined 16 DTs from FAP-associated and sporadic cases, finding that all 16/16 tumors expressed stem cell markers whereas matching normal stromal tissues were uniformly negative. DTs also contained a subclass of fibrocytes linked to wound healing, angiogenesis and fibrosis. Using a MSC cell line derived from a FAP-associated DT, we confirmed an expected loss in the expression of APC and the transcriptional repressor BMI-1 while documenting the co-expression of markers for chondrocytes, adipocytes and osteocytes. Together, our findings argue that DTs result from the growth of MSCs in a wound healing setting that is associated with deregulated Wnt signaling due to APC loss. The differentiation potential of these MSCs combined with expression of BMI-1, a transcriptional repressor downstream of Hedgehog and Notch signaling, suggests that DTs may respond to therapies targeting these pathways.
Keywords: Desmoid tumor, mesenchymal stromal cells, wound healing, stem cell niche
Introduction
DTs, also known as aggressive fibromatosis, are mesenchymal tumors that occur sporadically or are associated with the heritable colorectal cancer syndrome, FAP. DTs exhibit locally destructive growth, with a dense infiltrative character that can produce disfigurement, functional deficits, and death (1). Treatment often involves surgical excision, which is associated with high recurrence rates (2). Although several studies reported successful regression of DTs with drug treatments and radiation therapy, effective systemic therapy remains elusive, in large part due to the limited understanding of its pathophysiology (3, 4, 5).
A common feature of DTs is deregulated Wnt signaling via β-catenin-dependent activation of latent Tcf/Lef transcription factors. This pathway is critical in adult stem cell survival and self-renewal during wound healing (6). DTs arising in patients with FAP show loss of APC tumor suppressor function (7, 8). This leads to high intracellular β-catenin levels and is correlated with constitutive activation of Wnt signaling. In sporadic DTs, specific point mutations in the CTNNB1 gene that stabilize β-catenin protein achieve a similar result (9, 10).
DTs can arise at sites of wound healing and demonstrate histologic features observed in dermal fibroproliferative disorders such as keloids and hypertrophic scarring (11, 12). Normal wound healing is a tightly-regulated, self-limited process that produces rapid defect coverage to protect from further harm, then regenerates and remodels tissues at the injury site. In response to tissue stress or injury, mesenchymal cells from various sources are mobilized and recruited to wounds, where they engraft and promote healing (13, 14, 15). These cells include hematopoietic stem cell (HSC)-derived monocyte precursors, which comprise a small fraction of circulating nucleated cells that also home to sites of tissue injury, engraft, and differentiate into CD34+ fibrocytes. During wound healing, these pluripotent cells execute tissue remodeling and differentiate into endothelial cells, fibroblasts, and myofibroblasts (16, 17, 18). During the resolution phase of normal wound healing, recruited stem/progenitor cells undergo terminal differentiation or apoptosis. However, under conditions of chronic inflammation or tumor progression, these activated cells persist. For example, both MSCs and fibrocytes are found in keloids and hypertrophic scars (19, 20, 21). Together, these multipotent cells cooperate synergistically to support angiogenesis, a hallmark of accelerated wound healing and fibrosis (22, 23, 24). DTs exhibit features consistent with chronic wound healing, including increased angiogenesis and proliferation of fibroblast-like cells within a collagen matrix (25, 26). DTs also express genes characteristic of myofibroblasts, further indicating that persistent recruitment of monocyte precursors and defective wound healing resolution play significant roles in DT neoplasia (27).
Because of their association with wound healing, MSCs are implicated in DT formation. Primary fibroblast cell lines have been derived from DTs; however, the growth conditions employed did not specifically select or expand MSCs (28, 29). A recent report described the culture of putative MSCs from mouse DTs; however, these cells were not fully characterized (30, 31). We hypothesized that DTs arise after MSCs acquire somatic mutations during the proliferative phase of wound healing in genes that increase the transcriptional potential of β-catenin-Tcf/Lef. To explore this idea, we examined the expression of stem cell markers in archived human DT specimens and established a DT-derived MSC line from a patient with FAP. Our findings implicate MSCs in the etiology of DT and suggest novel targets for the systemic treatment of this disease.
Materials & Methods
Human DT specimens and normal MSCs
Human DTs and matching adjacent fascia from a subset of the collection were obtained from surgical specimens excised from patients under an approved protocol by the Institutional Review Board at the Brigham and Women’s Hospital (BWH). Tumors were fixed in 10% buffered formalin and embedded in paraffin for immunohistochemical analysis. Normal first passage adipose-derived MSCs were a generous gift from Dr. Martin L. Yarmush, Shriners Hospital for Children, Boston, MA.
Immunohistochemistry
Serial sections (4 μm) of preserved DTs were de-paraffinized in a xylene and graded ethanol series. Antigen retrieval was performed in pH 6.0 citrate buffer (Millipore) by heating for 2 min in a decloaking chamber (Biocare Medical). Peroxidase activity was quenched using the Dual Endogenous Enzyme Block (Dako North America, Inc.) for 15 min at room temperature. All primary antibody reactions were performed overnight at 4°C. Antibodies are listed in Supplementary Table 1. Biotinylated horse anti-mouse antibodies (IgG H+L) (Vector Laboratory) were used at room temperature for 30 min. Antibodies were diluted in Antibody Diluent Reagent Solution (Invitrogen). ABC Reagents (Vector Laboratory) were applied for enhancement for 15 min. Positive staining was detected with diaminobenzidine using the Liquid DAB+ Substrate Chromagen System (Dako North America, Inc.) and counter-stained with hematoxylin. Dehydrated slides were cover-slipped with Permount (Fisher). Images were acquired using an Olympus BX40 microscope. CD73+ and CD34+ cells were counted at 4 representative high-powered fields (40X) using Image J software. Student’s t-test was used for statistical analysis.
For immunofluorescent staining, cells were plated on 4-well Lab-Tek II Chamber slides and allowed to attach for 2 days. Cells were fixed in 4% formaldehyde for 30 min at room temperature, then washed and blocked with Blocking Buffer (1X PBS, 5% normal goat serum, 0.3% Triton X-100) for 1 h at room temperature. Reactions used antibodies placed in Antibody Buffer (1X PBS, 1% BSA, 0.3% Triton X100). Cells were washed and incubated in fluorochrome-conjugated secondary antibodies (Invitrogen) for 1 h at room temperature in the dark. Slides were cover-slipped with Prolong Gold Antifade Reagent (Invitrogen). Similar procedures were used after de-paraffinizing and rehyrdrating sectioned DTs. Imaging used a Nikon Eclipse TE2000-S inverted microscope with a Spot RT Slider Camera and Spot Software (Version 4.6 for Windows™) (Diagnostic Instruments, Inc.).
Cell culture & cell morphology analysis
To establish a DT cell line, a fresh tumor sample was immediately placed in Hepes-buffered saline supplemented with 15% FBS and antibiotics. In the laboratory, the tumor was minced and placed in tissue culture dishes submerged in MesenPRO RS Medium and incubated in a humidified 5% CO2 incubator. Cell culture medium and related reagents were purchased from Invitrogen Corp. Tumor debris and unattached cells were discarded after 4 days. A single attached cell was isolated and expanded with fresh medium provided every 4–5 days. At ~80% confluence, cells were trypsinized and expanded. Characterizations were performed on passage 1–3 cells. Cell density and viability (Trypan Blue exclusion) were determined using a hemocytometer. To characterize the morphology of the DT-derived cells, phase contrast imaging used a Nikon TE2000-E microscope and a Hamamatsu OrcaER camera at 10X magnification.
Cell differentiation
Cells were plated on 4-well Lab-Tek II Chamber Slides (Fisher) and incubated for 2 days in MesenPro MSC medium. For adipogenic differentiation, cells were placed in StemPro Adipogenesis Differentiation Medium. For osteogenic and chondrogenic differentiation, cells were incubated in either StemPro Osteogenesis Differentiation Medium or StemPro Chondrogenesis Medium. The DT-derived cells required 5–6 weeks for differentiation and were provided fresh medium every 3–4 days. For osteogenic differentiation, immunofluorescence histochemistry used OPN and RUNX2 antibodies. Similar procedures were executed for chondrogenic or adipocyte differentiation but used CTGF and SOX9, or PPARγ and c/EPBα antibodies, respectively. After fixing cells in 4% formaldehyde for 10 min, standard von Kossa, Alcian Blue, and Oil Red O staining reactions were performed.
Fluorescent Activated Cell Sorting analysis (FACS)
Cells were grown to near confluence and passaged, as described. After centrifugation, cell pellets were suspended in 4% formaldehyde and fixed for 10 min at 37°C, chilled on ice for 1 min, and permeabilized for 30 min at 4°C by adding ice-cold 100% methanol to pre-chilled cells for a final concentration of 90%. 5×105 cells in separate aliquots were washed and pelleted by centrifugation in Dilution Buffer (0.5% BSA in 1X PBS). Primary antibody was added at appropriate dilutions; reactions proceeded for 1 h at room temperature then were washed in Dilution Buffer. Fluorochrome-conjugated secondary antibodies diluted in Dilution Buffer were added appropriately and reactions were incubated for 30 min at room temperature. Cells suspended in PBS were analyzed using an Accuri C6 Flow Cytometer; data analysis was performed with CFlow Software.
Results
Human DTs express stem cell markers
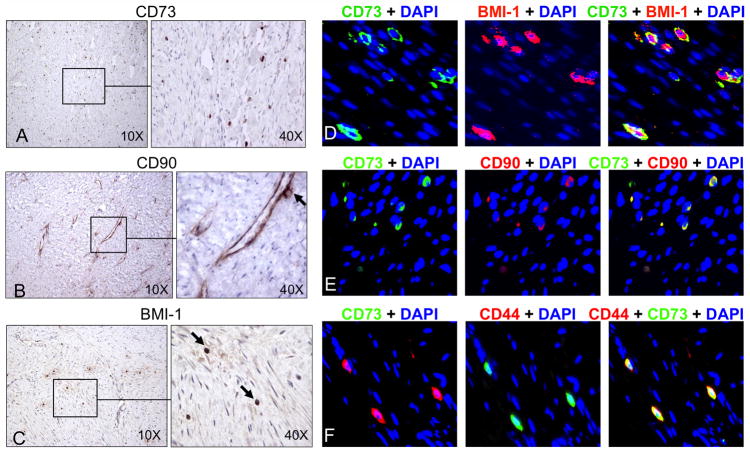
To investigate the presence of MSCs in DTs, immunohistochemical analysis was performed on 16 different paraffin-embedded tumor specimens and 5 matched normal adjacent tissues. Clinical features of the DT patients are provided in Supplementary Table 2. Within all 16 tumors, cells were positively stained for the obligate MSC markers, CD73 (Ecto-5′-nucleotidase) and CD90 (Thy-1) (Fig. 1A and B). Cells in DTs also expressed the stem cell marker and Polycomb group transcriptional repressor, BMI-1 (Fig. 1C). Staining for stem cell markers in matched normal connective tissue yielded negative results (Supplementary Fig. 1). Immunohistochemistry and immunofluorescent histochemistry of paraffin-embedded DTs also showed co-expression of CD73, CD90, BMI-1, and CD44 within the same cells (Fig. 1D–F). CD44 is a stem cell marker that directs MSC homing to anatomical locations other than bone marrow (32). The number of CD73+ cells varied among DT specimens, but the difference was not statistically significant (Supplementary Fig. 2).
Figure 1. Immunohistochemical staining demonstrated stem cell marker expression in DTs.
Sixteen archived DTs were stained for stem cell markers CD73 (A), CD90 (B), and BMI-1 (C), and representative images demonstrated the presence of abundant MSCs (black arrows) within tumors (original magnification 10X or 40X). Fluorescent immunohistochemistry of paraffin-embedded DTs showed co-expression of the MSC markers CD73 (green) and BMI-1 (red) (D), CD73 (green) and CD90 (red) (E), as well as CD73 (green) and CD44 (red) (F). Co-expression of both markers produced a yellow overlay. Original magnification was 40X. In this and subsequent fluorescent immunohistochemistry, nuclei were counterstained blue with DAPI.
DT-derived cells demonstrate characteristics of MSCs
Freshly removed DT cells were cultured in MSC-specific medium. First passage DT-derived cells had a large, flat polyploid morphology (Supplementary Fig. 3A). Prominent cytoskeletal structures spanning the length of cells were visible under the light microscope. These cells grew more slowly and were more resistant to detachment by Trypsin-EDTA (0.05%) treatment than normal human fat-derived MSCs (data not shown). Supplementary Fig. 3B and C show first passage DT-derived cells at increasing densities. At higher density, cells aligned with one another in a polarized fashion forming long ridges with cell bodies stretched in opposite directions. At this time, cell clusters spontaneously differentiated into neuronal-like cells, a result consistent with reports that both DTs and MSCs express neuronal genes (Supplementary Fig. 3D) (27, 33). Upon passage, the neuronal-like cells did not attach and were subsequently lost. While all subsequent characterizations were performed using DT-derived cells passaged less than 3 times, this cell line was maintained for at least 10 population doublings and appeared virtually immortal consistent with the self-renewal capability of stem cells.
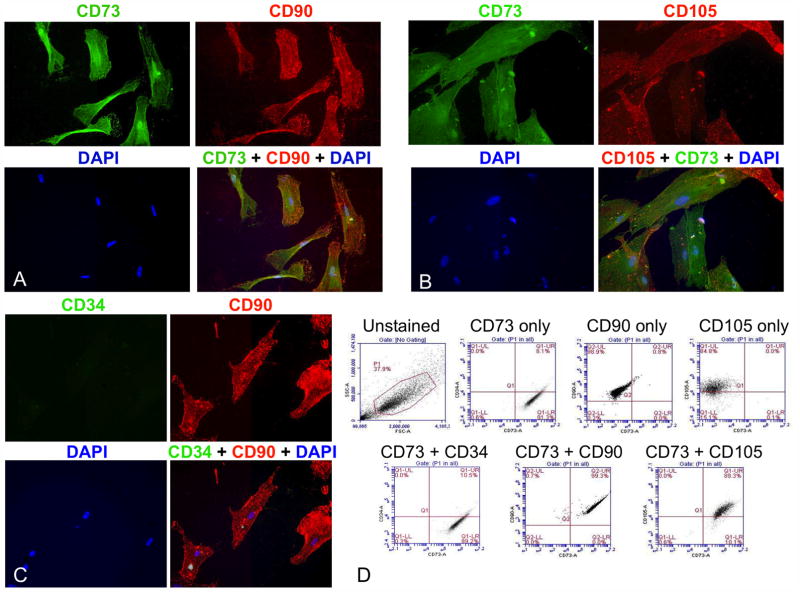
Cultured DT cells were characterized by fluorescent immunohistochemistry to assess obligate MSC markers. The cell line co-expressed CD73, CD90, and CD105 (Endoglin) (Fig. 2A and B). Appropriately, the transmembrane endothelial precursor marker, CD34, was not expressed in the DT-derived cells (Fig. 2C). Additionally, the DT-derived cells were positively stained with antibodies for TGFβ1, 2, 3 and the active form of integrin β1, and displayed a prominent F-actin cytoskeleton upon staining with Alexa Fluor 568-labeled phalloidin (Supplementary Fig. 4A and B). To prove that these DT-derived cells were authentic MSCs, fluorescence-activated cell sorting (FACS) analysis of surface antigen profiles confirmed that ~90% of the population co-expressed MSC positive, but not negative markers (Fig. 2D).
Figure 2. DT-derived MSCs co-expressed positive but not negative MSC markers.
Fluorescent immunohistochemistry was performed on early passage DT-derived MSCs demonstrating co-expression of the MSC markers CD73 (green) and CD90 (red) (A) as well as CD73 (green) and CD105 (red) (B), but not expression of the negative endothelial precursor marker, CD34 (C). Original magnification was 40X. FACS analysis of these cells showed co-expression of the obligate MSC markers CD73, CD90, and CD105, but not CD34 (D). The gated population is outlined in red on the top, left dot plot. Sorting showed that: 99.1% of cells were CD73+, CD34−; 99.3% were CD73+, CD90+; and 89.3% were CD73+, CD105+.
CD34+ fibrocytes also populate DTs
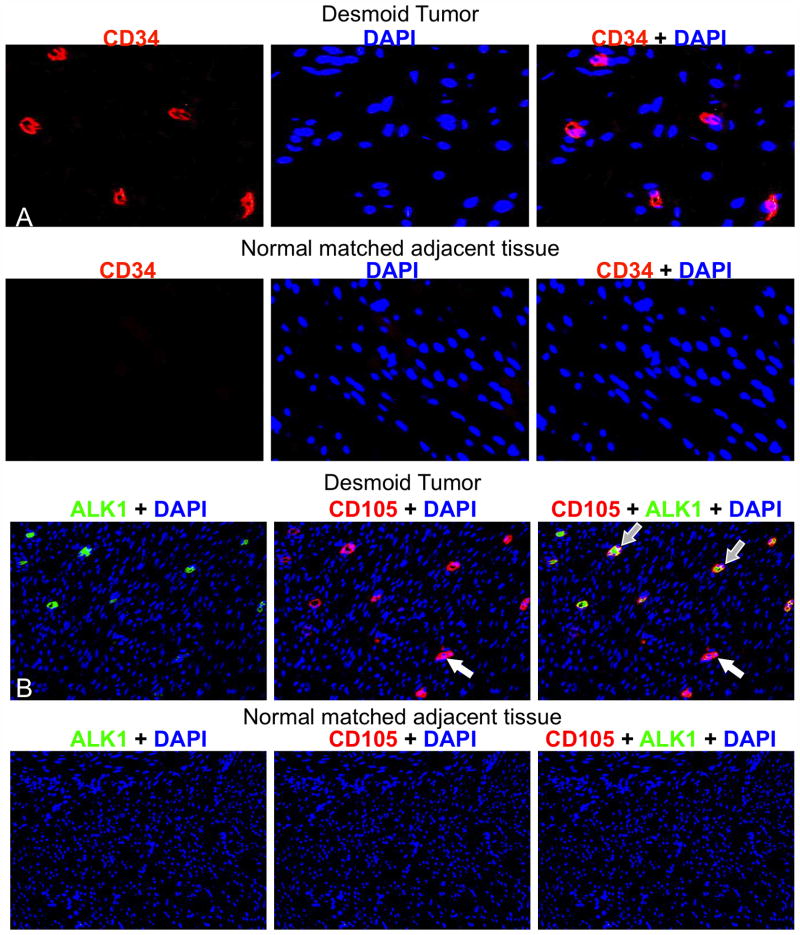
Monocyte precursors home to sites of tissue injury and differentiate into CD34+ fibrocytes (17). Because MSCs and HSC-derived progenitors maintain close physical contacts, we anticipated that CD34+ fibrocytes would be found in DTs. We performed fluorescent immunohistochemistry for CD34 on our archived DT collection and the matching set of adjacent normal tissues. Consistent with a wound healing origin, CD34+ fibrocytes were present in tumors but not in matched normal tissue (Fig. 3A). The number of CD34+ cells did not vary significantly between FAP and sporadic DTs (Supplementary Fig. 2).
Figure 3. DTs contained CD34+, CD105+, and ALK1+ cells.
Representative fluorescent immunohistochemistry images are shown of formalin-fixed, paraffin-embedded DTs stained for CD34 (red) (A, Top). Matched normal adjacent tissue did not display any CD34+ cells (A, Bottom) (original magnification 40X). Representative fluorescent immunohistochemistry images of formalin-fixed paraffin-embedded archival DTs stained for ALK1 (red) and CD105 (green) (B, Top). Matched normal adjacent tissue showed only AKL1− and CD105− cells (B, Bottom). Open arrows indicate CD105+ ALK1− cells; grey filled arrows indicate dual positive cells. The original magnification was 20X.
CD34+ fibrocytes give rise to endothelial cells that stimulate angiogenesis. The TGFβ signaling receptor, ALK1, and CD105 accessory protein are co-expressed in endothelial cells and promote angiogenesis during embryonic development (34). Expression of ALK1 in normal adult vasculature is minimal, but is induced during wound healing and tumor progression (35). Fluorescent immunohistochemistry assessed ALK1 and CD105 co-expression in DTs (Fig. 3B). Among many cells expressing CD105, a subset co-expressed ALK1. This result is consistent with the observation that fibrocytes express both CD34 and CD105, whereas MSCs lack CD34 expression (24). As expected, neither CD105 nor ALK1 were expressed in the matched normal tissues from DT patients (Fig. 3B).
Tri-lineage mesenchymal differentiation of DT-derived MSCs
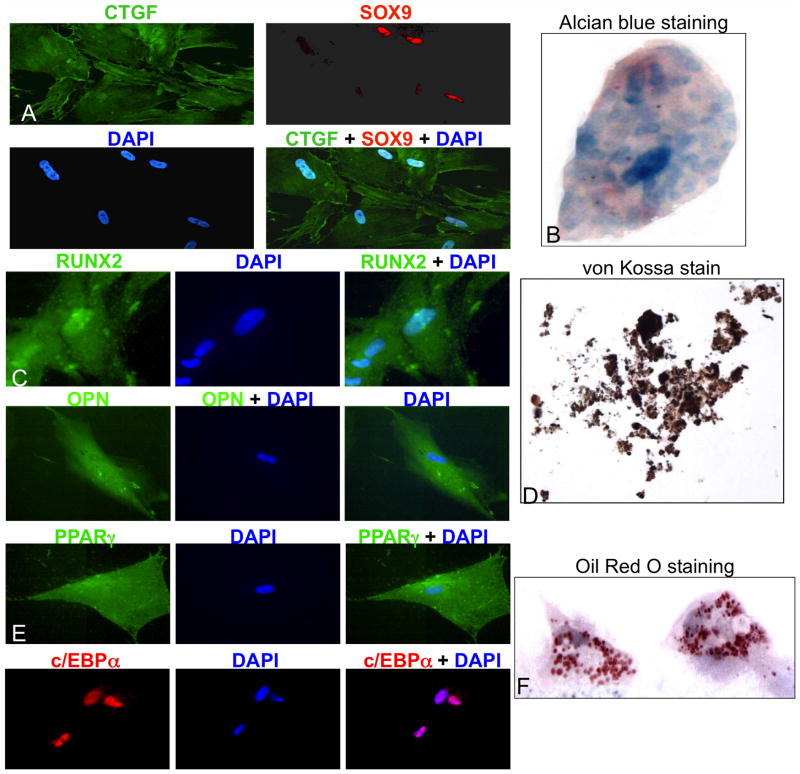
In addition to FACS, multipotency must be demonstrated to confirm MSC status. The DT-derived cells were cultured for 5–6 weeks in differentiation medium, and then assayed for lineage-specific markers to confirm differentiation into chondrocytes, adipocytes, and osteocytes (Fig. 4). Chondrocyte differentiation requires activity of the transcription factor, SOX9, and chondrocytes specifically express Connective Tissue Growth Factor (CTGF). Fluorescent immunohistochemistry showed that the DT-derived cells grown in chondrocyte differentiation medium co-expressed these markers, and confirmed nuclear localization of SOX9 (Fig. 4A). Also, collagen proteoglycans present in the chondrocytes yielded the appropriate result when fixed DT-derived cells were subjected to Alcian Blue staining (Fig. 4B). Osteocyte differentiation requires activity of the osteoblast-specific transcription factor RUNX2, and osteopontin (OPN) is a glycoprotein selectively secreted by osteocytes. Fluorescent immunohistochemistry showed that the DT-derived cells grown in osteocyte differentiation medium expressed these markers with nuclear and cytoplasmic localization of RUNX2 (Fig. 4C). In addition, a black precipitate characteristic of osteocyte mineralization was evident when these cells were subjected to von Kossa staining (Fig. 4D). Adipocyte differentiation requires the activities of transcription regulators PPARγ and c/EBPα. Fluorescent immunohistochemistry showed that the DT-derived cells grown in adipocyte differentiation medium expressed these markers with nuclear localization of c/EPBα. In addition, lipid droplets were present with Oil Red O staining (Fig. 4E and F). Taken together these data confirmed development of a multipotent MSC line from a human DT.
Figure 4. DT-derived MSCs exhibited tri-potent differentiation capability.
DT-derived cells were incubated in differentiation media, and confirmation of differentiation was obtained by expression of chondrocyte markers CTGF (green) and Sox9 (red), overlay shows yellow stain (A) plus positive Alcian Blue staining (B); osteocyte markers RUNX2 (green) and OPN (green) (C) plus positive von Kossa staining (D); and adipocyte markers PPARγ (green) and c/EBPα (red) (E) plus positive Oil Red O staining (F). Original magnification was 40X.
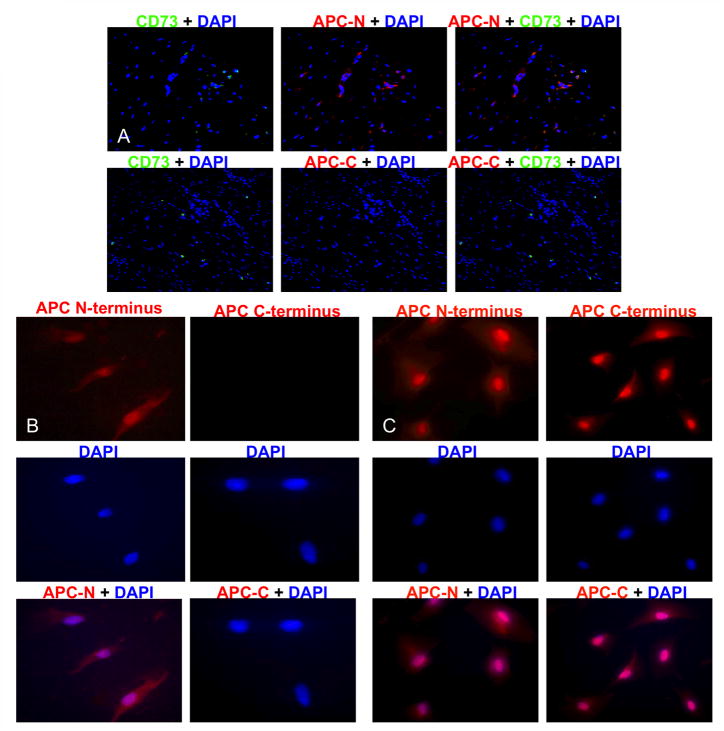
MSCs expressed the N-terminus but not the C-terminus of APC protein
The germline of patients with FAP contains a mutation in one APC gene that typically truncates the carboxy-terminus of the encoded protein. Deregulated Wnt signaling occurs in APC-associated tumors including DTs when tumor-engendering stem cells sustain loss of heterozygosity (LOH) of the wild-type APC allele (7). We confirmed that MSCs from both the original DT and the DT-derived cell line were APC-deficient by performing fluorescent immunohistochemistry to detect the carboxy- vs. amino-terminus of APC. This assay showed that although the truncated APC product was present, MSCs in both the DT and the cell line lacked the APC carboxy terminus antigen, consistent with somatic LOH (Fig. 5A and B). As a positive control, immunohistochemistry performed in parallel on first passage normal human adipose-derived MSCs confirmed the presence of both terminal ends of APC protein in these non-tumor cells (Fig. 5C).
Figure 5. MSCs expressed only the APC-N terminus consistent with somatic APC+ LOH.
Fluorescent immunohistochemistry was performed on paraffin-embedded DTs and DT-derived cells for both the APC (N-15) and (C-20) termini. Normal human adipose-derived MSCs were used as controls. In MSCs from both the DT (A) and cell line (B), staining was positive for the N-terminus (red) but not the C-terminus. In contrast, staining was positive for both N- and C-termini of APC protein in normal human adipose-derived MSCs (C). Original magnification was 10X and 20X. Overlay shows a purple nuclear stain.
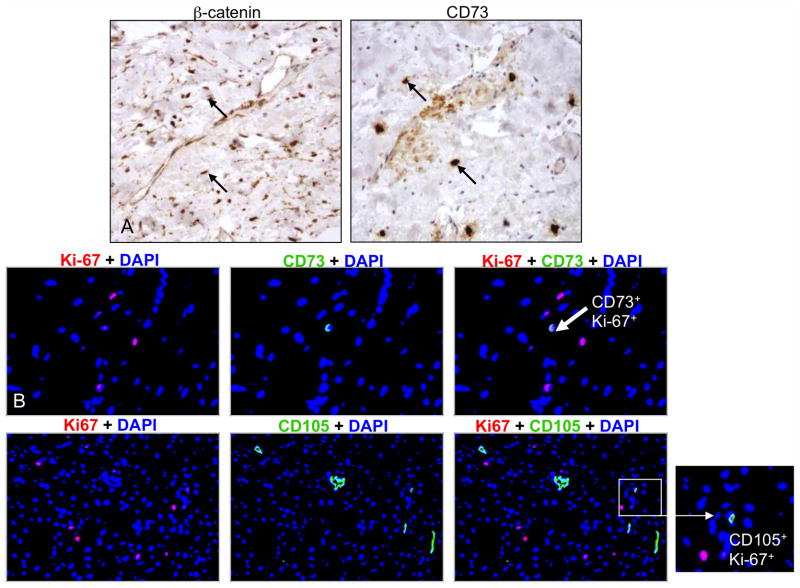
Wnt signaling is activated in APC-deficient MSCs
APC loss leads to activation of Wnt signaling as demonstrated by the accumulation of nuclear β-catenin. Previously, we showed that abundant nuclear β-catenin was present in our archived DT library (36). Nuclear β-catenin was also detected in DT-embedded MSCs, suggesting β-catenin stabilization (Fig. 6A). Immunostaining of DTs for the proliferation marker, Ki-67, showed that CD73+ MSCs were among the Ki-67+ population (Fig. 6B).
Figure 6. MSCs demonstrated nuclear β-catenin localization.
Immunohistochemistry was performed on paraffin-embedded DTs and showed accumulation of nuclear β-catenin in MSCs, indicating active Wnt signaling (A). Original magnification was 10X and 40X. Fluorescent immunohistochemistry of DT sections stained for Ki-67 (red) and CD73 or CD105 (green) identified dually positive cells (B).
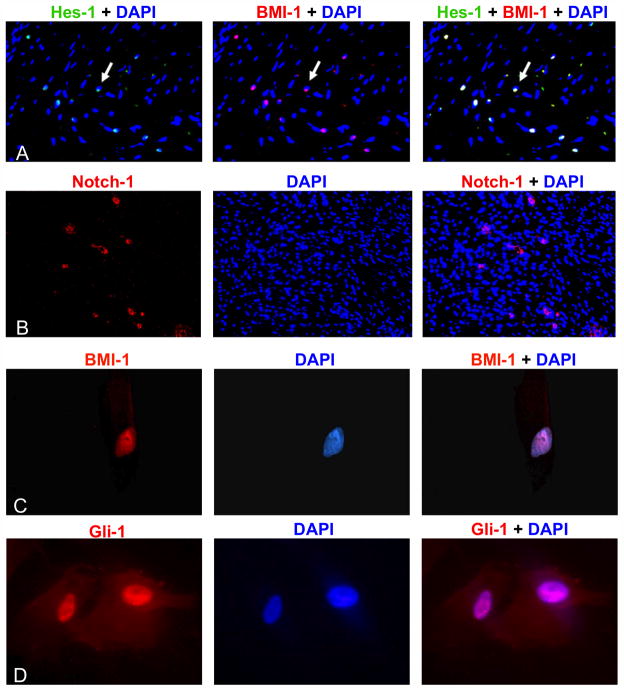
Notch and Hedgehog pathways are activated in DT-derived MSCs
Activation of Notch and Hedgehog signaling is implicated in the development of multiple cancers. To investigate the presence of Notch signaling in desmoid tumorigenesis, immunostaining of DTs showed that MSCs expressed Notch-1 and its activation target, Hes-1 (Fig. 7A and B). Consistent with our findings in tumor specimens (Fig 1C and D), we also found that the DT-derived cells expressed BMI-1, as well as its upstream Hedgehog activation target, Gli-1 (Fig. 7C and D). Notch signaling is presumed to play a positive role in regulating BMI-1 function since transcriptional repression of this target is relieved by γ-secretase inhibitors.
Figure 7. Notch and Hedgehog pathways were upregulated in DTs.
Fluorescent immunohistochemistry was performed on paraffin-embedded DTs and showed positive expression of the Notch target gene Hes-1 (green) (A) and Notch-1 (red) (B). Co-localization with BMI-1 (red) produced yellow nuclei (A). DT-derived cells also expressed the transcriptional repressor BMI-1 (C). Co-localization of red staining of BMI-1 with the blue counter-stain produced purple-colored nuclei. Similarly, purple nuclei were evident when the MSCs were stained with antibodies for the Hedgehog transcriptional activator, Gli-1 (red) (D).
Discussion
In this study, we examined a tumor of mesenchymal origin to obtain insight into stromal cell interactions and desmoid tumorigenesis. We showed that both MSCs and HSCs are present in human DTs but not in matching adjacent normal tissues. We also produced a human DT-derived MSC line and showed that both the original tumor and cell line contained truncated APC protein. Furthermore, we demonstrated the presence of nuclear β-catenin within these APC-deficient MSCs, denoting active Wnt signaling. Taken together, our findings suggest that DTs may arise from MSCs following APC mutation and subsequent Wnt signaling deregulation.
DTs are relatively acellular neoplasms that do not metastasize (1, 2). They are composed of cells of fibroblast lineage, and exhibit a marked fibrotic response with significant vascularity. Importantly, their histological characteristics are indistinguishable from that of non-neoplastic lesions associated with abnormal wound healing, including hypertrophic scars or dermal keloids (19, 20, 21). There are data suggesting that a wound healing response may induce DT formation (11, 12). Indeed, clinicians treating patients with FAP have noted a tendency for surgery to increase DT growth, and it is not uncommon for sporadic DTs to present initially within surgical scars.
Tumorigenesis shares characteristics observed in wound healing. For instance, symmetrical stem cell division, a process yielding stem cell duplication rather than the stem cell and its committed progenitor, is a prominent feature of the proliferative phase of normal wound healing. Tumors are initiated by symmetrical stem cell division that entails fixation of a mutation inactivating a critical tumor suppressor gene, such as APC, or activating a specific oncogene, such as CTNNB1 (7, 8, 9, 10, 11, 12). Tumor growth proceeds with continued self-renewal of the mutant stem cell and expansion of its progeny. Since DTs have a monoclonal origin (25, 26), it is feasible that this initiating mutation occurs in a MSC recruited or activated in situ during the proliferative response to tissue stress or injury. In this scenario, the specific mutation allows stem cell growth without the normal instructive positional, spatial, and mechanical cues provided by the tissue microenvironment (37). In particular, signaling via Wnt, Hedgehog, and Notch pathways governs normal adult stem cell behavior in vivo. Appropriate responses in MSCs to input from these physical or paracrine cues may be compromised specifically by APC loss and contribute to DT etiology.
MSCs are multipotent cells that reside in most adult tissues. While normally quiescent, they serve in normal tissue homeostasis by contributing to the maintenance of stromal elements. During wound healing, these multipotent cells aid tissue regeneration. HSC-derived monocyte precursors circulate in the blood, and are distinguished from MSCs by expression of CD34, a marker found on short term reconstituting HSCs and their multipotent progenitors (16, 17). MSCs and HSCs cohabit the same niche in bone marrow, and a relationship of MSC-dependent support of HSCs is illustrated in co-culture studies, which show that expansion of HSCs in vitro is stimulated by the addition of MSCs (38, 39). In a systemic response, MSCs and HSC-derived monocyte precursors are recruited to sites of tissue injury where both cell types proliferate. In a wound healing context, monocyte precursors differentiate into multipotent CD34+ fibrocytes, and, depending on the availability of certain cytokines, differentiate into endothelial cells or myofibroblasts (24). Fibrocytes and myofibroblasts produce extracellular matrix components such as collagens and express matrix metalloproteinases, factors needed for tissue remodeling during wound healing (16, 17). In circumstances where the proliferative phase of wound healing fails to terminate, myofibroblasts also express fibrogenic factors such as TGFβ and inflammatory mediators such as the prostaglandin, PGE2.
Here, we show that MSCs are present in DTs and may contribute to the etiology of these neoplasms. This latter claim is supported by the absence of APC+/+ protein in both the FAP-associated DT and its derived MSC line, indicating clonal expansion of a cell that acquired an APC loss of heterozygosity (Fig. 5). Absent APC function, β-catenin is constitutively stabilized (Fig. 6). Although we previously noted abundant nuclear β-catenin in DTs and stromal cells, here we observed few proliferating MSCs (Ki67+) (36). Hoos et al. corroborated our findings and showed that Ki-67 expression was not upregulated in DTs, in contrast with other malignant tumors (40). Although our data suggest that Wnt signaling does not drive DT proliferation, we propose that excess Wnt signaling maintains MSCs in an immature state. Ross et al. demonstrated that enforced Wnt signaling inhibits terminal differentiation of pre-adipocytes (MSC progenitors) and a similar conclusion was found in HSCs (41, 42).
Our DT-derived MSC line also exhibited stem cell behavior (Fig. 2 and 4). Therefore, notwithstanding APC loss or stabilized β-catenin protein, mutant MSCs associated with DT formation presumably remain non-autonomous for growth and maintenance, and require both supporting cells and a niche microenvironment. Similar to conditions in the bone marrow, we found that MSCs resided with CD34+ fibrocytes in DTs, suggesting that these latter cells may provide support for the MSC tumor cells (38). MSCs and CD34+ fibrocytes also co-exist in keloids and hypertrophic scars, which DTs resemble histologically (19, 20, 21). Our findings suggest that DT etiology resembles defective wound healing, and the presence of immature stromal progenitor cells in both dermal tumors and DTs may reflect Wnt signaling-dependent inhibition of differentiation (41, 42). Furthermore, studies show that homing of HSC-derived progenitors to peripheral tissues under conditions of chronic inflammation causes fibrosis (43). It is therefore possible that the high levels of fibrosis and angiogenesis found in DTs derive from a tumor-associated inflammatory response, with persistent recruitment of CD34+ fibrocytes.
Under conditions of tissue homeostasis, canonical Wnt signaling is tightly controlled, permitting proliferation of committed progeny from adult stem cells, differentiation, and niche exit. However, it is likely that deficient negative regulation of Wnt signaling increases the self-renewal of tumor-initiating MSCs that arise in FAP-associated DTs with APC loss and in sporadic DTs with stabilizing CTNNB1 mutations (7, 8, 9, 10, 11, 12). Three Wnt target genes are relevant. Secreted Frizzled-related protein1 (sFRP1) is expressed in DTs and encodes a soluble Wnt receptor antagonist (27). Although its loss allows constitutive Wnt signaling and drives cancer growth, SFRP1 gene expression, resulting in canonical Wnt pathway inhibition, increases non-cancerous stem cell self-renewal (44). SFRP1 expression is downstream of Gli-1 activity, which we showed was expressed in DT-derived MSCs (Fig. 7D). Importantly, SFRP1 expression regulates β-catenin activity in HSCs, and in DTs may promote the retention and maintenance of CD34+ fibrocytes (45). We also showed that DTs contained CD73+ MSCs expressing CD44 (Fig. 1F). CD44, a hyaluronic acid adhesion receptor, is up-regulated in APC-deficient cells and potently affects stem cell niche production and homing of HSC-derived progenitors (46).
Our main goal is to identify aspects of DT biology that will define effective therapies for this challenging disease. Our results enable predictions about potential therapies for DTs that target either MSCs or CD34+ fibrocytes. We found that DT-derived MSCs expressed BMI-1 and the Hedghog signaling effector, Gli-1 (Fig. 7C and D). BMI-1 is a transcriptional repressor downstream of Hedgehog and Notch pathways. Drugs for these targets include Notch pathway γ-secretase inhibitors and Hedgehog inhibitors. Endothelial cells specifically utilize the TGFβ pathway to stimulate angiogenesis via CD105, ALK1, and BMP9 under conditions of wound healing and tumor progression (35). We showed that within DTs, a population of cells co-expressed ALK1 and CD105 (Fig. 3B); therefore, survival of CD34+ fibrocyte-derived endothelial cells in DTs may be inhibited by anti-angiogenesis therapies directed against ALK1 (47). If DTs depend on maintenance and proximity of both pluripotent MSCs and CD34+ fibrocytes, then therapies aimed at differentiating or reprogramming these cells might disturb the niche requirements of one or both. We showed that DT-derived APC-deficient MSCs were capable of terminal differentiation. Therefore, differentiation therapies such as PPARγ agonists or retinoids may be effective (48, 49). Finally, our work shows that tumor MSC lines can be generated from human DTs, thereby providing a resource that can accelerate preclinical drug testing.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by The Eleanor and Miles Shore/Robert T. Osteen Junior Faculty Research Grant from the Department of Surgery at Brigham and Women’s Hospital awarded to N.L.C., and K05-CA131504 awarded to M.M.B.
We thank Drs. Jonathan Fletcher and Adrian Marino-Enriquez for technical support. This work was supported by The Eleanor and Miles Shore/Robert T. Osteen Junior Faculty Research Grant from the Department of Surgery at Brigham and Women’s Hospital awarded to N.L.C., and K05-CA131504 awarded to M.M.B.
Abbreviations
- DTs
Desmoid tumors
- MSCs
mesenchymal stromal cells
- FAP
familial adenomatous polyposis
- HSCs
hematopoietic stem cells
- FACS
fluorescence-activated cell sorting
- APC
Adenomatous polyposis coli
- OPN
Osteopontin
- CTGF
Connective tissue growth factor
- sFRP-1
secreted Frizzled-related protein-1
Footnotes
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Klemmer S, Pascoe L, DeCosse J. Occurrence of desmoids in patients with familial adenomatous polyposis of the colon. Am J Med Genet. 1987;28:385–392. doi: 10.1002/ajmg.1320280217. [DOI] [PubMed] [Google Scholar]
- 2.Rodriquez-Bigas MA, Mahoney MC, Karakousis CP, Petrelli NJ. Desmoid tumors in patients with familial adenomatous polyposis. Cancer. 1994;74:1270–1274. doi: 10.1002/1097-0142(19940815)74:4<1270::aid-cncr2820740415>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 3.Hansmann A, Adolph C, Vogel T, Unger A, Moeslein G. High-dose tamoxifen and sulindac as first-line treatment for desmoid tumors. Cancer. 2004;100:612–620. doi: 10.1002/cncr.11937. [DOI] [PubMed] [Google Scholar]
- 4.Mace J, Sybil Biermann J, Sondak V, McGinn C, Hayes C, Thomas D, et al. Response of extra-abdominal desmoid tumors to therapy with imatinib mesylate. Cancer. 2002;95:2373–2379. doi: 10.1002/cncr.11029. [DOI] [PubMed] [Google Scholar]
- 5.Bertagnolli MM, Morgan JA, Fletcher CD, Raut CP, Dileo P, Gill RR, et al. Multimodality treatment of mesenteric desmoid tumors. Eur J Cancer. 2008;44:2404–2410. doi: 10.1016/j.ejca.2008.06.038. [DOI] [PubMed] [Google Scholar]
- 6.Alfaro MP, Pagni M, Vincent A, Atkinson J, Hill MF, Cates J, et al. The Wnt modulator sFRP2 enhances mesenchymal stem cell engraftment, granulation tissue formation and myocardial repair. Proc Natl Acad Sci USA. 2008;105:18366–18371. doi: 10.1073/pnas.0803437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Latchford A, Volikos E, Johnson V, Rogers P, Suraweera N, Tomlinson I, et al. APC mutations in FAP-associated desmoid tumours are non-random but not “just right”. Hum Mol Genet. 2007;16:78–82. doi: 10.1093/hmg/ddl442. [DOI] [PubMed] [Google Scholar]
- 8.Salas S, Chibon F, Noguchi T, Terrier P, Ranchere-Vince D, Lagarde P, et al. Molecular characterization by array comparative genomic hybridization and DNA sequencing of 194 desmoid tumors. Genes Chromosomes Cancer. 2010;49:560–568. doi: 10.1002/gcc.20766. [DOI] [PubMed] [Google Scholar]
- 9.Tejpar S, Nollet F, Li C, Wunder JS, Michils G, dal Cin P, et al. Predominance of β-catenin mutations and β-catenin dysregulation in sporadic aggressive fibromatosis (desmoid tumor) Oncogene. 1999;18:6873–6882. doi: 10.1038/sj.onc.1203041. [DOI] [PubMed] [Google Scholar]
- 10.Lazar AJ, Tuvin D, Hajibashi S, Habeeb S, Bolshakov S, Mayordomo-Aranda E, et al. Specific mutations in the β-catenin gene (CTNNB1) correlate with local recurrence in sporadic desmoid tumors. Am J Pathol. 2008;173:1518–1527. doi: 10.2353/ajpath.2008.080475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mills BG, Frausto A, Brien E. Cytokines associated with the pathophysiology of aggressive fibromatosis. J Orthop Res. 2000;18:655–662. doi: 10.1002/jor.1100180419. [DOI] [PubMed] [Google Scholar]
- 12.Cheon SS, Cheah AY, Turley S, Nadesan P, Poon R, Clevers H, et al. β-Catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proc Natl Acad Sci USA. 2002;99:6973–6978. doi: 10.1073/pnas.102657399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 14.Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A, et al. Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells. 2004;22:812–822. doi: 10.1634/stemcells.22-5-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang G, Bunnell BA, Painter RG, Quiniones BC, Tom S, Lanson NA, et al. Adult stem cells from the bone marrow stroma differentiate into airway epithelial cells: potential therapy for cystic fibrosis. Proc Natl Acad Sci USA. 2005;102:186–191. doi: 10.1073/pnas.0406266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87:858–870. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 17.Metz CN. Fibrocytes: a unique cell population implicated in wound healing. Cell Mol Life Sci. 2003;60:1342–1350. doi: 10.1007/s00018-003-2328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Opalenik SR, Davidson JM. Fibroblast differentiation of bone marrow-derived cells during wound repair. FASEB J. 2005;19:1561–1563. doi: 10.1096/fj.04-2978fje. [DOI] [PubMed] [Google Scholar]
- 19.Yang L, Scott PG, Medina A, Jiao H, Shankowsky HA, Ghahary A, et al. Identification of fibrocytes in postburn hypertrophic scar. Wound Repair Regen. 2005;13:398–404. doi: 10.1111/j.1067-1927.2005.130407.x. [DOI] [PubMed] [Google Scholar]
- 20.Moon JH, Kwak SS, Park G, Jung HY, Yoon BS, Park J, et al. Isolation and characterization of multipotent human keloid-derived mesenchymal stem cells. Stem Cells Dev. 2008;17:713–724. doi: 10.1089/scd.2007.0210. [DOI] [PubMed] [Google Scholar]
- 21.Akino K, Akita S, Yakabe A, Mineda Y, Hayashi T, Hirano A. Human mesenchymal stem cells may be involved in keloid pathogenesis. Int J Dermatol. 2008;47:1112–1117. doi: 10.1111/j.1365-4632.2008.03380.x. [DOI] [PubMed] [Google Scholar]
- 22.Hartlapp I, Abe R, Saeed RW, Peng T, Voelter W, Bucala R, et al. Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. FASEB J. 2001;15:2215–2224. doi: 10.1096/fj.01-0049com. [DOI] [PubMed] [Google Scholar]
- 23.Moioli EK, Clark PA, Chen M, Dennis JE, Erickson HP, Gerson SL, et al. Synergistic actions of hematopoietic and mesenchymal stem/progenitor cells in vascularizing bioengineered tissues. PLoS One. 2008;3(12):e3922. doi: 10.1371/journal.pone.0003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Y, Zhao RC, Tredget EE. Concise review: bone marrow-derived stem/progenitor cells in cutaneous repair and regeneration. Stem Cells. 2010;28:901–915. doi: 10.1002/stem.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Middleton SB, Frayling IM, Phillips RKS. Desmoids in familial adenomatous polyposis are monoclonal proliferations. Br J Cancer. 2000;82:827–832. doi: 10.1054/bjoc.1999.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li M, Cordon-Cardo C, Gerald WL, Rosai J. Desmoid fibromatosis is a clonal process. Hum Pathol. 1996;27:939–943. doi: 10.1016/s0046-8177(96)90221-x. [DOI] [PubMed] [Google Scholar]
- 27.Bacac M, Migliavacca E, Stehle JC, McKee T, Delorenzi M, Coindre JM, et al. A gene expression signature that distinguishes desmoid tumours from nodular fasciitis. J Pathol. 2006;208:543–553. doi: 10.1002/path.1915. [DOI] [PubMed] [Google Scholar]
- 28.Denys H, De Wever O, Nusgens B, Kong Y, Sciot R, Le AT, et al. Invasion and MMP expression profile in desmoid tumours. Br J Cancer. 2004;90:1443–1449. doi: 10.1038/sj.bjc.6601661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nik SA, Ebrahim RP, Dam KV, Cassiman JJ, Teipar S. TGF-β modulates β-catenin stability and signaling in mesenchymal proliferations. Exp Cell Res. 2007;313:2887–2895. doi: 10.1016/j.yexcr.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 30.Wu C, Nik-Amini S, Nadesan P, Stanford WL, Alman BA. Aggressive fibromatosis (desmoid tumor) is derived from mesenchymal progenitor cells. Cancer Res. 2010;70:7690–7698. doi: 10.1158/0008-5472.CAN-10-1656. [DOI] [PubMed] [Google Scholar]
- 31.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 32.Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CP, et al. Ex vivo gylcan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14:181–187. doi: 10.1038/nm1703. [DOI] [PubMed] [Google Scholar]
- 33.Deng J, Petersen BE, Steindler DA, Jorgensen ML, Laywell ED. Mesenchymal stem cells spontaneously express neural proteins in culture and are neurogenic after transplantation. Stem Cells. 2006;24:1054–1064. doi: 10.1634/stemcells.2005-0370. [DOI] [PubMed] [Google Scholar]
- 34.Roelen BA, van Rooijen MA, Mummery CL. Expression of ALK1, a type 1 serine/threonine kinase receptor, coincides with sites of vasculogenesis and angiogenesis in early mouse development. Dev Dyn. 1997;209:418–430. doi: 10.1002/(SICI)1097-0177(199708)209:4<418::AID-AJA9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 35.Cunha SI, Pietras K. ALK1 as an emerging target for antiangiogenic therapy. Blood. 2011;117(26):6999–7006. doi: 10.1182/blood-2011-01-330142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho NL, Carothers AM, Rizvi H, Hasson RM, Redston M, Bertagnolli MM. Immunohistochemical and molecular analysis of tyrosine kinase activity in desmoid tumors. J Surg Res. 2010 Nov 26; doi: 10.1016/j.jss.2010.10.037. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.Vazin T, Schaffer DV. Engineering strategies to emulate the stem cell niche. Trends Biotechnol. 2009;28:117–124. doi: 10.1016/j.tibtech.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson SN, Ng J, Niu T, Yang H, McMannis JD, Karandish S, et al. Superior ex vivo cord blood expansion following co-culture with bone marrow-derived mesenchymal stem cells. Bone Marrow Transpl. 2006;37:359–366. doi: 10.1038/sj.bmt.1705258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoos A, Lewis JJ, Antonescu CR, Dudas ME, Leon L, Woodruff JM, et al. Characterization of molecular abnormalities in human fibroblastic neoplasms: a model for genotype-phenotype association in soft tissue tumors. Cancer Res. 2001;61:3171–3175. [PubMed] [Google Scholar]
- 41.Ross SE, Erickson RL, Gerin I, DeRose PM, Bajnok L, Longo KA, et al. Microarray analyses during adipogenesis: Understanding the effects of Wnt signaling on adipogenesis and the roles of liver X receptor α in adipocyte metabolism. Mol Cell Biol. 2002;22:5989–5999. doi: 10.1128/MCB.22.16.5989-5999.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luis TC, Naber BAE, Roozen PPC, Brugman MH, de Haas EFE, Ghazvini M, et al. Canonical Wnt signaling regulates hematopoiesis in a dosage-dependent fashion. Cell Stem Cell. 2011;9:345–356. doi: 10.1016/j.stem.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 43.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Chen WD, et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. 2004;36:417–422. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- 45.Renström J, Istvanffy R, Gauthier K, Shimono A, Mages J, Jardon-Alvarez A, et al. Secreted Frizzled-related protein 1 extrinsically regulates cycling activity and maintenance of hematopietic stem cells. Cell Stem Cell. 2009;5:157–167. doi: 10.1016/j.stem.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 46.Wielenga VJM, Smits R, Korinek V, Smit L, Kielman M, Fodde R, et al. Expression of CD44 in Apc and Tcf mutant mice implies regulation by the Wnt pathway. Am J Pathol. 1999;154:515–523. doi: 10.1016/S0002-9440(10)65297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitchell D, Pobre EG, Mulivor AW, Grinberg AV, Castonguay R, Monnell TE, et al. ALK1 inhibits multiple mediators of angiogenesis and suppresses tumor growth. Mol Cancer Ther. 2010;9:379–388. doi: 10.1158/1535-7163.MCT-09-0650. [DOI] [PubMed] [Google Scholar]
- 48.Styner M, Sen B, Xie Z, Case N, Rubin J. Indomethacin promotes adipogenesis of mesenchymal stem cells through a cyclooxygenase independent mechanism. J Cell Biochem. 2011;111:1042–1050. doi: 10.1002/jcb.22793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gudas LJ, Wagner JA. Retinoids regulate stem cell differentiation. J Cell Physiol. 2011;226:322–330. doi: 10.1002/jcp.22417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.