Abstract
Objective: We aimed to perform a preliminary study of the association between induced pluripotent stem cell (iPS)-related genes and biological behavior of human colorectal cancer (CRC) cells, and the potential for developing anti-cancer drugs targeting these genes. Methods: We used real-time reverse transcriptase polymerase chain reaction (RT-PCR) to evaluate the transcript levels of iPS-related genes NANOG, OCT4, SOX2, C-MYC and KLF4 in CRC cell lines and cancer stem cells (CSCs)-enriched tumor spheres. NANOG was knockdowned in CRC cell line SW620 by lentiviral transduction. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays, plate colony formation, and a mouse xenograft model were used to evaluate alterations in biological behavior in NANOG-knockdown SW620 cells. Also, mock-knockdown and NANOG-knockdown cells were treated with 5-fluorouracil (5-FU) and survival rate was measured by MTT assay to evaluate drug sensitivity. Results: A significant difference in the transcript levels of iPS-related genes between tumor spheres and their parental bulky cells was observed. NANOG knockdown suppressed proliferation, colony formation, and in vivo tumorigenicity but increased the sensitivity to 5-FU of SW620 cells. 5-FU treatment greatly inhibited the expression of the major stemness-associated genes NANOG, OCT4, and SOX2. Conclusions: These results collectively suggest an overlap between iPS-related genes and CSCs in CRC. Quenching a certain gene NANOG may truncate the aggressiveness of CRC cells.
Keywords: Induced pluripotent stem cell, Cancer stem cell, Colorectal cancer, NANOG, 5-Fluorouracil
1. Introduction
The hypothesis that malignant tumors are driven by a minor group of tumor-initiating cells, namely cancer stem cells (CSCs), was proposed long ago and has been evolving for several decades. Prominent parallels have been observed between cancer cells and stem cells, such as self-renewal and similar signaling pathways and surface markers (Lapidot et al., 1994; Reya et al., 2001; Galli et al., 2004; Singh et al., 2004; Bjerkvig et al., 2005; Morrison and Kimble, 2006; Wang et al., 2009; Korkaya and Wicha, 2010). There is growing evidence suggesting that CSCs are indispensable for tumor initiation in a variety of tumor types (Lapidot et al., 1994; Galli et al., 2004; Singh et al., 2004; Wang et al., 2009), though the CSC theory is still controversial (Kelly et al., 2007; Quintana et al., 2008; Shackleton et al., 2009). Besides hunting for valid surface markers or regulators for CSCs in a certain type of tumor, increasing efforts have been made to determine the origin of CSCs and the overall mechanisms controlling oncogenesis driven by CSCs.
The successful artificial de-differentiation of adult somatic cells (Takahashi et al., 2007; Yu et al., 2007; Park et al., 2008) and cancer cells (Carette et al., 2010; Miyoshi et al., 2010) to an embryonic stem cell-like state by defined reprogramming factors, including OCT4, SOX2, KLF4, C-MYC, NANOG, and LIN28, has drawn growing attention to the putative resemblance between the laboratory generation of induced pluripotent stem/cancer (iPS/iPC) cells in vitro and the spontaneous generation of CSCs in vivo. We speculate that partial reactivation of the reprogramming factor set and inappropriate micro-environments may lead to a disordered self-renewal and differentiation capacity rather than regulated pluripotency. Increasing evidence shows that reprogramming factors are over-expressed in a variety of malignant tumors, including colorectal cancer (CRC), and participate in tumor progression (Almstrup et al., 2004; Ben-Porath et al., 2008; Saiki et al., 2009; Bae et al., 2010; Fang et al., 2010; Meng et al., 2010). However, most studies have been based on analysis of the bulky cells from cell lines or patient samples. In this study, for the first time, we used CSCs-enriched tumor spheres as a subject to investigate the relationship between these reprogramming factors and CSCs in human CRC cell lines.
CSCs are suspected to be responsible for relapse, metastasis, and drug-resistance in malignant tumors (Li et al., 2007; Lacerda et al., 2010; Singh and Settleman, 2010). Anti-cancer agents targeting CSCs and which can be used as potential adjuvants to conventional chemotherapy have yet to be developed (Tang et al., 2007). In a preliminary attempt to explore the potential of these reprogramming factors as anti-CSC targets, we chose the most promising one—NANOG, to evaluate its role in the biological behavior of CRC cells. Loss-of-function studies demonstrated that NANOG was important in tumor proliferation, tumorigenicity and sensitivity to standard chemotherapy agents. The relationship between reprogramming factor silencing and standard chemotherapy needs further research.
2. Materials and methods
2.1. Cell culture
SW620, SW480 and HT29 CRC cell lines were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA) and cultured according to ATCC’s protocols. Tumor spheres were obtained from these cell lines as follows: cells were trypsinized, washed twice with phosphate buffered saline (PBS), and added to low-attachment tissue culture plates; cells were maintained in serum-free (Leibovitz’s) L-15 (for SW620 and SW480) or McCoy’s 5a (for HT29) growth medium containing 4 U/L insulin, 20 ng/L basic fibroblast growth factor (b-FGF), 20 ng/L epidermal growth factor (EGF), 0.1% bovine serum albumin (BSA). Medium was changed every 2 d and cells were split at a 1:2 ratio.
2.2. Isolation of RNA and real-time reverse transcriptase polymerase chain reaction (RT-PCR) analysis
Total RNA from cell lines and tumor spheres was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The transcript levels of NANOG, SOX2, OCT4, KLF4, and C-MYC were determined by real-time PCR using the Applied Biosystems StepOne™ Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA). The PCR reactions were carried out in a total volume of 20 μl per well containing SYBR master mix reagent kit (Applied Biosystems) using published primers (Yu et al., 2007; Park et al., 2008). Human glyceraldehyde phosphate dehydrogenase (GAPDH) was amplified as an endogenous control.
2.3. Establishment of stable NANOG-knockdown SW620 cell clones
Small hairpin RNA (shRNA) lentiviral particles used for NANOG knockdown (sc-43958-v) and mock knockdown (sc-108080) were purchased from Santa Cruz (Santa Cruz, CA, USA). The viral particles were used to infect SW620 cells following the manufacturer’s instructions. The infected cells were selected with 3 μg/ml puromycin dihydrochloride 72 h after transduction. The medium was changed every 3‒4 d until puromycin-resistant colonies were evident. Surviving colonies were pooled and dispensed into 96-well plates at a density of 0.5 cell/well. About two weeks later, single colonies evident in some wells were picked into 24-well plates, cultured with puromycin selection medium and evaluated for NANOG mRNA expression using real-time RT-PCR.
2.4. Cell proliferation assay
Cells were prepared at a concentration of 8×103 cells/200 μl and then distributed in 96-well plates at 200 µl/well and cultured overnight. MTT assays were performed every day for up to 5 d. Briefly, 20 μl of 5 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma, St. Louis, MO, USA) was added to each well; plates were incubated at 37 °C for 4 h and the supernatants were removed carefully; 150 μl of dimethyl sulfoxide (DMSO) was added to each well and the plates were agitated on a shaker for 5 min. The optical density (OD) was measured with a microplate reader (BioRad, Hercules, CA, USA) at 570 nm. Experiments were performed in triplicate.
2.5. Plate colony formation assay
Cell colony formation rate was measured using a plate colony formation assay. About 2 000 cells were added to each well of a 6-well plate. Plates were incubated at 37 °C in an incubator for two weeks and colonies containing at least fifty cells were counted under a microscope.
2.6. Mouse xenograft model
Our animal protocol was approved and performed strictly in accordance with the relevant ethics regulations of Zhejiang Chinese Medical University. SW620 mock-knockdown cells and SW620 NANOG-knockdown cells were cultured until 80%‒90% confluence before harvesting. Cells were trypsinized, washed twice with PBS, and resuspended in serum-free (Leibovitz’s) L-15 medium to a concentration of 2×105, 1×106, or 5×106 per 200 μl. Then, some 200 μl of cells were injected subcutaneously into the dorsal flanks of 5-week-old female nude mice. Tumor sizes were measured in two dimensions with calipers twice a week and tumor volumes (mm3) were calculated as L×W 2×0.5 (L is tumor length and W is tumor width).
2.7. Statistical analysis
For continuous variables, data were expressed as mean±standard error (SE). Results of cell proliferation, plate colony formation assays, and in vivo tumorigenicity assays were analyzed by analysis of variance (ANOVA), with P<0.05 in all cases considered statistically significant.
3. Results and discussion
3.1. Differential expression of iPS-related genes in human CRC tumor spheres and bulky cells
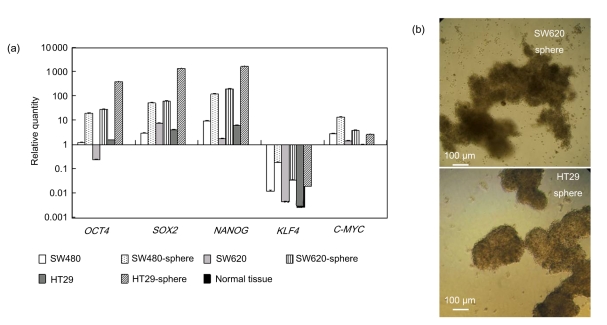
To obtain CSCs-enriched samples from CRC cell lines, we used a spheroid-culture system which has been extensively used for various tumor types, including CRC, and has proved to be suitable for enriching cancer-initiating cells (Todaro et al., 2007; Yeung et al., 2010; Cao et al., 2011; Fan et al., 2011; Yin et al., 2011). Undifferentiated tumor spheres derived from human CRC cell lines SW620, SW480, and HT-29 were cultured in serum-free medium containing EGF and FGF-2 (Dontu et al., 2003; Ma et al., 2010) (Fig. 1b). We performed real-time RT-PCR to evaluate mRNA expression of the reprogramming factors NANOG, SOX2, OCT4, KLF4, and C-MYC in both tumor spheres and their parental bulky cells (Fig. 1a). Normal human colon epithelial tissue RNA was used as a normal control (NC). Bulky cells from CRC cell lines showed relatively high expression of NANOG, SOX2, and OCT4 compared with NC. However, this alteration was almost negligible compared to the striking elevation in their sphere-like descendants. We did not see significant changes in mRNA levels of the oncogene C-MYC, which is involved mostly in cell proliferation and is dispensable in the reprogramming process. These data suggest that the minor CSC subsets may contribute a majority, if not all, of the expression of reprogramming factors in malignant tumors, as previously reported (Almstrup et al., 2004; Ben-Porath et al., 2008; Jeter et al., 2009; Saiki et al., 2009; Bae et al., 2010; Fang et al., 2010; Meng et al., 2010), which was in accordance with our hypothesis that tumor initiation might be achieved by “dysregulated-reprogramming”.
Fig. 1.
iPS reprogramming factors expressed differentially in human CRC tumor spheres and bulky cells
(a) A bar chart showing mRNA levels of NANOG, OCT4, SOX2, C-MYC, and KLF4 in CRC tumor spheres and their parental cell lines (Y-axis: relative expression levels normalized to normal colon epithelial tissue; P<0.05); (b) Morphology of tumor spheres
KLF4 was found to decrease substantially, which was predictable, since KLF4 is considered to be a normal regulator in the gastrointestinal tract epithelium (Wei et al., 2006; Yori et al., 2010) and a potential tumor suppressor in CRC (Zhao et al., 2004). Note that KLF4 expression in tumor spheres was relatively close to normal levels, implying a context-dependent property rather than stemness significance in colon cancer.
Spheroid culture has a heterogeneous property: several studies have shown that the cells in the inner region of a tumor sphere are quiescent (Freyer and Sutherland, 1980; Sherar et al., 1987; Tindall and Please, 2007) and more resistant to chemotherapeutics compared with those in the more proliferative outer layer (Wibe, 1980; Waleh et al., 1995). Since the mechanism of sphere formation is still elusive, it will be of great interest to find out which part of the tumor sphere expresses the high level of iPS-related genes. It is more likely that the quiescent inner cells express these “stemness” genes, considering that embryonic stem cells are low-proliferating. Some novel techniques, such as the use of a thermo-reversible CyGEL™ reagent that stabilizes live 3D tumor spheroids (Robertson et al., 2010), may help to locate the region highly expressing iPS-related genes.
3.2. Impaired proliferation, colony formation ability and tumorigenicity in NANOG-knockdown cells
The roles of reprogramming factors OCT3/4, SOX2, and NANOG in the progression of CRC and other tumor types have been studied extensively (Jeter et al., 2009; Saiki et al., 2009; Bae et al., 2010; Fang et al., 2010; Meng et al., 2010), although there has been limited research on the potential use of anti-CSC agents targeting these genes. We aimed to conduct a preliminary study on the significance of developing a therapeutic agent against a certain reprogramming factor. We chose to focus on NANOG because it is scarcely expressed in normal organs or tissues in the human body compared with OCT4 and SOX2, which implies that side effects could be more amenable in prospective therapeutic use. Although we found a prominent increase of NANOG in tumor spheres, we used parental bulk cells because the objective of our study was to target the general CRC cells in the human body, rather than only CSCs. CRC cell line SW620 was an ideal cell line to use because of its metastatic property and high tumorigenicity in mouse transplantation.
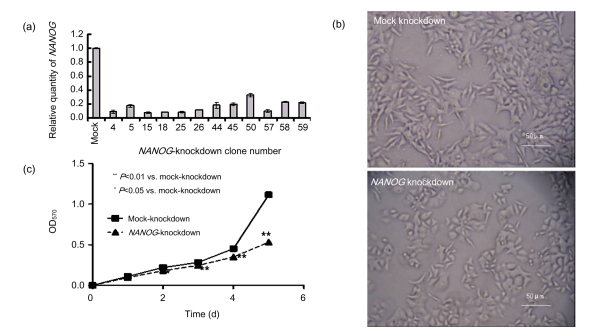
We established stable NANOG-knockdown SW620 cell clones using lentivirus-mediated shRNA gene silencing technology. NANOG mRNA expression levels were determined by real-time RT-PCR (Fig. 2a). Clones that showed less than 20% NANOG expression compared to the parental SW620 cell line were expanded individually for future use. Clones were mixed together equally before experiments. Interestingly, we noticed that NANOG-knockdown cells underwent remarkable alterations in their morphology (Fig. 2b) compared with parental SW620 cells. A typical clone displayed a flatter and rounder shape, while mock cells were protuberant and spindle-like, resembling typical SW620 cells. This alteration may predict attenuated motility, which is consistent with the previous finding that over-expression of NANOG could increase the migratory ability of SW480 cells in a wound healing assay (Meng et al., 2010). MTT assays illustrated a decreased proliferation in NANOG-knockdown cells (Fig. 2c).
Fig. 2.
Changes of cell morphology and growth rate of NANOG-knockdown SW620 cells
(a) Expression profile of NANOG knockdown clones; (b) Alterations of morphology in NANOG-knockdown SW620 cells; (c) Effect of NANOG knockdown on cell proliferation determined by MTT assay
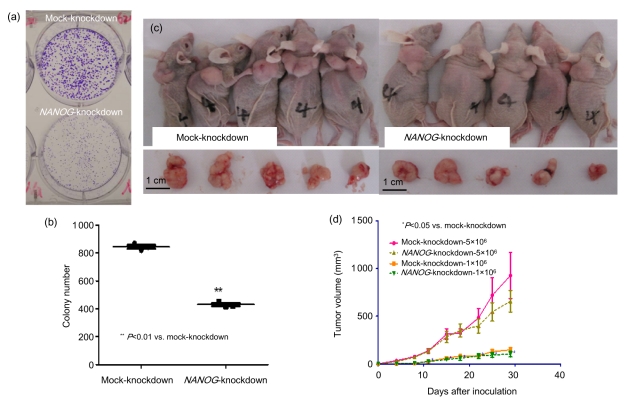
NANOG silencing significantly impaired the ability of SW620 cells to form colonies in the plate colony formation assay. NANOG-knockdown cells generated colonies with an apparent reduction in size and number (Figs. 3a and 3b). Moreover, the xenograft model showed that NANOG played a negative role in tumorigenesis of SW620 cells in vivo. Equal numbers of mock-knockdown and NANOG-knockdown cells were injected into the dorsal flanks of 5-week-old female nude mice. Tumor growth curves indicated that NANOG knockdown inhibits proliferation of SW620 cells in vivo (Fig. 3d). Furthermore, mice injected with mock-knockdown cells exhibited a morbid status both physically and mentally whereas mice in the NANOG-knockdown group looked healthier and more vigorous (Fig. 3c). This study provided a prophylactic model of NANOG interference in vivo. We will try to establish a therapeutic model using shRNA gene silencing after tumor cell inoculation in a future study.
Fig. 3.
Suppression of plate colony formation ability and in vivo tumorigenicity of NANOG-knockdown SW620 cells
(a) Representative pictures of colonies on plates; (b) Number of colonies formed from 2 000 cells (data are representatives of three independent experiments); (c) Representative pictures of tumor samples; (d) Growth curve of mock-knockdown and NANOG-knockdown cells in in vivo mouse model
3.3. Synergism of chemotherapy agent 5-FU treatment and NANOG silencing in suppressing SW620 cells in vitro
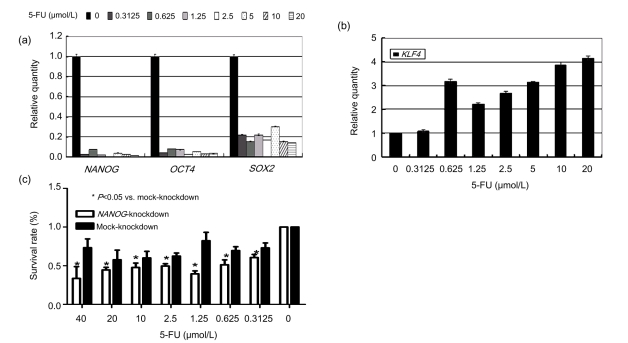
To investigate the effect of the standard chemotherapy agent 5-FU on stemness-associated genes, we treated SW620 cells with 5-FU at different concentrations (Fig. 4). After 5 d, mRNA levels of NANOG, SOX2, OCT4, and KLF4 were measured by real-time RT-PCR. We speculated that administration of 5-FU would kill the common bulky cells and enrich stem-like cells. Surprisingly, the levels of NANOG, SOX2, and OCT4 decreased dramatically, especially those of NANOG and SOX2 (Fig. 4a), whereas that of KLF4 went up slightly (Fig. 4b). These results collectively imply that a 5-d 5-FU treatment will suppress these stemness-associated genes.
Fig. 4.
Synergism of 5-FU treatment and NANOG silencing in suppressing SW620 cells in vitro
Transcript levels of (a) NANOG, OCT4, SOX2, and (b) KLF4 after a 5-d 5-FU treatment (Y-axis: relative expression levels normalized to SW620 cells without 5-FU treatment; P<0.05); (c) Survival rates of NANOG-knockdown and mock-knockdown cells after 72 h 5-FU treatment determined by MTT assay (Y-axis: relative absorbance normalized to NANOG-knockdown or mock-knockdown cells without 5-FU treatment)
This may be because once CSCs sense adverse changes in the niche, they will trigger a self-protecting system which allows them to stop self-renewal, inactivate most non-essential transcript activities, and step into dormancy. Another possibility is that 5-FU is able to interfere with the transcription stability of these genes. Several studies have indicated that chemotherapeutic agents like 5-FU are likely to enrich cancer cells with a CSC phenotype, based on elevated CSC surface marker levels or clonogenic ability (Dylla et al., 2008; Dallas et al., 2009). According to these findings, we could speculate that it is more likely that 5-FU disturbs the stability of these genes rather than shrinks the CSC population. This hypothesis needs support from in vivo experiments since a lack of a stem cell niche, such as in angiogenesis, could also explain the instability of CSCs. Another interesting study showed that the levels of SOX2 and OCT4 in residual cancer cells from patients after chemoradiotherapy were positively associated with distant recurrence (Saigusa et al., 2009). This result implies that the impact of chemoradiotherapy on cancer cells in vivo is a more context-dependent and patient-specific process. It will be more meaningful to conduct further studies on multiple primary cancer cell lines than on established cell lines.
The suppression of the stemness-associated gene NANOG raised the sensitivity of SW620 cells to 5-FU. We treated both mock-knockdown cells and NANOG-knockdown cells with 5-FU for 72 h and determined the survival rate by MTT assay. NANOG-knockdown cells displayed a decreased survival rate compared to mock cells when exposed to the same concentration of 5-FU.
These results suggest that regular chemotherapy and silencing of the stemness-associated factor NANOG act in synergy, and are not simply complementary. The addition of anti-CSC agents to regular chemotherapy may greatly enhance therapeutic efficiency and reduce the drug dose, which means fewer side effects. Systemic administration of anti-CSC agents after chemotherapy may be used as a strategy to prevent tumor relapse and metastasis.
The antimetabolite 5-FU is one of the standard chemotherapy regimens for colon cancer. Adjuvant chemotherapy has also been conducted with oxaliplatin and irinotecan in the last decade. However, persuasive evidence for including molecular targeted therapy is still lacking. Current molecular targeted therapy agents, like bevacizumab and cetuximab, function mainly by inhibiting angiogenesis. As a putative prime candidate for refractoriness of malignant cancer, CSCs warrant more attention. We have cast a brick to attract jade, hopefully encouraging the emergence of valuable research on therapy targeting CSCs in the coming decade.
4. Conclusions
For the first time, we have found that transcript levels of iPS-related genes OCT4, SOX2, and NANOG are strikingly high in CSCs-enriched human CRC tumor cells. RNA interference of NANOG could truncate the aggressiveness of CRC cells by suppressing proliferation, invasion, tumorigenicity and by increasing their sensitivity to 5-FU. Therefore, these reprogramming factors may play an important role in tumor progression. Strategies to interfere with these genes may have great clinical significance. Hopefully, we can draw more attention to anti-cancer therapies targeting these reprogramming factors.
Acknowledgments
We thank Drs. Yong-ming FANG, Jun YE, Hai LIU, Xue-feng FANG, and Zhi-gang CHEN (Cancer Institute, the Second Affiliated Hospital, School of Medicine, Zhejiang University, China) for their technical assistance, and the entire laboratory for fruitful discussions. We also thank Dr. Adebusola Abosede ALAGBALA (Center for Cell and Gene Therapy, Department of Pathology and Immunology, Baylor College of Medicine, USA) for writing assistance.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 30973382) and the Zhejiang Provincial International Scientific Technology Collaboration Key Project (No. 2009C14010)
References
- 1.Almstrup K, Hoei-Hansen CE, Wirkner U, Blake J, Schwager C, Ansorge W, Nielsen JE, Skakkebaek NE, Rajpert-de Meyts E, Leffers H. Embryonic stem cell-like features of testicular carcinoma in situ revealed by genome-wide gene expression profiling. Cancer Res. 2004;64(14):4736–4743. doi: 10.1158/0008-5472.CAN-04-0679. [DOI] [PubMed] [Google Scholar]
- 2.Bae KM, Su Z, Frye C, McClellan S, Allan RW, Andrejewski JT, Kelley V, Jorgensen M, Steindler DA, Vieweg J, et al. Expression of pluripotent stem cell reprogramming factors by prostate tumor initiating cells. J Urol. 2010;183(5):2045–2053. doi: 10.1016/j.juro.2009.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, Weinberg RA. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40(5):499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjerkvig R, Tysnes BB, Aboody KS, Najbauer J, Terzis AJ. Opinion: the origin of the cancer stem cell: current controversies and new insights. Nat Rev Cancer. 2005;5(11):899–904. doi: 10.1038/nrc1740. [DOI] [PubMed] [Google Scholar]
- 5.Cao L, Zhou Y, Zhai B, Liao J, Xu W, Zhang R, Li J, Zhang Y, Chen L, Qian H, et al. Sphere-forming cell subpopulations with cancer stem cell properties in human hepatoma cell lines. BMC Gastroenterol. 2011;11(1):71. doi: 10.1186/1471-230X-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carette JE, Pruszak J, Varadarajan M, Blomen VA, Gokhale S, Camargo FD, Wernig M, Jaenisch R, Brummelkamp TR. Generation of iPSCs from cultured human malignant cells. Blood. 2010;115(20):4039–4042. doi: 10.1182/blood-2009-07-231845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dallas NA, Xia L, Fan F, Gray MJ, Gaur P, van Buren GII, Samuel S, Kim MP, Lim SJ, Ellis LM. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res. 2009;69(5):1951–1957. doi: 10.1158/0008-5472.CAN-08-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17(10):1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dylla SJ, Beviglia L, Park IK, Chartier C, Raval J, Ngan L, Pickell K, Aguilar J, Lazetic S, Smith-Berdan S, et al. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS One. 2008;3(6):e2428. doi: 10.1371/journal.pone.0002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan X, Ouyang N, Teng H, Yao H. Isolation and characterization of spheroid cells from the HT29 colon cancer cell line. Int J Colorectal Dis. 2011;26(10):1279–1285. doi: 10.1007/s00384-011-1248-y. [DOI] [PubMed] [Google Scholar]
- 11.Fang X, Yu W, Li L, Shao J, Zhao N, Chen Q, Ye Z, Lin SC, Zheng S, Lin B. ChIP-seq and functional analysis of the SOX2 gene in colorectal cancers. OMICS. 2010;14(4):369–384. doi: 10.1089/omi.2010.0053. [DOI] [PubMed] [Google Scholar]
- 12.Freyer JP, Sutherland RM. Selective dissociation and characterization of cells from different regions of multicell tumor spheroids. Cancer Res. 1980;40(11):3956–3965. [PubMed] [Google Scholar]
- 13.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, de Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64(19):7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 14.Jeter CR, Badeaux M, Choy G, Chandra D, Patrawala L, Liu C, Calhoun-Davis T, Zaehres H, Daley GQ, Tang DG. Functional evidence that the self-renewal gene NANOG regulates human tumor development. Stem Cells. 2009;27(5):993–1005. doi: 10.1002/stem.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317(5836):337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- 16.Korkaya H, Wicha MS. Cancer stem cells: nature versus nurture. Nat Cell Biol. 2010;12(5):419–421. doi: 10.1038/ncb0510-419. [DOI] [PubMed] [Google Scholar]
- 17.Lacerda L, Pusztai L, Woodward WA. The role of tumor initiating cells in drug resistance of breast cancer: implications for future therapeutic approaches. Drug Resist Updat. 2010;13(4-5):99–108. doi: 10.1016/j.drup.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 19.Li F, Tiede B, Massague J, Kang Y. Beyond tumorigenesis: cancer stem cells in metastasis. Cell Res. 2007;17(1):3–14. doi: 10.1038/sj.cr.7310118. [DOI] [PubMed] [Google Scholar]
- 20.Ma S, Tang KH, Chan YP, Lee TK, Kwan PS, Castilho A, Ng I, Man K, Wong N, To KF, et al. miR-130b Promotes CD133+ liver tumor-initiating cell growth and self-renewal via tumor protein 53-induced nuclear protein 1. Cell Stem Cell. 2010;7(6):694–707. doi: 10.1016/j.stem.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Meng HM, Zheng P, Wang XY, Liu C, Sui HM, Wu SJ, Zhou J, Ding YQ, Li JM. Overexpression of Nanog predicts tumor progression and poor prognosis in colorectal cancer. Cancer Biol Ther. 2010;9(4):295–302. doi: 10.4161/cbt.9.4.10666. [DOI] [PubMed] [Google Scholar]
- 22.Miyoshi N, Ishii H, Nagai K, Hoshino H, Mimori K, Tanaka F, Nagano H, Sekimoto M, Doki Y, Mori M. Defined factors induce reprogramming of gastrointestinal cancer cells. PNAS. 2010;107(1):40–45. doi: 10.1073/pnas.0912407107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441(7097):1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- 24.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451(7175):141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 25.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456(7222):593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 27.Robertson FM, Ogasawara MA, Ye Z, Chu K, Pickei R, Debeb BG, Woodward WA, Hittelman WN, Cristofanilli M, Barsky SH. Imaging and analysis of 3D tumor spheroids enriched for a cancer stem cell phenotype. J Biomol Screen. 2010;15(7):820–829. doi: 10.1177/1087057110376541. [DOI] [PubMed] [Google Scholar]
- 28.Saigusa S, Tanaka K, Toiyama Y, Yokoe T, Okugawa Y, Ioue Y, Miki C, Kusunoki M. Correlation of CD133, OCT4, and SOX2 in rectal cancer and their association with distant recurrence after chemoradiotherapy. Ann Surg Oncol. 2009;16(12):3488–3498. doi: 10.1245/s10434-009-0617-z. [DOI] [PubMed] [Google Scholar]
- 29.Saiki Y, Ishimaru S, Mimori K, Takatsuno Y, Nagahara M, Ishii H, Yamada K, Mori M. Comprehensive analysis of the clinical significance of inducing pluripotent stemness-related gene expression in colorectal cancer cells. Ann Surg Oncol. 2009;16(9):2638–2644. doi: 10.1245/s10434-009-0567-5. [DOI] [PubMed] [Google Scholar]
- 30.Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138(5):822–829. doi: 10.1016/j.cell.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 31.Sherar MD, Noss MB, Foster FS. Ultrasound backscatter microscopy images the internal structure of living tumour spheroids. Nature. 1987;330(6147):493–495. doi: 10.1038/330493a0. [DOI] [PubMed] [Google Scholar]
- 32.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29(34):4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 35.Tang C, Ang BT, Pervaiz S. Cancer stem cell: target for anti-cancer therapy. FASEB J. 2007;21(14):3777–3785. doi: 10.1096/fj.07-8560rev. [DOI] [PubMed] [Google Scholar]
- 36.Tindall MJ, Please CP. Modelling the cell cycle and cell movement in multicellular tumour spheroids. Bull Math Biol. 2007;69(4):1147–1165. doi: 10.1007/s11538-006-9110-z. [DOI] [PubMed] [Google Scholar]
- 37.Todaro M, Alea MP, di Stefano AB, Cammareri P, Vermeulen L, Iovino F, Tripodo C, Russo A, Gulotta G, Medema JP, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1(4):389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Waleh NS, Brody MD, Knapp MA, Mendonca HL, Lord EM, Koch CJ, Laderoute KR, Sutherland RM. Mapping of the vascular endothelial growth factor-producing hypoxic cells in multicellular tumor spheroids using a hypoxia-specific marker. Cancer Res. 1995;55(24):6222–6226. [PubMed] [Google Scholar]
- 39.Wang X, Kruithof-de Julio M, Economides KD, Walker D, Yu H, Halili MV, Hu YP, Price SM, Abate-Shen C, Shen MM. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature. 2009;461(7263):495–500. doi: 10.1038/nature08361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei D, Kanai M, Huang S, Xie K. Emerging role of KLF4 in human gastrointestinal cancer. Carcinogenesis. 2006;27(1):23–31. doi: 10.1093/carcin/bgi243. [DOI] [PubMed] [Google Scholar]
- 41.Wibe E. Resistance to vincristine of human cells grown as multicellular spheroids. Br J Cancer. 1980;42(6):937–941. doi: 10.1038/bjc.1980.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeung TM, Gandhi SC, Wilding JL, Muschel R, Bodmer WF. Cancer stem cells from colorectal cancer-derived cell lines. PNAS. 2010;107(8):3722–3727. doi: 10.1073/pnas.0915135107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin BB, Wu SJ, Zong HJ, Ma BJ, Cai D. Preliminary screening and identification of stem cell-like sphere clones in a gallbladder cancer cell line GBC-SD. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2011;12(4):256–263. doi: 10.1631/jzus.B1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yori JL, Johnson E, Zhou G, Jain MK, Keri RA. Kruppel-like factor 4 inhibits epithelial-to-mesenchymal transition through regulation of E-cadherin gene expression. J Biol Chem. 2010;285(22):16854–16863. doi: 10.1074/jbc.M110.114546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 46.Zhao W, Hisamuddin IM, Nandan MO, Babbin BA, Lamb NE, Yang VW. Identification of Kruppel-like factor 4 as a potential tumor suppressor gene in colorectal cancer. Oncogene. 2004;23(2):395–402. doi: 10.1038/sj.onc.1207067. [DOI] [PMC free article] [PubMed] [Google Scholar]