Abstract
Objective: To explore the effects of insulin-like growth factor-1 (IGF-1) on migration, proliferation and differentiation of mesenchymal stem cells (MSCs). Methods: MSCs were obtained from Sprague-Dawley rats by a combination of gradient centrifugation and cell culture techniques and treated with IGF-1 at concentrations of 5–20 ng/ml. Proliferation of MSCs was determined as the mean doubling time. Expression of CXC chemokine receptor 4 (CXCR4) and migration property were determined by flow cytometry and transwell migration essay, respectively. mRNA expression of GATA-4 and collagen II was determined by reverse transcription-polymerase chain reaction (RT-PCR). Results: The mean doubling time of MSC proliferation was decreased, and the expression of CXCR4 on MSCs and migration of MSCs were increased by IGF-1, all in a dose-dependent manner, while the optimal concentration of IGF-1 on proliferation and migration was different. IGF-1 did not affect the expression of GATA-4 or collagen II mRNA. Conclusions: IGF-1 dose-dependently stimulated the proliferation of MSCs, upregulated the expression of CXCR4, and accelerated migration. There was no apparent differentiation of MSCs to cardiomyocytes or chondrocytes after culturing with IGF-1 alone.
Keywords: Mesenchymal stem cells (MSCs), Proliferation, Differentiation, Insulin-like growth factor-1 (IGF-1), CXC chemokine receptor 4 (CXCR4), Migration
1. Introduction
There is growing evidence that transplantation of mesenchymal stem cells (MSCs) is effective for the treatment of some cardiovascular diseases, particularly acute myocardial infarction (Tomita et al., 1999; Piao et al., 2005). While the clinical data are encouraging (Chen et al., 2004; Katritsis et al., 2005), controversies regarding the mechanisms responsible for the cardioprotective effects of MSCs remain. It was hypothesized that the paracrine effect of MSCs plays an important role in protecting cardiomyocytes and improving cardiac function post transplantation (Tang et al., 2005; Xu et al., 2007; Xiang et al., 2009). Insulin-like growth factor-1 (IGF-1) is one of the key cytokines secreted by MSCs post transplantation (Nagaya et al., 2005; Xu et al., 2007; Tao et al., 2010). Therefore, we hypothesized that IGF-1 may play an important role in proliferation, migration, and differentiation of MSCs during transplantation.
It has been shown that IGF-1 can promote the proliferation of many kinds of cells (Ren et al., 1999; Kaplan et al., 2005), but this effect is still not fully understood. Li et al. (2007) observed that MSCs cultured in vitro with IGF-1 at final concentrations of 2.5, 5.0 and 10.0 ng/ml for 48 h did not affect the rate of proliferation. However, this was not consistent with the phenomenon that IGF-1 promotes the proliferation of other cell types. Therefore, whether this result was due to inadequate concentrations of IGF-1 or the culture time was too short needs to be further investigated.
In terms of the migration of MSCs, Li et al. (2007) demonstrated that IGF-1 can upregulate the expression of CXC chemokine receptor 4 (CXCR4) on the membrane of cultured MSCs in a dose-dependent manner, which accelerates their migration. This was confirmed by Guo et al. (2008) who cultured cells with IGF-1 at concentrations of 2.5, 5.0 and 10.0 ng/ml for 8–48 h. They found that the expression of CXCR4 was upregulated in a dose- and time-dependent fashion. CXCR4 is the unique receptor for chemokine stromal cell-derived factor-1 (SDF-1) and is expressed on the membrane of stem cells. CXCR4 is essential for the homing and migration of MSCs (Guo et al., 2008). However, because each passage of cultured MSCs takes longer than 48 h, logically the duration of exposure to IGF-1 should exceed 48 h. Therefore, whether prolonging the duration of exposure to IGF-1 beyond 48 h can further enhance the expression of CXCR4 needs to be explored.
In terms of the differentiation of MSCs, Muguruma et al. (2003) showed that the combination of IGF-1, vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (bFGF) can induce MSCs to differentiate into cardiomyocytes. Recently, Bartunek et al. (2007) found that MSCs expressed cardiomyocyte markers such as desmin and Nkx2.5 after pre-treatment with IGF-1, bFGF, and bone morphogenetic protein-2 (BMP-2). However, it is unclear whether IGF-1 alone can stimulate the differentiation of MSCs.
On the other hand, MSCs are multipotent cells, which can differentiate into chondrocytes after treatment with IGF-1 and transforming growth factor-β (TGF-β), and express collagen II. However, the role of IGF-1 alone on the expression of collagen II is still controversial (Kawamura et al., 2005; Sakimura et al., 2006). Longobardi et al. (2006) cultured MSCs in pellets with IGF-1 alone or IGF-1 in combination with TGF-β for 7 d and found that collagen II was expressed in both groups, although it was much weaker with IGF-1 alone. These are considered to be signs for dropout differentiation of MSCs after being engrafted into a damaged heart.
In this study, we cultured MSCs in vitro and incubated with different concentrations of IGF-1 for 7 d to investigate the effects of IGF-1 on proliferation, migration and differentiation properties of MSCs, thus exploring the role of IGF-1 on MSC activity after transplantation into a damaged heart.
2. Materials and methods
2.1. Isolation and culture of MSCs
Healthy Sprague-Dawley rats weighing 150–200 g were obtained from the Experimental Animal Center of Sun Yat-sen University, Guangzhou, China. Three rats were used in each of three individual experiments. The study was approved by the Ethic Committee of Sun Yat-sen University. All animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council. Bone marrow cells were obtained as described by Piao et al. (2005). After rats were anesthetized with 20% sodium urethane (0.2 g/ml; 1.0 g/kg intraperitoneal injection), bone marrow cells were extracted from the tibias and femurs. The cell suspension from three rats was mixed together and cells were dispersed by pipetting and then centrifuged at 1 100×g for 4 min at 37 °C. The supernatant and adipose tissue were removed. The cell suspension was transferred to a 15-ml centrifuge tube containing 5 ml of Percoll (1.073 g/ml, Sigma Corp., St. Louis, Missouri, USA). Cells were dispersed by pipetting again and centrifuged at 1 500×g for 30 min. The mononuclear cells in the middle layer were obtained, washed three times with phosphate buffered saline (PBS) and then suspended in low-glucose Dulbecco’s modified Eagle’s medium (L-DMEM; Invitrogen, Paisley, UK) with 20% heat-inactivated fetal bovine serum (FBS; Gibco BRL, Gaithersburg, MD, USA), 100 U/ml penicillin G, and 100 µg/ml streptomycin. Cells were then placed in 25-cm2 flasks (Corning, MA, USA) and incubated with 95% air and 5% CO2 at 37 °C. The medium was replaced every 4 d. Unattached cells were discarded and adherent cells were retained. Each primary culture was replaced in three new flasks when MSCs grew to approximately 70%–80% confluence.
2.2. Detection of membrane markers of MSCs
MSCs of the fourth passage at 70%–80% confluence were trypsinized with 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA, 2.5 g/L) and the cell suspension was centrifuged at 1 100×g for 4 min. The MSCs were diluted by PBS to a concentration of 1×106 cells/ml. Then, 50 μl of the cell suspension was added to each tube, and 10 μl of mouse anti-rat CD34, CD45, CD44 or CD29 IgG (1:500; Santa Cruz, California, USA) was added to each tube. The cell suspensions were incubated for 45 min at 4 °C and 5 ml of PBS was then added to each tube. Tubes were centrifuged at 1 100×g for 4 min and supernatant was discarded. The pellet was washed three times with PBS, and 10 μl of fluorescein isothiocyanate (FITC)-labeled goat-anti-mouse IgG (1:200; Jackson ImmunoResearch Lab, Maryland, USA) was added and incubated shielding from light for 45 min at 4 °C. MSCs were then washed three times with PBS and resuspended in PBS at a concentration of 1×106 cells/ml for flow cytometry (Becton Dickinson, USA). Fluorescent intensity was detected at excitation and emission wavelengths of 488 and 515 nm, respectively (Yang et al., 2007). This experiment was performed in triplicate.
2.3. Growth curve of MSCs cultured with IGF-1
MSCs of the fourth passage at 70%–80% confluence were harvested and seeded into eight 48-well plates (1×104 cells/well). All wells contained L-DMEM. The wells were assigned to the control and IGF-1 groups, with two plates for each concentration. MSCs in the IGF-1 groups were then cultured with IGF-1 (Sigma Corp., USA) at final concentrations of 5, 10 and 20 ng/ml. The control cells only received DMEM. The number of cells was counted with a hemocytometer every day for 8 d to calculate the mean±standard deviation (SD) of six wells in each group. Cell growth curves were drawn and the doubling time of MSCs was calculated. The doubling time of MSCs in the logarithmic growth phase was calculated using the formula: td=Δt×lg2/(lgNt−lgN0), where t d is the doubling time, Δt is the duration of the logarithmic growth (from Day 3 to Day 6; 72 h), N 0 is the number of cells at the start of the logarithmic grow phase, and Nt is the number of cells after t (d) of logarithmic growth.
2.4. Detection of CXCR4 expression by flow cytometry
After incubated with IGF-1 for 7 d, the expression of CXCR4 was detected in MSCs of the fourth passage as the mean±SD of six wells of each group. This was performed, essentially as described above for membrane markers, except that 10 μl of mouse anti-rat CXCR4 (R&D Systems, Minneapolis, Minnesota, USA) was added at 1:200.
2.5. Detection of migration of MSCs
Migration was calculated from the number of cells found to have passed through an 8-μm pore size polycarbonate membrane (Corning, MA, USA). In brief, the 24-well transwell migration system was pre-incubated with L-DMEM without FBS (for each well, 600 μl at lower chamber and 100 μl at upper chamber) at 37 °C with 5% CO2 for 24 h before migration essay. After pre-incubation, the medium was removed and the transwell system was ready for use. The fourth passage of MSCs in the control and the group incubated with IGF-1 was used in this experiment. After being harvested and washed with L-DMEM, cell concentration was adjusted to 2.0×105 cells/ml in L-DMEM. A total of 600 μl L-DMEM without FBS and 5 μl 100 μg/ml rat SDF-1α (Prospec, USA; final concentration 100 nmol/L) were added into the lower chamber of each well. A total of 100 μl of cell suspension was added onto the upper chamber. Then the inserts were placed onto the wells. The whole system was incubated at 37 °C with 5% CO2 for 6 h, and then the upper surface of the membrane was scraped to remove non-migrated cells. The inserts were fixed with 11% glutaraldehyde (0.11 g/ml; Sigma Corp., USA) for 10 min at room temperature, washed twice with double distilled water (2 min for each time), stained with crystal violet solution (Sigma Corp., USA) for 15 min at room temperature, then washed with double distilled water, and observed under a microscope. The number of cells migrated was counted on 10 random high power fields (200× magnification) of each group.
2.6. Detection of markers of chondrocytes and cardiomyocytes
The expression of markers for chondrocytes (collagen II) and cardiomyocytes (GATA-4) was assessed by reverse transcription-polymerase chain reaction (RT-PCR) in six wells from each group and glyceraldehyde phosphate dehydrogenase (GAPDH) was used as an internal control. Cardiomyocytes and chondrocytes obtained from Sprague-Dawley rats were used as a positive control for GATA-4 and collagen II, respectively. Total RNA was extracted with Trizol. PCR amplification was performed in 25 ml of reaction mixture. Each cycle consisted of denaturation for 40 s at 94 °C, annealing for 40 s (collagen II, GATA-4, GAPDH at 55 °C), extension for 1 min at 72 °C, and final extension for 5 min at 72 °C, followed by 30 cycles of PCR amplification. Each PCR product was separated by electrophoresis on 1.5% agarose gel (0.015 g/ml). The density of each PCR band was analyzed with a densitometer (ImageMaster VDS, Vilber Lourmat, Germany). The primer sequences were shown in Table 1.
Table 1.
Sequences of primers
| Gene | Primer sequence | Product length (bp) |
| Collagen II | Forward: 5′-GCGAGTCCTCTTTGTCCGCGGGACAAC-3′ | 518 |
| Reverse: 5′-TGAAGTCGATACCGCTACC-3′ | ||
| GATA-4 | Forward: 5′-ACGGAAGCCCAAGAATC-3′ | 179 |
| Reverse: 5′-CTGCTGTGCCCATAGTGA-3′ | ||
| GAPDH | Forward: 5′-TGAGGGTGAGAAGGTGGAA-3′ | 280 |
| Reverse: 5′-CCTTTCGACACCGCACTACCGGCAC-3′ |
2.7. Statistical analysis
Data are expressed as mean±SD. Statistical analysis was performed using SPSS 13.0 software (SPSS Inc., Chicago, Illinois, USA) for Windows. Differences among groups were analyzed by one-way analysis of variance (ANOVA) or χ 2 tests. P<0.05 was regarded as statistically significant.
3. Results
3.1. Expression of cell markers in MSCs
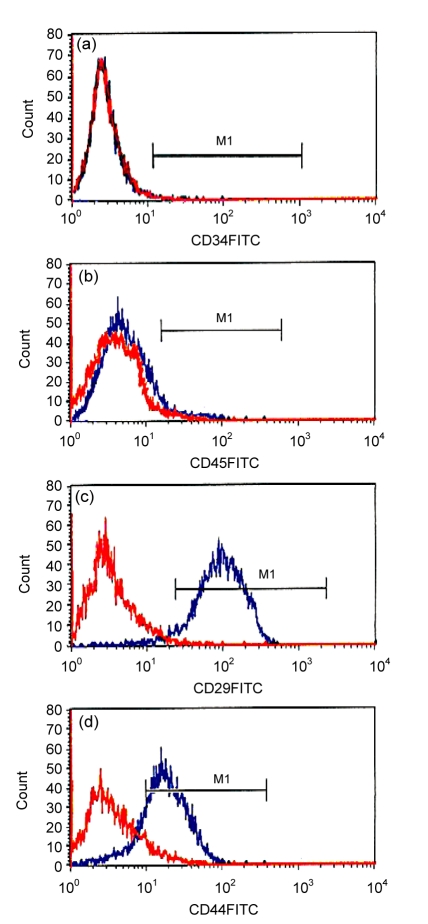
The expression of CD29, CD34, CD44, and CD45 in MSCs was detected using flow cytometry. The positive rates of CD29 and CD44 expression were (96.34±2.15)% and (78.64±3.26)%, respectively, while those of CD34 and CD45 were only (0.28±0.12)% and (1.98±0.43)%, respectively (Fig. 1). This confirmed that the MSCs isolated in this study expressed cell markers consistently with that reported previously (Majumdar et al., 2003; Pittenger and Martin, 2004). MSCs strongly express adherence factors such as CD29 and CD44 but weakly express markers of hematopoietic stem cells (HSCs) as CD34 and CD45.
Fig. 1.
Expression of cell markers in MSCs
MSCs at the fourth passage were trypsinized and diluted by PBS to a concentration of 1×106 cells/ml and then incubated with mouse anti-rat CD34, CD45, CD29 or CD44 antibodies (1:500), followed by FITC-labeled goat-anti-mouse IgG (1:200) for 45 min at 4 °C. Fluorescent intensity was detected by flow cytometry. Each group was assessed in triplicate. (a) CD34, (0.28±0.12)%; (b) CD45, (1.98±0.43)%; (c) CD29, (96.34±2.15)%; (d) CD44, (78.64±3.26)%
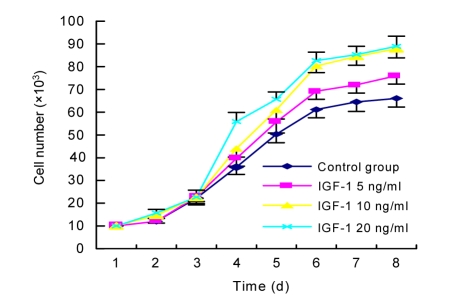
3.2. Effect of IGF-1 on proliferation of MSCs
The number of cells in six wells per group was counted every day for 8 d to generate a growth curve for each treatment (Fig. 2). The first 2 d corresponded to the latent phase, while the logarithmic growth phase lasted from Days 3 to 6, and Days 7 to 8 represented the plateau phase. Compared with the control group, the mean doubling time of MSCs in the logarithmic grow phase was significantly shorter for cells cultured in IGF-1 (P<0.05), and showed a dose-dependent effect of IGF-1 at concentrations of 5 and 10 ng/ml. The accelerating effect of IGF-1 on proliferation did not increase further at concentrations over 10 ng/ml (Table 2).
Fig. 2.
Growth curves for MSCs cultured with or without IGF-1
MSCs at the fourth passage were trypsinized, replaced and cultured with 5, 10 or 20 ng/ml IGF-1 for 7 d. The number of cells in each well was counted using a hemocytometer and cell growth curves were drawn. The growth curve for each group showed similar ‘S’ shape. The first 2 d represented the latent phase. The logarithmic growth phase was from Days 3 to 6. Days 7–8 represented the plateau phase
Table 2.
Effects of IGF-1 on MSC proliferation and migration
| Group | Doubling time (h) | CXCR4 expression (%) | Number of Migrated cells |
| Control | 49.26±4.80 | 10.38±1.29 | 33.3±4.14 |
| IGF-1 5 ng/ml | 44.63±3.24† | 18.35±2.68† | 43.9±6.41† |
| IGF-1 10 ng/ml | 39.31±4.07†* | 28.62±3.42†* | 59.8±7.42†* |
| IGF-1 20 ng/ml | 38.58±3.15†* | 38.94±3.93†*# | 105.0±11.54†*# |
Values represent means±SEM
P<0.05 vs. control group
P<0.05 vs. IGF-1 5 ng/ml group
P<0.05 vs. IGF-1 10 ng/ml group
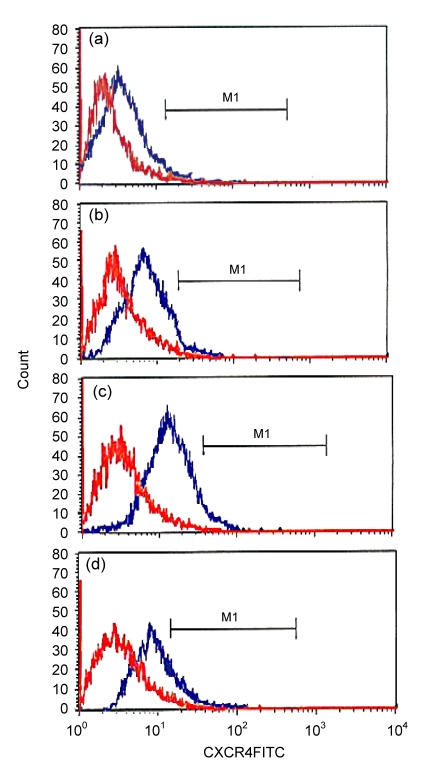
3.3. Effects of IGF-1 on expression of CXCR4 in MSCs
The positive expression rate of CXCR4 in the control cells and cells treated with 5, 10, and 20 ng/ml IGF-1 was (10.38±1.29)%, (18.35±2.68)%, (28.62±3.42)%, and (38.94±3.93)%, respectively. Therefore, the expression of CXCR4 was increased markedly, in a dose-dependent manner, after treatment with IGF-1, indicating that IGF-1 has a strong stimulatory effect on the expression of CXCR4 in MSCs (Table 2, Fig. 3).
Fig. 3.
Expression of CXCR4 in MSCs
After culture, the MSCs were trypsinized and diluted in PBS. Mouse anti-rat CXCR4 (1:200) and FITC-labeled goat-anti-mouse IgG (1:200) were added to the cell suspension and incubated for 45 min at 4 °C. Fluorescent intensity was detected by flow cytometry. (a) Control group, (10.38±1.29)%; (b) IGF-1 5 ng/ml group, (18.35±2.68)%; (c) IGF-1 10 ng/ml group, (28.62±3.42)%; (d) IGF-1 20 ng/ml group, (38.94±3.93)%
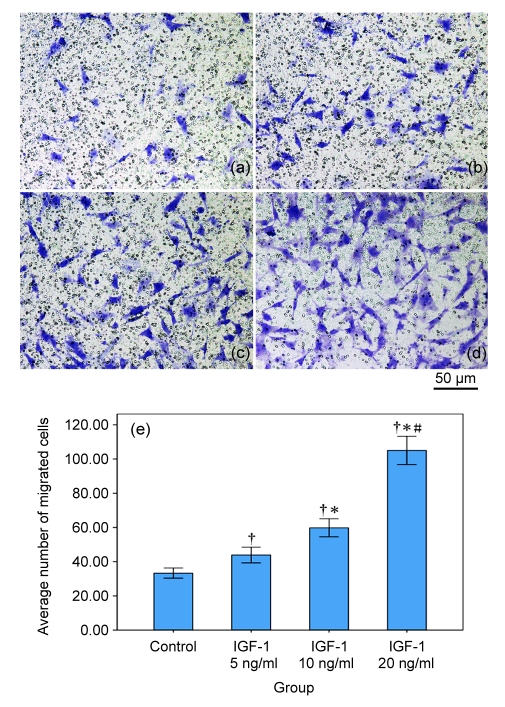
3.4. Effects of IGF-1 on migratory ability of MSCs
Amongst all the groups, the difference between negative control and IGF-1 pre-induced groups was statistically significant, and the migratory ability of MSCs was enhanced by pre-incubation with IGF-1 in a dose-dependent manner (all P<0.05). MSCs pre-induced with higher concentrations of IGF-1 showed a stronger ability of migration (Table 2, Fig. 4).
Fig. 4.
MSC migration assay by transwell
The number of cells migrated through pores was counted under 10 random high power fields for each group. IGF-1 enhanced the migratory ability of MSCs in a dose-dependent manner. (a–d) Pictures of migrated MSCs: (a) Control group; (b) IGF-1 5 ng/ml group; (c) IGF-1 10 ng/ml group; (d) IGF-1 20 ng/ml group. (e) Average number of migrated cells in each group. † P<0.05 vs. control group; * P<0.05 vs. IGF-1 5 ng/ml group; # P<0.05 vs. IGF-1 10 ng/ml group
3.5. Effects of IGF-1 on expression of GATA-4 and collagen II mRNA in MSCs
The mRNA expression of GATA-4 and collagen II was detected by RT-PCR. MSCs cultured with IGF-1 did not express GATA-4 or collagen II mRNA (Fig. 5), indicating that IGF-1 in the absence of other growth factors did not promote differentiation of MSCs to cardiomyocytes or chondrocytes.
Fig. 5.
Expression of GATA-4 and collagen II mRNA in MSCs cultured with IGF-1
The expression of markers for chondrocytes (collagen II) and cardiomyocytes (GATA-4) was assessed by RT-PCR. Cardiomyocytes and chondrocytes were used as positive controls for GATA-4 and collagen II, respectively. GAPDH was used as internal control. The PCR products for collagen II, GATA-4 and GAPDH were 518, 179 and 280 bp, respectively. Lane 1: DNA ladder DL2000 (bp); Lane 2: control group; Lane 3: IGF-1 5 ng/ml group; Lane 4: IGF-1 10 ng/ml group; Lane 5: IGF-1 20 ng/ml group; Lane 6: chondrocytes; Lane 7: cardiomyocytes; Lane 8: negative control
4. Discussion
MSCs are multipotential stem cells that can differentiate into multiple interstitial cell types, including adipocytes, chondrocytes, myocytes, osteocytes, and cardiomyocytes in suitable microenvironments in vivo or in vitro. This pluripotent property means that MSCs are very important in regenerative medicine (Pittenger and Martin, 2004). In our study, MSCs were isolated by a combination of gradient centrifugation and adherent culture screening. This improves the purity of MSCs and avoids potential adverse effects on cell viability, which are common after flow-cytometric sorting and immunomagnetic bead selection. Mononuclear cells obtained from the bone marrow were transferred into the Percoll solution layer (1.073 g/ml) by gradient centrifugation. Mononuclear cells were then obtained and purified by adherent culture screening. High-purity MSCs were obtained in this manner and their viability was also well preserved.
There is still no consensus on the specific markers of MSCs, but most researchers agree that MSCs express c-kit, Sca, CD44, CD13, CD90, CD106, Stro-1, CD29, CD71, CD120a, CD124, SH2, SH3, and SH4, but not CD34 or CD45, which are both expressed on HSCs (Majumdar et al., 2003; Pittenger and Martin, 2004). In our study, the isolated cells expressed CD29 and CD44, but not CD34 or CD45, which is consistent with previously reported expression profiles of MSCs. The positive expression rates for CD34 and CD45 were only 0.28% and 1.98%, suggesting that 98% of the HSCs were removed by the two-step isolation process.
Some studies suggest that IGF-1 can promote growth, proliferation and differentiation of many cell types, including cardiomyocytes and vascular smooth muscle cells, in vivo and in vitro, and inhibit cell apoptosis and necrosis (Ren et al., 1999; Kaplan et al., 2005). Xu et al. (2007) reported that MSCs cultured in vitro could secret IGF-1. Furthermore, Nagaya et al. (2005) demonstrated that the secretion of IGF-1 level was upregulated in conjunction with improvements in cardiac function after MSCs were transplanted into an injured heart. However, the effects of IGF-1 on proliferation, migration, and differentiation of MSCs are poorly understood.
Compared with the finding reported by Li et al. (2007), we found that IGF-1 could promote the proliferation of MSCs. Li et al. (2007) cultured MSCs obtained from Lewis rats with IGF-1 at concentrations of 2.5, 5.0, 10.0 and 20.0 ng/ml for 8, 16, 24 and 48 h, but found no stimulatory effect on cell proliferation. The differences in findings between their and our studies might be due to differences in culture duration. In our study, we found that IGF-1 did not affect proliferation until cultured for at least 3 d. Rapid proliferation period was from Days 4 to 6. We also found that the stimulatory effect was dose-dependent at IGF-1 concentrations less than 10 ng/ml. Concentrations exceeding 10 ng/ml did not further enhance proliferation.
Furthermore, we found that IGF-1 dose-dependently upregulated the expression of CXCR4 in MSCs. The effect was also dose-dependent at concentrations ranging from 5 to 20 ng/ml, and was similar to that reported by Guo et al. (2008). They cultured rat MSCs in vitro with IGF-1 at concentrations of 2.5, 5.0 and 10.0 ng/ml and infused the cells into rats with acute myocardial infarction via the tail vein. They found that IGF-1 treatment had time- and dose-dependent effects on CXCR4 expression in MSCs in vitro. Transplantation efficacy was also improved by pretreating MSCs with IGF-1. The mechanism might be that more MSCs were attracted to the infarcted area by upregulated expression of CXCR4. CXCR4 is considered to be the specific receptor for SDF-1, and MSCs could home to injured tissues when SDF-1 binds to CXCR4 (Ponte et al., 2007). In our study, we used IGF-1 to culture MSCs at a wider concentration range (5–20 ng/ml) and found that expression of CXCR4 was upregulated, and migration ability was enhanced in a dose-dependent manner, even at the concentration of 20 ng/ml. Our study indicated that the optimal concentration of IGF-1 for stimulating proliferation and migration was different, suggesting that the optimal IGF-1 concentration for culturing MSCs in vitro should vary depending on the intended use of MSCs.
MSCs are multipotential stem cells that can differentiate into multiple interstitial cell types in response to different inducing conditions in vitro. Therefore, controlling the inducing condition to promote MSC differentiation into the target cell type is a prerequisite for MSC therapy. It is important to explore the mechanisms involved in inducing differentiation and dropout of differentiation of MSCs to optimize the culture conditions, as to ensure MSC differentiation into the required cell types, and avoid differentiation dropout. However, there is currently no consensus about the effect of IGF-1 on the differentiation of MSCs.
Muguruma et al. (2003) showed that IGF-1, VEGF, and bFGF could induce MSCs to differentiate into cardiomyocytes under physiologic conditions. Recently, Bartunek et al. (2007) reported that MSCs expressed cardiomyocyte markers such as desmin and Nkx2.5 after pre-treatment with IGF-1, bFGF, and BMP-2. These studies demonstrated that IGF-1 in combination with other growth factors can promote MSCs to differentiate into cardiomyocytes. However, Sakimura et al. (2006) reported that MSCs differentiated into chondrocytes after being cultured with IGF-1 and TGF-β. Longobardi et al. (2006) cultured mouse MSCs in high-density pellets with IGF-1 alone or IGF-1 plus TGF-β for 7 d and found that collagen II was expressed in both groups, although it was much weaker in the IGF-1 alone group. These results prompted us to consider that, when transplanting MSCs pretreated with IGF-1 to cure cardiovascular diseases, we must find a way that prevents MSCs from differentiating into chondrocytes.
In our study, MSCs did not express GATA-4, a specific marker for cardiomyocytes, after treatment with IGF-1. Therefore, it is still unclear whether MSCs can differentiate towards cardiomyocytes after treatment with IGF-1 alone. Moreover, the MSCs treated with IGF-1 did not express collagen II either, suggesting that MSCs would not differentiate into chondrocytes in vitro after treatment with IGF-1 alone. Therefore, based on our findings and reports by Muguruma et al. (2003) and Bartunek et al. (2007) the differentiation of MSCs into cardiomyocytes seems to require multiple cytokines, not merely IGF-1 alone. As multiple growth factors regulate cell differentiation in a complex network, different growth factors interacting with one another could have distinct effects on the differentiation of MSCs.
Of note, MSCs cultured with IGF-1 alone did not differentiate into chondrocytes, unlike in Longobardi et al. (2006)’s study, although the expression of collagen II was weaker in MSCs cultured in IGF-1 alone than in MSCs cultured with IGF-1 plus TGF-β. This may be due to the differences in cell properties used. In our study, the cells seeded in the medium in the original passage were dispersed by pipetting, whereas Longobardi et al. (2006) used a pellet-culture method. Therefore, MSCs may be more likely to differentiate into chondrocytes when cultured in high-density pellets. This possibility needs to be evaluated in future studies.
In brief, the key findings of this study are as follows. (1) IGF-1 promotes proliferation of MSCs in a dose-dependent manner at concentrations below 10 ng/ml, but concentrations exceeding 10 ng/ml do not stimulate proliferation further. (2) IGF-1 upregulates the expression of CXCR4 in a dose- and time-dependent manner, even at concentrations as high as 20 ng/ml. The optimal concentration of IGF-1 for proliferation and migration is different, suggesting that the optimal concentration for culturing MSCs in vitro should vary according to the intended use of the MSCs. (3) There was no apparent effect of IGF-1 alone on differentiation of MSCs towards chondrocytes. Therefore, MSCs cultured with IGF-1 alone, as in our study, could be used as donor cells for cellular transplantation into an injured heart.
Footnotes
Project supported by the Guangdong Provincial Natural Science Foundation of China (No. 8151008901000157), the Scientific Research Fund of Guangdong Province, China (Nos. 2008B030301045 and 2011B031800021), and the Medical Scientific Research Grant of the Health Ministry of Guangdong Province, China (No. B2011310)
References
- 1.Bartunek J, Croissant JD, Wijns W, Gofflot S, de Lavareille A, Vanderheyden M, Kaluzhny Y, Mazouz N, Willemsen P, Penicka M, et al. Pretreatment of adult bone marrow mesenchymal stem cells with cardiomyogenic growth factors and repair of the chronically infarcted myocardium. Am J Physiol Heart Circ Physiol. 2007;292(2):H1095–H1104. doi: 10.1152/ajpheart.01009.2005. [DOI] [PubMed] [Google Scholar]
- 2.Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94(1):92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 3.Guo J, Lin G, Bao C, Hu Z, Chu H, Hu M. Insulin-like growth factor 1 improves the efficacy of mesenchymal stem cells transplantation in a rat model of myocardial infarction. J Biomed Sci. 2008;15(1):89–97. doi: 10.1007/s11373-007-9207-x. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan RC, Strickler HD, Rohan TE, Muzumdar R, Brown DL. Insulin-like growth factors and coronary heart disease. Cardiol Rev. 2005;13(1):35–39. doi: 10.1097/01.crd.0000134914.10407.40. [DOI] [PubMed] [Google Scholar]
- 5.Katritsis DG, Sotiropoulou PA, Karvouni E, Karabinos I, Korovesis S, Perez SA, Voridis EM, Papamichail M. Transcoronary transplantation of autologous mesenchymal stem cells and endothelial progenitors into infarcted human myocardium. Catheter Cardiovasc Interv. 2005;65(3):321–329. doi: 10.1002/ccd.20406. [DOI] [PubMed] [Google Scholar]
- 6.Kawamura K, Chu CR, Sobajima S, Robbins PD, Fu FH, Izzo NJ, Niyibizi C. Adenoviral-mediated transfer of TGF-β1 but not IGF-1 induces chondrogenic differentiation of human mesenchymal stem cells in pellet cultures. Exp Hematol. 2005;33(8):865–872. doi: 10.1016/j.exphem.2005.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Yu X, Lin S, Li X, Zhang S, Song YH. Insulin-like growth factor 1 enhances the migratory capacity of mesenchymal stem cells. Biochem Biophys Res Commun. 2007;356(3):780–784. doi: 10.1016/j.bbrc.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 8.Longobardi L, O′Rear L, Aakula S, Johnstone B, Shimer K, Chytil A, Horton WA, Moses HL, Spagnoli A. Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-β signaling. J Bone Miner Res. 2006;21(4):626–636. doi: 10.1359/jbmr.051213. [DOI] [PubMed] [Google Scholar]
- 9.Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, Mosca JD. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci. 2003;10(2):228–241. doi: 10.1007/BF02256058. [DOI] [PubMed] [Google Scholar]
- 10.Muguruma Y, Reyes M, Nakamura Y, Sato T, Matsuzawa H, Miyatake H, Akatsuka A, Itoh J, Yahata T, Ando K, et al. In vivo and in vitro differentiation of myocytes from human bone marrow-derived multipotent progenitor cells. Exp Hematol. 2003;31(12):1323–1330. doi: 10.1016/j.exphem.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, Fujii T, Uematsu M, Ohgushi H, Yamagishi M, et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112(8):1128–1135. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- 12.Piao H, Youn TJ, Kwon JS, Kim YH, Bae JW, Bora-Sohn, Kim DW, Cho MC, Lee MM, Park YB. Effects of bone marrow derived mesenchymal stem cells transplantation in acutely infarcting myocardium. Eur J Heart Fail. 2005;7(5):730–738. doi: 10.1016/j.ejheart.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95(1):9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 14.Ponte AL, Marais E, Gallay N, Langonne A, Delorme B, Herault O, Charbord P, Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25(7):1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 15.Ren J, Samson WK, Sowers JR. Insulin-like growth factor I as a cardiac hormone: physiological and pathophysiological implications in heart disease. J Mol Cell Cardiol. 1999;31(11):2049–2061. doi: 10.1006/jmcc.1999.1036. [DOI] [PubMed] [Google Scholar]
- 16.Sakimura K, Matsumoto T, Miyamoto C, Osaki M, Shindo H. Effects of insulin-like growth factor I on transforming growth factor β1 induced chondrogenesis of synovium-derived mesenchymal stem cells cultured in a polyglycolic acid scaffold. Cells Tissues Organs. 2006;183(2):55–61. doi: 10.1159/000095509. [DOI] [PubMed] [Google Scholar]
- 17.Tang YL, Zhao Q, Qin X, Shen L, Cheng L, Ge J, Phillips MI. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg. 2005;80(1):229–236. 236–237. doi: 10.1016/j.athoracsur.2005.02.072. [DOI] [PubMed] [Google Scholar]
- 18.Tao ZW, Li LG, Geng ZH, Dang T, Zhu SJ. Growth factors induce the improved cardiac remodeling in autologous mesenchymal stem cell-implanted failing rat hearts. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2010;11(4):238–248. doi: 10.1631/jzus.B0900244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, Sakai T, Jia ZQ. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100(s19):I247–I256. doi: 10.1161/01.cir.100.suppl_2.ii-247. [DOI] [PubMed] [Google Scholar]
- 20.Xiang MX, He AN, Wang JA, Gui C. Protective paracrine effect of mesenchymal stem cells on cardiomyocytes. J Zhejiang Univ-Sci B. 2009;10(8):619–624. doi: 10.1631/jzus.B0920153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu M, Uemura R, Dai Y, Wang Y, Pasha Z, Ashraf M. In vitro and in vivo effects of bone marrow stem cells on cardiac structure and function. J Mol Cell Cardiol. 2007;42(2):441–448. doi: 10.1016/j.yjmcc.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J, Zhou W, Zheng W, Ma Y, Lin L, Tang T, Liu J, Yu J, Zhou X, Hu J. Effects of myocardial transplantation of marrow mesenchymal stem cells transfected with vascular endothelial growth factor for the improvement of heart function and angiogenesis after myocardial infarction. Cardiology. 2007;107(1):17–29. doi: 10.1159/000093609. [DOI] [PubMed] [Google Scholar]