Abstract
Constipation is one of the most common functional digestive complaints worldwide. We investigated the laxative effects of figs (Ficus carica L) in a beagle model of constipation induced by high protein diet and movement restriction. The experiments were consecutively conducted over 9 weeks divided into 3 periods of 3 weeks each. All 15 beagles were subjected to a non-treatment (control) period, a constipation induction period, and a fig paste treatment period. We administered fig paste (12 g/kg daily, by gavage) for 3 weeks following a 3-week period of constipation induction in dogs. Segmental colonic transit time (CTT) was measured by counting radiopaque markers (Kolomark) using a radiograph performed every 6 h after feeding Kolomark capsules, until capsules were no longer observed. Fig paste significantly increased fecal quantity in constipated dogs, and segmental CTT was also reduced following fig paste administration. There were no significant differences in feed intake, water intake, body weight, or blood test results, between the constipation and fig paste administration periods. Our results demonstrate that fig is an effective treatment for constipation in beagles. Specifically, stool weight increased and segmental CTT decreased. Fig pastes may be useful as a complementary medicine in humans suffering from chronic constipation.
Keywords: Constipation, fig paste, segmental colonic transit time, Kolomark, beagle dog
Constipation is prevalent in modern societies and is a common gastrointestinal symptom in clinical practice, affecting 5 to 20% of the general population [1]. Constipation is typically defined as stool frequency of less than 3 bowel movements per week [2]. Constipation is thought to be partially caused by psychosocial and behavioral factors, such as decreased mobility, inadequate caloric intake, and anorectal sensation changes. Furthermore, it has a multifactorial etiology, including co-morbid illness and medication side effects, including those induced by narcotic analgesics, anticonvulsants, antidepressants, and anticancer drugs [3-5]. In addition, repeated use of a purgative medicine can cause chronic constipation, diarrhea, enteritis, and colorectal dysfunction, and is a risk factor for colorectal neoplasm [6,7].
Although constipation is a symptom, rather than a disease, it may be the cause of severe secondary diseases resulting from enteral fermentation, inducing toxic gas, and therefore requires active prevention and proper treatment [8]. The most common functional food remedy for constipation is dietary fiber. There are various studies on the treatment of constipation using natural substances [9,10].
The common fig (Ficus carica L) is a deciduous broadleaf shrub belonging to the Moraceae family and is widely known as one of the first edible fruits cultivated by humans in areas with subtropical climate [11]. The fig originates from Carica in Asia Minor and the primary fig producers now are America and the Mediterranean [12]. Figs are high in natural and simple sugars, minerals, water, and fiber. They contain substantial levels of potassium, calcium, magnesium, iron, copper, manganese, and sodium, while they are low in fat [13,14]. They are a good source of flavonoids and polyphenols as well as being rich in the phytosterols lanosterol and stigmasterol [12]. Several reports have shown that the leaf, stem, and woody tissue contain antioxidants and antibiotics [15,16].
The purpose of the present study was to evaluate the laxative effect of figs by measuring stool weight and colonic transit time (CTT) after fig paste administration for 3 weeks in beagles with constipation induced by a high protein diet and exercise restriction.
Materials and Methods
Materials
Figs were supplied by the Black Raspberry Research Institute (Gochang, Korea) and made into a paste at the Research Center for Industrial Development of BioFood Materials (Chonbuk University, Jeonju, Korea).
Animals
Fifteen healthy beagle dogs (4 years old; Marshall Beijing, Beijing, China) with a weight range of 10-13 kg were purchased from Orient Co (Seongnam, Korea). All animals were subjected to a physical examination and were quarantined. The animals were housed individually in clean cages (H100×W120×L120 cm) placed in a well-ventilated house with a controlled temperature (25℃), 12/12-h light/dark cycle, and illumination of 200 lux. All experiments complied with ethical standards and were approved by the Animal Ethics Committee at Wonkwang University (Iksan, Korea).
Constipation model
We created a canine model of constipation with 15 beagles who were individually housed in general cage (H100×W120×L120 cm). In order to induce a constipation condition, the dogs were restrained in a small cage (H38×W60×L50 cm) for 1 week and moved to indoor kennels.
The experiment was performed in the same 15 dogs and separated to three time sections which were control period, constipation period (constipation induction in the small cage), and +fig paste period (fig paste administration), for 3 weeks each. In control period, dogs were given feed containing 23% of proteins at the rate of 3% of weight and 100 mL/kg of water. Being changed to over 40% of high proteins (Feline feed, Purina Korea, Seoul, Korea) in constipation and +fig paste periods, feed was cut down to 2% of weight to inhibit the movement of digestive duct. Water also reduced to 80 mL/kg. Dogs during constipation and +fig paste periods were individually housed in small cage to restrict movement. Dogs during +fig paste period were administered 12 g/kg of fig paste.
Measurement of body weight, fecal weight, and feed and water intakes
We measured body weights and stool weights each week during the experimental periods. Feed and water intakes were measured for each 24-h period.
Blood biochemical analysis
We analyzed serum chemical parameters for the assessment of fig paste's safety in the experimental beagles. Venous blood was collected at the last day of each experimental period. The whole blood was centrifuged at 2,000 g for 20 min at 4℃ to obtain serum. Blood lipids including total cholesterol (TC), high-density lipoproteins (HDL) and triglycerides (TG) were analyzed using commercial kits (Sigma Diagnostics, St. Louis, MO, USA). Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) as hepatotoxicity parameters were measured using automated techniques at the Department of Diagnostic Analysis, Wonkwang University Hospital (Iksan, Korea).
Measurement of segmental CTT
Based on preliminary tests, in which normal CTT was less than 6 h, 1 capsule of Kolomark (20 Ring; Konsyl Pharmaceuticals, Easton, MD, USA) containing 20 radiopaque rings, was ingested at 09:00, and simple abdominal radiographs were taken with PET 325 (Medinet, Gwangju, Korea) were taken in the ventro-dorsal position at 6-h intervals until the Kolomark was egested. The localization of markers on abdominal films relied on identifying bony structures as suggested by Arhan et al. [17]. Markers located to the right of the vertebral spinous processes above a line from the seventh lumbar vertebra to the right pelvic outlet were assigned to the right colon, markers to the left of the vertebral spinous processes and above an imaginary line from the seventh lumbar vertebra to the anterior superior iliac crest were assigned to the left colon, and markers inferior to a line from the pelvic brim on the right and superior iliac crest on the left were judged to be in the rectosigmoid colon and rectum. CTT was calculated by monitoring the Kolomark ring movement in the entire or segmental colon [17].
Statistical analysis
Results are given as mean ± standard error (SE). Differences were tested for significance by one-way ANOVA (Duncan's multiple-range test). Differences were considered significant at error probabilities smaller than 0.05.
Results
Feed and water intakes and body weights
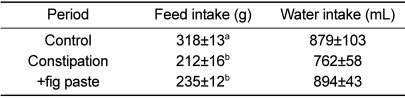
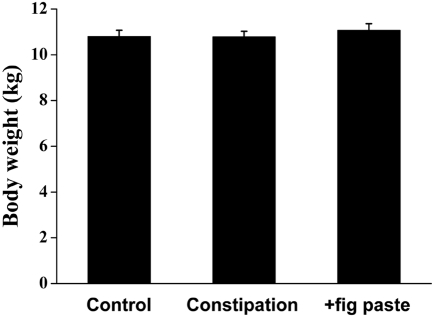
Feed intake was 318±13, 212±16, and 235±12 g during the control, constipation, and +fig paste periods, respectively (Table 1). This showed a significant tendency to decrease feed intake between the constipation and +fig paste periods compared with the control period, although the difference in feed intake was not statistically significant between constipation and +fig paste periods. There were no changes in water intake or body weight among the 3 periods (Table 1 and Figure 1).
Table 1.
Effects of fig paste in change of feed and water intakes (n=15)
Column values with different superscripts are significantly different (P<0.05).
Figure 1.
Effects of fig paste on change in body weight in each beagle experimental period (n=15).
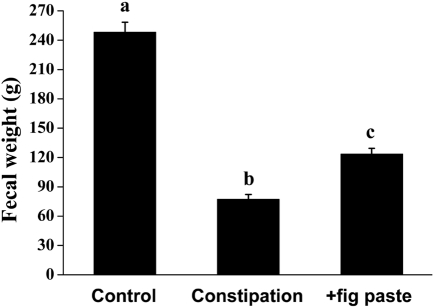
Fecal weights
To examine the laxative effect of fig paste on constipation, the wet weight of stools was measured and compared. Figure 2 shows that fecal weight significantly decreased during the constipation period compared with control period, while it was significantly increased by fig administration (Figure 2, P<0.05). However, after 24 h of drying, there was no difference in water content (data not shown).
Figure 2.
Effects of fig paste on fecal weight in each experimental period (n=15). Bars with different letters from the control are significantly different (P<0.05).
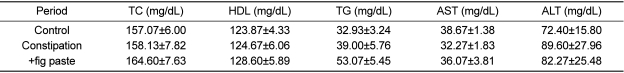
Blood biochemistry
Serum concentrations of blood lipids and hepatotoxicity parameters such as TC, HDL, TG, AST, and ALT were not significantly different among the 3 experimental periods (Table 2). The results of all experimental period were within the normal range, and no abnormal symptoms were observed.
Table 2.
Effects of fig paste on blood biochemical parameters (n=15)
TC: total cholesterol, HDL: high-density lipoproteins, TG: triglycerides, AST: aspartate aminotransferase, ALT: alanine aminotransaminase.
Segmental CTT
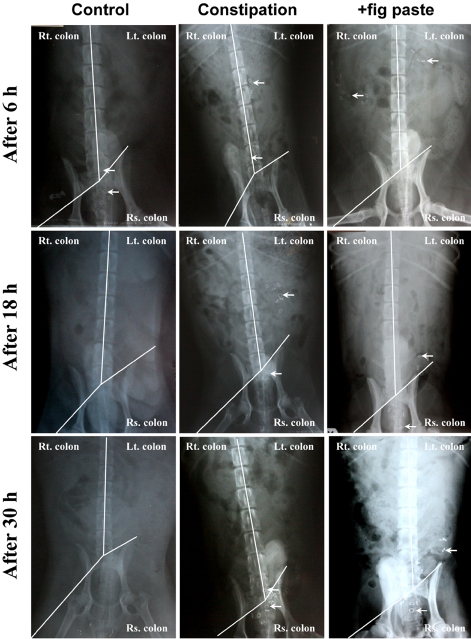
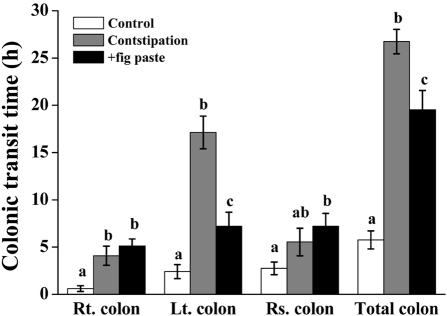
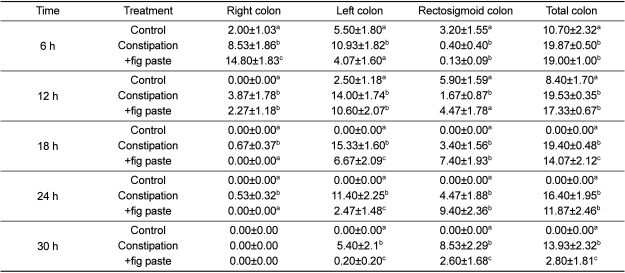
We observed the movement of Kolomark rings to assess intestinal activity using time-dependant radiography (Figure 3). Kolomark rings migrated from the right colon to the rectosigmoid colon over a period of time. The transit time of Kolomark rings in the gastrointestinal tract was shortest over constipation period and was shorter during the +fig paste period than during constipation period. There was no remarkable findings on the abdominal radiography. We counted Kolomark rings using radiography (Figure 4, P<0.05). The numbers of Kolomark rings in the right colon were higher during +fig paste period than during constipation period after 6 h, but the numbers of Kolomark rings in the right, left, rectosigmoid and total colons were fewer during +fig paste period than during constipation period after 18 h (Table 3, P<0.05). There were no Kolomark rings in the right colon during the control period after 12 h, and all rings had been egested after 18 h. The CTT of +fig paste period was 18 h, which was shorter than 30 h during constipation period. The final Kolomark ring was egested after 48 h during constipation period (data not shown). The CTT in the right colon, left colon, rectosigmoid colon, and total colon during control period was significantly faster than during constipation and +fig paste periods. Whereas the CTT in the left colon and total colon during +fig paste period was significantly shorter than during constipation period, there were no statistically significant differences in the CTT in the right and rectosigmoid colons between constipation and +fig paste periods.
Figure 3.
Effects of fig paste on radiographic measurement of Kolomark transit in each experimental period (n=15). Rt.: right, Lt.: left, Rs.: rectosigmoid.
Figure 4.
Effects of fig paste on change in segmental CTT for each experimental period (n=15). Bars with different letters from the control are significantly different (P<0.05). Rt.: right, Lt.: left, Rs.: rectosigmoid.
Table 3.
Effects of fig paste on radiographic measurement of Kolomark transit for each experimental period (n=15)
Data are numbers of markers present. Column values with different superscripts are significantly different (P<0.05).
Discussion
The Rome criteria define chronic constipation on the basis of the presence of 2 or more of a list of 5 symptoms related to the ability to defecate over a period of 3 months or more, such as a frequency of 2 or less bowel movements per week, stools of hard mass over 25%, uncomfortable feeling after evacuation, fecal weight below 35 g, and excessive abdominal press at defecation [18]. Chronic constipation can be caused by peripheral neuropathy (Chagas disease or Hirschsprung's disease), obstructive disease (colon cancer, hernia, or intestinal stricture), endocrine disease (hypothyroidism, diabetes mellitus, or hypokalemia), drugs (morphine, anticholinergic compounds, or calcium) and idiopathic factors [19].
Recently, the use of herbal remedies as constipation treatments has become a common practice in many countries. This study clearly demonstrates that fig extract or paste has a laxative activity. Figs contain numerous components: vitamins, minerals, cellulose, and amino acids [20,21]. They are reported to have antioxidant effects, as well as beneficial effects on cardiovascular, respiratory, and inflammatory diseases [22,23].
Figs contain cellulose, and beneficial effects of cellulose have been reported for cardiac disease, hypertension, diabetes, obesity, gastrointestinal disease, hyperlipidemia, and immune function [24-30]. Lee and Hwang [31] reported that cellulose increases fecal excretion by increasing water content and bulk, and elevating viscosity, and that both water-soluble and insoluble cellulose increases fecal egestion [32]. Fecal water content and volume are increased by eating fiber that does not decompose and is not digested by coliform bacilli [33,34]. Fig paste administration significantly decreased the feed intake both during constipation and +fig paste periods compared with control period. Our results show a tendency to increase during +fig paste period compared with constipation period, but this difference was not statistically significant. There were no changes in water intake and body weight (Table 1 and Figure 1). All serum chemistry results were also in the normal range following fig administration, indicating that fig paste is safe to use (Table 2). The weight of stools significantly decreased during constipation period compared with control period, which was increased by fig paste administration, although the water content of stools was not increased by fig paste (Figure 2).
CTT is the basic test used to assess movement disorder in the diagnosis of chronic constipation and irritable bowel syndrome [35]. It involves the use of radiography to count the number of markers remaining after a certain time period following administration of a gelatin capsule that contains 20 radiopaque markers; segmental CTT is also reported by Arhan et al. [17] and Metcalf et al. [36].
In the present study, considering that CTT in dogs is faster than in humans, we took abdominal radiographs every 6 h until there was no remaining Kolomark in the body (Figure 3) and measured CTT by counting the number of Kolomark rings in the intestinal tract (Table 3 and Figure 4). CTT was shortest during the control period, of all 3 periods. CTT in the left colon was faster during +fig paste period than during constipation period, whereas in the right colon and rectosigmoid colon, there was no difference between constipation period and +fig paste period. In our study, we confirmed that total CTT was fastest during control period followed by +fig paste period, and this result indicates that CTT was decreased by fig administration. Soluble cellulose accelerates peristaltic activity by maintaining higher acidity, because fermented cellulose results in acid production by coliform bacilli in rats [37]. Furthermore, Gordon [38] reported that cellulose shortens large intestine transit time, so that fecal excretion time decreases accordingly. Similar to our results, Spiller et al. [39] reported that intestinal transit time was diminished following administration of food-mixed cellulose and 5% guar gum, compared with rats given normal food [39]. Moreover, Jenkins et al. [40] reported that cellulose increases fecal egestion and reduces intestinal transit time, as well as reducing fecal sojourn time within intestines [40-42]. The shortened CTT indicates that fig paste stimulates intestinal peristalsis and accelerates fecal movement. Recently, intestinal ileum peristalsis was reported to be enhanced in rats by treatment with a low concentration of fig extract, but reduced by a high concentration [43]. Similarly, Baldassano et al. [44] reported that intestinal ileum peristalsis declined in a dose-dependent manner in the isolated mouse intestinal ileum after treatment with 10-320 mg/mL fig extract. Although these results differ from ours, this might be due to different experimental conditions with regard to animal species, type of figs used, and dosage.
In conclusion, feeding fig paste increased fecal weight, which had been decreased by diet-induced constipation, and shortened CTT. The results of serum chemistry showed that oral administration of fig paste is safe, and we did not observe any abnormal symptoms in experimental animals. Therefore, fig paste may be suitable for human patients suffering from constipation, particularly where it is due to diet. In addition, our findings on the use of beagles as a constipation model could be useful in other constipation studies.
Acknowledgments
This work was supported by the Yeong-Am Figs of Food Cluster project of the Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea (Grant: 2008-001).
References
- 1.Drossman DA, Richter JE, Talley NS. The Functional Gastrointestinal Disorders. 1st ed. Boston: Brown and Company; 1994. pp. 217–263. [Google Scholar]
- 2.Read NW, Timms JM, Barfield LJ, Donnelly TC, Bannister JJ. Impairment of defecation in young women with severe constipation. Gastroenterology. 1986;90(1):53–60. doi: 10.1016/0016-5085(86)90074-0. [DOI] [PubMed] [Google Scholar]
- 3.Elliot DL, Watts WJ, Girard DE. Constipation. Mechanisms and management of a common clinical problem. Postgrad Med. 1983;74(2):143–149. doi: 10.1080/00325481.1983.11698384. [DOI] [PubMed] [Google Scholar]
- 4.Enck RE. Constipation: etiologies and management. Am J Hosp Care. 1988;5(5):17–19. [PubMed] [Google Scholar]
- 5.Rolig E. Diet and constipation. J Fam Pract. 1985;21(5):337–338. [PubMed] [Google Scholar]
- 6.Burkitt DP. Epidemiology of cancer of the colon and rectum. Dis Colon Rectum. 1993;36(11):1071–1082. doi: 10.1007/BF02047303. [DOI] [PubMed] [Google Scholar]
- 7.Di Gregorio C, Losi L, Fante R, Modica S, Ghidoni M, Pedroni M, Tamassia MG, Gafà L, Ponz de Leon M, Roncucci L. Histology of aberrant crypt foci in the human colon. Histopathology. 1997;30(4):328–334. doi: 10.1046/j.1365-2559.1997.d01-626.x. [DOI] [PubMed] [Google Scholar]
- 8.Kirjavainen PV, Ouwehand AC, Isolauri E, Salminen SJ. The ability of probiotic bacteria to bind to human intestinal mucus. FEMS Microbiol Lett. 1998;167(2):185–189. doi: 10.1111/j.1574-6968.1998.tb13226.x. [DOI] [PubMed] [Google Scholar]
- 9.Ewe K, Ueberschaer B, Press AG. Influence of senna, fibre, and fibre+senna on colonic transit in loperamide-induced constipation. Pharmacology. 1993;47(Suppl 1):242–248. doi: 10.1159/000139864. [DOI] [PubMed] [Google Scholar]
- 10.Wintola OA, Sunmonu TO, Afolayan AJ. The effect of Aloe ferox Mill. in the treatment of loperamide-induced constipation in Wistar rats. BMC Gastroenterol. 2010;10:95. doi: 10.1186/1471-230X-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams DC, Sgarbieri VC, Whitaker JR. Proteolytic activity in the genus ficus. Plant Physiol. 1968;43(7):1083–1088. doi: 10.1104/pp.43.7.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vinson JA. The functional food properties of figs. Cereal Food World. 1999;44(2):82–87. [Google Scholar]
- 13.Kim KH. Chemical components of Korean figs and its storage stability. Korean J Food Sci Technol. 1981;13(2):165–169. [Google Scholar]
- 14.Kim SS, Lee CH, Oh SL, Chung DH. Chemical components in the two cultivars of Korean figs (Ficus carica L.) Agric Chem Biotechnol. 1992;35(1):51–54. [Google Scholar]
- 15.Moon CK, Kim YG, Kim MY. Studies on the bioactivities of the extractives from Ficus carica. J Inst Agric Res Util. 1997;31:69–79. [Google Scholar]
- 16.Ryu SR, Cho H, Jung JS, Jung ST. The study on the separation, antitumor activity as new substances in fig. J Applied Chem. 1998;2(2):961–964. [Google Scholar]
- 17.Arhan P, Devroede G, Jehannin B, Lanza M, Faverdin C, Dornic C, Persoz B, Tétreault L, Perey B, Pellerin D. Segmental colonic transit time. Dis Colon Rectum. 1981;24(8):625–629. doi: 10.1007/BF02605761. [DOI] [PubMed] [Google Scholar]
- 18.Drossman DA, Thompson WG, Talley NJ, Funch-Jensen P, Jassens J, Whiteheal WE. Identification of functional gastrointestinal disorders. Gastroenterol Int. 1990;3:159–172. [Google Scholar]
- 19.Haubrich WS, Schaffner F, Berk JE. Bockus Gastroenterology. 5th ed. Philadephia: W.B. Saunders; 1995. pp. 102–112. [Google Scholar]
- 20.Solomon A, Golubowicz S, Yablowicz Z, Grossman S, Bergman M, Gottlieb HE, Altman A, Kerem Z, Flaishman MA. Antioxidant activities and anthocyanin content of fresh fruits of common fig (Ficus carica L.) J Agric Food Chem. 2006;54(20):7717–7723. doi: 10.1021/jf060497h. [DOI] [PubMed] [Google Scholar]
- 21.Veberic R, Colaric M, Stampar F. Phenolic acids and flavonoids of fig fruit Ficus carica in the northern Mediterranean region. Food Chem. 2008;106(1):153–157. [Google Scholar]
- 22.Guarrera PM. Traditional phytotherapy in Central Italy (Marche, Abruzzo, and Latium) Fitoterapia. 2005;76(1):1–25. doi: 10.1016/j.fitote.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Jeong WS, Lachance PA. Phytosterols and fatty acids in fig (Ficus carica, var. Mission) fruit and tree components. J Food Sci. 2001;66(2):278–281. [Google Scholar]
- 24.Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr. 1999;69(1):30–42. doi: 10.1093/ajcn/69.1.30. [DOI] [PubMed] [Google Scholar]
- 25.Montonen J, Knekt P, Järvinen R, Aromaa A, Reunanen A. Whole-grain and fiber intake and the incidence of type 2 diabetes. Am J Clin Nutr. 2003;77(3):622–629. doi: 10.1093/ajcn/77.3.622. [DOI] [PubMed] [Google Scholar]
- 26.Lairon D, Arnault N, Bertrais S, Planells R, Clero E, Hercberg S, Boutron-Ruault MC. Dietary fiber intake and risk factors for cardiovascular disease in French adults. Am J Clin Nutr. 2005;82(6):1185–1194. doi: 10.1093/ajcn/82.6.1185. [DOI] [PubMed] [Google Scholar]
- 27.Liu S, Stampfer MJ, Hu FB, Giovannucci E, Rimm E, Manson JE, Hennekens CH, Willett WC. Whole-grain consumption and risk of coronary heart disease: results from the Nurses' Health Study. Am J Clin Nutr. 1999;70(3):412–419. doi: 10.1093/ajcn/70.3.412. [DOI] [PubMed] [Google Scholar]
- 28.Petruzziello L, Iacopini F, Bulajic M, Shah S, Costamagna G. Review article: uncomplicated diverticular disease of the colon. Aliment Pharmacol Ther. 2006;23(10):1379–1391. doi: 10.1111/j.1365-2036.2006.02896.x. [DOI] [PubMed] [Google Scholar]
- 29.Watzl B, Girrbach S, Roller M. Inulin, oligofructose and immunomodulation. Br J Nutr. 2005;93(Suppl 1):S49–S55. doi: 10.1079/bjn20041357. [DOI] [PubMed] [Google Scholar]
- 30.Whelton SP, Hyre AD, Pedersen B, Yi Y, Whelton PK, He J. Effect of dietary fiber intake on blood pressure: a meta-analysis of randomized, controlled clinical trials. J Hypertens. 2005;23(3):475–481. doi: 10.1097/01.hjh.0000160199.51158.cf. [DOI] [PubMed] [Google Scholar]
- 31.Lee HJ, Hwang EH. Effects of alginic acid, cellulose and pectin level on bowel function in rats. Korean J Nutr. 1997;30(5):465–477. [Google Scholar]
- 32.Lupton JR, Morin JL, Robinson MC. Barley bran flour accelerates gastrointestinal transit time. J Am Diet Assoc. 1993;93(8):881–885. doi: 10.1016/0002-8223(93)91526-v. [DOI] [PubMed] [Google Scholar]
- 33.Gordon DT. The importance of total dietary cellulose in human nutrition and health. Korean J Nutr. 1992;25(1):75–76. [Google Scholar]
- 34.Nyman M, Schweizer TF, Tyrén S, Reimann S, Asp NG. Fermentation of vegetable fiber in the intestinal tract of rats and effects on fecal bulking and bile acid excretion. J Nutr. 1990;120(5):459–466. doi: 10.1093/jn/120.5.459. [DOI] [PubMed] [Google Scholar]
- 35.Wald A. Colonic transit and anorectal manometry in chronic idiopathic constipation. Arch Intern Med. 1986;146(9):1713–1716. [PubMed] [Google Scholar]
- 36.Metcalf AM, Phillips SF, Zinsmeister AR, MacCarty RL, Beart RW, Wolff BG. Simplified assessment of segmental colonic transit. Gastroenterology. 1987;92(1):40–47. doi: 10.1016/0016-5085(87)90837-7. [DOI] [PubMed] [Google Scholar]
- 37.Lee SI. Treatment of constipation. Korean J Gastroenterol. 2002;40:37S–47S. [Google Scholar]
- 38.Gordon DT. Functional properties vs physiological action of total dietary fiber. Cereal Foods World. 1989;34(7):517–525. [Google Scholar]
- 39.Spiller GA, Chernoff MC, Hill RA, Gates JE, Nassar JJ, Shipley EA. Effect of purified cellulose, pectin, and a low-residue diet on fecal volatile fatty acids, transit time, and fecal weight in humans. Am J Clin Nutr. 1980;33(4):754–759. doi: 10.1093/ajcn/33.4.754. [DOI] [PubMed] [Google Scholar]
- 40.Jenkins DJ, Reynolds D, Leeds AR, Waller AL, Cummings JH. Hypocholesterolemic action of dietary fiber unrelated to fecal bulking effect. Am J Clin Nutr. 1979;32(12):2430–2435. doi: 10.1093/ajcn/32.12.2430. [DOI] [PubMed] [Google Scholar]
- 41.Park EY, Lee SS. Effect of dietary fiber on the serum lipid level and bowel function in aged rat. Korean J Nutr. 1996;29(9):934–942. [Google Scholar]
- 42.Vahouny GV, Kritchevsky D. Dietary Fiber in Health and Disease. New York: Plenum Press; 1982. pp. 263–415. [Google Scholar]
- 43.Amos S, Binda L, Chindo B, Akah P, Abdurahman M, Danmallam HU, Wambebe C, Gamaniel K. Evaluation of methanolic extract of Ficus platyphylla on gastrointestinal activity. Indian J Exp Biol. 2001;39(1):63–67. [PubMed] [Google Scholar]
- 44.Baldassano S, Tesoriere L, Rotondo A, Serio R, Livrea MA, Mule F. Inhibition of the mechanical activity of mouse ileum by cactus pear (Opuntia ficus indica, L, Mill.) fruit extract and its pigment indicaxanthin. J Agric Food Chem. 2010;58(13):7565–7571. doi: 10.1021/jf100434e. [DOI] [PubMed] [Google Scholar]