Abstract
The effects of a fermentation filtrate of Ganoderma lucidum (FGL) on carbon tetrachloride (CCl4)-induced hepatic fibrosis were investigated in rats. Male Sprague-Dawley rats were orally administered with FGL (20 or 100 mg/kg) for 33 days, and orally administered with CCl4 (1.0 mL/kg; 2 mL/kg of 50% in corn oil) at 3-day intervals 1 h after FGL treatment. Body and liver weights, blood and histopathological findings in accordance with hydroxyproline concentrations were analyzed. Chronic exposure to CCl4 reduced the body weight gain, but increased liver weights and fibrosis, resulting in 3.35-fold increase in hydroxyproline level. Although FGL did not significantly reduce the CCl4-induced body and liver weight changes, it attenuated the increases in the hepatic fibrosis and hydroxyproline contents. Taken together, it is suggested that FGL might prevent hepatic fibrosis, and that FGL or its ingredient could be a potential candidate for the prevention of chronic hepatic disorders.
Keywords: Carbon tetrachloride (CCl4), hepatic fibrosis, Ganoderma lucidum, fermentation filtrate
Hepatitis, a high incidence ailment in modern societies, is caused by hepatitis viruses, chronic alcohol intake, lipid peroxidative products and various drugs [1]. Chronic liver injury results in hepatic fibrosis and end stage cirrhosis. This is one of the major public health problems, related to life-threatening complications of portal hypertension, liver failure and finally increased incidence of hepatocellular carcinoma [2].
The relationship between oxidative damage and hepatocellular injury was observed in many previous studies showing strong hepatoprotective activities of antioxidative Oriental traditional herbs, such as Scutellaria radix [1], Ginkgo biloba [3] and Ganoderma lucidum (GL) [4]. GL, a traditional Oriental medicinal mushroom, has been widely used for the treatment of chronic hepatopathy of various etiologies with little or no side effects [5]. Polysaccharide and triterpenoid components in GL have been proposed as the bioactive constituents responsible for the protective activities against toxin- and ethanol-induced liver injuries [5,6]. It was also reported that triterpenoids and peptides isolated from GL protect against carbon tetrachloride (CCl4)- and D-galactosamine-induced hepatic injuries through its antioxidative and free radical-scavenging abilities [7-9]. In addition, we showed that culture extract of GL exerted anti-allergic and anti-atopic effects [10,11].
Notably, it is well known that fermentation of natural products leads to increases in active ingredients and to production of novel compounds. Based on the antioxidative, anti-inflammatory and anti-allergic, and acute hepatoprotective activities of GL, a fermentation filtrate of Ganoderma lucidum (FGL) was expected to improve chronic active hepatic fibrosis. In the present study, we investigate the antifibrotic effects of FGL in CCl4-induced chronic hepatic fibrosis model.
Dried GL was minced, macerated, and adjusted to 25-30% precipitates by adding purified water. Into the homogenate, cultivated Saccharomyces (strain HM 2104) was added up to 4% and fermented at 45℃ and pH 5.5 for 30-40 h. The crude culture precipitates were removed by centrifugation and microfiltration (pore size, 0.05 µm), and the alcohol produced during fermentation was fully removed in a vacuum evaporator [12].
Seven-week-old male Sprague-Dawley rats were orally administered with FGL (20 or 100 mg/kg) for 33 days, and, 1 h later, orally administered with CCl4 at a dose of 1.0 mL/kg (50% in corn oil, 2 mL/kg) at 3-day intervals [13]. Body weights were recorded throughout the experimental period, and twenty four h after the final treatment, liver weights were measured. Liver tissues were fixed in 10% formalin, and paraffin-embedded sections (4 µm) were stained with hematoxylin-eosin for microscopic examination.
A part of liver tissue was homogenized in a 6 N HCl solution to make 3% homogenate, and boiled at 120℃ for 20 min [14,15]. Into an aliquot (50 µL) of the homogenate, chloramine-T solution (400 µL) was added and incubated for 25 min at room temperature. Again, Ehrlich's solution (500 µL) was added, incubated in a 65℃ water bath for 20 min, and the absorbance was read at 550 nm [13].
Data were expressed as the mean±SEM. Statistical analysis was performed using an analysis of variance (ANOVA) with the aid of SPSS for Windows v.10.0 (Chicago, Illinois, USA). A P value <0.05 was considered statistically significant.
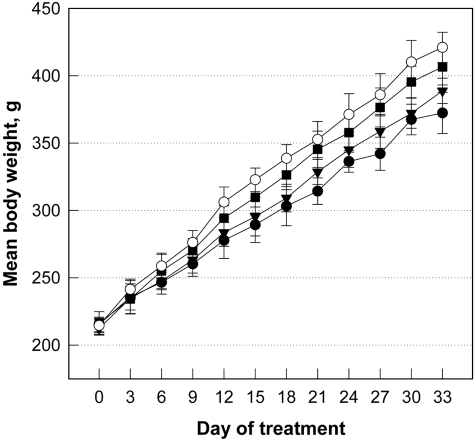
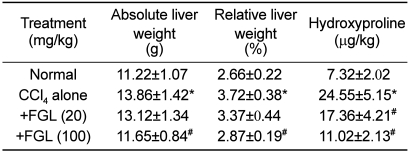
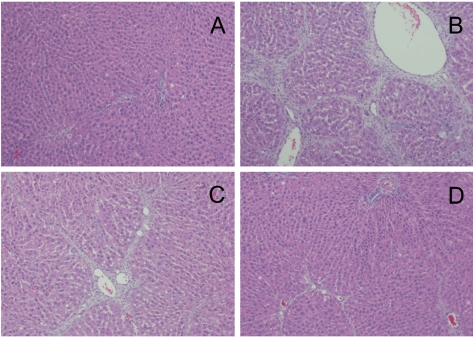
CCl4 (1.0 mL/kg) treatment at 3-day intervals decreased the body weight gain by 12%, which was attenuated by treatment with FGL in a dose-dependent manner (Figure 1). Especially, daily treatment with a high dose (100 mg/kg) of FGL recovered the CCl4-induced decrease in the body weight gain by 70%. In contrast to the decrease in the body weight gain, CCl4 not only increased liver weight by 24%, but also enhanced the hydroxyproline contents to 3.35 folds control level (Table 1). The increased hydroxyproline content was confirmed by microscopic findings, exhibiting severe fibrosis (Figure 2). The liver weights increased by exposure to CCl4 were attenuated by FGL in a dose-dependent manner, especially to the near-normal level in a high dose (100 mg/kg). Notably, the increased hydroxyproline contents were significantly recovered by FGL, in accordance with the microscopic observations.
Figure 1.
Change in body weights of rats administered with carbon tetrachloride (CCl4; 1 mL/kg) for 33 days at 3-day intervals and/or a fermentation filtrate of Ganoderma lucidum (FGL; 20 or 100 mg/kg daily). ○, normal; •, CCl4 alone; ▾, CCl4+20 mg/kg FGL; ▪, CCl4+100 mg/kg FGL.
Table 1.
Effect of a fermentation filtrate of Ganoderma lucidum (FGL) on the liver weights and hepatic fibrosis induced by treatment with carbon tetrachloride (CCl4, 1 mL/kg) for 33 days at 3-day intervals
*Significantly different from normal control (P<0.05). #Significantly different from CCl4 alone (P<0.05).
Figure 2.
Representative hepatic fibrosis of the liver of rats administered with carbon tetrachloride (CCl4, 1 mL/kg) for 33 days at 3-day intervals and/or a fermentation filtrate of Ganoderma lucidum (FGL; 20 or 100 mg/kg daily). A, normal; B, CCl4 alone; C, CCl4+20 mg/kg FGL; D, CCl4+100 mg/kg FGL.
In spite of tremendous strides in the modern medicine, there are not much drugs available for the treatment of liver disorders [16]. Liver fibrosis results from the fibrogenic activation of fibrocytes and hepatic stellate cells, called Ito cells, following continuous hepatocytic injury and cholestasis, sometimes leading to liver cirrhosis and hepatocellular carconoma [2,17-19]. In the present study, repeated administration of CCl4 also induced serious hepatic fibrosis, in parallel with the increased hydroxyproline contents [13].
Since oxidative stress is one of the most-causative factors of tissue fibrosis, the antifibrogenic effect of FGL was expected. Moreover, such effects also were inferred from that polysaccharides, triterpenoids and peptides of GL exerted protective effects against acute hepatic injury induced by toxins, CCl4 or D-galactosamine [5,7-9]. More recently, it was demonstrated that an ethanol extract of GL suppressed thioacetamide-induced hepatic fibrosis by enhancing collagenase activity [20].
In the present preliminary study, FGL was confirmed to possess strong anti-fibrogenic activity. Although active ingredients and the action mechanisms remain to be clarified, it is suggested that FGL could be a potential candidate for the improvement of chronic hepatic disorders.
Acknowledgments
This work was supported by Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0094035).
References
- 1.Hwang JM, Tseng TH, Tsai YY, Lee HJ, Chou FP, Wang CJ, Chu CY. Protective effects of baicalein on tert-butyl hydroperoxide-induced hepatic toxicity in rat hepatocytes. J Biomed Sci. 2005;12(2):389–397. doi: 10.1007/s11373-005-1572-8. [DOI] [PubMed] [Google Scholar]
- 2.Zou Y, Yang Y, Li J, Li W, Wu Q. Prevention of hepatic injury by a traditional Chinese formulation, BJ-JN, in mice treated with Bacille-Calmette-Guérin and lipopolysaccharide. J Ethnopharmacol. 2006;107(3):442–448. doi: 10.1016/j.jep.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Ozenirler S, Dinçer S, Akyol G, Ozoğul C, Oz E. The protective effect of Ginkgo biloba extract on CCl4-induced hepatic damage. Acta Physiol Hung. 1997;85(3):277–285. [PubMed] [Google Scholar]
- 4.Lin JM, Lin CC, Chen MF, Ujiie T, Takada A. Radical scavenger and antihepatotoxic activity of Ganoderma formosanum, Ganoderma lucidum and Ganoderma neojaponicum. J Ethnopharmacol. 1995;47(1):33–41. doi: 10.1016/0378-8741(95)01251-8. [DOI] [PubMed] [Google Scholar]
- 5.Gao YH, Huang M, Lin ZB, Zhou SF. Hepatoprotective Activity and the Mechanisms of Action of Ganoderma lucidum (Curt.:Fr.) P. Karst. (Ling Zhi, Reishi Mushroom) (Aphyllophoromycetideae) Inter J Medic Mushrooms. 2003;5:111–131. [Google Scholar]
- 6.Zhou CY, Jia W, Yang Y, Bai YQ. Experimental studies on prevention of several kinds of fungi polysaccharides against alcohol-induced hepatic injury. Edible Fungi. 2002;24:36–37. [Google Scholar]
- 7.Wang MY, Liu Q, Che QM, Lin ZB. Effects of total Triterpenoids Extract from Ganoderma Iucidum (Curt: Fr) P Karst (Reishi mushroom) on experimental liver injury models induced by carbon tetrachloride or D-galactosamine in mice. Acta Pharmaceut Sin. 2000;35:326–329. [Google Scholar]
- 8.Sun J, He H, Xie BJ. Novel antioxidant peptides from fermented mushroom Ganoderma lucidum. J Agric Food Chem. 2004;52(21):6646–6652. doi: 10.1021/jf0495136. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y, Sun J, He H, Guo H, Zhang S. Hepatoprotective effects of Ganoderma lucidum peptides against D-galactosamine-induced liver injury in mice. J Ethnopharmacol. 2008;117(3):415–419. doi: 10.1016/j.jep.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 10.Kwon SC, Shin S, Jeon JH, Park D, Jang MJ, Kim JJ, Kim CH, Jeong JH, Kim YB. Anti-allergic effects of rose petal extract and Ganoderma lucidum culture on mast cell-mediated allergy model. Lab Anim Res. 2008;24:93–97. [Google Scholar]
- 11.Jeon JH, Kwon SC, Park D, Shin S, Jang MJ, Joo SS, Kang H, Kim SH, Oh JY, Jeong JH, Kim YB. Effects of red and white rose petal extracts and Ganoderma lucidum culture on ovalbumin-induced atopic dermatitis. Lab Anim Res. 2008;24(3):347–354. [Google Scholar]
- 12.Jeon JH, Shin S, Park D, Jang JY, Choi BI, Kang JK, Joo SS, Hwang SY, Kim JC, Kim BY, Kim MR, Kim YB. Fermentation filtrates of Rubus coreanus relax the corpus cavernosum and increase sperm count and motility. J Med Food. 2008;11(3):474–478. doi: 10.1089/jmf.2007.0070. [DOI] [PubMed] [Google Scholar]
- 13.Kim EJ, Shin S, Jang JY, Choi B, Park D, Jeon JH, Park JH, Hwang SY, Kim YB. Effects of Hwalgidan® on hepatic injury induced by carbon tetrachloride in rats. Lab Anim Res. 2007;23(1):59–67. [Google Scholar]
- 14.Fort J, Oberti F, Pilette C, Veal N, Gallois Y, Douay O, Rousselet MC, Rosenbaum J, Calès P. Antifibrotic and hemodynamic effects of the early and chronic administration of octreotide in two models of liver fibrosis in rats. Hepatology. 1998;28(6):1525–1531. doi: 10.1002/hep.510280612. [DOI] [PubMed] [Google Scholar]
- 15.Yorozu K, Fujii E, Teruya S, Ogawa Y, Ito H, Suzuki M, Sugimoto T. Lobular difference in fibrotic changes in rat cirrhosis model induced by carbon tetrachloride. J Toxicol Pathol. 2004;17(4):267–274. [Google Scholar]
- 16.Bhandarkar MR, Khan A. Antihepatotoxic effect of Nymphaea stellata willd., against carbon tetrachloride-induced hepatic damage in albino rats. J Ethnopharmacol. 2004;91(1):61–64. doi: 10.1016/j.jep.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Sherlock S, Dooley J. Diseases of the Liver and Biliary System. 10th ed. Oxford: Blackwell Science Press; 1997. pp. 303–384. [Google Scholar]
- 18.Anthony PP, Ishak KG, Nayak NC, Poulsen HE, Scheuer PJ, Sobin LH. The morphology of cirrhosis: definition nomenclature and classification. Bull World Health Organ. 1977;55(4):521–540. [PMC free article] [PubMed] [Google Scholar]
- 19.Anthony PP, Ishak KG, Nayak NC, Poulsen HE, Scheuer PJ, Sobin LH. The morphology of cirrhosis. Recommendations on definition, nomenclature, and classification by a working group sponsored by the World Health Organization. J Clin Pathol. 1978;31(5):395–414. doi: 10.1136/jcp.31.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu YW, Fang HL, Lin WC. Post-treatment of Ganoderma lucidum reduced liver fibrosis induced by thioacetamide in mice. Phytother Res. 2010;24(4):494–499. doi: 10.1002/ptr.2949. [DOI] [PubMed] [Google Scholar]