Abstract
Background
Prophylactic central neck dissection (CND) remains controversial in papillary thyroid carcinoma (PTC). Because postsurgical stimulated thyroglobulin (sTg) level is a good surrogate for recurrence, the study aimed to evaluate the impact of prophylactic CND on preablative and postablative sTg levels after total thyroidectomy.
Methods
Of the 185 patients retrospectively analyzed, 82 (44.3%) underwent a total thyroidectomy and prophylactic CND (CND-positive group) while 103 (55.7%) underwent total thyroidectomy only (CND-negative group). All patients had no preoperative or intraoperative evidence of lymph node metastases. Clinicopathological characteristics, postoperative outcomes, and preablative and postablative sTg levels were compared between the two groups. Preablative sTg level was taken at the time of radioiodine ablation, while postablative sTg level was taken 6 months after ablation. A multivariable analysis was conducted to identify factors for preablative athyroglobulinemia (sTg < 0.5 μg/L).
Results
Relative to the CND-negative group, the CND-positive group had larger tumors (15 mm vs. 10 mm, P < 0.005), more extrathyroidal extension (26.8% vs. 14.6%, P < 0.003), more tumor, node, metastasis system stage III disease (32.9% vs. 9.7%, P < 0.001), and more temporary hypoparathyroidism (18.3% vs. 8.7%, P = 0.017). Fourteen patients (17.1%) in the CND-positive group were upstaged from stages I/II to III as a result of prophylactic CND. The CND-positive group experienced lower median preablative sTg (<0.5 μg/L vs. 6.7 μg/L, P < 0.001) and a higher rate of preablative athyroglobulinemia (51.2% vs. 22.3%, P = 0.024), but these differences were not observed 6 months after ablation. Prophylactic CND was the only independent factor for preablative athyroglobulinemia.
Conclusions
Although performing prophylactic CND in total thyroidectomy may offer a more complete initial tumor resection than total thyroidectomy alone by minimizing any residual microscopic disease, such a difference becomes less noticeable 6 months after ablation.
Papillary thyroid carcinoma (PTC) is the most common type of differentiated thyroid carcinoma. Its age-adjusted incidence has doubled over the last 25 years.1 Despite its relatively good prognosis with a 10-year cancer-specific survival above 90%, locoregional recurrence is common.2 With recognition of the concept of stepwise progression of lymph node metastasis originating from the central (level VI) to the lateral compartment (levels II–V), a growing number of surgeons are performing routine prophylactic central neck dissection (CND) at the time of the thyroidectomy for PTC.3 However, its routine practice remains controversial.4–10 The arguments for it include improved pathological staging accuracy, preoperative failure to reliably detect central lymph node metastasis, and avoidance of central compartment reoperations.3,4,11 On the other hand, arguments against it include increased surgical morbidities such as hypoparathyroidism and recurrent laryngeal nerve (RLN) injury; most important of all, prophylactic CND has not been shown to reduce recurrence or mortality rate.9,10,12–14 There have been no randomized, controlled studies showing that prophylactic CND reduces recurrence or improves survival.10 Furthermore, most studies to date have been relatively underpowered with short follow-up periods, and a recent meta-analysis also failed to demonstrate lower recurrence rates in patients who routinely underwent prophylactic CND.5
To overcome these problems, some authors have compared the postoperative stimulated thyroglobulin (sTg) level between those who had prophylactic CND and those who did not.6,15,16 Because Tg is produced solely by differentiated thyroid follicular cells, measuring serum sTg could potentially help in the detection of residual, recurrent, or metastatic disease. Studies have found postsurgical sTg levels could reliably predict disease outcomes.4,17–19 However, to our knowledge, there have been few studies assessing the impact of prophylactic CND on postsurgical sTg, and their results had not been consistent.6,15,16 Furthermore, few studies have evaluated the relationship between preablative and postablative sTg in the setting of patients who routinely undergo prophylactic CND.6,16,20
We hypothesized that patients who underwent a total thyroidectomy and prophylactic CND would have lower preablative sTg levels as a reflection of the better surgical completeness than those who underwent total thyroidectomy alone. Our study aimed to evaluate the impact of prophylactic CND on preablative and postablative sTg in PTC after total thyroidectomy.
Patients and Methods
Patients
From 2004 to 2010, a total of 257 consecutive patients with PTC underwent surgery at our institution. All patients were operated by the same team of endocrine surgeons. For the purpose of the study, patients who required a concomitant lateral neck dissection (n = 32) or were diagnosed with clinically occult microcarcinoma (n = 40) were excluded. After excluding these patients, 185 patients were retrospectively analyzed. All patients had no evidence of central lymph node metastases preoperatively on ultrasound (US) or at the time of operation. Patients with obviously enlarged central lymph nodes requiring a therapeutic CND were not included. All operations were performed by two endocrine surgeons. The decision for a prophylactic unilateral CND was based on the personal preference of the operating surgeon and not based on tumor size or other tumor characteristics. Nevertheless, there was an increasing tendency to perform more prophylactic CND in the latter part of the study period.
In terms of patient characteristics, most were women (78.4%) and ethnic Chinese (96.8%). The median age of operation was 51.0 (range 17.1–90.6) years, and the median follow-up period was 26.0 (range 9.7–101.2) months. Eighty-two patients (44.3%) underwent a total thyroidectomy and prophylactic CND (CND-positive group), while 103 (55.7%) underwent a total thyroidectomy only (CND-negative group). All relevant clinical, laboratory, radiologic, and perioperative data were collected prospectively, and follow-up data were regularly updated in a computerized thyroid database. The present study protocol was approved by the local institutional review board. Patient demographics, biochemical profile, operative findings, surgical approach, and postoperative outcomes were compared between the two groups.
Management of PTC
Details of surgical treatment, criteria for radioiodine (RAI) ablation, postoperative care, and follow-up protocol had been described previously.21 In brief, total thyroidectomy has been the preferred procedure for all patients with a preoperative diagnosis of PTC. A prophylactic CND was performed immediately after the completion of the total thyroidectomy and consisted of the removal of all nodes and fibro-fatty tissue extending vertically from the hyoid bone to the thoracic inlet and laterally from the medial border of common carotid artery to the midline of the trachea. A frozen section of central lymph nodes was not routinely performed. The ipsilateral RLN was mobilized and skeletonized along its entire cervical course. An intraoperative nerve stimulator was used to confirm the functional integrity of RLN.22 Autotransplantation of any inadvertently removed parathyroid glands was readily performed. sTg was defined as a Tg level measured in the presence of thyroid-stimulating hormone (TSH) of >30 mIU/L either by 4-week thyroxine withdrawal or recombinant TSH injections. Postoperative sTg levels were taken approximately 2 months after surgery (or at the time of RAI ablation) and again 8–9 months after surgery (or 6 months after RAI ablation, usually at the time of the whole-body scan). Tg autoantibodies were measured at the same time. The decision for RAI ablation was based on the presence of risk factors including tumor size >1 cm, lymph node metastasis, age >45 years old, extrathyroidal extension, macroscopic postoperative residual disease in the neck, and distant metastasis. It was not based on the preablative sTg level. Three gigabecquerels or 80 mCi I131 was the standard fixed ablative dose, while subsequent I131 therapy was performed with 5.5 GBq (or 150 mCi). TSH suppression to below <0.1 μg/L was recommended for high- and intermediate-risk patients.
Postoperative Care and Follow-up Protocol
Serum calcium and phosphate levels were measured within 6 h and every 12 h after the operation until stabilized. Calcium with or without vitamin D supplements was prescribed for symptomatic hypocalcemia or a calcium level <1.70 mmol/L. Those who could discontinue supplements in the presence of normocalcemia within 6 months after surgery were categorized as having temporary hypoparathyroidism, whereas those needed supplements for >6 months together with a below-normal serum parathyroid hormone level were considered to have permanent hypoparathyroidism. Perioperative direct laryngoscopy was performed before and within 1 week after the operation to assess vocal cord function. RLN palsy lasting >6 months after thyroidectomy as documented by direct laryngoscopy was regarded as permanent. To calculate transient and permanent RLN palsy rates, the number of nerves at risk was used.
After surgery, all patients were followed up within 4 weeks in a specialized combined surgical oncology clinic. A follow-up visit was conducted at 3-month intervals in the first 2 years, 6-month intervals in the subsequent 3 years, and annually thereafter. Clinical examination, neck US, and nonstimulated Tg levels were assessed during follow-up visits. Locoregional recurrence was defined as macroscopic disease at clinical examination or US that was not present at the initial presentation.
Laboratory Methods
All postoperative serum Tg levels were measured at the same laboratory with the same immunometric assay. The assay used was the Immulite 2000 (Diagnostic Products; Roche, Los Angeles, CA). This was calibrated against the CRM-457 standard. A sTg level of <0.5 μg/L was considered undetectable or athyroglobulinemia. Normal reference range was <0.5–55 μg/L, and sensitivity was <0.2 μg/L.
Statistical Analysis
Statistical analysis was performed by the chi-square test or by Fisher’s exact test to compare categorical variables, and the Mann–Whitney U-test was used to compare continuous variables between groups. Continuous variables were expressed as medians with ranges. Variables that were significant in the univariate analysis were entered into multivariate analysis. To improve clinical utility, before entering into the multivariate analysis, significant continuous variables were converted into binary variables by using the overall median value as the cutoff. Binary logistic regression analysis with a variable entrance criterion of 0.05 or less was conducted to identify factors associated with preablative athyroglobulinemia after surgery. To evaluate the correlation between the number of lymph nodes retrieved and preablative sTg level, the Spearman rank correlation test was performed. All statistical analyses were performed by SPSS software, version 18.0 (SPSS, Chicago, IL).
Results
Table 1 shows a comparison of patient clinicopathological features, tumor, node, metastasis system (TNM) tumor stages, and MACIS (metastases, age, completeness of surgery, invasion, and size) score between the CND-positive and CND-negative groups. Age at operation, sex, and clinical presentation were similar between the two groups. When compared to the CND-negative group, the median tumor size was significantly larger (15 mm vs. 10 mm, P < 0.005) and the incidence of extrathyroidal extension was significantly higher in the CND-positive group (26.8% vs. 14.6%, P < 0.003). However, the incidence of tumor multifocality, capsular invasion, coexisting thyroiditis, and MACIS scores were comparable in the two groups. In terms of TNM stages, there was a significantly higher proportion belonging to tumor stage III (32.9% vs. 9.7%, P < 0.001) but a smaller proportion belonging to tumor stage I (48.8% vs. 76.7%, P < 0.001) in the CND-positive group. As expected, the median number of central lymph nodes retrieved, number of central lymph nodes involved, and proportion of patients with metastatic central compartment lymph nodes (pN1a) were significantly greater in the CND-positive group. Five patients in the CND-negative group had one perithyroidal lymph node removed, and all (100%) turned out to be metastatic, whereas in the CND-positive group, 45 patients (54.9%) had pN1a-positive tumor. Ectopic thyroid tissue or thyroid rests were not found in any of the central compartment specimens examined. Among those belonging to TNM stage III, there were 14 patients (17.1%) in the CND-positive group and 4 patients (3.9%) in the CND-negative group who had pT1 or pT2 on histology. This meant that as a result of prophylactic CND, tumors of 12.7% of patients were upstaged from TNM stages I/II to III. Table 2 compares postoperative complications and incidence of parathyroid autotransplantation between the CND-positive and CND-negative groups. The rate of temporary hypoparathyroidism was significantly higher in the CND-positive group (18.3% vs. 8.7%, P = 0.017). Rates of RLN injury, permanent hypoparathyroidism, bleeding, and wound infection were similar between the two groups. The percentage of parathyroid autotransplantation was significantly higher in the CND-positive group (37.8% vs. 19.4%, P = 0.008)
Table 1.
Comparison of patient clinicopathologic features, TNM stage, and MACIS score between those who underwent a routine CND (CND-positive group) and those who did not (CND-negative group)
| Characteristic | CND-positive group (n = 82) | CND-negative group (n = 103) | P value |
|---|---|---|---|
| Age at operation (y) | 52.0 (17.1–79.2) | 50.0 (26.2–90.8) | 0.304 |
| Sex | 1.000 | ||
| Male | 18 (22.0) | 22 (21.4) | |
| Female | 64 (78.0) | 81 (78.6) | |
| Presented as a palpable swelling | 56 (68.3) | 79 (76.7) | 0.201 |
| Tumor characteristics | |||
| Tumor size (mm) | 15 (2–90) | 10 (2–110) | 0.005 |
| Multifocality | 30 (36.6) | 28 (27.2) | 0.179 |
| Capsular invasion | 18 (22.0) | 25 (24.3) | 0.750 |
| Extrathyroidal extension | 22 (26.8) | 15 (14.6) | 0.033 |
| Coexisting thyroiditis | 16 (19.5) | 12 (11.7) | 0.352 |
| TNM PTC stage | <0.001 | ||
| I | 40 (48.8) | 79 (76.7) | |
| II | 7 (8.5) | 6 (5.8) | |
| III | 27 (32.9) | 10 (9.7) | |
| IV | 8 (9.8) | 8 (7.8) | |
| No. of central LNs retrieved | 5 (2–18) | 0 (0–1) | <0.001 |
| No. of positive central LNs excised | 1 (0–8) | 0 (0–1) | <0.001 |
| No. of patients with metastatic central compartment LNs (pN1a) | 45 (54.9) | 5 (4.9) | <0.001 |
| MACIS score | 4.84 (2.18–11.34) | 4.55 (3.13–9.80) | 0.089 |
Continuous variables are expressed as median (range); categorical variables are expressed as n (%)
PTC papillary thyroid carcinoma, TNM tumor, node, metastasis system (6th edition), LN lymph node, MACIS metastases, age, completeness of surgery, invasion, and size
Table 2.
Comparison of postoperative complications and incidence of parathyroid autotransplantation between those who underwent a routine CND (CND-positive group) and those who did not (CND-negative group)
| Complication | CND-positive group (n = 82), n (%) | CND-negative group (n = 103), n (%) | P value |
|---|---|---|---|
| RLN injurya | |||
| Temporary | 3 (1.8) | 0 (0.0) | 0.324 |
| Permanent | 1 (0.6) | 1 (0.5) | 0.443 |
| Hypoparathyroidism | |||
| Temporary | 15 (18.3) | 9 (8.7) | 0.017 |
| Permanent | 2 (2.4) | 1 (1.0) | 1.000 |
| Bleeding | 0 (0.0) | 1 (1.0) | 1.000 |
| Wound infection | 0 (0.0) | 0 (0.0) | 1.000 |
| Parathyroid autotransplantation | 31 (37.8) | 20 (19.4) | 0.008 |
CND central neck dissection, RLN recurrent laryngeal nerve
aCalculated on the basis of the number of nerves at risk
Table 3 shows a comparison of RAI ablation, postoperative Tg levels, follow-up period, and recurrence rate between the CND-positive and CND-negative groups. In the CND-positive group, there was a significantly higher proportion of patients receiving RAI ablation (75.6% vs. 68.0%, P = 0.023). The CND-positive group had a significantly lower median preablative sTg level (<0.5 μg/L vs. 6.7 μg/L, P < 0.001) and a higher rate of preablative athyroglobulinemia (51.2% vs. 22.3%, P = 0.024). Furthermore, there was a significant inverse association between the number of central lymph nodes retrieved and the preablative sTg level (ρ = −0.252, P = 0.020). However, 6 months after RAI ablation, the median sTg level and the proportion of athyroglobulinemia became comparable between the two groups. The proportion of detectable sTg 6 months after ablation was similar between the two groups (P = 0.292). Follow-up duration and recurrence rate were comparable in the two groups. In both groups, all six locoregional recurrences occurred in the lateral (or noncentral) neck compartment. The time to recurrence ranged 28.3–56.9 months. There was one distant recurrence detected in the lung on fludeoxyglucose–positron emission tomographic scan in the CND-negative group at 30.7 months.
Table 3.
Comparison of radioiodine ablation, postoperative thyroglobulin levels, follow-up period, and recurrence rate between those who underwent a routine CND (CND-positive group) and those who did not (CND-negative group)
| Characteristic | CND-positive group (n = 82) | CND-negative group (n = 103) | P value |
|---|---|---|---|
| RAI ablation | 0.023 | ||
| Provided | 62 (75.6) | 63 (68.0) | |
| Not provided | 20 (24.4) | 40 (32.0) | |
| Preablation (2 months after surgery) | |||
| TSH level (mIU/L) | 51.0 (30.5–331.0) | 61.5 (33.2–145.0) | 0.959 |
| Stimulated Tg level (μg/L) | <0.5 (<0.5–480.0) | 6.70 (<0.5–140.0) | <0.001 |
| No. of athyroglobulinemia | 42 (51.2) | 23 (22.3) | 0.024 |
| Postablation (8 months after surgery) | |||
| TSH level (mIU/L) | 66.5 (33.8–129.5) | 73.5 (33.3–126.5) | 0.125 |
| Stimulated Tg level (μg/L) | <0.5 (<0.5–480.0) | <0.5 (<0.5–800.0) | 0.787 |
| No. of athyroglobulinemia | 63 (76.8) | 72 (69.9) | 0.292 |
| Nonstimulated Tg level on last visit (μg/L) | <0.5 (<0.5–325.0) | <0.5 (<0.5–495.0) | 0.304 |
| Follow-up period (mo) | 25.5 (9.7–77.1) | 27.1 (10.7–101.2) | 0.307 |
| First recurrence site | 1.000 | ||
| Locoregional | 3 (3.7) | 3 (2.9) | |
| Distant | 0 (0.0) | 1 (1.0) | |
Continuous variables are expressed as median (range); categorical variables are expressed as n (%)
CND central neck dissection, Tg thyroglobulin level, TSH thyroid-stimulating hormone
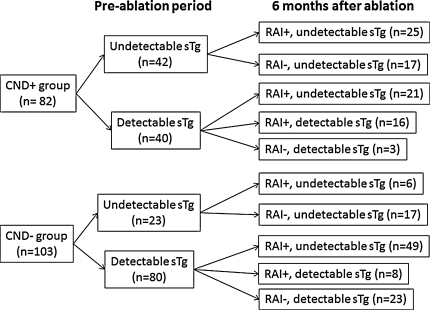
Figure 1 shows a breakdown of the number of undetectable and detectable sTg level in the CND-positive and CND-negative groups before and 6 months after ablation. Among patients with detectable sTg before ablation, those in the CND-negative group were significantly more likely to become athyroglobulinemia than those in the CND-positive group (49/57 vs. 21/37, P = 0.003)
Fig. 1.
Chart showing the breakdown of the number of undetectable and detectable stimulated thyroglobulin (sTg) between those who underwent a prophylactic central neck dissection (CND-positive group) and those who did not (CND-negative group) in the preablation period and 6 months after radioiodine (RAI) ablation. RAI+ RAI ablation provided, RAI− no RAI ablation provided
Table 4 shows the multivariable analysis for undetectable sTg level after surgery before ablation. Although both tumor size and extrathyroidal extension were significant in the univariate analysis, they were not entered into the multivariable analysis because they were already represented by TNM stages. After adjusting for other significant factors, prophylactic CND turned out to be the only independent factor for preablative athyroglobulinemia (odds ratio 3.60, 95% confidence interval 1.12–11.55).
Table 4.
Multivariable analysis of preoperative clinicopathological factors for preablative undetectable stimulated thyroglobulin level or athyroglobulinemia after surgery
| Covariate | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| Age at operation (y) | 0.652–4.533 | 0.273 | |
| <50 | 1 | ||
| ≥50 | 1.719 | ||
| Sex | 0.737–8.110 | 0.144 | |
| Male | 1 | ||
| Female | 2.445 | ||
| Stage of PTC by TNM | 0.317–2.438 | 0.804 | |
| Tumor stages I/II | 1 | ||
| Tumor stages III/IV | 0.804 | ||
| Prophylactic central neck dissection | 1.124–11.549 | 0.031 | |
| No | 1 | ||
| Yes | 3.603 | ||
| No. of central lymph nodes retrieved | 0.305–3.049 | 0.951 | |
| ≤4 | 1 | ||
| >4 | 0.965 |
PTC papillary thyroid carcinoma, TNM tumor, node, metastasis system (6th edition)
Discussion
Despite its increasing popularity, adopting routine prophylactic CND remains controversial because studies have not demonstrated a clear advantage of prophylactic CND in improving disease outcomes.4–10 Given the relatively slow tumor growth and excellent prognosis associated with PTC, a prospective study of sufficient size would be required to evaluate the impact of prophylactic CND on disease outcomes. Both preablative and postablative sTg levels have been shown to be excellent surrogate markers and predictors for disease-free emission and disease-specific survivals in PTC.4,17–19 Preablative sTg was shown to have potential application in modulating RAI ablation and stratifying follow-up strategy.19 Furthermore, disease recurrence has been redefined as disease measurable by elevated Tg levels and/or on US and not solely on the basis of whole-body scan.4 As a result of this paradigm shift, achieving athyroglobulinemia has become an important issue for patients with PTC after surgery.4 Sywak et al. examined the association between prophylactic CND and sTg and found that prophylactic CND could lower postablative sTg and achieve a higher rate of athyroglobulinemia than those who did not undergo prophylactic CND.15 The same group subsequently showed that the number of central lymph nodes retrieved significantly inversely correlated with sTg levels 1 year after ablation.16 However, because the postablative sTg level not only depends on the completeness of the initial surgery but also on the ablative effect of RAI on residual microscopic disease present within the central compartment as well as areas outside it, our study aimed to specifically evaluate the impact of additional prophylactic CND on preablative and postablative sTg levels.6
Despite the far larger size and more advanced stages of PTC, our study found that the CND-positive group had a much lower median preablative sTg level and experienced a higher rate of preablative athyroglobulinemia than the CND-negative group. In fact, about 50% of patients had undetectable sTg level in the CND-positive group before ablation as compared to 22.3% in the CND-negative group. This implied that the addition of prophylactic CND may provide a more complete surgical excision by leaving less amount of residual microscopic disease behind as reflected by the lower sTg level. Although detectable levels of sTg without ablation could potentially come from the presence of thyroid remnants or thyroid rests not removed at the time of initial surgery rather than from the residual microscopic disease, we believe that because the same thyroidectomy procedure was performed during the study period, regardless of whether or not prophylactic CND was performed, the volume of the thyroid remnant would be comparable between the two groups. Furthermore, we did not find the presence of any ectopic thyroid tissue or thyroid rests in any of the central compartment specimen, although this was believed to be present in up to 20% of cases.23 Perhaps this might be because the thymus was not removed in our prophylactic CND. Therefore, we postulated that perhaps prophylactic CND was able to remove any subclinical or microscopic disease present in the central lymph nodes leading to the lower level of preablative sTg. This was somewhat supported by the fact that >50% of the central lymph nodes excised contained metastatic PTC; similar rates have been reported by other authors.6–8,14 In further support of this finding, our data found a statistically significant inverse correlation between the number of lymph nodes retrieved and preablative sTg levels, and in the multivariable analysis, after adjusting for age, sex, tumor stage, and number of lymph nodes retrieved, prophylactic CND was an independent significant factor for achieving preablative athyroglobulinemia. We believed that these findings may provide indirect evidence that the addition of prophylactic CND at the time of total thyroidectomy offers a more complete local resection of the PTC by removing subclinical or microscopic residual disease, presumably harbored within the central compartment. However, it was interesting to note that 6 months after ablation, the median sTg level and rate of athyroglobulinemia became comparable in the two groups, and among those with detectable preablative sTg who received RAI ablation (n = 120), the CND-negative group were significantly more likely to become athyroglobulinemic than those the CND-positive group (P = 0.003). Perhaps this means that the residual disease not removed in the central compartment in the CND-negative group was still able to be completely ablated and thus a similar overall rate of postablative athyroglobulinemia was experienced as the CND-positive group. However, the question of whether RAI ablation could make up for the less complete surgery (i.e., without CND) is debatable and is beyond the scope of the study.
Another interesting observation was tumor upstaging as a result of prophylactic CND, leading to higher doses and frequency of RAI ablation, as reported by other authors.6–8 Although a difference in RAI dose per patient was not observed because we adopted a fixed-dose protocol, we did observe a far higher frequency of RAI ablation in the CND-positive group. This was presumably related to the higher incidence of pN1a in the CND-positive group, leading to upstaging of 12.7% PTC from stages I/II to III. This was in concordance with other authors.6–8,24
Although prophylactic CND resulted in a far higher temporary hypoparathyroidism rate, we believe that the unilateral prophylactic CND was associated with acceptable level of complications and was a good compromise between achieving completeness of surgery and reducing patient morbidity because this has been advocated by other groups.15,16 We believe that performing bilateral prophylactic CND may further increase patient morbidities because of the risk of damaging the contralateral inferior parathyroid gland and RLN. In our opinion, a more liberal use of parathyroid autotransplantation at the time of prophylactic CND could reduce the rate of permanent hypoparathyroidism.25 An alternative strategy to reduce the overall surgical morbidity would be to adopt a selective approach, where only patients with certain prognostic factors associated with subclinical central lymph node metastasis would undergo prophylactic CND. A recent study found that male sex, tumor multifocality, and extrathyroidal extension were predictors for subclinical central lymph node metastasis.26
Our data should be interpreted cautiously because this was a relatively moderate-size retrospective study, which tends to limit the power of the study to identify smaller effects such as rate of recurrence and permanent hypoparathyroidism. Also, because this was not a randomized study, it was prone to selection biases. One important selection bias was that it was difficult to know whether the CND-positive group also included some patients with slightly suspicious intraoperative central lymph nodes because the decision was at the discretion of the operating surgeon. Also, it would be interesting to evaluate the difference in significance between micro- and macrometastases in the central lymph nodes in the future. Although sTg is a good surrogate marker, the clinical significance of mildly detectable sTg remains unknown, and it was observed that such detectable levels may spontaneously decrease over time.27,28
Despite having larger-size and more advanced PTCs, the CND-positive group had a much lower median sTg level and a higher rate of athyroglobulinemia before ablation than the CND-negative group, but 6 months after ablation, these differences were no longer observed. On the basis of these findings, although the addition of prophylactic CND for PTC may provide a more complete initial resection than total thyroidectomy alone by minimizing the amount of microscopic residual disease, differences between the two procedures become less noticeable 6 months after RAI ablation.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Cancer incidence and mortality in Hong Kong, 1983–2006. Hong Kong Cancer Registry, Hong Kong. http://www3.ha.org.hk/cancereg/e_stat.asp.
- 2.Lang BH, Lo CY, Chan WF, Lam KY, Wan KY. Prognostic factors in papillary and follicular thyroid carcinoma: implications for cancer staging. Ann Surg Oncol. 2007;14:730–738. doi: 10.1245/s10434-006-9207-5. [DOI] [PubMed] [Google Scholar]
- 3.Machens A, Hauptmann S, Dralle H. Lymph node dissection in the lateral neck for completion in central node-positive papillary thyroid cancer. Surgery. 2009;145:176–181. doi: 10.1016/j.surg.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Cooper DS, Doherty GM, Hauger BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 5.Zetoune T, Keutgen X, Buitrago D, et al. Prophylactic central neck dissection and local recurrence in papillary thyroid cancer: a meta-analysis. Ann Surg Oncol. 2010;17:3287–3293. doi: 10.1245/s10434-010-1137-6. [DOI] [PubMed] [Google Scholar]
- 6.Hughes DT, White ML, Miller BS, Gauger PG, Burney RE, Doherty GM. Influence of prophylactic central lymph node dissection on postoperative thyroglobulin levels and radioiodine treatment in papillary thyroid cancer. Surgery. 2010;148:1100–1107. doi: 10.1016/j.surg.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Moo TA, McGill J, Allendorf J, Lee J, Fahey T, Zarnegar R. Impact of prophylactic central neck lymph node dissection on early recurrence in papillary thyroid carcinoma. World J Surg. 2010;34:1187–1191. doi: 10.1007/s00268-010-0418-3. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet S, Hartl D, Leboulleux S, et al. Prophylactic lymph node dissection for papillary thyroid cancer less than 2 cm: implications for radioiodine treatment. J Clin Endocrinol Metab. 2009;94:1162–1167. doi: 10.1210/jc.2008-1931. [DOI] [PubMed] [Google Scholar]
- 9.Forest VI, Clark JR, Ebrahimi A, et al. Central compartment dissection in thyroid papillary carcinoma. Ann Surg. 2011;253:123–130. doi: 10.1097/SLA.0b013e3181fc9644. [DOI] [PubMed] [Google Scholar]
- 10.Carling T, Long WD, Udelsman R. Controversy surrounding the role for routine central lymph node dissection for differentiated thyroid cancer. Curr Opin Oncol. 2010;22:30–34. doi: 10.1097/CCO.0b013e328333ac97. [DOI] [PubMed] [Google Scholar]
- 11.Ito Y, Tomoda C, Uruno T, et al. Clinical significance of metastasis to the central compartment from papillary microcarcinoma of the thyroid. World J Surg. 2006;30:91–99. doi: 10.1007/s00268-005-0113-y. [DOI] [PubMed] [Google Scholar]
- 12.Shen WT, Ogawa L, Ruan D, Suh I, Duh QY, Clark OH. Central neck lymph node dissection for papillary thyroid cancer: the reliability of surgeon judgment in predicting which patients will benefit. Surgery. 2010;148:398–403. doi: 10.1016/j.surg.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 13.Rosenbaum MA, McHenry CR. Central neck dissection for papillary thyroid cancer. Arch Otolaryngol Head Neck Surg. 2009;135:1092–1097. doi: 10.1001/archoto.2009.158. [DOI] [PubMed] [Google Scholar]
- 14.Roh JL, Park JY, Park CI. Prevention of postoperative hypocalcemia with routine oral calcium and vitamin D supplements in patients with differentiated papillary thyroid carcinoma undergoing total thyroidectomy plus central neck dissection. Cancer. 2009;115:251–258. doi: 10.1002/cncr.24027. [DOI] [PubMed] [Google Scholar]
- 15.Sywak M, Cornford L, Roach P, Stalberg P, Sidhu S, Delbridge L. Routine ipsilateral level VI lymphadenectomy reduces postoperative thyroglobulin levels in papillary thyroid carcinoma. Surgery. 2006;140:1000–1007. doi: 10.1016/j.surg.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Low TH, Delbridge L, Sidhu S, et al. Lymph node status influences follow-up thyroglobulin levels in papillary thyroid cancer. Ann Surg Oncol. 2008;15:2827–2832. doi: 10.1245/s10434-008-0049-1. [DOI] [PubMed] [Google Scholar]
- 17.Kim TY, Kim WB, Kim ES, et al. Serum thyroglobulin levels at the time of 131I remnant ablation just after thyroidectomy are useful for early prediction of clinical recurrence in low-risk patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2005;90:1440–1445. doi: 10.1210/jc.2004-1771. [DOI] [PubMed] [Google Scholar]
- 18.Heemstra KA, Liu YY, Stokkel M, et al. Serum thyroglobulin concentrations predict disease-free remission and death in differentiated thyroid carcinoma. Clin Endocrinol (Oxf) 2007;66:58–64. doi: 10.1111/j.1365-2265.2006.02685.x. [DOI] [PubMed] [Google Scholar]
- 19.Giovanella L, Ceriani L, Suriano S, Ghelfo A, Maffioli M. Thyroglobulin measurement before rh-TSH-aided 131I ablation in detecting metastases form differentiated thyroid carcinoma. Clin Endocrinol (Oxf) 2008;69:659–663. doi: 10.1111/j.1365-2265.2008.03244.x. [DOI] [PubMed] [Google Scholar]
- 20.So YK, Seo MY, Son YI. Prophylactic central lymph node dissection for clinically node-negative papillary thyroid microcarcinoma: influence on serum thyroglobulin level, recurrence rate, and postoperative complications. Surgery. doi:10.1016/j.surg.2011.02.004. [DOI] [PubMed]
- 21.Lang BH, Lo CY, Chan WF, Lam KY, Wan KY. Staging systems for papillary thyroid carcinoma: a review and comparison. Ann Surg. 2007;245:366–378. doi: 10.1097/01.sla.0000250445.92336.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan WF, Lang BH, Lo CY. The role of intraoperative neuromonitoring of recurrent laryngeal nerve during thyroidectomy: a comparative study on 1000 nerves at risk. Surgery. 2006;140:866–873. doi: 10.1016/j.surg.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Sackett WR, Reeve TS, Barraclough B, Delbridge L. Thyrothymic thyroid rest: incidence and relationship to the thyroid gland. J Am Coll Surg. 2002;195:635–640. doi: 10.1016/S1072-7515(02)01319-4. [DOI] [PubMed] [Google Scholar]
- 24.Lang B, Lo CY, Chan WF, Lam KY, Wan KY. Restaging of differentiated thyroid carcinoma by the sixth edition AJCC/UICC TNM staging system: stage migration and predictability. Ann Surg Oncol. 2007;14:1551–1559. doi: 10.1245/s10434-006-9242-2. [DOI] [PubMed] [Google Scholar]
- 25.Lo CY. Parathyroid autotransplantation during thyroidectomy. ANZ J Surg. 2002;72:902–907. doi: 10.1046/j.1445-2197.2002.02580.x. [DOI] [PubMed] [Google Scholar]
- 26.So YK, Son YI, Hong SD, et al. Subclinical lymph node metastasis in papillary thyroid microcarcinoma: a study of 551 resections. Surgery. 2010;148:526–531. doi: 10.1016/j.surg.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Smallridge RC, Meek SE, Morgan MA, et al. Monitoring thyroglobulin in a sensitive immunoassay has comparable sensitivity to recombinant human TSH-stimulated thyroglobulin in follow-up of thyroid cancer patients. J Clin Endocrinol Metab. 2007;92:82–87. doi: 10.1210/jc.2006-0993. [DOI] [PubMed] [Google Scholar]
- 28.Kloos RT, Mazzaferri EL. A single recombinant human thyrotrophin–stimulated serum thyroglobulin measurement predicts differentiated thyroid carcinoma metastases three to five years later. J Clin Endocrinol Metab. 2005;90:5047–5057. doi: 10.1210/jc.2005-0492. [DOI] [PubMed] [Google Scholar]