Abstract
AIM: To investigate appropriate therapeutic strategies for graft-vs-host disease (GVHD) following liver transplantation.
METHODS: Four patients who developed GVHD after liver transplantation in West China Hospital were included in this study. Therapeutic strategies with augmentation or withdrawal of immunosuppressants combined with supportive therapy were investigated in these patients. In addition, a literature review of patients who developed GVHD after liver transplantation was performed.
RESULTS: Although a transient response to initial treatment was detected, all four patients died of complications from GVHD: one from sepsis with multiple organ failure, one from gastrointestinal bleeding, and the other two from sepsis with gastrointestinal bleeding. Few consensuses for the treatment of GVHD after liver transplantation have been reached.
CONCLUSION: New and effective treatments are required for GVHD after liver transplantation to improve the prognosis of patients with this diagnosis.
Keywords: Graft-vs-host disease, Immunosuppressant, Immunosuppression, Liver transplantation, Treatment
INTRODUCTION
Although the liver is recognized as an immunologically privileged organ, acute graft-vs-host disease (GVHD) may occur in 1%-2% of patients after liver transplantation (LT), and the mortality rate of patients with GVHD is very high (> 85%)[1-3]. The donor lymphocytes remaining in the portal tracts and the parenchyma of the donor liver graft after flushing with cold preservative solution[4,5] colonize the recipient, recognizing the host tissue antigens as foreign and react against the host tissue. The typical clinical presentations of GVHD include fever, skin rash (Figures 1, 2, 3 and 4), diarrhea, and pancytopenia beginning 2 to 6 wk after LT[1,2,6]. The diagnosis is usually made according to the typical clinical manifestations mentioned above, with the exclusion of other phenomena such as infection, drug allergies or rejection, which share the same clinical features as GVHD. A rapid diagnosis by the detection of lymphocyte macrochimerism through DNA-short tandem repeat (STR) has been recommended[7], while human leukocyte antigen (HLA)-typing has also proven critical in confirming GVHD and elucidating its cause[8].
Figure 1.
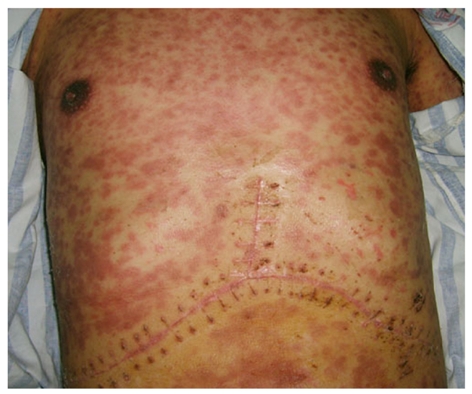
The maculopapular rash on the skin of the patient’s chest wall and abdominal wall.
Figure 2.
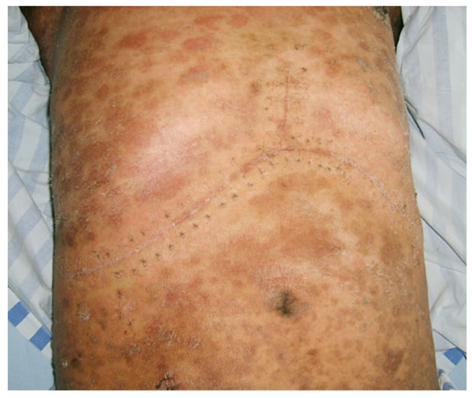
The skin underwent desquamation and pigmentation (the same patient as in Figure 1).
Figure 3.
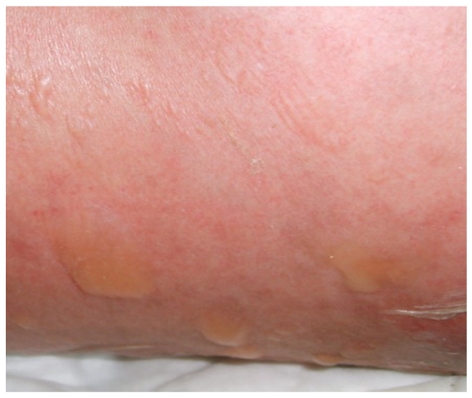
Skin blisters and rash on the flank and back of the patient.
Figure 4.
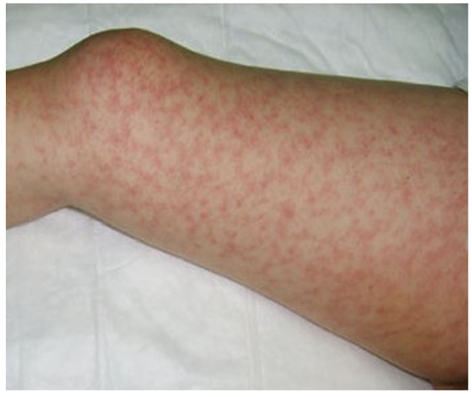
Maculopapular rash on the leg.
Treatment of GVHD typically consists of increasing immunosuppression using antibody preparations, such as OKT3 or antithymocyte globulin, to eliminate the donor lymphocytes and supporting myelopoiesis through the use of cytokines. However, this treatment has been unsuccessful; the majority of GVHD patients died of native bone marrow failure, resulting in fatal sepsis[1,9,10]. In one case, immunosuppressant withdrawal to control GVHD was suggested by Chinnakotla et al[11]. In their report, two of three patients exhibited the rapid loss of donor T-cell chimerism and resolution of their symptoms; however, the remaining patient continued to progress to severe GVHD and subsequently died.
In this study, we describe the unsuccessful results of different treatment regimens involving augmentation or withdrawal of immunosuppressant therapy in four patients with established GVHD after LT.
MATERIALS AND METHODS
From January 1999 to December 2010, 836 cases of LT were performed in West China Hospital. A total of four recipients developed postoperative GVHD (Table 1). Each of these patients was given a pathological diagnosis of GVHD following skin rash biopsy; HLA typing and polymerase chain reaction (PCR)-STR were not performed. The various treatments in these patients are shown in Table 2. One patient received increased immunosuppressive therapy (methylprednisolone 500 mg, iv, qd, for 3 d). This therapy was then augmented by consecutive use of cyclosporine A (CsA) and mycophenolate mofetil, while granulocyte colony-stimulating factor (G-CSF) was subsequently administered as a supportive treatment. The other three patients also initially received increased immunosuppressive therapy (methylprednisolone 500 mg, iv, qd, for 2 d); however, this was followed by the complete withdrawal of immunosuppressant therapy combined with the administration of supportive treatment including G-CSF (0.075 mg, bid), intravenous immunoglobulin (20 g, qd) or thymosin α1 (Zadaxin 1.6 mg, qd). Due to immune dysfunction, the patient who received increased immunosuppressive therapy eventually developed a severe infection. Enterobacter cloacae (a yeast-like organism) and Candida albicans were identified through repeat sputum and buccal swab culture in this patient. Subsequently, many different antibiotics (imipenem/cilastatin sodium, azithromycin, moxifloxacin, meropenem and teicoplanin) and an anti-fungal agent (fluconazole) were administered. Broad-spectrum antibiotics with or without anti-fungal medicines were also administered in the other three patients after GVHD onset to control infection or as a routine prophylaxis (Table 2). A flowchart of treatment is shown in Figure 5.
Table 1.
Clinic data of recipients with graft-vs-host disease
| Case | Age (yr)/sex | Etiology | HCC criteria | MELD score | Onset day | Presenting symptoms | Changes in PLT count | Changes in WBC count |
| 1 | 48/M | HCC/LC/HBV | UCSF | 28 | POD 23 | Fever, rash, diarrhea, BMS, gastrointestinal bleeding | 111 × 109/L to 12 × 109/L | 8.69 × 109/L to 0.28 × 109/L |
| 2 | 43/M | HCC/LC/HBV | Milan | 21 | POD 21 | Fever, rash, diarrhea, BMS, gastrointestinal bleeding | 124 × 109/L to 45 × 109/L | 7.59 × 109/L to 0.11 × 109 /L |
| 3 | 55/M | HCC/LC/HBV | Milan | 23 | POD 20 | Fever, rash, diarrhea, BMS, gastrointestinal bleeding | 273 × 109/L to 18 × 109/L | 4.39 × 109/L to 0.03 × 109/L |
| 4 | 56/F | LC/HBV | - | 27 | POD 21 | Fever, rash, diarrhea, BMS, gastrointestinal bleeding | 150 × 109/L to 1 × 109/L | 5.12 × 109/L to 0.16 × 109/L |
M: Male; F: Female; HCC: Hepatocellular carcinoma; LC: Liver cirrhosis; HBV: Hepatitis B virus; UCSF: University of California, San Francisco criteria for liver transplantation; BMS: Bone marrow suppression; POD: Post-operation day; MELD: Model for End-Stage Liver Disease; PLT: Platelet; WBC: White blood cell. Normal value for PLT is (100-300) × 109/L; Normal value for WBC is (4-10) × 109/L.
Table 2.
Treatment for graft-vs-host disease and outcome
| Case | Immunosuppression and supportive therapy | Time of death | Cause of death |
| 1 | Increased immunosuppressant (methylprednisolone 500 mg, iv, qd, for 3 d), then CsA and mycophenolate mofetil + G-CSF (0.075 mg, bid, for 11 d) + antibiotics (imipenem/cilastatin sodium, azithromycin, moxifloxacin, meropenem and teicoplanin) + anti-fungal (fluconazole) | POD 34 | MOF and sepsis |
| 2 | Increased immunosuppressant (methylprednisolone 500 mg, iv, qd, for 2 d), then withdrawal (POD 29) of immunosuppressant + G-CSF (0.075 mg, bid, for 9 d) + IVIG (20 g, qd, for 9 d) + broad spectrum antibiotics (cefuroxime, ceftriaxone and imipenem/cilastatin) + anti-fungal (fluconazole) | POD 38 | Gastrointestinal bleeding |
| 3 | Increased immunosuppressant (methylprednisolone 500 mg, qd, for 2 d), then withdrawal (POD 29) of immunosuppressant + thymosin α1 (Zadaxin 1.6 mg, qd, for 7 d) + G-CSF (0.075 mg, bid, for 7 d) + broad spectrum antibiotics (cefminox) | POD 35 | Gastrointestinal bleeding and sepsis |
| 4 | Increased immunosuppressant (methylprednisolone 500 mg, iv, qd, for 2 d), then withdrawal (POD 30) of immunosuppressant + IVIG (20 g, qd, for 7 d) + thymosin α1 (Zadaxin 1.6 mg, qd, for 7 d) + G-CSF (0.075 mg, bid, for 5 d) + broad spectrum antibiotics (cefminox, cefoperazone/sulbactam) | POD 36 | Gastrointestinal bleeding and sepsis |
CsA: Cyclosporine A; G-CSF: Granulocyte colony-stimulating factor; IVIG: Intravenous immunoglobulin therapy; MOF: Multiple organ failure; POD: Post-operation day.
Figure 5.
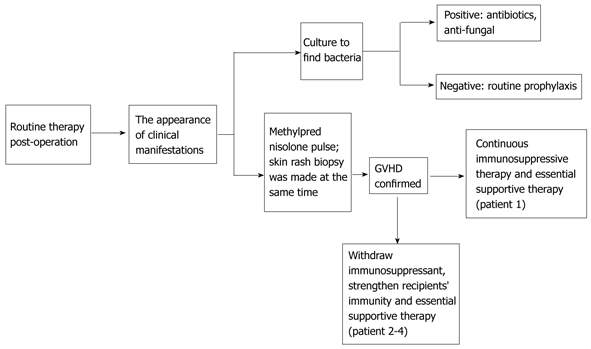
Flowchart of the management of graft-vs-host disease after liver transplantation in our center. GVHD: Graft-vs-host disease.
RESULTS
All patients with confirmed pathological diagnoses of GVHD developed fever, skin rash, diarrhea, severe bone marrow suppression and gastrointestinal bleeding. In response to the initial treatment, the rash faded, the skin underwent desquamation and pigmentation (Figure 2), and the temperature displayed a transient decrease. However, diarrhea persisted, bone marrow failure and gastrointestinal bleeding developed, and the levels of white blood cells (WBC) and platelets dropped. The most conspicuous changes in platelet number and white blood cell count were observed in cases 4 and 3, from a normal level to 1 × 109/L and from a normal level to 0.03 × 109/L, respectively (Table 1). All of these patients died of complications from GVHD: one from sepsis with multiple organ failure (MOF), one from gastrointestinal bleeding, and the other two from sepsis with gastrointestinal bleeding (Table 2). The average survival time from onset of GVHD was 14.25 d.
DISCUSSION
GVHD following LT is an uncommon but fatal complication that poses a major diagnostic and therapeutic challenge. Research has shown that GVHD involves activation of donor T lymphocytes by antigen-presenting cells (APCs), causing an alloreactive T-cell response to recipient tissues mediated by cytotoxic T-cells and inflammatory cytokines[12], such as tumor necrosis factor-alpha (TNF-α), interleukins 1, 2, 6 and 10, and interferon γ[13]. Of these cytokines, TNF-α is recognized as the key inflammatory cytokine involved in the pathogenesis of GVHD, which can activate APCs, recruit effector cells, and cause tissue damage[13,14]. Taking the role of TNF-α during GVHD into account, certain anti-TNF-α agents such as etanercept and infliximab have been used in steroid-refractory GVHD following hematopoietic stem cell transplantation (HSCT), and one case who developed GVHD after LT was reported to be successfully treated with infliximab[14,15]. Etanercept, a soluble recombinant human TNF-α receptor type II fusion protein, which is usually used to treat rheumatoid arthritis and inflammatory bowel disease, was reported to be used in acute GVHD with steroids. A substantial majority of patients displayed a complete response (CR), which was very promising[15,16].
At present, most cases of GVHD after LT are diagnosed by typical clinical manifestations. However, the following laboratory examinations can help with the diagnosis: (1) biopsy of the skin rash; (2) detection of donor peripheral blood leukocytic chimerism; (3) microsatellite phenotype; (4) detection of donor HLA types in the peripheral blood, mucous membrane, or skin by PCR; and (5) detection of donor lymphocytes using immunohistochemistry on the skin rash[17]. Once diagnosis is confirmed, treatment should follow. Due to the low incidence of the disease and difficulty in evaluating the efficacy of treatment modalities, the therapeutic schedule for GVHD after LT has not yet been standardized. Most treatment methods are derived from those experiences of GVHD management following HSCT. Most treatments focus on increasing immunosuppression, usually in the form of antibody preparations such as antithymocyte globulin (ATG) or OKT3, which are used to treat conventional GVHD in a bone marrow transplant recipient. Unfortunately, these experiences did not demonstrate any survival benefit[9,10,18-21].
CsA is the mainstay of pharmacologic prevention of acute GVHD[22], while high-dose corticosteroid is considered an important part of first-line treatment for acute GVHD after LT[1,3]. The use of corticosteroids for the treatment of GVHD after LT is derived from the HSCT experience: corticosteroids resolve symptoms in many patients. The mechanism by which glucocorticoids ameliorate acute GVHD is not completely clear, but it is likely related primarily to the suppression of lymphocytic activity. However, GVHD after LT is less responsive to corticosteroids than GVHD after stem cell transplantation. The literature includes reports on 12 patients who were treated primarily with corticosteroids and/or increased immunosuppressive medications following the diagnosis of GVHD after LT[1,3,9,10,21,23-27]. Of these patients, all adult patients died of GVHD-related complications during the subsequent 11 d; only two children[28,29] were alive at the time of the report. These results suggest that, at least in the adult patients, treatment of GVHD after LT exclusively with corticosteroids or increasing immunosuppression is an inadequate approach to long-term therapy. Although temporary symptom relief may be available, the resolution of GVHD cannot be expected.
In the first case in our series, high-dose methylprednisolone followed by CsA and mycophenolate mofetil were administered to treat GVHD. Although the fever improved transiently in this patient, diarrhea and bone marrow suppression did not respond to this treatment regimen; continuous diarrhea and bone marrow failure developed, and WBC and platelet counts progressively decreased. Furthermore, hyperpyrexia developed again after two days’ amelioration. This patient soon died of severe lung infection and MOF 11 d after GVHD onset.
Due to the unsuccessful outcome of acute GVHD in a liver allograft recipient following increased immunosuppression, reduction and even complete withdrawal of immunosuppressant therapy has been proposed as a treatment for GVHD after LT. Theoretically, such an approach should allow the recipient immune system to reject alloreactive donor lymphocytes mediating the GVHD[29-31]. Attempts at implementing this approach were met with an initial worsening of symptoms[2,32] and involved a risk of the donor liver being rejected[30]. These limitations would explain why few patients were treated primarily with the reduction or discontinuation of immunosuppression. Antilymphocyte globulin, ATG and OKT3 were also used in these patients, but there was no evidence that these agents altered the eventual outcome[9,10,8-21].
Six adult patients were reported to have been treated for GVHD after LT primarily by the reduction or discontinuation of immunosuppression before 2004[9,26,33-35]. Two of these patients were reported to be alive at the time of the report, with their recovery attributed to a reduction in immunosuppression[26,30]. Five children have been treated with this approach[1,29,31,33,36]. Of these children, three were alive, one was alive with chronic GVHD, and one child died. Chinnakotla et al[11] reported the success of immunosuppression withdrawal in three patients diagnosed with GVHD in 2007. This approach was successful in two of three cases, but the other patient died. Another successful approach to treatment involving improvement of the immunity of the patient was also reported by Lu et al [17].
In our series, three patients were treated first by increasing immunosuppression (high-dose methylprednisolone 500 mg once daily for 2 d + FK506 or CsA) upon the initial appearance of GVHD symptoms, such as hyperpyrexia and skin rash. Because the diagnosis of GVHD was confirmed by skin rash biopsy, the amelioration of fever, and progressive bone marrow cell proliferation suppression, complete withdrawal of the immunosuppressant was proposed. At the same time, supportive therapies such as immunoglobulin, thymosin α1 and G-CSF were administered. Unfortunately, these three patients did not respond effectively to this regimen of immunosuppression withdrawal combined with supportive therapy; one patient died of gastrointestinal bleeding, while two died of sepsis with gastrointestinal bleeding.
In sum, although two major strategies (augmentation or withdrawal of immunosuppressant therapy) have been proposed for the treatment of GVHD after LT, many novel strategies have been reported to be effective: anti-TNF-α with etanercept or infliximab, use of alefacept to elevate the blood cell count[36], pulse cyclophosphamide to treat the steroid-refractory hepatitis form of liver GVHD not associated with gut GVHD[37], interleukin 2-receptor antibody (basiliximab or daclizumab) therapy[7], and the preventive broad-spectrum chemokine-inhibitor NR58-3.14.3 (in animal experiments only)[38]. However, few consensuses have been reached. For non-typical manifestation at an early stage, which involve symptoms similar to those of a drug allergy or mimicking a more common clinical condition such as infection, GVHD can easily be misdiagnosed or even omitted. Moreover, as a rare post-transplant complication with no standardized treatment protocol hitherto, early diagnosis and effective treatment of GVHD would be complex, frustrating and laden with additional difficulties. Although a handful of successful cases have been reported, further research is necessary. It is therefore imperative to develop a de novo therapeutic schedule with definite effects.
COMMENTS
Background
Graft-vs-host disease (GVHD) following liver transplantation (LT) is a rare but fatal complication. The incidence is about 1%-2%, and the mortality rate is over 85%. People who develop GVHD after LT usually die of serious complications such as multiple organ failure, gastrointestinal bleeding and sepsis. So, a rapid diagnosis and treatment of GVHD is critical. At present, the consensuses of treatment methods for GVHD after LT have not been reached and the therapeutic experiences are derived from those of management following hematopoietic stem cell transplantation.Two major strategies (augmentation or withdrawal of immunosuppressant therapy) have been proposed as the treatment of GVHD after LT. Also, many novel strategies have been reported to be effective.
Research frontiers
A rapid diagnosis by the detection of lymphocyte macrochimerism through DNA-short tandem repeat has been recommended, while human leukocyte antigen- typing has also proven critical in confirming GVHD and elucidating its cause. Tumor necrosis factor-alpha (TNF-α) is recognized as the key inflammatory cytokine involved in the pathogenesis of GVHD, which can activate antigen presenting cells, recruit effector cells and cause tissue damage. So, certain anti-TNF-α agents such as etanercept and infliximab have been used in steroid-refractory GVHD.
Innovations and breakthroughs
The authors herein report four patients who developed GVHD after liver transplantation. Therapeutic strategies with augmentation or withdrawal of immunosuppressant combined with supportive therapy were investigated in these patients and a literature review of patients who developed GVHD after liver transplantation was performed in this article to investigate appropriate therapeutic strategies for GVHD following LT.
Applications
This article describes the clinical manifestations and diagnostic method of GVHD after LT and summarizes different therapeutic strategies, which would be helpful to the diagnosis or cure of this disease.
Terminology
GVHD: An immunological disorder that affects many organ systems, including the gastrointestinal tract, liver, skin and lungs, is a common and serious complication of transplantation where there is a reaction of donated organ/bone marrow against a patient's own tissue. It is an incompatibility reaction when donor lymphocytes or a graft containing lymphocytes that immunologically competent are given to a patient that has low immunological competence. Due to antibodies from the donor against antigens in the host, it can produce lymphocyte clones that will react by a variety of processes against the host and cause damage. Immunosuppressant: The agent administrated by patients who accept organ/bone marrow transplantation that decrease the immunity of the patients.
Peer review
The manuscript is well written and summarizes the treatment methods of GVHD after LT.
Footnotes
Peer reviewer: Rui Marinho, Professor, Department of Gastroenterology and Hepatology, Rua Prof. Aires de Sousa, 1 r/c A, Lisboa 1600-590, Portugal
S- Editor Sun H L- Editor Webster JR E- Editor Li JY
References
- 1.Smith DM, Agura E, Netto G, Collins R, Levy M, Goldstein R, Christensen L, Baker J, Altrabulsi B, Osowski L, et al. Liver transplant-associated graft-versus-host disease. Transplantation. 2003;75:118–126. doi: 10.1097/00007890-200301150-00022. [DOI] [PubMed] [Google Scholar]
- 2.Taylor AL, Gibbs P, Bradley JA. Acute graft versus host disease following liver transplantation: the enemy within. Am J Transplant. 2004;4:466–474. doi: 10.1111/j.1600-6143.2004.00406.x. [DOI] [PubMed] [Google Scholar]
- 3.Taylor AL, Gibbs P, Sudhindran S, Key T, Goodman RS, Morgan CH, Watson CJ, Delriviere L, Alexander GJ, Jamieson NV, et al. Monitoring systemic donor lymphocyte macrochimerism to aid the diagnosis of graft-versus-host disease after liver transplantation. Transplantation. 2004;77:441–446. doi: 10.1097/01.TP.0000103721.29729.FE. [DOI] [PubMed] [Google Scholar]
- 4.Norris S, Collins C, Doherty DG, Smith F, McEntee G, Traynor O, Nolan N, Hegarty J, O’Farrelly C. Resident human hepatic lymphocytes are phenotypically different from circulating lymphocytes. J Hepatol. 1998;28:84–90. doi: 10.1016/s0168-8278(98)80206-7. [DOI] [PubMed] [Google Scholar]
- 5.Domiati-Saad R, Klintmalm GB, Netto G, Agura ED, Chinnakotla S, Smith DM. Acute graft versus host disease after liver transplantation: patterns of lymphocyte chimerism. Am J Transplant. 2005;5:2968–2973. doi: 10.1111/j.1600-6143.2005.01110.x. [DOI] [PubMed] [Google Scholar]
- 6.Pirenne J, Benedetti E, Dunn DL. Graft versus host response: clinical and biological relevance after transplantation of solid organs. Transplant Rev. 1996;10:46–48. [Google Scholar]
- 7.Guo ZY, He XS, Wu LW, Zhu XF, Ju WQ, Wang DP, You S, Ma Y, Wang GD, Huang JF. Graft-verse-host disease after liver transplantation: a report of two cases and review of literature. World J Gastroenterol. 2008;14:974–979. doi: 10.3748/wjg.14.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grosskreutz C, Gudzowaty O, Shi P, Rodriguez-Laiz G, Malone A, Isola L. Partial HLA matching and RH incompatibility resulting in graft versus host reaction and Evans syndrome after liver transplantation. Am J Hematol. 2008;83:599–601. doi: 10.1002/ajh.21067. [DOI] [PubMed] [Google Scholar]
- 9.Roberts JP, Ascher NL, Lake J, Capper J, Purohit S, Garovoy M, Lynch R, Ferrell L, Wright T. Graft vs. host disease after liver transplantation in humans: a report of four cases. Hepatology. 1991;14:274–281. [PubMed] [Google Scholar]
- 10.Romagnuolo J, Jewell LD, Kneteman NM, Bain VG. Graft-versus-host disease after liver transplantation complicated by systemic aspergillosis with pancarditis. Can J Gastroenterol. 2000;14:637–640. doi: 10.1155/2000/678043. [DOI] [PubMed] [Google Scholar]
- 11.Chinnakotla S, Smith DM, Domiati-Saad R, Agura ED, Watkins DL, Netto G, Uemura T, Sanchez EQ, Levy MF, Klintmalm GB. Acute graft-versus-host disease after liver transplantation: role of withdrawal of immunosuppression in therapeutic management. Liver Transpl. 2007;13:157–161. doi: 10.1002/lt.20982. [DOI] [PubMed] [Google Scholar]
- 12.Mawad R, Hsieh A, Damon L. Graft-versus-host disease presenting with pancytopenia after en bloc multiorgan transplantation: case report and literature review. Transplant Proc. 2009;41:4431–4433. doi: 10.1016/j.transproceed.2009.06.229. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piton G, Larosa F, Minello A, Becker MC, Mantion G, Aubin F, Deconinck E, Hillon P, Di Martino V. Infliximab treatment for steroid-refractory acute graft-versus-host disease after orthotopic liver transplantation: a case report. Liver Transpl. 2009;15:682–685. doi: 10.1002/lt.21793. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara JL. Novel strategies for the treatment and diagnosis of graft-versus-host-disease. Best Pract Res Clin Haematol. 2007;20:91–97. doi: 10.1016/j.beha.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Levine JE, Paczesny S, Mineishi S, Braun T, Choi SW, Hutchinson RJ, Jones D, Khaled Y, Kitko CL, Bickley D, et al. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood. 2008;111:2470–2475. doi: 10.1182/blood-2007-09-112987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu Y, Wu LQ, Zhang BY, Cao JY. Graft-versus-host disease after liver transplantation: successful treatment of a case. Transplant Proc. 2008;40:3784–3786. doi: 10.1016/j.transproceed.2008.03.164. [DOI] [PubMed] [Google Scholar]
- 18.Burdick JF, Vogelsang GB, Smith WJ, Farmer ER, Bias WB, Kaufmann SH, Horn J, Colombani PM, Pitt HA, Perler BA. Severe graft-versus-host disease in a liver-transplant recipient. N Engl J Med. 1988;318:689–691. doi: 10.1056/NEJM198803173181107. [DOI] [PubMed] [Google Scholar]
- 19.Jamieson NV, Joysey V, Friend PJ, Marcus R, Ramsbottom S, Baglin T, Johnston PS, Williams R, Calne RY. Graft-versus-host disease in solid organ transplantation. Transpl Int. 1991;4:67–71. doi: 10.1007/BF00336399. [DOI] [PubMed] [Google Scholar]
- 20.Joysey VC, Wood H, Ramsbottom S, Morgan H, Ford C, Horsford J, Jamieson N, Friend P, Alexander G, Calne RY. Lymphocyte chimaerism after organ transplantation. Transplant Proc. 1992;24:2519–2522. [PubMed] [Google Scholar]
- 21.Collins RH, Anastasi J, Terstappen LW, Nikaein A, Feng J, Fay JW, Klintmalm G, Stone MJ. Brief report: donor-derived long-term multilineage hematopoiesis in a liver-transplant recipient. N Engl J Med. 1993;328:762–765. doi: 10.1056/NEJM199303183281104. [DOI] [PubMed] [Google Scholar]
- 22.Oshima K, Kanda Y, Nakasone H, Arai S, Nishimoto N, Sato H, Watanabe T, Hosoya N, Izutsu K, Asai T, et al. Decreased incidence of acute graft-versus-host disease by continuous infusion of cyclosporine with a higher target blood level. Am J Hematol. 2008;83:226–232. doi: 10.1002/ajh.21087. [DOI] [PubMed] [Google Scholar]
- 23.Mazzaferro V, Andreola S, Regalia E, Poli F, Doci R, Bozzetti F, Gennari L. Confirmation of graft-versus-host disease after liver transplantation by PCR HLA-typing. Transplantation. 1993;55:423–425. [PubMed] [Google Scholar]
- 24.Schmuth M, Vogel W, Weinlich G, Margreiter R, Fritsch P, Sepp N. Cutaneous lesions as the presenting sign of acute graft-versus-host disease following liver transplantation. Br J Dermatol. 1999;141:901–904. doi: 10.1046/j.1365-2133.1999.03166.x. [DOI] [PubMed] [Google Scholar]
- 25.Schrager JJ, Vnencak-Jones CL, Graber SE, Neff AT, Chari RS, Wright KJ, Pinson CW, Stewart JH, Gorden DL. Use of short tandem repeats for DNA fingerprinting to rapidly diagnose graft-versus-host disease in solid organ transplant patients. Transplantation. 2006;81:21–25. doi: 10.1097/01.tp.0000190431.94252.3f. [DOI] [PubMed] [Google Scholar]
- 26.Starzl TE, Demetris AJ, Trucco M, Murase N, Ricordi C, Ildstad S, Ramos H, Todo S, Tzakis A, Fung JJ. Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance. Hepatology. 1993;17:1127–1152. [PMC free article] [PubMed] [Google Scholar]
- 27.Whalen JG, Jukic DM, English JC. Rash and pancytopenia as initial manifestations of acute graft-versus-host disease after liver transplantation. J Am Acad Dermatol. 2005;52:908–912. doi: 10.1016/j.jaad.2005.01.126. [DOI] [PubMed] [Google Scholar]
- 28.Cattral MS, Langnas AN, Wisecarver JL, Harper JC, Rubocki RJ, Bynon JS, Fox IJ, Heffron TG, Shaw BW. Survival of graft-versus-host disease in a liver transplant recipient. Transplantation. 1994;57:1271–1274. doi: 10.1097/00007890-199404270-00024. [DOI] [PubMed] [Google Scholar]
- 29.Pinna AD, Weppler D, Berho M, Masetti M, DeFaria W, Kato T, Thompson J, Ricordi C, Tzakis AG. Unusual presentation of graft-versus-host disease in pediatric liver transplant recipients: evidence of late and recurrent disease. Pediatr Transplant. 1999;3:236–242. doi: 10.1034/j.1399-3046.1999.00043.x. [DOI] [PubMed] [Google Scholar]
- 30.Lehner F, Becker T, Sybrecht L, Lück R, Schwinzer R, Slateva K, Blasczyk R, Hertenstein B, Klempnauer J, Nashan B. Successful outcome of acute graft-versus-host disease in a liver allograft recipient by withdrawal of immunosuppression. Transplantation. 2002;73:307–310. doi: 10.1097/00007890-200201270-00030. [DOI] [PubMed] [Google Scholar]
- 31.Dunn SP, Krueger LJ, Butani L, Punnett H. Late onset of severe graft-versus-host disease in a pediatric liver transplant recipient. Transplantation. 2001;71:1483–1485. doi: 10.1097/00007890-200105270-00022. [DOI] [PubMed] [Google Scholar]
- 32.Comenzo RL, Malachowski ME, Rohrer RJ, Freeman RB, Rabson A, Berkman EM. Anomalous ABO phenotype in a child after an ABO-incompatible liver transplantation. N Engl J Med. 1992;326:867–870. doi: 10.1056/NEJM199203263261305. [DOI] [PubMed] [Google Scholar]
- 33.Hahn AB, Baliga P. Rapid method for the analysis of peripheral chimerism in suspected graft-versus-host disease after liver transplantation. Liver Transpl. 2000;6:180–184. doi: 10.1002/lt.500060214. [DOI] [PubMed] [Google Scholar]
- 34.Kuball J, Theobald M, Ferreira EA, Hess G, Burg J, Maccagno G, Barreiros AP, Lüth S, Schimanski CC, Schuchmann M, et al. Control of organ transplant-associated graft-versus-host disease by activated host lymphocyte infusions. Transplantation. 2004;78:1774–1779. doi: 10.1097/01.tp.0000144183.77279.ec. [DOI] [PubMed] [Google Scholar]
- 35.Walling HW, Voigt MD, Stone MS. Lichenoid graft vs. host disease following liver transplantation. J Cutan Pathol. 2004;31:179–184. doi: 10.1111/j.0303-6987.2004.00146.x. [DOI] [PubMed] [Google Scholar]
- 36.Stotler CJ, Eghtesad B, Hsi E, Silver B. Rapid resolution of GVHD after orthotopic liver transplantation in a patient treated with alefacept. Blood. 2009;113:5365–5366. doi: 10.1182/blood-2009-02-207431. [DOI] [PubMed] [Google Scholar]
- 37.Mayer J, Krejci M, Pospisil Z, Doubek M, Janikova A, Zackova D, Racil Z, Smardova L, Navratil M, Kamelander J. Successful treatment of steroid-refractory hepatitic variant of liver graft-vs-host disease with pulse cyclophosphamide. Exp Hematol. 2009;37:767–773. doi: 10.1016/j.exphem.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Miklos S, Mueller G, Chang Y, Bouazzaoui A, Spacenko E, Schubert TE, Grainger DJ, Holler E, Andreesen R, Hildebrandt GC. Preventive usage of broad spectrum chemokine inhibitor NR58-3.14.3 reduces the severity of pulmonary and hepatic graft-versus-host disease. Int J Hematol. 2009;89:383–397. doi: 10.1007/s12185-009-0272-y. [DOI] [PubMed] [Google Scholar]


