Abstract
No vaccine candidate has induced antibodies (Abs) that efficiently neutralize multiple primary isolates of HIV-1. Preexisting high titers of neutralizing antibodies (NAbs) are essential, because the virus establishes infection before anamnestic responses could take effect. HIV-1 infection elicits Abs against Env, Gag, and other viral proteins, but of these only a subset of the anti-Env Abs can neutralize the virus. Whereas the corresponding proteins from other viruses form the basis of successful vaccines, multiple large doses of HIV-1 Env elicit low, transient titers of Abs that are not protective in humans. The inaccessibility of neutralization epitopes hinders NAb induction, but Env may also subvert the immune response by interacting with receptors on T cells, B cells, monocytes, macrophages, and dendritic cells. Here, we discuss evidence from immunizations of different species with various modified Env constructs. We also suggest how the divergent Ab responses to Gag and Env during infection may reflect differences in B cell regulation. Drawing on these analyses, we outline strategies for improving Env as a component of a vaccine aimed at inducing strong and sustained NAb responses.
The Neutralizing Antibody Response as a Block to HIV Type 1 Transmission
The envelope glycoprotein complex (Env) studs the HIV-1 virion and mediates entry into target cells. Env consists of trimers of heterodimers, with each unit of the transmembrane protein, gp41, noncovalently attached to a monomer of the receptor-binding surface glycoprotein, gp120. The Env complex is the only target for neutralizing antibodies (NAbs), i.e., Abs that block productive viral entry.1,2 Other structural components (e.g., Gag) are immunogenic during infection, but the resulting Abs are not neutralizing, because the target proteins are inaccessible within the virion. Passive immunization of macaques with NAbs protects against virus transmission,3–5 whereas Env-binding, nonneutralizing Abs, depending on the epitope to which they are directed, are completely inert or markedly less effective6 (D.R. Burton and J.P. Moore, unpublished observations). Although we consider NAbs crucial to protection, much of what we discuss below applies also to nonneutralizing Abs.
Antigenicity and immunogenicity are both important aspects of the Ab response to Env. Antigenicity, the capacity to be recognized by an immune response, is problematic for Env, because many Abs bind to denatured, disassembled, or incompletely processed Env components but not to the functional trimer. Flexibility of the Env complex can impede interactions with Abs.7 In addition, the virus rapidly mutates its env gene, thereby escaping from NAbs by changing its antigenicity.8,9 Immunogenicity, the capacity to elicit an immune response, is also restricted for the Env complex: much of its surface is shielded by high-mannose glycans, rendering it largely immunosilent.2 Furthermore, gp120 specifically binds to important immune-system molecules: CD4, chemokine receptors, mannose-binding C-type lectin receptors (MCLRs), and the homing integrin α4β7,10 thereby potentially perturbing key players in the immune response such as T and B lymphocytes, monocytes, macrophages, and dendritic cells. There are cross-reactive neutralization epitopes on gp41, but NAbs against them are rarely elicited during infection and never to date by engineered protein mimics.11,12 Other gp41 epitopes are immunodominant but bind nonneutralizing Abs.13 The gp41 subunit, like its counterparts from other retroviruses, has been implicated in immunosuppression, at least in vitro.14
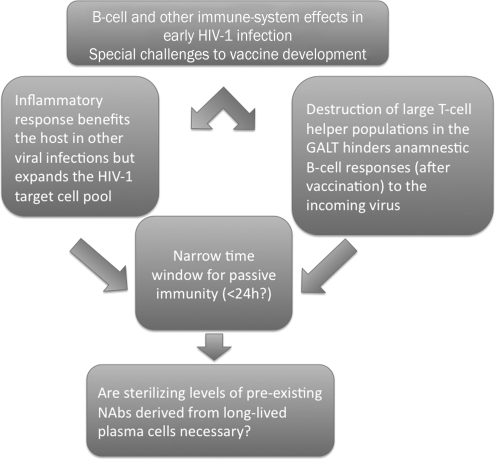
How HIV-1 establishes infection in a new host poses particular challenges to vaccination. Immune reactions to incoming viruses normally benefit the host, but they can perversely favor HIV-1 by increasing target cell numbers.15,16 Furthermore, HIV-1 destroys large populations of T-helper (TH) cells and inductive sites for B cells in the intestinal mucosa during acute infection.17,18 The damage might undermine any vaccine-primed anamnestic responses to an incoming virus. In the macaque model, passive immunization by NAb transfer is protective when started 6 h, but not 24 h, after virus inoculation.19 By analogy, anamnestic immune responses in vaccinees may not have time to outpace the incoming virus.20 Together, the disadvantages of immune activation, the early pathogenic effects, and the time constraints emphasize the importance of eliciting preexisting high titers of NAbs (Fig. 1).
FIG. 1.
The early effects of HIV-1 infection on the immune system pose atypical demands on a vaccine. On the one hand, the inflammatory response benefits the virus by providing an expanded pool of susceptible cells (e.g., lymphocyte activation leads to increased CCR5 expression). Conversely, CD4+ T lymphocytes are rapidly destroyed in the gut-associated lymphoid tissue (GALT), which deprives the B cell response of much early T cell help. Studies of the timing of postexposure passive immunization after SHIV challenge of macaques show that to prevent infection, the Ab must be given earlier than 24 h after the virus. The time window for immune prevention of the establishment of infection may be similar, and hence too short for an anamnestic response to be effective; the most feasible safety net would be preexisting high titers of neutralizing antibodies.
The NAb response that eventually matures in a minority of HIV-1-infected patients is stronger and broader than anything that has yet been accomplished by Env vaccination.21 Compared with cellular immune responses, NAbs do little to curb viral loads, but do they protect against superinfection? The evidence, though inconclusive, is not encouraging. The incidence of superinfection varies from 50% to 100% of the primary-infection rate in uninfected control cohorts,22–24 and superinfection occurs in the face of substantial cross-neutralizing titers, also specifically against early posttransmission forms of the virus.22 However, the minority of subjects with broad and strong NAb responses may be too small to make a detectable impact, and the NAb titers may even more rarely reach the high levels found experimentally to be required for protection.
What Env vaccine could then induce better NAb responses than infection does? The critical structural aspects of Env vaccine design have been well addressed elsewhere,2,25 as have adjuvant strategies based on innate immunity.26,27 Here, we discuss which features of Env may limit its immunogenicity. We compare Env with the nonglycosylated core protein Gag, as well as with viral proteins used in other vaccines, and we outline how Env may suppress and divert precisely the kind of Ab responses that an effective vaccine must elicit. Many, often adverse, effects of gp120 and gp41 components on immune-system cells have been studied in vitro. The required concentrations of the proteins or peptides can be so high that the observed effects are of questionable relevance to pathogenesis.28 However, the local Env concentrations soon after vaccination could be in a range that makes some in vitro studies pertinent to immunization strategies.
The B Cell Response to Env in HIV-1 Infection
B cell responses have many dimensions, including thymus-dependent (TD) vs. thymus-independent (TI) stimulation; Ab-isotype profiles, partly reflecting T-helper (TH) polarization; memory B cells vs. long-lived plasma cells; and high vs. low antigen-concentration dependence. In discussing the Ab response to HIV-1 infection, we focus on how these characteristics relate to vaccine research. For a more detailed discussion of HIV-1-induced B cell abnormalities and pathogenic mechanisms, other reviews should be consulted.29,30
Hypergammaglobulinemia (hyper-GG) is a hallmark of untreated HIV-1 infection.31,32 The excess IgG is directed against Ags from various microorganisms, including HIV-1, and some of it cross-reacts with autoantigens.17,33–35 HIV-1 also causes B cell depletion.30,36 Both the hyperstimulation of B cells and their loss can hinder NAb production. Inductive anatomical niches and survival factors may be scarce commodities — not least for long-lived plasma cell differentiation37— easily squandered on the nonprotective hyperproliferation that contributes to hyper-GG. Losing naive B cells may remove NAb-producing precursors at an early stage, while depleting memory B cells could reduce somatic hypermutation (SHM), thereby compromising the development of broadly active NAbs.38–41
Differential responses to Gag and Env
Within weeks of HIV-1 infection, humans seroconvert to multiple HIV-1 Ags, most prominently to Gag, Pol, and Env. While Ab titers to gp120 and gp41 remain high, those to the Gag protein p24 decline with disease progression42,43 (Table 1). Why do anti-Gag and anti-Env titers diverge? Although p24 antigenemia rises concomitantly, absorption of Abs by Ag does not explain the loss of anti-Gag Abs. First, the molar excess of Ab over Ag is vast.28,42 Second, Ag drives Ab production,44–46 e.g., NAb titers are higher in people with elevated Ag levels than in long-term nonprogressors and effectively treated patients.29,47,48 Third, Gag can be less abundant than Env, at least in lymph nodes.49
Table 1.
Paradoxes of the Antibody Response to HIV-1
| In spite of immune pathology, the neutralization response is generally higher and broader during infection than after immunization with gp120. |
| Cross-neutralizing responses are slow to develop (months, years), slower than other Abs to both Gag and Env, but often directed to single, dominant epitopes. |
| NAb titers are generally lower in patients with undetectable than in those with high viral loads. |
| During clinical progression Ab levels to Gag, but not Env, decline. Absorption by Ag is not responsible. Are Gag and Env responses regulated differently? |
Ab, antibody; NAb, neutralizing antibody; Ag, antigen.
An alternative explanation is that Env and Gag Ab responses are stimulated differently. The Gag response may be strongly TD and therefore particularly sensitive to CD4+ T cell losses.42,50 In contrast, the Env response may have a TI component, may depend strongly on Ag levels, and could be enhanced by continuous sequence evolution, the latter mechanism being less applicable to the more conserved Gag protein.23,36 In other words, the Env-directed Ab response, which is sustained in the face of declining T cell help, may reflect continual stimulation by evolving antigenic variants of Env rather than the continued production of Ab elicited by the Env protein of the originally infecting virus. How do these hypotheses agree with clinical and experimental observations?
When CD20+ B cells were depleted to treat a lymphoplasmacytoid lymphoma in an HIV-1-positive patient, the autologous-virus NAb titer dropped 3-fold over the following 3 months while viral loads increased 50-fold.51 During this period, the virus became more neutralization sensitive; NAb titers were eventually restored and viral loads were again suppressed. Although we cannot generalize from a single case, the dynamic changes suggest that NAbs can suppress HIV-1 replication. Of particular note is that plasma cells do not express CD20. Hence, long-lived plasma cells may not have been responsible for the NAb titers in this patient. The generation of long-lived plasma cells is typically a TD process. Overall, in this clinical case, the Ab response to virus-derived Env may have resembled the transient responses to Env protein immunization (see below).
Only 17 cases of seronegative HIV-1 infection have been described, all with otherwise normal Ig levels but high viral loads.52 It seems likely that these patients never developed an Ab response to the virus, rather than doing so early on and then seroreverting.53 In principle, early viral damage to TH cells could account for the failure to seroconvert, but so could more direct adverse effects on B cells. Thus, severe losses of activated memory B cells during acute SIV infection of macaques are associated with lack of seroconversion; blocking the Programmed-Death-1 (PD-1) pathway of apoptosis prevents these losses and restores the suppressed Ab titers to both Gag and Env.54,55 When antiretroviral therapy (ART) suppressed viremia in six of the seronegative humans, four seroconverted to the major antigens within 1–9 months, one did so only to Env, and one remained completely seronegative.52 The increase in Ab production could reflect the restoration of TH function, which might indicate that both the Gag and Env responses are TD. Alternatively, recovery of B cells may be directly responsible, since ART normalizes the numbers of naive and resting memory B cells.56 Overall, these various studies are inconclusive as to the relative TD and TI contributions to the Gag and Env Ab responses. More direct evidence came from a study in which TH cell costimulation of B cells was blocked in SIV-infected macaques. The intervention delayed the Ab response to viral antigens, including NAbs against a sensitive test virus.57 This suggests that at least the initial anti-Env Ab response is largely TD, although a TI component cannot be excluded.
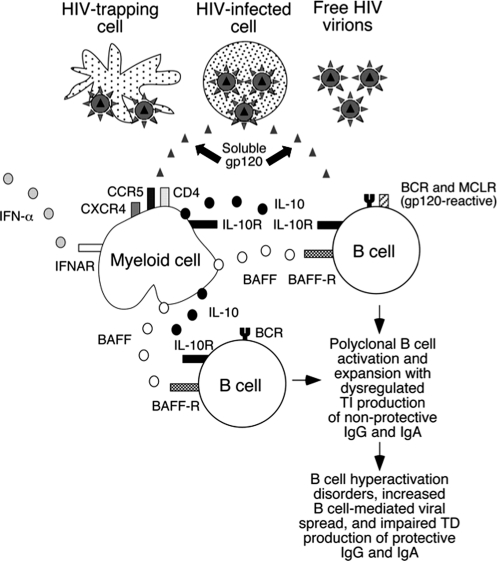
There is indeed evidence that the anti-Env Ab response may have a TI element. Thus, Env-expressing virus-like particles can induce anti-Env Ab production in CD4 knockout mice,58 and so can gp120-pulsed DCs in CD4+ T cell-depleted mice.59 HIV-1, and Env in particular, can activate B cells in both an Ag-specific manner and by polyclonal, nonspecific mechanisms.33,60–62 These reactions are reminiscent of TI-1 responses, which involve both mature and immature B cells. Pathogen-associated molecular patterns (PAMPs), including those on viral envelope glycoproteins, often elicit TI-2 responses, which activate only mature B cells.63 Some PAMPs directly activate B cells through interactions with pattern-recognition receptors and stereotyped B cell receptors (BCRs).64,65 If soluble or virion-bound gp120 interacts with B cells in this way, there could be synergy with its binding to MCLRs. Together these stimuli may elicit secretion of interleukin (IL)-10 and B cell-activating factor of the TNF family (BAFF) and then CD40-independent class switch recombination (CSR) as well as Ab secretion65 (Fig. 2). Overall, however, the relative contributions of TI and TD mechanisms to the anti-Env response remain unknown. As there are implications for Env vaccine responses (see below), this gap in our knowledge should be filled.
FIG. 2.
The interplay among gp120, interferon (IFN)-α, interleukin (IL)-10, B cell-activating factor of the TNF family (BAFF), and the receptors CD4, CXCR4, CCR5, IFN-α-R, BAFF-R, mannose-binding C-type lectin receptors (MCLRs), IL-10R, and B cell receptors (BCRs). gp120 (or other forms of Env) can be present on infected cells, on the virion surface, as a soluble protein, or attached to MCLR- or CD4-expressing cells. gp120 and IL-10 together promote IgG and IgA class switch recombination (CSRs), but Ab secretion requires BAFF and BCR engagement; gp120 may induce IL-10 secretion from B cells, monocytes, and dendritic cells via MCLR binding, and it triggers BAFF secretion from monocytes and macrophages. IL-10 and IFN-α also induce BAFF, which can upregulate MCLRs. Env proteins may also engage stereotypical BCRs. The net result of this CD40L-independent circuitry may be impaired development of high-affinity Abs to Env, particularly of the rare cross-neutralizing specificities. (Copyright 2006. The American Association of Immunologists, Inc.)
How do the Ab responses to Gag and Env vary with Ag concentration and T-helper cell levels? When people who have already seroconverted begin ART, both anti-Gag and anti-Env Ab responses generally decline as viremia is suppressed, although on rare occasions they rise.42,44,46,50,66,67 Both Ab responses seem, therefore, to be more responsive to Ag concentration changes than they are to the restored T cell help. A unique case for comparison is the possible eradication of HIV-1 through transplantation of bone marrow from a CCR5-Δ32-homozygous donor. Before transplantation, ART had suppressed the recipient's viral load to undetectable levels, whereas 20 months afterward the virus remained undetectable in the absence of ART. At that time, his previously strong Ab response to Gag had disappeared but anti-Env Abs, although declining, were still detectable.68 Whether this difference was due to a longer half-life of Env- than Gag-specific plasma cells, or a slower turnover of Env than Gag Ag, cannot be determined.
Stores of nonreplicating Ag may help maintain HIV-1-specific Ab responses after ART suppresses viral replication. Both Gag and Env proteins can persist for months on follicular dendritic cells in lymph node germinal centers, in the absence of detectable viral RNA.67 MCLRs expressed by these cells might trap Env, but other mechanisms such as Fc-receptor-mediated capture of immune complexes must also contribute to the maintenance of Ag depots, particularly for nonglycosylated Gag proteins that are often present at higher levels than Env.49,67 Hence, individual differences in the amounts of trapped Gag and Env protein may contribute to the variation in the corresponding Ab levels in ART recipients.44,67
The cross-neutralizing Ab response during infection appears to be Ag concentration dependent in that it correlates with viremia,47,48,69–71 particularly with the early levels of antigenemia.72 This broad neutralization response takes 2–3 years to develop,71 during which time multiple Env variants are presented to the immune system. Paradoxically, the substantial heterogeneity in Env may by default boost the response to the most conserved epitopes. Similarly, superinfection, which presents new sets of Env variants, is also followed by increased cross-neutralization.22,23 However, it is not only the amount of Ag or how much it varies that may favor the development of broad neutralization responses. Some variants appear to have a stronger propensity to induce such responses: amino acid signatures in Env, particularly in the CD4-binding site, have been identified as predictors of the strength and breadth of the responses that will be elicited by infection with a particular HIV-1 variant.73 Furthermore, neutralization breadth correlates with the degree of activation of CD4+ T cells, which suggests that this component of the Ab response to Env is TD.71 It is important to ascertain whether the broad neutralization response is more T-helper cell dependent than the general Ab response to Env.
TH1 or TH2 polarization is not clearly reflected in the human IgG subclass profile of the response to HIV-1 since IgG1 Abs, which are not associated with either T-helper-cell subset,74 dominate the response to all the viral antigens.75–78 IgG2 responses can be TD, and when they are, they are linked to TH1 polarization.74 However, IgG2 Abs can also be elicited as a TI response to repetitive carbohydrate epitopes. Among asymptomatic HIV-1-positive patients, IgG2 Abs specific for gp41 in Western blot were associated with low viral load and weak neutralizing titers, whereas IgG1 Env reactivity correlated positively with viral load.79 Together, HIV-1-specific TH1 T cell responses and anti-gp41 IgG2 Abs were reported to provide an excellent prognostic marker for lack of clinical progression.80 In a cross-sectional study, IgG2 reactivity with glycosylated gp41 was also linked to asymptomatic infection, but IgG2 reactive with nonglycosylated gp41 showed no such association.78 In line with the latter finding, when gp41-specific IgG2 was detected with nonglycosylated peptides, no difference in reactivity between viremic and virus-controlling patients was seen.75 To what extent IgG2 can be elicited against glycosylation-dependent epitopes on Env in a TI fashion remains to be determined. IgG4 Abs, associated with TH2 polarization in humans, are rarely reactive with HIV-1 proteins, although more frequently with Gag than Env.76–78
Overall, the B cell responses to Gag and Env are not easily categorized. Both responses fluctuate with Ag concentration; they may both be largely TD, but it remains a viable hypothesis that Env also elicits responses with prominent TI components (Fig. 2). Any TI responses that Env may trigger are highly relevant to vaccination and deserve further scrutiny. Perhaps the most striking difference between the two viral proteins, the extent to which they vary in sequence, provides the best clue to how they differ as immunogens during infection. The extraordinary variation in Env, partly driven by NAb selection pressure,81 may repeatedly prime new Ab responses rather than boost the already formed specificities. The repeated stimulation by Env neoepitopes could be what sustains anti-Env Ab levels when anti-Gag Abs are lost during untreated HIV-1 infection. As a complication, the extreme and prolonged antigenic stimulation may also, in the end, lead to B cell exhaustion. Of course any stimulatory mechanisms of the Ab response that depend on Env hypervariability will not apply when sequence-invariant Env vaccines are used. Nevertheless, the transience of the Ab responses to Env vaccines, by analogy to what was seen in a case of iatrogenic B cell depletion during HIV-1 infection,51 may be attributable to a deficiency in the generation of long-lived plasma cells.
The Ab Response to Env Vaccines
How do Ab responses to Env differ between infection and vaccination? The lack of protection by Env subunit vaccines allows vaccine- and infection-induced Ab responses to be compared in the same individuals. Thus, 12 people who had been immunized at least three times with gp120 (eight with MN and four with SF-2) had a low range of peak mid-point titers of Ab to heterologous gp120 (100–13,000 as reciprocal titers, also given henceforth). Stable titers against the same antigens in the same individuals after infection were higher (10,000–1,000,000), as were the titers in 16 other infected individuals (62,000–94,000).82 Another comparison found end-point titers to gp120 of 300–200,000 in gp120-vaccinated volunteers and 500–30,000,000 in infected people.83 The wide spread of titers could reflect genetic influences on the gp120-specific responses to infection as well as to immunization.84
The kinetics of the Ab response to Env vaccines
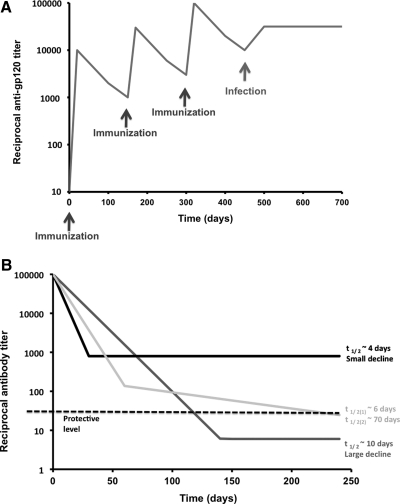
Both gp120-protein-only and DNA-prime with gp120-protein-boost immunizations of humans induce Ab responses that fade away within 2 years. Anti-gp120 titers increase after every injection, but less each time, and then drop rapidly with initial half-lives of ∼50 days82,85–87 (Fig. 3A). In one study, titers fell during the year after a prime-boost regimen as rapidly as they rose during the immunization process and although the decline decelerated, the titers trended toward undetectable levels88 (Fig. 3B). The titer kinetics in nonhuman primates given HIV-1 or SIV Env proteins resemble those of the human response.89,90 In contrast, when infection occurs in gp120 vaccinees, anti-gp120 titers jump to high levels that are sustained throughout infection.82 The decay in the anti-gp120 titer when ART suppresses HIV-1 replication in infected people is biphasic; a rapid but small decline is followed by a more stable phase44 (Fig. 3B). When cynomolgus macaques were simultaneously immunized with HIV-1 Env trimers (in one leg) and influenza HA monomers (in the other leg), IgG titers to each antigen declined at similar rates after the second immunization. The half-lives were around 2 weeks for both IgG responses during an initial phase, which was followed by a more stable second phase (C. Sundling and G. Kanlsson Hedestam, personal communication). After gp120 vaccination of humans, this second phase is usually either absent or the initial response is too weak to allow its detection. Whether vaccine recipients have any detectable anti-Env plasma Abs several years after immunization is not known: Long-term follow-up studies have not been described. Nor is it known whether the magnitude of the peak response to Env vaccines (which varies substantially among individuals) predicts the long-term outcome. Are strong initial responders more likely to sustain their responses for longer? Or does everyone's response decay at much the same rate and to the same eventual extent? Does the Ab response to some viral envelope glycoproteins decline more rapidly than to other soluble protein immunogens? The long-term response to Env vaccines might reflect specific aspects of the underlying B cell biology.
FIG. 3.
(A) The characteristic saw-tooth pattern of anti-gp120 titers after vaccination is shown on a log scale as a function of time. After each immunization (black arrows) with typically 200 μg of purified, recombinant gp120 in Alum, the binding-Ab titer rises by a log. The titers then decline rapidly. When infection occurs (gray arrow), the titers tend to stabilize at a high plateau. The kinetics of neutralization titers against a hypersensitive HIV-1 strain such as a TCLA virus or SF162 would look similar to the saw-tooth pattern of the first part of the curve, but there would be little or no cross-neutralization of primary isolates. However, several months after infection, significant titers of primary virus-neutralizing Abs do often develop. In (B), three schematic curves illustrate distinct features of immune responses to viral vaccines and infections. Time 0 can represent the time of completion of an immunization series or the initiation of antiretroviral therapy (ART) during infection. In the latter case phases with different rates of decline have been observed; in the former they are hypothetical. The magnitude of the antibody titer decline to a stable level is at least as important as the half-life of the early response. The black curve shows the response with the shortest half-life in the initial phase, ∼4 days, but it also has the smallest decline. The dark gray curve shows a longer initial half-life (10 days), but the decline is large. The light gray curve shows a biphasic decline with an initial intermediate half-life of ∼6 days, and ∼70 days in the second phase. If a minimal protective level equal to a reciprocal titer of 30 (dashed black line) is postulated, the plateau of the black curve falls above that cut-off, the dark gray curve below it. Thus, only the black curve with the initial shortest half-life remains safely above minimal protective levels (although it should be noted that no vaccine-induced Ab titer has been proven to confer protection against HIV-1). This diagram illustrates that the maximum degree of decline can be more important than the initial half-life in determining whether residual Ab levels are protective.
The average half-life of IgG titers in human serum after passive immunization is ∼21 days,91 but it can vary widely among recipients of the same IgG preparation.92 The half-life of anti-gp120 IgG after immunization, ∼40–60 days, is 2- to 3-fold longer,85,88 which suggests some continuous production of Abs occurs. In the short term, the declining Ab levels presumably also reflect how Ag concentrations diminish over time after vaccination. Long-lived plasma cells, which have down-modulated both BCR and MHC class II, are no longer sensitive to fluctuations in Ag levels, and they have substantially longer half-lives, of several months.93 The Ab response to gp120 vaccination thus conforms to a pattern in which Ag-concentration-sensitive memory B cells convert to short-lived plasma cells with half-lives of days.94 The pattern of titer decay suggests that Env-specific, long-lived plasma cells are not generated, which would hinder the creation of a protective vaccine that requires only a few immunizations rather than regular boosting. Hence, there is a need to investigate whether these very important long-lived plasma cells are elicited by Env vaccination and, if not, why not.
A recent model of imprinted lifespan of plasma cells suggests how to prolong humoral responses.95 The model proposes that the duration of humoral immunity is determined only at the priming stage of the immune response, and not by subsequent boosting, by the persistence of trapped Ag after the germinal-center reaction,96 by intermittent polyclonal activation, nor by the mere availability of bone marrow niches for plasma cell survival. Accordingly, different immunogens may elicit responses of characteristically distinct durations.95,97 The lifespan of the plasma cell would be proportional to the cumulative signals received by B cells during the critical priming phase of the immune response. This signaling would be mediated by T cell help, the cross-linking of B cell receptors, TLR activation by PAMPs, and stimuli from cytokines such as BAFF and APRIL. If an immunogen possesses only some of these features, responses might be improved by supplying missing elements. Given the brevity of the anti-Env vaccine response, this new model is well worth considering.
How much Env does an optimal immune response require?
The gp120 dose used in human trials is 200–600 μg, the number of immunizations ranging from two after DNA priming to seven over 30 months in a protein-only regimen.86,98,99 When three 200-μg immunizations were followed by a fourth of either 200 or 800 μg, the higher dose elicited a 10-fold higher titer.98 However, the functional activity of gp120, defined as CD4-binding capacity, varies several-fold from one affinity-purified preparation to another.100 All gp120 preparations contain misfolded or denatured protein and the proportion of nonnative forms may be greater with trimeric Env, which tends to dissociate and aggregate.101 After immunization, Env could be degraded further, a process that might be exacerbated by some adjuvants. Native Env molecules may, therefore, constitute only a small fraction of what the immune system encounters after vaccine administration; their quantity could indeed be suboptimal, for it is the functional activity rather than the total gp120 concentration that most influences the NAb response.100 The nonnative forms of Env may compromise the induction of NAbs by competing for T cell help and B cell survival factors.102,103
Immunosuppression through Env–receptor interactions?
gp120, like some other viral vaccine immunogens, specifically binds receptors on cells that have key immune functions. When 300 μg of gp120 in 1 ml is injected, the local concentration is initially 2.5 μM, which would, e.g., give >95% occupancy of CD4. For comparison, the highest level of gp120 found in lymph nodes of HIV-1-infected patients is 70 pM, i.e., ∼40,000-fold lower. Moreover, no gp120-specific Abs are present immediately after immunization to block gp120–receptor interactions, whereas they are usually in vast excess over gp120 during infection. It is therefore possible that gp120 could have effects near the injection site similar to those observed in cell culture systems,28 such as inducing apoptosis of T cells, arresting the differentiation of dendritic cells, reducing IL-12 production, and increasing IL-10 and BAFF secretion.65,104–107 By interacting with CD4 and coreceptors on monocytes, gp120 elicits the secretion of BAFF, which cooperates with gp120 in TI B cell activation65 (Fig. 2). The resulting TI cascades could harm the vaccine response by terminally differentiating and exhausting B cells, thereby reducing the number available for the TD responses that yield high-affinity Abs and long-lived plasma cells, which sustain high levels of Ab secretion. Furthermore, both the low-affinity Env-specific and the polyclonally activated B cells might compete with the few NAb-producing B cells for survival factors.
Env immunogens can be modified to eliminate potentially adverse receptor interactions, although a complication is the overlap between receptor-binding sites and important neutralization epitopes. For example, the V2 region of gp120 interacts with the activated form of the homing integrin α4β7 and overlaps the epitope for the potent cross-neutralizing Abs PG9 and PG16.108 Preserving the latter structures is important, and yet Env interactions with homing receptors could be detrimental to immunogenicity. Thus, gp120 may block the intestinal homing of CD4+ T cells by binding to integrin α4β710 and of B cells by interacting with CCR5 and CXCR4.109 If this occurs in vivo, intestinal B cells would be deprived of essential T cell help, and IgA-switched B cells diverted from the intestine, thereby compromising NAb-mediated protection of mucosal surfaces. The gp120–CD4 interaction has been proposed to impair T-helper responses.110 Mutating the CD4-binding site of gp120, to avoid such effects, reduced NAb titers in primates to that site and to CD4-induced epitopes. However, neither the overall anti-gp120 titers nor the Env-specific CD4 T cell responses were affected, arguing against a suppressive effect of CD4 binding in vivo.111 Similarly, gp120 does not induce markedly superior Ab responses in rodents, despite being unable to bind CD4 and coreceptors in those species. It is therefore doubtful whether the CD4-binding capacity needs to be eliminated from Env vaccines.
The mannose residues on gp120 mediate binding to MCLRs on dendritic cells, thereby potentially affecting TH1, TH2, TH17, and Treg responses and tolerance.112,113 gp120 also binds to BDCA-2 (CD303), an MCLR specifically expressed on plasmacytoid dendritic cells, thereby suppressing tumor necrosis factor (TNF)-α and interferon (IFN)-α secretion.114 In addition, mannose residues can enhance clearance of proteins by the liver.115 Demannosylating mammalian-cell-expressed gp120 for immunization of mice increased Ab titers by a factor of 1083 and blocking the mannose moieties by a lectin has a similar effect (K. Banerjee, K. Palmer, J.P. Moore, and P.J. Klasse, unpublished observations). How countering mannose residues increases Env immunogenicity remains to be explained, but one possible mechanism is by eliminating gp120–MCLR interactions that trigger IL-10 secretion, which in turn selectively suppresses effector and memory CD4+ T cell responses.107,116 Whether the specific mannose-mediated interactions or the mimicry of self-antigens by the glycan shield117,118 most reduce Env immunogenicity remains to be understood.
The epitope for the HIV-1 cross-neutralizing NAb 2G12 encompasses mannosyl residues, i.e., components of the glycan shield.119–122 Although the 2G12 epitope is present on gp120 variants from many divergent HIV-1 isolates, it is immunogenic only exceptionally. Of note is that if NAb responses to the 2G12 epitope were frequent, the plasticity of the glycan shield would probably allow facile viral escape, and as a result this particular epitope would have disappeared from the HIV-1 strains of the pandemic.123,124 Hence the poor immunogenicity of the 2G12 epitope during HIV-1 infection, combined with its widespread antigenicity, makes it an important target for vaccine design.125 Arguably, the same might apply to other, hypothetical epitopes formed by components of the glycan shield.
The block to the immunogenicity of the 2G12 epitope is not explained merely by its similarity to self-antigens: Abs recognizing the same Manα1-2 Man motif are abundant in human sera, and even greater numbers of such Abs can be induced by mannose-rich immunogens resulting from blocked carbohydrate processing.126 Unfortunately, these Abs do not neutralize HIV-1.118,127 What, then, is the origin of the rare 2G12 MAb specificity that does efficiently neutralize many HIV-1 strains? 2G12 has as high an affinity for mannose-dependent epitopes on two species of the fungus Candida (albicans and tropicalis) as it does for gp120, an affinity that is unusually high for an anticarbohydrate Ab.123 It is possible that the 2G12 specificity was elicited by Candida and not HIV-1 Env.123 Whether this hypothesis is correct or not, the fungal molecules might be better immunogens than Env, if their other epitopes are less immunodominant than some on gp120, and if they are less immunosuppressive.
Broadly neutralizing Abs to another cluster of epitopes on the HIV-1 Env complex also show some cross-reactivity with autoantigens. NAbs 2F5 and 4E10, both recognizing amino acid motifs in the membrane-proximal external region (MPER) of gp41, have shown different degrees of cross-reactivity with endogenous phospholipids, such as cardiolipin.128–131 The cross-reactivity is more marked for 4E10 than 2F5.130,131 It has been suggested that this recognition of “self” would eliminate B cells with such specificities through tolerance mechanisms.128 However, similar polyspecificity occurs in several viral infections, and this particular cross-reactivity with phospholipids resembles those antiphospholipid responses of infections more than they do the autoimmune counterpart.38,130,132 The phospholipid epitopes on living, as opposed to apoptotic or necrotic, cells are largely occluded, which means that Abs with these specificities may not mediate pathogenic, autoimmune effects. Indeed, the host could benefit from Ab-mediated clearance of cellular and microbial debris.132 If elimination of autoreactive Abs is not the explanation for the poor immunogenicity of the MPER epitopes, the contribution of the contiguous lipids to the MPER epitopes may nevertheless explain why linear MPER peptide sequences, even when presented on optimized scaffolds, fail to induce strong NAb responses.11,12
Hypermannosylated gp120 from the YU2 primary isolate, made in insect cells, induced only weak Ab responses in mice and rabbits.133 In contrast, gp120 produced from the T cell line-adapted clone HXBc2 in the same cells yielded high titers and a strong TH response. The immunogenicity of the primary-isolate gp120 was improved in various ways: by making the protein in mammalian instead of insect cells, thereby increasing carbohydrate processing and reducing its mannose content; by using Freund's complete adjuvant instead of Ribi; and by denaturing the protein. The last two strategies, which disrupt gp120 structure and are therefore counterproductive, may facilitate protein processing for presentation of internal TH epitopes.134 The resulting T cell help would promote Ab responses to both the native and denatured proteins that are present in any gp120 preparation. A final strategy to improve the B cell response was grafting a universal MHC class II TH epitope onto the gp120 C-terminus. This strengthened the TH2 response at the expense of TH1, as assessed by the IgG subclass profile and cytokine induction.133
When DNA vaccine encoding soluble HIV-1 gp120 or membrane-anchored influenza hemagglutinin was given to mice, the IgG titers against the two immunogens were similar but the gp120 response was more TH2 biased. IL-10- or IL-4-defective mice responded to gp120 with a cytokine profile and an IgG subclass pattern that were more TH1 polarized.135 Likewise, demannosylation of mammalian-cell-expressed gp120 tipped the balance modestly toward TH1 responses.83 Another aspect of carbohydrate processing that can affect both gp120 antigenicity and immunogenicity is its sialic acid content; nonsialylated gp120 exposed CD4bs and CD4i epitopes better and induced stronger Ab responses than its sialylated counterpart.136 Sialyl residues on the Fc portion mediate the binding of IgG to SIGN-R1, the murine orthologue of DC-SIGN, thereby initiating an antiinflammatory cascade.137 If the sialyl residues on gp120 have similar effects, a certain redundancy is noteworthy since gp120 mannosylation also confers DC-SIGN binding.
Although interactions with DC-SIGN and other MCLRs may hamper the immunogenicity of an Env-based immunogen, directing it to dendritic cells by other means could be beneficial. For example, tagging multiple α-galactose (α-Gal) epitopes to either p24 or gp120 increased their immunogenicity 10–100 times in mice that raise Abs to α-Gal because their α-1,3-galactosyltransferase gene has been knocked out.138 Humans, like these engineered mice, do produce α-Gal Abs.139 Natural Abs like these may help direct immunogens to follicular dendritic cells via CR2 and FcRγ.102 Similarly, precomplexing gp120 with nonneutralizing Abs increased anti-gp120 titers in rabbits (S. Phogat and W. Koff, personal communication). This particular strategy could promote Env presentation to TH cells while reducing unwanted interactions with BCRs specific for selected nonneutralization epitopes.
A recent study involving gp120 immunization of mice showed that silencing the ubiquitin-editing enzyme A20 offered several advantages: a preferential activation of T-helper over Treg responses, an increased expression of the gut-homing receptors integrin α4β7 and CCR9 on T and B lymphocytes in secondary lymphoid tissues, and increased titers of gp120-specific Abs, both serum IgG and mucosal sIgA. The mechanism of these changes is suggested by the role of A20 as an Ag-presentation attenuator that acts through feedback inhibition of the TNFR-, retinoic acid-inducible-gene I-, and TLR-signaling pathways.59 Previously, improved Ab responses to gp120 and resistance to its immunosuppressive effects were achieved by silencing another Ag-presentation attenuator, the suppressor of cytokine signaling 1 (SOCS-1).140 Even when these natural systems of brakes are not specifically activated by HIV-1 Env, countering them might be warranted to improve the problematic immunogenicity of that protein. Overall, safe pharmacological means of arousing the immune system temporarily by inhibiting the inhibitors could assist vaccination.141
In summary, the capacity of Env to signal via immunologically important receptors does not seem to compromise its immunogenicity, with the possible exception of MCLR interactions. However, a caveat is that usually only the short-lived, peak anti-gp120 response is measured in human and animal studies of Env immunizations. We noted above how the limited ability of gp120s to induce the TD responses that are important for the generation of long-lived plasma cells may be a severe hindrance to the development of sustained, high-level NAb responses. Whether the various receptor-binding capacities of Env play a role here is not known. It is also unknown whether the putative immunosuppressive motif in gp4114 affects the short- or longer-term immunogenicity of any Env immunogens that include this element. A systematic elimination of receptor-binding and other potentially immunosuppressive motifs from both gp120 and gp41 might elucidate whether these features influence the transient or sustained Ab responses to Env immunogens.
How and Why Anti-Env Responses Differ from Those to Other Immunogens
In a rare side-by-side comparison, volunteers were primed by four immunizations at one of four different mucosal sites, or both mucosally and intramuscularly, with a canarypox vector expressing either HIV-1 pseudovirus particles or the rabies virus glycoprotein.99 They were then boosted twice intramuscularly with gp120 or the rabies vaccine Imovax. The serum IgG and IgA and the mucosal IgA responses to gp120 were all rarer than the corresponding responses to the rabies glycoprotein and canarypox proteins after priming, although a caveat is that expression levels and Ab detection sensitivities may have differed among the antigens. The study showed that combined systemic and mucosal priming was better than mucosal priming alone, and that IgA responses can be induced by a prime-boost strategy. But a more fundamental observation is that gp120 was less able to induce IgG and IgA responses than similarly delivered canarypox and rabies proteins.
The contrast between the responses to the human papilloma virus (HPV) vaccine and to HIV-1 vaccine candidates may be instructive. The HPV vaccine has shown 96–100% efficacy in preventing infection in a 5-year follow-up of women.142 It consists of the L1 protein from each of the four most pathogenically important strains (HPV-6, −11, −16, and −18), produced in yeast and forming virus-like particles. The Ab response is mostly of the IgG class with negligible IgA levels in the female genital tract.143 IgG normally dominates at that site, unlike in other mucosae where IgA preponderates.144 At least in women then, the HPV-protective Abs are likely to be IgG that transudates from plasma, although some may be actively transported or locally produced.145 In the vaccine, 120 μg of HPV L1 (30 μg per variant) is adsorbed onto a variant of Alum adjuvant in 0.5 ml, and is given three times over 6 months. Thus, lower amounts of L1 are administered less often than gp120 (viz., 200–600 μg per immunization, up to six times). The titers to all four HPV antigens drop ∼10-fold over 30 months after the last immunization but then stabilize above baseline. For comparison, anti-gp120 titers fall 5- to 10-fold over 1 year, becoming undetectable.88 Furthermore, the Ab responses to HPV vaccination and infection are equally strong,142 again in contrast to gp120 and HIV-1. The range of titers among the HPV vaccinees, <2-fold within the 95% confidence interval around the geometric mean, is also narrower than the corresponding range of anti-gp120 titers, ∼10-fold.75,82 In addition, there may be a small group of particularly poor responders to gp120.82,146
Immunization with hepatitis B virus (HBV) protein induces rapidly declining Ab responses but provides long-lasting protection. Boosting 5–22 years after initial immunization can raise titers ∼100-fold, through a vigorous memory response.147 The half-life of Ab levels is around 1 month, i.e., at least as short as for gp120. And, as with gp120, the range of titers among vaccinees can be wide.148 Nonetheless, the majority of healthy, actively vaccinated subjects are protected even if they only develop weak Ab responses.147 In contrast, after passive immunization, protection is rapidly lost as the anti-HBV Ab levels decline. To conclude, the successful HBV vaccine appears to rely on anamnestic responses, a mode of protection that is implausible in HIV-1 vaccination.
The long-term kinetics of protective Ab levels have been comprehensively studied for other pathogens. For example, Ab titers in response to a replicating vaccine (vaccinia), naturally infecting viruses (measles, mumps, rubella, varicella-zoster, and Epstein–Barr virus), and two nonreplicating bacterial vaccines (tetanus and diphtheria proteins) were monitored in 45 individuals over 26 years. A few recipients of vaccines against the nonpersistent measles, mumps, and rubella viruses were also included and resembled the infected subjects in Ab kinetics. The half-lives, reflecting second, slower phase of titer decay, were impressively long for the various viruses: 50 years for varicella-zoster and much longer than human life spans for the others, but they were shorter, around a decade, for the two bacterial antigens.97 A separate study found that protective Ab levels to vaccinia were stable over 75 years, while the T cell responses to the virus declined with half-lives of 8–15 years.149 Still, generalization about the types of immunization and the kinetics of the responses is not warranted: a comparison of multiple studies on replicating and inert viral immunogens revealed no associations between the rate of Ab decline and the vaccine type.150 However, the new theory of imprinted lifespan points to the totality of different B cell signals at the inductive stage of the immune response as the principal determinant of plasma-cell longevity and, hence, of Ab-titer kinetics.95
Effective vaccines are available against flaviviruses causing yellow fever, Japanese encephalitis, and tick-borne encephalitis. Natural adaptive immunity and promising steps toward vaccine development have been recorded for other flaviviruses, such as the hepatitis C, dengue, and West Nile viruses.151 In other words, the obstacles to protective NAb responses collectively appear less insurmountable for this virus family than for lentiviruses such as HIV-1. Yet the neutralization targets on flaviviruses are also proteins that interact with various important surface molecules on immune system cells, some of which serve as entry receptors: CD81, CD14, MCLRs including DC-SIGN and the mannose receptor, and integrins.151 The attenuated yellow fever vaccine confers protection to 80–90% of human vaccinees. Neutralization titers vary without a clear demarcation of what is protective. A gene-expression signature that predicts a strong NAb response with 100% accuracy has been identified. This signature includes elevated expression of the tumor-necrosis-factor-receptor-superfamily member 17 (TNFRSF17), which is a receptor for the B cell growth factor BLyS-BAFF152 that potentiates Ab responses (see above). The immunogenicity of this receptor-binding viral protein thus strongly correlates with the host's expression of a B cell-regulating factor. HIV-1 Env might be similar in this regard, in that BAFF may heighten the amount of Env-specific Abs produced,65 although it is uncertain whether this would promote or hinder protection. With the yellow fever vaccine, in contrast, even some of the weaker immune responses are protective.
Concluding Remarks
The demands on B cell responses for preventing HIV-1 infection are formidable. Anamnestic responses would arise too late to protect, which places the onus squarely on high, preexisting NAb titers, preferably at mucosal sites; although cytotoxic T cell responses may also contribute by eliminating cells that get infected when virus slips through the neutralizing barrier. Anti-Env Abs elicited by HIV-1 infection and by vaccination differ in both quantity and quality. During infection, Ab responses to Env can be strong, and somatic hypermutation over years sometimes leads to high cross-neutralizing Ab titers.38–41 This affinity maturation occurs despite widespread pathological effects of the virus, such as B cell hyperactivation, losses of naive and memory B cells, and impaired T cell helper functions. Despite these immune defects, the NAb response to HIV-1 infection overshadows what Env vaccines have elicited to date. Not only is NAb induction by Env vaccination difficult, but mucosal Ab responses to Env are particularly hard to elicit.99 Also during chronic infection, only low amounts of HIV-1-specific IgA can be found in either sera or external secretions.153 Given that IgA is normally the dominant Ab class in the gastrointestinal and some other mucosae, the deficient anti-gp120 IgA response in those locations is significant.144,154,155 The goals of long-lasting responses and mucosal targeting may even be linked in that long-lived plasma cells often express IgA and mucosal-homing factors.37,156,157 Such cells appear to be inefficiently induced in response to Env vaccines, and yet they are critical to the production of stable, high levels of NAbs. We need to understand how to generate these cells in large numbers (Table 2).
Table 2.
Outstanding Questions in Neutralizing Antibody Induction and the Immunogenicity of Env
| • Is Env immunosuppressive, specifically for B cell responses, either directly or indirectly? |
| • To what extent is the Env response TD or TI? What kind of response should be the aim of vaccination? |
| • Is Env a B cell mitogen? Are PAMP properties beneficial or harmful? |
| • How can TH, particularly TFH (IgA), T cell responses to Env be harnessed? |
| • How can the conditions in infection sometimes leading to high affinity and cross-neutralization through SHM be mimicked by vaccination? |
| • How can long-lived plasma-cell responses be induced by Env? |
TD, thymus dependent; TI, thymus independent; PAMP, pathogen-associated molecular pattern; TH, T-helper; TFH, T-follicular helper; SHM, somatic hypermutation.
Env vaccine candidates require antigenic optimization, but whatever forms of Env are finally developed, their immunogenicity may also need to be improved. Thus, Env administered at high doses could conceivably compromise its own immunogenicity by interacting with cell-surface receptors on key immune cells. This may not affect the initial, transient response that is most commonly measured in vaccine trials, but the longer-term effects have not been studied. The hyperglycosylation of Env may have adverse effects on immunogenicity that go beyond the shielding of peptidic epitopes. Env glycans could be problematic by interacting with MCLRs, by mimicking self-antigens, or by creating PAMPS that elicit short-lived, nonneutralizing TI responses, which would compete with the desired generation of NAbs via TD processes. Our task must be to pinpoint the rare conditions during infection that yield strong, broad neutralization responses, and then to mimic them by engineering Env constructs on which nonneutralization epitopes are minimally immunogenic and from which immunosuppressive functions have been eliminated. Such immunogens may need to be complemented by TLR stimulators and other adjuvants, as well as inhibitors of Ag-presentation attenuators.
Acknowledgments
We are grateful to Dennis Burton, Gunilla Karlsson Hedestam, Wayne Koff, and Richard Wyatt for comments on the manuscript. This work was supported by NIH Grants AI 36082 and AI 45463 as well as by The International AIDS Vaccine Initiative (IAVI).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Klasse PJ. Sattentau QJ. Occupancy and mechanism in antibody-mediated neutralization of animal viruses. J Gen Virol. 2002;83:2091–2108. doi: 10.1099/0022-1317-83-9-2091. [DOI] [PubMed] [Google Scholar]
- 2.Pantophlet R. Burton DR. GP120: Target for neutralizing HIV-1 antibodies. Annu Rev Immunol. 2006;24:739–769. doi: 10.1146/annurev.immunol.24.021605.090557. [DOI] [PubMed] [Google Scholar]
- 3.Hessell AJ. Poignard P. Hunter M. Hangartner L. Tehrani DM. Bleeker WK. Parren PW. Marx PA. Burton DR. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med. 2009;15:951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mascola JR. Lewis MG. Stiegler G. Harris D. VanCott TC. Hayes D. Louder MK. Brown CR. Sapan CV. Frankel SS. Lu Y. Robb ML. Katinger H. Birx DL. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parren PW. Marx PA. Hessell AJ. Luckay A. Harouse J. Cheng-Mayer C. Moore JP. Burton DR. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol. 2001;75:8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibata R. Igarashi T. Haigwood N. Buckler-White A. Ogert R. Ross W. Willey R. Cho MW. Martin MA. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 7.Kwong PD. Doyle ML. Casper DJ. Cicala C. Leavitt SA. Majeed S. Steenbeke TD. Venturi M. Chaiken I. Fung M. Katinger H. Parren PW. Robinson J. Van Ryk D. Wang L. Burton DR. Freire E. Wyatt R. Sodroski J. Hendrickson WA. Arthos J. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature. 2002;420:678–682. doi: 10.1038/nature01188. [DOI] [PubMed] [Google Scholar]
- 8.Barouch DH. Korber B. HIV-1 vaccine development after STEP. Annu Rev Med. 2010;61:153–167. doi: 10.1146/annurev.med.042508.093728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei X. Decker JM. Wang S. Hui H. Kappes JC. Wu X. Salazar-Gonzalez JF. Salazar MG. Kilby JM. Saag MS. Komarova NL. Nowak MA. Hahn BH. Kwong PD. Shaw GM. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 10.Arthos J. Cicala C. Martinelli E. Macleod K. Van Ryk D. Wei D. Xiao Z. Veenstra TD. Conrad TP. Lempicki RA. McLaughlin S. Pascuccio M. Gopaul R. McNally J. Cruz CC. Censoplano N. Chung E. Reitano KN. Kottilil S. Goode DJ. Fauci AS. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. 2008;9:301–309. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- 11.Correia BE. Ban YE. Holmes MA. Xu H. Ellingson K. Kraft Z. Carrico C. Boni E. Sather DN. Zenobia C. Burke KY. Bradley-Hewitt T. Bruhn-Johannsen JF. Kalyuzhniy O. Baker D. Strong RK. Stamatatos L. Schief WR. Computational design of epitope-scaffolds allows induction of antibodies specific for a poorly immunogenic HIV vaccine epitope. Structure. 2010;18:1116–1126. doi: 10.1016/j.str.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Ofek G. Guenaga FJ. Schief WR. Skinner J. Baker D. Wyatt R. Kwong PD. Elicitation of structure-specific antibodies by epitope scaffolds. Proc Natl Acad Sci USA. 2010;107:17880–17887. doi: 10.1073/pnas.1004728107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson C. Reitz MS., Jr. Aldrich K. Klasse PJ. Blomberg J. Gallo RC. Robert-Guroff M. The site of an immune-selected point mutation in the transmembrane protein of human immunodeficiency virus type 1 does not constitute the neutralization epitope. J Virol. 1990;64:3240–3248. doi: 10.1128/jvi.64.7.3240-3248.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cianciolo GJ. Copeland TD. Oroszlan S. Snyderman R. Inhibition of lymphocyte proliferation by a synthetic peptide homologous to retroviral envelope proteins. Science. 1985;230:453–455. doi: 10.1126/science.2996136. [DOI] [PubMed] [Google Scholar]
- 15.Douek DC. Brenchley JM. Betts MR. Ambrozak DR. Hill BJ. Okamoto Y. Casazza JP. Kuruppu J. Kunstman K. Wolinsky S. Grossman Z. Dybul M. Oxenius A. Price DA. Connors M. Koup RA. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 16.Staprans SI. Barry AP. Silvestri G. Safrit JT. Kozyr N. Sumpter B. Nguyen H. McClure H. Montefiori D. Cohen JI. Feinberg MB. Enhanced SIV replication and accelerated progression to AIDS in macaques primed to mount a CD4 T cell response to the SIV envelope protein. Proc Natl Acad Sci USA. 2004;101:13026–13031. doi: 10.1073/pnas.0404739101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levesque MC. Moody MA. Hwang KK. Marshall DJ. Whitesides JF. Amos JD. Gurley TC. Allgood S. Haynes BB. Vandergrift NA. Plonk S. Parker DC. Cohen MS. Tomaras GD. Goepfert PA. Shaw GM. Schmitz JE. Eron JJ. Shaheen NJ. Hicks CB. Liao HX. Markowitz M. Kelsoe G. Margolis DM. Haynes BF. Polyclonal B cell differentiation and loss of gastrointestinal tract germinal centers in the earliest stages of HIV-1 infection. PLoS Med. 2009;6:e1000107. doi: 10.1371/journal.pmed.1000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veazey RS. Marx PA. Lackner AA. The mucosal immune system: Primary target for HIV infection and AIDS. Trends Immunol. 2001;22:626–633. doi: 10.1016/s1471-4906(01)02039-7. [DOI] [PubMed] [Google Scholar]
- 19.Nishimura Y. Igarashi T. Haigwood NL. Sadjadpour R. Donau OK. Buckler C. Plishka RJ. Buckler-White A. Martin MA. Transfer of neutralizing IgG to macaques 6 h but not 24 h after SHIV infection confers sterilizing protection: Implications for HIV-1 vaccine development. Proc Natl Acad Sci USA. 2003;100:15131–15136. doi: 10.1073/pnas.2436476100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMichael AJ. Borrow P. Tomaras GD. Goonetilleke N. Haynes BF. The immune response during acute HIV-1 infection: Clues for vaccine development. Nat Rev Immunol. 2010;10:11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stamatatos L. Morris L. Burton DR. Mascola JR. Neutralizing antibodies generated during natural HIV-1 infection: Good news for an HIV-1 vaccine? Nat Med. 2009;15:866–870. doi: 10.1038/nm.1949. [DOI] [PubMed] [Google Scholar]
- 22.Blish CA. Dogan OC. Derby NR. Nguyen MA. Chohan B. Richardson BA. Overbaugh J. Human immunodeficiency virus type 1 superinfection occurs despite relatively robust neutralizing antibody responses. J Virol. 2008;82:12094–12103. doi: 10.1128/JVI.01730-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powell RL. Kinge T. Nyambi PN. Infection by discordant strains of HIV-1 markedly enhances the neutralizing antibody response against heterologous virus. J Virol. 2010;84:9415–9426. doi: 10.1128/JVI.02732-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith DM. Wong JK. Hightower GK. Ignacio CC. Koelsch KK. Daar ES. Richman DD. Little SJ. Incidence of HIV superinfection following primary infection. JAMA. 2004;292:1177–1178. doi: 10.1001/jama.292.10.1177. [DOI] [PubMed] [Google Scholar]
- 25.Walker LM. Burton DR. Rational antibody-based HIV-1 vaccine design: Current approaches and future directions. Curr Opin Immunol. 2010;22:358–366. doi: 10.1016/j.coi.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bachmann MF. Jennings GT. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10:787–796. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 27.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 28.Klasse PJ. Moore JP. Is there enough gp120 in the body fluids of HIV-1-infected individuals to have biologically significant effects? Virology. 2004;323:1–8. doi: 10.1016/j.virol.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Doria-Rose NA. Connors M. Antibody-secreting B cells in HIV infection. Curr Opin HIV AIDS. 2009;4:426–430. doi: 10.1097/COH.0b013e32832d9fac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moir S. Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9:235–245. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adalid-Peralta L. Godot V. Colin C. Krzysiek R. Tran T. Poignard P. Venet A. Hosmalin A. Lebon P. Rouzioux C. Chene G. Emilie D. Stimulation of the primary anti-HIV antibody response by IFN-alpha in patients with acute HIV-1 infection. J Leukoc Biol. 2008;83:1060–1067. doi: 10.1189/jlb.1007675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lane HC. Masur H. Edgar LC. Whalen G. Rook AH. Fauci AS. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983;309:453–458. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- 33.Shirai A. Cosentino M. Leitman-Klinman SF. Klinman DM. Human immunodeficiency virus infection induces both polyclonal and virus-specific B cell activation. J Clin Invest. 1992;89:561–566. doi: 10.1172/JCI115621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ditzel HJ. Itoh K. Burton DR. Determinants of polyreactivity in a large panel of recombinant human antibodies from HIV-1 infection. J Immunol. 1996;157:739–749. [PubMed] [Google Scholar]
- 35.Ditzel HJ. Barbas SM. Barbas CF., 3rd Burton DR. The nature of the autoimmune antibody repertoire in human immunodeficiency virus type 1 infection. Proc Natl Acad Sci USA. 1994;91:3710–3714. doi: 10.1073/pnas.91.9.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bussmann BM. Reiche S. Bieniek B. Krznaric I. Ackermann F. Jassoy C. Loss of HIV-specific memory B-cells as a potential mechanism for the dysfunction of the humoral immune response against HIV. Virology. 2010;397:7–13. doi: 10.1016/j.virol.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Dorner T. Radbruch A. Antibodies and B cell memory in viral immunity. Immunity. 2007;27:384–392. doi: 10.1016/j.immuni.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Mouquet H. Scheid JF. Zoller MJ. Krogsgaard M. Ott RG. Shukair S. Artyomov MN. Pietzsch J. Connors M. Pereyra F. Walker BD. Ho DD. Wilson PC. Seaman MS. Eisen HN. Chakraborty AK. Hope TJ. Ravetch JV. Wardemann H. Nussenzweig MC. Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature. 2010;467:591–595. doi: 10.1038/nature09385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheid JF. Mouquet H. Feldhahn N. Seaman MS. Velinzon K. Pietzsch J. Ott RG. Anthony RM. Zebroski H. Hurley A. Phogat A. Chakrabarti B. Li Y. Connors M. Pereyra F. Walker BD. Wardemann H. Ho D. Wyatt RT. Mascola JR. Ravetch JV. Nussenzweig MC. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458:636–640. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 40.Zwick MB. Komori HK. Stanfield RL. Church S. Wang M. Parren PW. Kunert R. Katinger H. Wilson IA. Burton DR. The long third complementarity-determining region of the heavy chain is important in the activity of the broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2F5. J Virol. 2004;78:3155–3161. doi: 10.1128/JVI.78.6.3155-3161.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zwick MB. Parren PW. Saphire EO. Church S. Wang M. Scott JK. Dawson PE. Wilson IA. Burton DR. Molecular features of the broadly neutralizing immunoglobulin G1 b12 required for recognition of human immunodeficiency virus type 1 gp120. J Virol. 2003;77:5863–5876. doi: 10.1128/JVI.77.10.5863-5876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Binley JM. Klasse PJ. Cao Y. Jones I. Markowitz M. Ho DD. Moore JP. Differential regulation of the antibody responses to Gag and Env proteins of human immunodeficiency virus type 1. J Virol. 1997;71:2799–2809. doi: 10.1128/jvi.71.4.2799-2809.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weber JN. Clapham PR. Weiss RA. Parker D. Roberts C. Duncan J. Weller I. Carne C. Tedder RS. Pinching AJ, et al. Human immunodeficiency virus infection in two cohorts of homosexual men: Neutralising sera and association of anti-gag antibody with prognosis. Lancet. 1987;1:119–122. doi: 10.1016/s0140-6736(87)91964-7. [DOI] [PubMed] [Google Scholar]
- 44.Bonsignori M. Moody MA. Parks RJ. Holl TM. Kelsoe G. Hicks CB. Vandergrift N. Tomaras GD. Haynes BF. HIV-1 envelope induces memory B cell responses that correlate with plasma antibody levels after envelope gp120 protein vaccination or HIV-1 infection. J Immunol. 2009;183:2708–2717. doi: 10.4049/jimmunol.0901068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guan Y. Sajadi MM. Kamin-Lewis R. Fouts TR. Dimitrov A. Zhang Z. Redfield RR. DeVico AL. Gallo RC. Lewis GK. Discordant memory B cell and circulating anti-Env antibody responses in HIV-1 infection. Proc Natl Acad Sci USA. 2009;106:3952–3957. doi: 10.1073/pnas.0813392106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morris L. Binley JM. Clas BA. Bonhoeffer S. Astill TP. Kost R. Hurley A. Cao Y. Markowitz M. Ho DD. Moore JP. HIV-1 antigen-specific and -nonspecific B cell responses are sensitive to combination antiretroviral therapy. J Exp Med. 1998;188:233–245. doi: 10.1084/jem.188.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pereyra F. Palmer S. Miura T. Block BL. Wiegand A. Rothchild AC. Baker B. Rosenberg R. Cutrell E. Seaman MS. Coffin JM. Walker BD. Persistent low-level viremia in HIV-1 elite controllers and relationship to immunologic parameters. J Infect Dis. 2009;200:984–990. doi: 10.1086/605446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sather DN. Armann J. Ching LK. Mavrantoni A. Sellhorn G. Caldwell Z. Yu X. Wood B. Self S. Kalams S. Stamatatos L. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J Virol. 2009;83:757–769. doi: 10.1128/JVI.02036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santosuosso M. Righi E. Lindstrom V. Leblanc PR. Poznansky MC. HIV-1 envelope protein gp120 is present at high concentrations in secondary lymphoid organs of individuals with chronic HIV-1 infection. J Infect Dis. 2009;200:1050–1053. doi: 10.1086/605695. [DOI] [PubMed] [Google Scholar]
- 50.Notermans DW. de Jong JJ. Goudsmit J. Bakker M. Roos MT. Nijholt L. Cremers J. Hellings JA. Danner SA. de Ronde A. Potent antiretroviral therapy initiates normalization of hypergammaglobulinemia and a decline in HIV type 1-specific antibody responses. AIDS Res Hum Retroviruses. 2001;17:1003–1008. doi: 10.1089/088922201300343681. [DOI] [PubMed] [Google Scholar]
- 51.Huang KH. Bonsall D. Katzourakis A. Thomson EC. Fidler SJ. Main J. Muir D. Weber JN. Frater AJ. Phillips RE. Pybus OG. Goulder PJ. McClure MO. Cooke GS. Klenerman P. B-cell depletion reveals a role for antibodies in the control of chronic HIV-1 infection. Nat Commun. 2010;1:102. doi: 10.1038/ncomms1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spivak AM. Sydnor ER. Blankson JN. Gallant JE. Seronegative HIV-1 infection: A review of the literature. AIDS. 2010;24:1407–1414. doi: 10.1097/QAD.0b013e32833ac65c. [DOI] [PubMed] [Google Scholar]
- 53.Roy MJ. Damato JJ. Burke DS. Absence of true seroreversion of HIV-1 antibody in seroreactive individuals. JAMA. 1993;269:2876–2879. [PubMed] [Google Scholar]
- 54.Titanji K. Velu V. Chennareddi L. Vijay-Kumar M. Gewirtz AT. Freeman GJ. Amara RR. Acute depletion of activated memory B cells involves the PD-1 pathway in rapidly progressing SIV-infected macaques. J Clin Invest. 2010;120:3878–3890. doi: 10.1172/JCI43271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Velu V. Titanji K. Zhu B. Husain S. Pladevega A. Lai L. Vanderford TH. Chennareddi L. Silvestri G. Freeman GJ. Ahmed R. Amara RR. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moir S. Malaspina A. Ho J. Wang W. Dipoto AC. O'Shea MA. Roby G. Mican JM. Kottilil S. Chun TW. Proschan MA. Fauci AS. Normalization of B cell counts and subpopulations after antiretroviral therapy in chronic HIV disease. J Infect Dis. 2008;197:572–579. doi: 10.1086/526789. [DOI] [PubMed] [Google Scholar]
- 57.Garber DA. Silvestri G. Barry AP. Fedanov A. Kozyr N. McClure H. Montefiori DC. Larsen CP. Altman JD. Staprans SI. Feinberg MB. Blockade of T cell costimulation reveals interrelated actions of CD4+ and CD8+ T cells in control of SIV replication. J Clin Invest. 2004;113:836–845. doi: 10.1172/JCI19442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yao Q. Zhang R. Guo L. Li M. Chen C. Th cell-independent immune responses to chimeric hemagglutinin/simian human immunodeficiency virus-like particles vaccine. J Immunol. 2004;173:1951–1958. doi: 10.4049/jimmunol.173.3.1951. [DOI] [PubMed] [Google Scholar]
- 59.Hong B. Song XT. Rollins L. Berry L. Huang XF. Chen SY. Mucosal and systemic anti-HIV immunity controlled by A20 in mouse dendritic cells. J Clin Invest. 2011;121:739–751. doi: 10.1172/JCI42656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amadori A. Zamarchi R. Ciminale V. Del Mistro A. Siervo S. Alberti A. Colombatti M. Chieco-Bianchi L. HIV-1-specific B cell activation. A major constituent of spontaneous B cell activation during HIV-1 infection. J Immunol. 1989;143:2146–2152. [PubMed] [Google Scholar]
- 61.Rieckmann P. Poli G. Fox CH. Kehrl JH. Fauci AS. Recombinant gp120 specifically enhances tumor necrosis factor-alpha production and Ig secretion in B lymphocytes from HIV-infected individuals but not from seronegative donors. J Immunol. 1991;147:2922–2927. [PubMed] [Google Scholar]
- 62.Schnittman SM. Lane HC. Higgins SE. Folks T. Fauci AS. Direct polyclonal activation of human B lymphocytes by the acquired immune deficiency syndrome virus. Science. 1986;233:1084–1086. doi: 10.1126/science.3016902. [DOI] [PubMed] [Google Scholar]
- 63.Szomolanyi-Tsuda E. Welsh RM. T-cell-independent antiviral antibody responses. Curr Opin Immunol. 1998;10:431–435. doi: 10.1016/s0952-7915(98)80117-9. [DOI] [PubMed] [Google Scholar]
- 64.Fagarasan S. Honjo T. T-Independent immune response: New aspects of B cell biology. Science. 2000;290:89–92. doi: 10.1126/science.290.5489.89. [DOI] [PubMed] [Google Scholar]
- 65.He B. Qiao X. Klasse PJ. Chiu A. Chadburn A. Knowles DM. Moore JP. Cerutti A. HIV-1 envelope triggers polyclonal Ig class switch recombination through a CD40-independent mechanism involving BAFF and C-type lectin receptors. J Immunol. 2006;176:3931–3941. doi: 10.4049/jimmunol.176.7.3931. [DOI] [PubMed] [Google Scholar]
- 66.Moir S. Fauci AS. Immunology. Salmonella susceptibility. Science. 2010;328:439–440. doi: 10.1126/science.1189088. [DOI] [PubMed] [Google Scholar]
- 67.Popovic M. Tenner-Racz K. Pelser C. Stellbrink HJ. van Lunzen J. Lewis G. Kalyanaraman VS. Gallo RC. Racz P. Persistence of HIV-1 structural proteins and glycoproteins in lymph nodes of patients under highly active antiretroviral therapy. Proc Natl Acad Sci USA. 2005;102:14807–14612. doi: 10.1073/pnas.0506857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hutter G. Nowak D. Mossner M. Ganepola S. Mussig A. Allers K. Schneider T. Hofmann J. Kucherer C. Blau O. Blau IW. Hofmann WK. Thiel E. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 69.Doria-Rose NA. Klein RM. Daniels MG. O'Dell S. Nason M. Lapedes A. Bhattacharya T. Migueles SA. Wyatt RT. Korber BT. Mascola JR. Connors M. Breadth of human immunodeficiency virus-specific neutralizing activity in sera: Clustering analysis and association with clinical variables. J Virol. 2010;84:1631–1636. doi: 10.1128/JVI.01482-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simek MD. Rida W. Priddy FH. Pung P. Carrow E. Laufer DS. Lehrman JK. Boaz M. Tarragona-Fiol T. Miiro G. Birungi J. Pozniak A. McPhee DA. Manigart O. Karita E. Inwoley A. Jaoko W. Dehovitz J. Bekker LG. Pitisuttithum P. Paris R. Walker LM. Poignard P. Wrin T. Fast PE. Burton DR. Koff WC. Human immunodeficiency virus type 1 elite neutralizers: Individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J Virol. 2009;83:7337–7348. doi: 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mikell I. Sather DN. Kalams SA. Altfeld M. Alter G. Stamatatos L. Characteristics of the earliest cross-neutralizing antibody response to HIV-1. PLoS Pathog. 2011;7:e1001251. doi: 10.1371/journal.ppat.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Piantadosi A. Panteleeff D. Blish CA. Baeten JM. Jaoko W. McClelland RS. Overbaugh J. Breadth of neutralizing antibody response to human immunodeficiency virus type 1 is affected by factors early in infection but does not influence disease progression. J Virol. 2009;83:10269–10274. doi: 10.1128/JVI.01149-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gnanakaran S. Daniels MG. Bhattacharya T. Lapedes AS. Sethi A. Li M. Tang H. Greene K. Gao H. Haynes BF. Cohen MS. Shaw GM. Seaman MS. Kumar A. Gao F. Montefiori DC. Korber B. Genetic signatures in the envelope glycoproteins of HIV-1 that associate with broadly neutralizing antibodies. PLoS Comput Biol. 2010;6:e1000955. doi: 10.1371/journal.pcbi.1000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spellberg B. Immunoglobulin G2 antibodies and HIV-infected long-term nonprogressors: What is the mechanism? J Infect Dis. 2006;193:1047. doi: 10.1086/500837. author reply 1048. [DOI] [PubMed] [Google Scholar]
- 75.Banerjee K. Klasse PJ. Sanders RW. Pereyra F. Michael E. Lu M. Walker BD. Moore JP. IgG subclass profiles in infected HIV type 1 controllers and chronic progressors and in uninfected recipients of Env vaccines. AIDS Res Hum Retroviruses. 2010;26:445–458. doi: 10.1089/aid.2009.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khalife J. Guy B. Capron M. Kieny MP. Ameisen JC. Montagnier L. Lecocq JP. Capron A. Isotypic restriction of the antibody response to human immunodeficiency virus. AIDS Res Hum Retroviruses. 1988;4:3–9. doi: 10.1089/aid.1988.4.3. [DOI] [PubMed] [Google Scholar]
- 77.Klasse J. Blomberg J. Patterns of antibodies to human immunodeficiency virus proteins in different subclasses of IgG. J Infect Dis. 1987;156:1026–1030. doi: 10.1093/infdis/156.6.1026. [DOI] [PubMed] [Google Scholar]
- 78.Lal RB. Heiba IM. Dhawan RR. Smith ES. Perine PL. IgG subclass responses to human immunodeficiency virus-1 antigens: Lack of IgG2 response to gp41 correlates with clinical manifestation of disease. Clin Immunol Immunopathol. 1991;58:267–277. doi: 10.1016/0090-1229(91)90141-v. [DOI] [PubMed] [Google Scholar]
- 79.Ngo-Giang-Huong N. Candotti D. Goubar A. Autran B. Maynart M. Sicard D. Clauvel JP. Agut H. Costagliola D. Rouzioux C. HIV type 1-specific IgG2 antibodies: Markers of helper T cell type 1 response and prognostic marker of long-term nonprogression. AIDS Res Hum Retroviruses. 2001;17:1435–1446. doi: 10.1089/088922201753197105. [DOI] [PubMed] [Google Scholar]
- 80.Martinez V. Costagliola D. Bonduelle O. N'go N. Schnuriger A. Theodorou I. Clauvel JP. Sicard D. Agut H. Debre P. Rouzioux C. Autran B. Combination of HIV-1-specific CD4 Th1 cell responses and IgG2 antibodies is the best predictor for persistence of long-term nonprogression. J Infect Dis. 2005;191:2053–2063. doi: 10.1086/430320. [DOI] [PubMed] [Google Scholar]
- 81.Frost SD. Wrin T. Smith DM. Kosakovsky Pond SL. Liu Y. Paxinos E. Chappey C. Galovich J. Beauchaine J. Petropoulos CJ. Little SJ. Richman DD. Neutralizing antibody responses drive the evolution of human immunodeficiency virus type 1 envelope during recent HIV infection. Proc Natl Acad Sci USA. 2005;102:18514–18519. doi: 10.1073/pnas.0504658102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Connor RI. Korber BT. Graham BS. Hahn BH. Ho DD. Walker BD. Neumann AU. Vermund SH. Mestecky J. Jackson S. Fenamore E. Cao Y. Gao F. Kalams S. Kunstman KJ. McDonald D. McWilliams N. Trkola A. Moore JP. Wolinsky SM. Immunological and virological analyses of persons infected by human immunodeficiency virus type 1 while participating in trials of recombinant gp120 subunit vaccines. J Virol. 1998;72:1552–1576. doi: 10.1128/jvi.72.2.1552-1576.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Banerjee K. Andjelic S. Klasse PJ. Kang Y. Sanders RW. Michael E. Durso RJ. Ketas TJ. Olson WC. Moore JP. Enzymatic removal of mannose moieties can increase the immune response to HIV-1 gp120 in vivo. Virology. 2009;389:108–121. doi: 10.1016/j.virol.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moore JP. Klasse PJ. Dolan MJ. Ahuja SK. AIDS/HIV. A STEP into darkness or light? Science. 2008;320:753–755. doi: 10.1126/science.1154258. [DOI] [PubMed] [Google Scholar]
- 85.Berman PW. Murthy KK. Wrin T. Vennari JC. Cobb EK. Eastman DJ. Champe M. Nakamura GR. Davison D. Powell MF. Bussiere J. Francis DP. Matthews T. Gregory TJ. Obijeski JF. Protection of MN-rgp120-immunized chimpanzees from heterologous infection with a primary isolate of human immunodeficiency virus type 1. J Infect Dis. 1996;173:52–59. doi: 10.1093/infdis/173.1.52. [DOI] [PubMed] [Google Scholar]
- 86.Gilbert PB. Peterson ML. Follmann D. Hudgens MG. Francis DP. Gurwith M. Heyward WL. Jobes DV. Popovic V. Self SG. Sinangil F. Burke D. Berman PW. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. J Infect Dis. 2005;191:666–677. doi: 10.1086/428405. [DOI] [PubMed] [Google Scholar]
- 87.McElrath MJ. Corey L. Montefiori D. Wolff M. Schwartz D. Keefer M. Belshe R. Graham BS. Matthews T. Wright P. Gorse G. Dolin R. Berman P. Francis D. Duliege AM. Bolognesi D. Stablein D. Ketter N. Fast P. A phase II study of two HIV type 1 envelope vaccines, comparing their immunogenicity in populations at risk for acquiring HIV type 1 infection. AIDS Vaccine Evaluation Group. AIDS Res Hum Retroviruses. 2000;16:907–919. doi: 10.1089/08892220050042846. [DOI] [PubMed] [Google Scholar]
- 88.Gupta K. Hudgens M. Corey L. McElrath MJ. Weinhold K. Montefiori DC. Gorse GJ. Frey SE. Keefer MC. Evans TG. Dolin R. Schwartz DH. Harro C. Graham B. Spearman PW. Mulligan M. Goepfert P. Safety and immunogenicity of a high-titered canarypox vaccine in combination with rgp120 in a diverse population of HIV-1-uninfected adults: AIDS Vaccine Evaluation Group Protocol 022A. J Acquir Immune Defic Syndr. 2002;29:254–261. doi: 10.1097/00126334-200203010-00005. [DOI] [PubMed] [Google Scholar]
- 89.Anderson KP. Lucas C. Hanson CV. Londe HF. Izu A. Gregory T. Ammann A. Berman PW. Eichberg JW. Effect of dose and immunization schedule on immune response of baboons to recombinant glycoprotein 120 of HIV-1. J Infect Dis. 1989;160:960–969. doi: 10.1093/infdis/160.6.960. [DOI] [PubMed] [Google Scholar]
- 90.Haynes BF. Torres JV. Langlois AJ. Bolognesi DP. Gardner MB. Palker TJ. Scearce RM. Jones DM. Moody MA. McDanal C, et al. Induction of HIVMN neutralizing antibodies in primates using a prime-boost regimen of hybrid synthetic gp120 envelope peptides. J Immunol. 1993;151:1646–1653. [PubMed] [Google Scholar]
- 91.Hopkins RJ. Kramer WG. Blackwelder WC. Ashtekar M. Hague L. Winker-La Roche SD. Berezuk G. Smith D. Leese PT. Safety and pharmacokinetic evaluation of intravenous vaccinia immune globulin in healthy volunteers. Clin Infect Dis. 2004;39:759–766. doi: 10.1086/422998. [DOI] [PubMed] [Google Scholar]
- 92.Scheiermann N. Kuwert EK. Uptake and elimination of hepatitis B immunoglobulins after intramuscular application in man. Dev Biol Stand. 1983;54:347–355. [PubMed] [Google Scholar]
- 93.Slifka MK. Ahmed R. Long-lived plasma cells: A mechanism for maintaining persistent antibody production. Curr Opin Immunol. 1998;10:252–258. doi: 10.1016/s0952-7915(98)80162-3. [DOI] [PubMed] [Google Scholar]
- 94.Traggiai E. Puzone R. Lanzavecchia A. Antigen dependent and independent mechanisms that sustain serum antibody levels. Vaccine. 2003;21(Suppl 2):S35–S37. doi: 10.1016/s0264-410x(03)00198-1. [DOI] [PubMed] [Google Scholar]
- 95.Amanna IJ. Slifka MK. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol Rev. 2010;236:125–138. doi: 10.1111/j.1600-065X.2010.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gatto D. Martin SW. Bessa J. Pellicioli E. Saudan P. Hinton HJ. Bachmann MF. Regulation of memory antibody levels: The role of persisting antigen versus plasma cell life span. J Immunol. 2007;178:67–76. doi: 10.4049/jimmunol.178.1.67. [DOI] [PubMed] [Google Scholar]
- 97.Amanna IJ. Carlson NE. Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 98.Gorse GJ. McElrath MJ. Matthews TJ. Hsieh RH. Belshe RB. Corey L. Frey SE. Kennedy DJ. Walker MC. Eibl MM. Modulation of immunologic responses to HIV-1MN recombinant gp160 vaccine by dose and schedule of administration. National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. Vaccine. 1998;16:493–506. doi: 10.1016/s0264-410x(97)80003-5. [DOI] [PubMed] [Google Scholar]
- 99.Wright PF. Mestecky J. McElrath MJ. Keefer MC. Gorse GJ. Goepfert PA. Moldoveanu Z. Schwartz D. Spearman PW. El Habib R. Spring MD. Zhu Y. Smith C. Flores J. Weinhold KJ. Comparison of systemic and mucosal delivery of 2 canarypox virus vaccines expressing either HIV-1 genes or the gene for rabies virus G protein. J Infect Dis. 2004;189:1221–1231. doi: 10.1086/382088. [DOI] [PubMed] [Google Scholar]
- 100.Dey B. Pancera M. Svehla K. Shu Y. Xiang SH. Vainshtein J. Li Y. Sodroski J. Kwong PD. Mascola JR. Wyatt R. Characterization of human immunodeficiency virus type 1 monomeric and trimeric gp120 glycoproteins stabilized in the CD4-bound state: Antigenicity, biophysics, and immunogenicity. J Virol. 2007;81:5579–5593. doi: 10.1128/JVI.02500-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Parren PW. Burton DR. Sattentau QJ. HIV-1 antibody—debris or virion? Nat Med. 1997;3:366–367. doi: 10.1038/nm0497-366d. [DOI] [PubMed] [Google Scholar]
- 102.Goodnow CC. Vinuesa CG. Randall KL. Mackay F. Brink R. Control systems and decision making for antibody production. Nat Immunol. 2010;11:681–688. doi: 10.1038/ni.1900. [DOI] [PubMed] [Google Scholar]
- 103.Victora GD. Schwickert TA. Fooksman DR. Kamphorst AO. Meyer-Hermann M. Dustin ML. Nussenzweig MC. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Conti L. Fantuzzi L. Del Corno M. Belardelli F. Gessani S. Immunomodulatory effects of the HIV-1 gp120 protein on antigen presenting cells: Implications for AIDS pathogenesis. Immunobiology. 2004;209:99–115. doi: 10.1016/j.imbio.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 105.Fantuzzi L. Purificato C. Donato K. Belardelli F. Gessani S. Human immunodeficiency virus type 1 gp120 induces abnormal maturation and functional alterations of dendritic cells: A novel mechanism for AIDS pathogenesis. J Virol. 2004;78:9763–9772. doi: 10.1128/JVI.78.18.9763-9772.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yoon V. Fridkis-Hareli M. Munisamy S. Lee J. Anastasiades D. Stevceva L. The GP120 molecule of HIV-1 and its interaction with T cells. Curr Med Chem. 2010;17:741–749. doi: 10.2174/092986710790514499. [DOI] [PubMed] [Google Scholar]
- 107.Shan M. Klasse PJ. Banerjee K. Dey AK. Iyer SP. Dionisio R. Charles D. Campbell-Gardener L. Olson WC. Sanders RW. Moore JP. HIV-1 gp120 mannoses induce immunosuppressive responses from dendritic cells. PLoS Pathog. 2007;3:e169. doi: 10.1371/journal.ppat.0030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Walker LM. Phogat SK. Chan-Hui PY. Wagner D. Phung P. Goss JL. Wrin T. Simek MD. Fling S. Mitcham JL. Lehrman JK. Priddy FH. Olsen OA. Frey SM. Hammond PW. Kaminsky S. Zamb T. Moyle M. Koff WC. Poignard P. Burton DR. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Badr G. Borhis G. Treton D. Moog C. Garraud O. Richard Y. HIV type 1 glycoprotein 120 inhibits human B cell chemotaxis to CXC chemokine ligand (CXCL) 12, CC chemokine ligand (CCL)20, and CCL21. J Immunol. 2005;175:302–310. doi: 10.4049/jimmunol.175.1.302. [DOI] [PubMed] [Google Scholar]
- 110.Hu H. Fernando K. Ni H. Weissman D. HIV envelope suppresses CD4+ T cell activation independent of T regulatory cells. J Immunol. 2008;180:5593–5600. doi: 10.4049/jimmunol.180.8.5593. [DOI] [PubMed] [Google Scholar]
- 111.Douagi I. Forsell MN. Sundling C. O'Dell S. Feng Y. Dosenovic P. Li Y. Seder R. Lore K. Mascola JR. Wyatt RT. Karlsson Hedestam GB. Influence of novel CD4 binding-defective HIV-1 envelope glycoprotein immunogens on neutralizing antibody and T-cell responses in nonhuman primates. J Virol. 2010;84:1683–1695. doi: 10.1128/JVI.01896-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pulendran B. Tang H. Manicassamy S. Programming dendritic cells to induce T(H)2 and tolerogenic responses. Nat Immunol. 2010;11:647–655. doi: 10.1038/ni.1894. [DOI] [PubMed] [Google Scholar]
- 113.Steinman RM. Hawiger D. Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 114.Martinelli E. Cicala C. Van Ryk D. Goode DJ. Macleod K. Arthos J. Fauci AS. HIV-1 gp120 inhibits TLR9-mediated activation and IFN-{alpha} secretion in plasmacytoid dendritic cells. Proc Natl Acad Sci USA. 2007;104:3396–3401. doi: 10.1073/pnas.0611353104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee SJ. Evers S. Roeder D. Parlow AF. Risteli J. Risteli L. Lee YC. Feizi T. Langen H. Nussenzweig MC. Mannose receptor-mediated regulation of serum glycoprotein homeostasis. Science. 2002;295:1898–1901. doi: 10.1126/science.1069540. [DOI] [PubMed] [Google Scholar]
- 116.Brooks DG. Walsh KB. Elsaesser H. Oldstone MB. IL-10 directly suppresses CD4 but not CD8 T cell effector and memory responses following acute viral infection. Proc Natl Acad Sci USA. 2010;107:3018–3023. doi: 10.1073/pnas.0914500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Astronomo RD. Burton DR. Carbohydrate vaccines: Developing sweet solutions to sticky situations? Nat Rev Drug Discov. 2010;9:308–324. doi: 10.1038/nrd3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dunlop DC. Bonomelli C. Mansab F. Vasiljevic S. Doores KJ. Wormald MR. Palma AS. Feizi T. Harvey DJ. Dwek RA. Crispin M. Scanlan CN. Polysaccharide mimicry of the epitope of the broadly neutralizing anti-HIV antibody, 2G12, induces enhanced antibody responses to self oligomannose glycans. Glycobiology. 2010;20:812–823. doi: 10.1093/glycob/cwq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Calarese DA. Lee HK. Huang CY. Best MD. Astronomo RD. Stanfield RL. Katinger H. Burton DR. Wong CH. Wilson IA. Dissection of the carbohydrate specificity of the broadly neutralizing anti-HIV-1 antibody 2G12. Proc Natl Acad Sci USA. 2005;102:13372–13377. doi: 10.1073/pnas.0505763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Calarese DA. Scanlan CN. Zwick MB. Deechongkit S. Mimura Y. Kunert R. Zhu P. Wormald MR. Stanfield RL. Roux KH. Kelly JW. Rudd PM. Dwek RA. Katinger H. Burton DR. Wilson IA. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science. 2003;300:2065–2071. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 121.Sanders RW. Venturi M. Schiffner L. Kalyanaraman R. Katinger H. Lloyd KO. Kwong PD. Moore JP. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J Virol. 2002;76:7293–7305. doi: 10.1128/JVI.76.14.7293-7305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Scanlan CN. Pantophlet R. Wormald MR. Ollmann Saphire E. Stanfield R. Wilson IA. Katinger H. Dwek RA. Rudd PM. Burton DR. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1→2 mannose residues on the outer face of gp120. J Virol. 2002;76:7306–7321. doi: 10.1128/JVI.76.14.7306-7321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dunlop DC. Ulrich A. Appelmelk BJ. Burton DR. Dwek RA. Zitzmann N. Scanlan CN. Antigenic mimicry of the HIV envelope by AIDS-associated pathogens. AIDS. 2008;22:2214–2217. doi: 10.1097/QAD.0b013e328314b5df. [DOI] [PubMed] [Google Scholar]
- 124.Trkola A. Kuster H. Rusert P. Joos B. Fischer M. Leemann C. Manrique A. Huber M. Rehr M. Oxenius A. Weber R. Stiegler G. Vcelar B. Katinger H. Aceto L. Gunthard HF. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med. 2005;11:615–622. doi: 10.1038/nm1244. [DOI] [PubMed] [Google Scholar]
- 125.Scanlan CN. Offer J. Zitzmann N. Dwek RA. Exploiting the defensive sugars of HIV-1 for drug and vaccine design. Nature. 2007;446:1038–1045. doi: 10.1038/nature05818. [DOI] [PubMed] [Google Scholar]
- 126.Scanlan CN. Ritchie GE. Baruah K. Crispin M. Harvey DJ. Singer BB. Lucka L. Wormald MR. Wentworth P., Jr. Zitzmann N. Rudd PM. Burton DR. Dwek RA. Inhibition of mammalian glycan biosynthesis produces non-self antigens for a broadly neutralising, HIV-1 specific antibody. J Mol Biol. 2007;372:16–22. doi: 10.1016/j.jmb.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 127.Agrawal-Gamse C. Luallen RJ. Liu B. Fu H. Lee FH. Geng Y. Doms RW. Yeast-elicited cross-reactive antibodies to HIV Env glycans efficiently neutralize virions expressing exclusively high-mannose N-linked glycans. J Virol. 2011;85:470–480. doi: 10.1128/JVI.01349-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Haynes BF. Fleming J. St Clair EW. Katinger H. Stiegler G. Kunert R. Robinson J. Scearce RM. Plonk K. Staats HF. Ortel TL. Liao HX. Alam SM. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 129.Scherer EM. Leaman DP. Zwick MB. McMichael AJ. Burton DR. Aromatic residues at the edge of the antibody combining site facilitate viral glycoprotein recognition through membrane interactions. Proc Natl Acad Sci USA. 2010;107:1529–1534. doi: 10.1073/pnas.0909680107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Scherer EM. Zwick MB. Teyton L. Burton DR. Difficulties in eliciting broadly neutralizing anti-HIV antibodies are not explained by cardiolipin autoreactivity. AIDS. 2007;21:2131–2139. doi: 10.1097/QAD.0b013e3282a4a632. [DOI] [PubMed] [Google Scholar]
- 131.Franquelim HG. Chiantia S. Veiga AS. Santos NC. Schwille P. Castanho MA. Anti-HIV-1 antibodies 2F5 and 4E10 interact differently with lipids to bind their epitopes. AIDS. 2011;25:419–428. doi: 10.1097/QAD.0b013e328342ff11. [DOI] [PubMed] [Google Scholar]
- 132.Alving CR. 4E10 and 2F5 monoclonal antibodies: Binding specificities to phospholipids, tolerance, and clinical safety issues. AIDS. 2008;22:649–651. doi: 10.1097/QAD.0b013e3282f51922. [DOI] [PubMed] [Google Scholar]
- 133.Grundner C. Pancera M. Kang JM. Koch M. Sodroski J. Wyatt R. Factors limiting the immunogenicity of HIV-1 gp120 envelope glycoproteins. Virology. 2004;330:233–248. doi: 10.1016/j.virol.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 134.Mirano-Bascos D. Steede NK. Robinson JE. Landry SJ. Influence of disulfide-stabilized structure on the specificity of helper T-cell and antibody responses to HIV envelope glycoprotein gp120. J Virol. 2010;84:3303–3311. doi: 10.1128/JVI.02242-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Daly LM. Johnson PA. Donnelly G. Nicolson C. Robertson J. Mills KH. Innate IL-10 promotes the induction of Th2 responses with plasmid DNA expressing HIV gp120. Vaccine. 2005;23:963–974. doi: 10.1016/j.vaccine.2004.03.072. [DOI] [PubMed] [Google Scholar]
- 136.Kong L. Sheppard NC. Stewart-Jones GB. Robson CL. Chen H. Xu X. Krashias G. Bonomelli C. Scanlan CN. Kwong PD. Jeffs SA. Jones IM. Sattentau QJ. Expression-system-dependent modulation of HIV-1 envelope glycoprotein antigenicity and immunogenicity. J Mol Biol. 2010;403:131–147. doi: 10.1016/j.jmb.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Anthony RM. Ravetch JV. A novel role for the IgG Fc glycan: The anti-inflammatory activity of sialylated IgG Fcs. J Clin Immunol. 2010;30(Suppl 1):S9–S14. doi: 10.1007/s10875-010-9405-6. [DOI] [PubMed] [Google Scholar]
- 138.Abdel-Motal U. Wang S. Lu S. Wigglesworth K. Galili U. Increased immunogenicity of human immunodeficiency virus gp120 engineered to express Galalpha1-3Galbeta1-4GlcNAc-R epitopes. J Virol. 2006;80:6943–6951. doi: 10.1128/JVI.00310-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Takeuchi Y. Porter CD. Strahan KM. Preece AF. Gustafsson K. Cosset FL. Weiss RA. Collins MK. Sensitization of cells and retroviruses to human serum by (alpha 1-3) galactosyltransferase. Nature. 1996;379:85–88. doi: 10.1038/379085a0. [DOI] [PubMed] [Google Scholar]
- 140.Song XT. Evel-Kabler K. Rollins L. Aldrich M. Gao F. Huang XF. Chen SY. An alternative and effective HIV vaccination approach based on inhibition of antigen presentation attenuators in dendritic cells. PLoS Med. 2006;3:e11. doi: 10.1371/journal.pmed.0030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Graham BS. New approaches to vaccine adjuvants: Inhibiting the inhibitor. PLoS Med. 2006;3:e57. doi: 10.1371/journal.pmed.0030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Villa LL. Costa RL. Petta CA. Andrade RP. Paavonen J. Iversen OE. Olsson SE. Hoye J. Steinwall M. Riis-Johannessen G. Andersson-Ellstrom A. Elfgren K. Krogh G. Lehtinen M. Malm C. Tamms GM. Giacoletti K. Lupinacci L. Railkar R. Taddeo FJ. Bryan J. Esser MT. Sings HL. Saah AJ. Barr E. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer. 2006;95:1459–1466. doi: 10.1038/sj.bjc.6603469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nardelli-Haefliger D. Wirthner D. Schiller JT. Lowy DR. Hildesheim A. Ponci F. De Grandi P. Specific antibody levels at the cervix during the menstrual cycle of women vaccinated with human papillomavirus 16 virus-like particles. J Natl Cancer Inst. 2003;95:1128–1137. doi: 10.1093/jnci/djg018. [DOI] [PubMed] [Google Scholar]
- 144.Mestecky J. Moldoveanu Z. Smith PD. Hel Z. Alexander RC. Mucosal immunology of the genital and gastrointestinal tracts and HIV-1 infection. J Reprod Immunol. 2009;83:196–200. doi: 10.1016/j.jri.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hocini H. Barra A. Belec L. Iscaki S. Preud'homme JL. Pillot J. Bouvet JP. Systemic and secretory humoral immunity in the normal human vaginal tract. Scand J Immunol. 1995;42:269–274. doi: 10.1111/j.1365-3083.1995.tb03653.x. [DOI] [PubMed] [Google Scholar]
- 146.Gilbert P. Wang M. Wrin T. Petropoulos C. Gurwith M. Sinangil F. D'Souza P. Rodriguez-Chavez IR. DeCamp A. Giganti M. Berman PW. Self SG. Montefiori DC. Magnitude and breadth of a nonprotective neutralizing antibody response in an efficacy trial of a candidate HIV-1 gp120 vaccine. J Infect Dis. 2010;202:595–605. doi: 10.1086/654816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.West DJ. Calandra GB. Vaccine induced immunologic memory for hepatitis B surface antigen: Implications for policy on booster vaccination. Vaccine. 1996;14:1019–1027. doi: 10.1016/0264-410x(96)00062-x. [DOI] [PubMed] [Google Scholar]
- 148.Gesemann M. Scheiermann N. Quantification of hepatitis B vaccine-induced antibodies as a predictor of anti-HBs persistence. Vaccine. 1995;13:443–447. doi: 10.1016/0264-410x(94)00010-k. [DOI] [PubMed] [Google Scholar]
- 149.Hammarlund E. Lewis MW. Hansen SG. Strelow LI. Nelson JA. Sexton GJ. Hanifin JM. Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 150.Amanna IJ. Slifka MK. Wanted, dead or alive: New viral vaccines. Antiviral Res. 2009;84:119–130. doi: 10.1016/j.antiviral.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mukhopadhyay S. Kuhn RJ. Rossmann MG. A structural perspective of the flavivirus life cycle. Nat Rev Microbiol. 2005;3:13–22. doi: 10.1038/nrmicro1067. [DOI] [PubMed] [Google Scholar]
- 152.Querec TD. Akondy RS. Lee EK. Cao W. Nakaya HI. Teuwen D. Pirani A. Gernert K. Deng J. Marzolf B. Kennedy K. Wu H. Bennouna S. Oluoch H. Miller J. Vencio RZ. Mulligan M. Aderem A. Ahmed R. Pulendran B. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2005;10:116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mestecky J. Jackson S. Moldoveanu Z. Nesbit LR. Kulhavy R. Prince SJ. Sabbaj S. Mulligan MJ. Goepfert PA. Paucity of antigen-specific IgA responses in sera and external secretions of HIV-type 1-infected individuals. AIDS Res Hum Retroviruses. 2004;20:972–988. doi: 10.1089/aid.2004.20.972. [DOI] [PubMed] [Google Scholar]
- 154.Israel ZR. Marx PA. Nonclassical mucosal antibodies predominate in genital secretions of HIV-1 infected chimpanzees. J Med Primatol. 1995;24:53–60. doi: 10.1111/j.1600-0684.1995.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 155.Schafer F. Kewenig S. Stolte N. Stahl-Hennig C. Stallmach A. Kaup FJ. Zeitz M. Schneider T. Lack of simian immunodeficiency virus (SIV) specific IgA response in the intestine of SIV infected rhesus macaques. Gut. 2002;50:608–614. doi: 10.1136/gut.50.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cerutti A. Immunology. IgA changes the rules of memory. Science. 2010;328:1646–1647. doi: 10.1126/science.1192488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cerutti A. Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008;28:740–750. doi: 10.1016/j.immuni.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]