Abstract
The topoisomerase I (Top 1) poison irinotecan is an important component of the modern treatment of colorectal cancer. By stabilising Top 1-DNA complexes, irinotecan generates Top 1-linked DNA single-strand breaks that can evolve into double-strand breaks and ultimately cause cell death. However, cancer cells may overcome cell killing by releasing the stalled topoisomerase from DNA termini, thereby reducing the efficacy of Top 1 poisons in clinics. Thus, understanding the DNA repair mechanisms involved in the repair of Top 1-mediated DNA damage provides a useful tool to identify potential biomarkers that predict response to this class of chemotherapy. Furthermore, targeting these pathways could enhance the therapeutic benefits of Top 1 poisons. In this review, we describe the cellular mechanisms and consequences of targeting Top 1 activity in cells. We summarise preclinical data and discuss the potential clinical utility of small-molecule inhibitors of the key proteins.
Keywords: irinotecan, colorectal cancer, biomarkers, topoisomerase I, TDP1, PARP
Clinical utility of topoisomerase I (Top 1) poisons
Colorectal cancer remains a significant cause of morbidity and mortality worldwide, with 39 000 new cases per year in the UK and 16 000 deaths. In North America, the figures are 177 000 and 58 000, respectively (http://globocan.iarc.fr/). Despite the development of biological agents targeting EGFR and VEGF signalling, defining subgroups of patients that derive maximal benefit has proved difficult. Combination chemotherapy consisting of 5-fluorouracil paired with either the third-generation platinum compound oxaliplatin or the Top 1 poison irinotecan remains the mainstay of treatment for metastatic disease. The efficacy of irinotecan in metastatic colorectal cancer was demonstrated in clinical trials conducted over a decade ago (Douillard et al, 2000; Saltz et al, 2000), with response rates to combination regimens of 30–50% and overall survival in some studies approaching 24 months (Fuchs et al, 2007). In the treatment-naive population, there is broad equivalence in tumour response between irinotecan and oxaliplatin when combined with 5-fluorouracil (Seymour et al, 2007). However, the observation that responses to both irinotecan and oxaliplatin occur in the second line setting after progression on the other drug indicates that individual tumours differ in their sensitivity to these drugs. Biomarkers are therefore required to optimise patient treatment.
Locally advanced rectal cancers are increasingly treated with neoadjuvant chemo-radiotherapy strategies to optimise surgical resection and reduce rates of local and distant relapse. Phase I/II studies incorporating irinotecan, 5FU and radiotherapy in rectal cancer have indicated improved efficacy over 5FU chemo-radiotherapy alone, and have proved to be deliverable in terms of acute toxicity (Glynne-Jones et al, 2007; Willeke et al, 2007; Gollins et al, 2011). Neoadjuvant strategies incorporating oxaliplatin are also being developed, and thus robust predictive markers are required to optimise patient selection and maximise clinical benefit. Beyond its role in colorectal cancer, which will be the main focus of this review, there is also growing interest in the use of irinotecan in small-cell lung cancer, where there is evidence of increased efficacy over etoposide regimens (Lima et al, 2010), and a range of other tumour types including glioblastoma.
Cellular biochemistry of Top 1
The compact and supercoiled nature of the DNA double helix requires topological modification during important cellular processes such as transcription, replication and repair. This modification is conducted by DNA topoisomerases and involves transient cleavage and re-ligation of the double-stranded DNA molecule. Topoisomerases are enzymes that cleave one or both of the sugar-phosphate backbones of double-stranded DNA without altering its chemical composition (hence the term ‘isomerase’). Type I topoisomerases (Top I, Wang, 1971) cut a single strand of DNA to allow relaxation of torsional stresses before re-annealing. Type II topoisomerases (Top II, Gellert et al, 1976) incise double-stranded DNA to facilitate the passage of an intact duplex through the gap before rejoining the cut DNA. This mode of catalysis involves an intermediate known as the cleavage complex, which comprises the topoisomerase enzyme attached to the cleaved DNA by a covalent phosphotyrosyl bond. Increased levels of Top I mRNA and protein are seen across human tumours (Husain et al, 1994), suggesting increased transcription or mRNA stability, although genomic amplification of Top I in colorectal cancer has been described and correlates with increased RNA and protein expression (Yu et al, 2008).
Top I is the target of the camptothecin derivatives irinotecan and topotecan, whereas Top II is targeted by etoposide and anthracyclins. Camptothecin is a naturally occurring cytotoxic quinolone alkaloid (derived from the bark of Camptotheca acuminata) that binds to and stalls Top 1 on DNA. Irinotecan is a semisynthetic analogue of camptothecin that is activated by hydrolysis to the active metabolite SN38, which is subsequently metabolised through glucoronidation by uridine diphosphate glucoronosyltransferase 1A1 (UGT1A1) and excreted in the bile. Patients with specific polymorphisms in UGT1A1 (UGT1A1*28) have impaired metabolism of SN38 and are predisposed to the major toxicities of irinotecan, which are diarrhoea and myelosuppression, particularly neutropenia (Innocenti et al, 2004; O’Dwyer and Catalano, 2006). More recently, it has been suggested that different polymorphisms in UGT1A might also modulate tumour response rates (Cecchin et al, 2009).
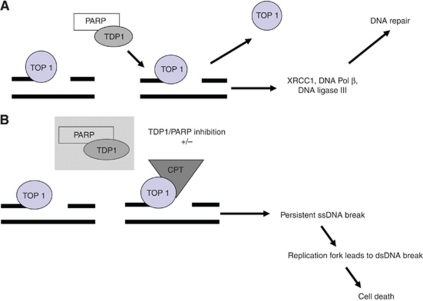
Irinotecan (predominantly in the form of SN38) binds to the Top I-DNA complex, stabilizing it and preventing re-ligation (Hsiang et al, 1985; Hsiang and Liu, 1988). Collision with advancing replication forks results in the formation of double-stranded DNA breaks. These breaks activate cell cycle arrest in G2 phase and, if unrepaired, can cause cell death (Figure 1). The requirement for DNA replication in this cytotoxic mechanism confers a degree of tumour specificity, with the major toxic effects arising in rapidly proliferating normal tissues. However, cell cycle-independent cytotoxicity may also occur through apoptosis, which is thought to be triggered by inhibition of Top I activity during DNA transcription (Morris and Geller, 1996). There is also recent evidence that activation of p38 MAPK may protect cells from irinotecan cytotoxicity (Paillas et al, 2011).
Figure 1.
(A) Top 1 cleavage complexes are ordinarily removed through TDP1- and PARP-dependent mechanisms, with the ssDNA breaks repaired through XRCC1 and DNA through polymerases/ligases. (B) Camptothecin (and irinotecan via SN38) stabilises the cleavage complexes, with the persistent ssDNA breaks converting to dsDNA lesions causing cell death, and expected synergy with TDP1/PARP inhibition.
Repair of irinotecan-associated DNA damage requires removal of the stalled Top 1 peptide and resolution of the associated DNA break (Figure 1). A number of repair proteins are involved in this process, some of which have clinical potential either as predictive biomarkers or as therapeutic targets. The most important factors will be briefly described in this section of the review.
Excision of the covalently linked topoisomerase is mandatory if subsequent repair steps are to be initiated; this can be achieved either by nonspecific nucleolytic cleavage of DNA, releasing the topoisomerase and a fragment of DNA, or by hydrolytic cleavage of the covalent phosphotyrosyl bond that links the topoisomerase to the DNA termini (reviewed in El-Khamisy, 2011). The prototype enzyme for the latter process was first identified in yeast and named tyrosyl DNA phosphodiesterase 1 (Yang et al, 1996). Tyrosyl DNA phosphodiesterase 1 (TDP1) catalyses hydrolysis of the phosphodiester bond between Top 1 and the 3′-phosphate of DNA, allowing resolution of the stalled Top I-DNA complexes (El-Khamisy et al, 2005; Interthal et al, 2005). In a similar manner, a second protein (TDP2) functions to remove Top II covalently bound to DNA double-strand breaks (Cortes Ledesma et al, 2009). Lack of TDP1 is associated with defects in the repair of Top 1-associated DNA strand breaks, and cells deficient in TDP1 accumulate DNA strand breaks when incubated with camptothecin.
In vitro, TDP1 can process a variety of 3′-oxidative termini, including 3′-phosphoglycolate moieties that are a common feature of DNA breaks induced by ionising radiation (IR; Zhou et al, 2005, 2009; El-Khamisy et al, 2007; Chiang et al, 2010). This observation points to a requirement for TDP1 for effective resolution of DNA damage associated with both Top 1 inhibition and IR, and indicates a potentially critical role for TDP1 in the cellular response to irinotecan-based chemoradiation. The clinical implications of this will be discussed later.
Poly(ADP-ribose) polymerase (PARP) also influences repair of Top I-mediated DNA damage. Inhibition of PARP sensitises cells to camptothecin, primarily by delaying DNA repair (Smith et al, 2005). As PARP inhibition does not confer additional sensitivity to camptothecin in TDP1 knockout cells (Zhang et al, 2011), it has been suggested that PARP and TDP1 are components of a single repair pathway for Top I–DNA complexes. In the same study, XPF and ERCC1 were also shown to be involved in repair of camptothecin-induced DNA damage; however, unlike TDP1, knockdown of XPF was synergistic with PARP inhibition in terms of camptothecin cytotoxicity (Zhang et al, 2011). PARP/TDP1 and XPF-ERCC1 may therefore comprise alternative pathways for repairing Top 1-mediated DNA damage.
Another candidate that has a role during the repair of Top 1 breaks is Aprataxin (APTX). APTX is the gene mutated in ataxia-ocular apraxia 1 (Moreira et al, 2001) and encodes a protein involved in the repair of single- (SSB) and double-stranded (DSB) DNA breaks. There is evidence that APTX participates in the repair of CPT-induced DNA damage (Mosesso et al, 2005), and a synergy between the actions of TDP1 and APTX has also been reported during the repair of specific types of DNA breaks (El-Khamisy et al, 2009).
Approximately 5% of colorectal cancers are associated with hereditary non-polyposis colon cancer, an inherited cancer predisposition syndrome caused by germ-line alteration of mismatch repair genes (MMR). Moreover, around 15% of sporadic cases demonstrate somatic loss of function of one or more MMR genes (most commonly MSH2 or MLH1). The MMR proteins recognise errors in the base sequence of DNA that occur during DNA replication (insertions, deletions or substitutions) and facilitate excision of the mismatched strand and restoration of base fidelity. Loss of function of one or more of these proteins results in microsatellite instability (MSI) – abnormally long or short microsatellites (repeated sequences of DNA) – which serves as a genetic signature for this phenotype. MMR-defective colorectal cancer lines exhibit increased sensitivity to CPT in vitro, which is reversed when wild-type gene expression is restored (Jacob et al, 2001). MMR proteins may also have additional roles in DSB repair and induction of apoptosis in response to DNA damage, and it is hypothesised that these actions may contribute to modulating the response to Top 1 response to topoisomerase poisons detailed below.
Therapeutic targets in the context of Top 1 inhibition
Much of our understanding of the proteins involved in repairing Top I cleavage complexes is derived from experimental strategies in which inhibition of those proteins potentiates the DNA damage sustained. Such proteins therefore constitute targets for therapeutic interventions aimed at improving the clinical efficacy of irinotecan.
Cell line and xenograft data demonstrate the potentiating effect of PARP inhibition on the cytotoxicity of irinotecan (Miknyoczki et al, 2003; Calabrese et al, 2004; Smith et al, 2005). Phase I studies of a number of PARP inhibitors, for example, veliparib (Abbott Laboratories, Abbott Park, IL, USA), iniparib (Sanofi-Aventis, Surrey, UK) and olaparib (AstraZeneca, London, UK), in combination with irinotecan are underway. The radiosensitising effects of PARP inhibition are also well documented (Calabrese et al, 2004; Chalmers et al, 2010; Efimova et al, 2010), and clinical trials in various tumour sites are in development (Verheij et al, 2010). Locally advanced rectal cancer may therefore provide an ideal opportunity to test combinations of irinotecan, radiotherapy and PARP inhibitors. Of critical importance will be whether meaningful improvements in tumour response can be achieved without unacceptable exacerbation of normal tissue toxicities, particularly bone marrow suppression and diarrhoea.
The rationale for developing inhibitors of TDP1 for subsequent combination with Top 1 poisons is similarly robust. In vitro, cells deficient in TDP1 accumulate an excess of DNA strand breaks when incubated with camptothecin (El-Khamisy et al, 2005; Interthal et al, 2005) or exposed to IR (El-Khamisy et al, 2007). This dual activity makes inhibition of TDP1 a compelling target for clinical studies in combination with Top I inhibitors and radiotherapy, especially when viewed in the context of the early success of PARP inhibition in clinical practice. Beyond its use in the management of colorectal cancer, the recently demonstrated activity of irinotecan in small-cell lung cancer and glioblastoma may give TDP1 a broader utility. Combining TDP1 inhibitors with topotecan in ovarian cancer may also prove synergistic. However, given similarities in function and pathways at a cellular level, it remains to be seen how similar or different PARP and TDP1 inhibition will prove and whether inhibiting both together would prove synergistic or mutually redundant.
Potential as predictive biomarkers
As described previously, there are a number of therapeutic options available for patients with colorectal cancer, and patient selection is a critical process that is currently sub-optimal. Our increasing knowledge of the mechanisms determining sensitivity to Top 1 inhibitors raises the possibility that some of the key molecules described above will have utility as biomarkers that predict response of tumours to treatment.
Top 1
As the cytotoxic effects of topoisomerase poisons are dependent on stabilisation of the topoisomerase–DNA complex, it is reasonable to predict that cellular sensitivity to these agents will be modulated by absolute Top I levels, although cell lines containing Top I mutations that alter Top I DNA or camptothecin interaction have been described that confer resistance to camptothecin (Li et al, 1996; Gongora et al, 2011). Repeated exposure of colorectal cancer xenografts to camptothecin resulted in downregulation of Top I levels (Giovanella et al, 1989) and the same effect has been observed in peripheral blood mononuclear cells after treatment with topotecan (Hochster et al, 1997). Clinically, tumour expression of Top I decreased (between pretreatment biopsy and subsequent surgical specimen) following neoadjuvant treatment of rectal cancer with chemoradiation comprising irinotecan and 5FU (Horisberger et al, 2009).
Top I is highly expressed in around half of the colorectal cancers, with one study demonstrating higher levels in rectal cancers (Boonsong et al, 2002). The observed broad range of expression supports the hypothesis that Top I expression will predict response to irinotecan. It has been suggested that higher levels of Top I expression may predict response to irinotecan-containing neoadjuvant chemoradiation in rectal cancer (Horisberger et al, 2009). In addition, the results of the MRC FOCUS study of 1313 patients with metastatic colorectal cancer indicated that tumours with moderate or high levels of Top I expression as determined by immunohistochemistry showed the greatest benefit from adding irinotecan or oxaliplatin to 5FU in the first-line metastatic setting (Braun et al, 2008). However, subsequent data from the similar ‘CAIRO’ study from the Dutch Colorectal Cancer Group (Koopman et al, 2007) failed to replicate these findings, with no association seen between Top 1 expression (by immunohistochemistry) and response to irinotecan and capecitabine in 545 patients (Koopman et al, 2009). These apparently contradictory findings suggest that although absolute Top I expression levels may play a part, it is likely that additional molecules contribute to irinotecan sensitivity in the clinic.
TDP1
The critical role of TDP1 in determining cellular responses to irinotecan makes it a promising biomarker. A number of studies have investigated polymorphisms in genes involved in irinotecan/Top 1-related DNA repair and response to treatment, and some of these have included TDP1. In one such study, 107 patients treated with irinotecan were screened for host polymorphisms in PARP, TDP1, Top 1 and XRCC1 (Hoskins et al, 2008). Univariate analysis suggested that specific polymorphisms in TDP1 and XRCC1 were linked with response to irinotecan, but on multivariate analysis only XRCC1 remained significant.
The available data indicate that TDP1 expression is increased in colorectal tumour samples compared with paired normal tissue (Yu et al, 2005). In the most relevant study to date, 52 metastatic colorectal cancer specimens were analysed by RT–PCR for expression of 24 genes hypothesised to be associated with response to irinotecan. TDP1 was one of eight genes (including ERCC1 – see above) that showed significantly higher levels of expression in tumours than in normal tissue. Expression of TDP1 grouped with other genes involved in DNA repair. Interrogation of oncomine (www.oncomine.org) supports this finding, with TDP1 expression levels appearing to be broadly increased in colorectal cancer specimens. Several microarray expression profiles for rectal adenocarcinomas have been published (NCBI GEO, EBI), but only one of these used a platform that included a probe for TDP1 (Snipstad et al, 2010). Our analysis of these data demonstrates increased levels of TDP1 in rectal cancers compared with normal tissues.
As detailed above, it has also been shown that TDP1 has a role in the repair of SSBs induced by IR (El-Khamisy et al, 2007). Specifically, cells deficient in TDP1 exhibit delayed repair of SSBs induced by IR (Katyal et al, 2007; Chiang et al, 2010). Although the cytotoxic effects of IR are predominately mediated through double-strand breaks, unrepaired SSBs can be converted to DSBs during DNA replication. This raises the intriguing possibility that TDP1 could be a dual biomarker for sensitivity to both irinotecan and radiotherapy. Although there is no published data to substantiate this claim, high quality tissue is available from several clinical trials that have tested irinotecan-based chemoradiation regimes, and these samples are currently being analysed for expression of TDP1 and other relevant DNA repair genes and proteins.
Finally, in vitro experiments using the PARP inhibitor ABT-888 (Zhang et al, 2011) show no further enhancement of camptothecin cytotoxicity in cell lines lacking TDP1, suggesting that PARP and TDP1 comprise a common repair pathway. Although this supports the rationale for either (but not both together) as therapeutic targets in potentiating topoisomerase poisons, it is possible that increased TDP1 expression levels might prove a biomarker in predicting benefit from the addition of PARP inhibitors to irinotecan or radiotherapy.
APTX
In vitro studies of colon cancer cell lines have shown an association between APTX expression levels and sensitivity to camptothecin (Mariadason et al, 2003), and there is also evidence to suggest that APTX modulates response to irinotecan in metastatic colorectal cancer, with higher protein expression associated with a lower likelihood of response. Tumour blocks from 135 patients with metastatic disease treated with a variety of irinotecan/5FU combination regimens were probed for APTX using immunohistochemistry (Dopeso et al, 2010). With a median follow-up of 4.6 years, patients with low levels of APTX had improved progression-free and overall survival (PFS 9.2 vs 5.5 months P=0.03, OS 36.7 vs 19 months P=0.008). These promising data require validation, but demonstrates the potential value of this class of biomarker.
MMR
Mismatch repair-deficient colorectal cancers have been reported to be resistant to 5FU (Ribic et al, 2003), but more recent evidence indicates that they may be sensitive to irinotecan. In the adjuvant setting, CALGB 89803 randomised 1264 patients with stage III colon cancer to weekly 5FU/leucovorin±irinotecan. Of all, 723 cases were retrospectively genotyped for MSI, and MMR protein expression was analysed by immunohistochemistry (Bertagnolli et al, 2009). Tumours with evidence of MMR deficiency showed improved 5-year disease-free survival when treated with irinotecan (0.76 vs 0.59, P=0.03), a difference that was not observed in the 5FU-treated arm. This effect has also been documented in the metastatic setting (Fallik et al, 2003). Here, 72 patients treated with irinotecan-containing regimens were analysed for loss of expression of hMLH1 and hMSH2 and genotyped for microsatellite instability. Four out of seven tumours with high levels of MSI responded to irinotecan as opposed to seven out of sixty-five patients with low-level MSI (P=0.009). However, unlike Top I, MLH1/MSH2 immunohistochemical analysis was not able to predict response to irinotecan (or oxaliplatin) within the FOCUS study (Braun et al, 2008), although with only 4.4% samples showing evidence of impaired mismatch repair the statistical power was low. As molecular subtyping of colorectal cancer improves, it is likely that MMR-deficient tumours will acquire specific treatment protocols. Current understanding of DNA repair mechanisms would place irinotecan at the centre of these, but more clinical data are required before such protocols are adopted.
Biomarkers of toxicity
Given the equivalent first-line efficacy of oxaliplatin and irinotecan regimens, the ability to predict toxicity would be of value in individualising treatment decisions. Here, germ-line polymorphisms in the genes discussed in this review may be more relevant than variations in tumour expression. The previously described study (Hoskins et al, 2008) genotyped 107 metastatic CRC patients treated with irinotecan regimens for single-nucleotide polymorphisms (SNP) in Top 1, CDC45L, NFKB1, PARP1, TDP1 and XRCC1. These SNPs were tested for association with the most frequent and significant side effects of irinotecan, namely grade three out of four diarrhoea and neutropenia. In univariate analysis, SNPs in both Top 1 and TDP1 were associated with grade three out of four neutropenia. However, multivariate analysis failed to demonstrate significant association, and the same authors failed to replicate these findings in a separate sample set (Hoskins et al, 2009). However, neither study was powered to detect relatively small effects, and consideration of the overlapping pathways involved in determining irinotecan response suggests that any modulation of toxicity is likely to be multifactorial.
Clinical application of potential biomarkers
There is increasing awareness of the potential value of molecular pathology in clinical decision making, and colorectal cancer is at the forefront of this vogue. The MRC FOCUS 3 trial is currently testing the feasibility of such a strategy in a study that stratifies patients with metastatic colorectal cancer into treatment groups based on Top 1 I levels in their tumour specimens (http://www.ctu.mrc.ac.uk/). Drawing on molecular data from the FOCUS study described above (Braun et al, 2008), and using combination irinotecan and 5FU as a control regimen, patients with low Top I-expressing tumours will be randomised to omit the irinotecan (i.e., receive 5FU alone), whereas tumours with high Top I will be randomised to add oxaliplatin to irinotecan/5FU. A further randomisation will be determined by the mutation status of KRAS and BRAF, with the addition of cetuximab being tested if KRAS/BRAF are both wild type and bevacizumab if either are mutated. If successful, this ambitious study will be extremely informative both from a clinical perspective and as an indicator of the feasibility of individualising treatment by virtue of molecular testing.
Increasing application of irinotecan in the neoadjuvant treatment of rectal cancer (Gollins et al, 2011) may provide opportunities for testing a range of the potential biomarkers discussed in this review. MRI and pathological response at definitive surgery provide robust and quantitative early-outcome measures, and the availability of pre- and post-treatment tissue samples makes this an ideal setting in which to investigate new drug combinations and associated biomarkers.
Potential utility beyond topoisomerase inhibitors
There is increasing interest in the use of small-molecule inhibitors of DNA repair enzymes to overcome resistance to conventional cytotoxic agents that kill cells predominantly by damaging DNA. PARP inhibitors are at the forefront of this field, and several clinical trials combining PARP inhibitors with radiotherapy and/or cytotoxic drugs are either underway or in development. As previously highlighted, there are functional parallels between TDP1 and PARP, with TDP1 having a role in the resolution of SSBs induced by Top 1 poisons and by ionising radiation. Hence, there is a biological rationale for combining TDP1 inhibitors with radiotherapy (El-Khamisy et al, 2007), either alone or in the context of chemoradiation schedules. Although relatively little cancer-specific research has been conducted, TDP1 is known to be expressed in a variety of tumour types (Liu et al, 2007). In addition to the compelling evidence for TDP1 as a therapeutic target in the treatment of rectal cancer, it is reasonable to predict that ongoing research will identify whether additional therapeutic applications exist for combination treatments comprising TDP1 inhibitors.
Conclusions
Although decades of basic scientific research has yielded a number of anti-cancer drugs that target signal transduction pathways, only recently has there been a resurgence of interest in understanding and exploiting the cellular mechanisms of DNA repair. This new knowledge promises to better explain clinical responses to conventional cytotoxic agents including radiotherapy, and to reveal biomarkers predictive of response and resistance (Table 1). Specifically, the mechanisms for repairing topoisomerase-associated DNA breaks that accumulate following treatment with Top I poisons, such as irinotecan, comprise proteins that can be targeted to modulate sensitivity to these agents. TDP1 has well-characterised roles in the repair of DNA-Top I intermediates and radiation-induced DNA breaks and shows significant promise as a biomarker. Furthermore, the clinical development of PARP inhibitors has demonstrated that this understanding can identify therapeutic targets, inhibitors of which might realistically be combined with irinotecan to yield clinically significant improvements in tumour response rates.
Table 1. Candidate therapeutic targets and biomarkers of response to irinotecan derived from an improved understanding of topoisomerase I activity and subsequent DNA repair mechanisms.
|
Potential clinical utility
|
|||
|---|---|---|---|
| Molecule | Therapeutic target | Biomarker of response | Reference |
| Top I | Mechanism of action of Irinotecan | Yes | Braun et al, 2008 |
| TDP1 | Yes | Proposed | El-Khamisy et al, 2005, 2007 |
| PARP | Yes | ? | Smith et al, 2005; Zhang et al, 2011 |
| Aprataxin | ? | Yes | Dopeso et al, 2010 |
| Mismatch repair | ? | Yes | Bertagnolli et al, 2009 |
Abbreviations: PARP=poly(ADP-ribose) polymerase; TDP1=tyrosyl DNA phosphodiesterase 1; Top I=type I topoisomerases.
The promise of the biomarkers described in this review should be comprehensively assessed by translational work on the plethora of clinical studies that have used irinotecan in colorectal cancer (and beyond). Retrospective work, however, will require the cooperation of treating departments in collecting meaningful sample sets. The development of trials that begin to match treatment arms to underlying molecular characteristics (e.g., FOCUS3) should be widely supported and further developed. Combining an improved molecular understanding of individual tumours with specific adjunctive therapies, Top 1 will remain a key target in the treatment of colorectal cancer.
References
- Bertagnolli MM, Niedzwiecki D, Compton CC, Hahn HP, Hall M, Damas B, Jewell SD, Mayer RJ, Goldberg RM, Saltz LB, Warren RS, Redston M (2009) Microsatellite instability predicts improved response to adjuvant therapy with irinotecan, fluorouracil, and leucovorin in stage III colon cancer: cancer and leukemia group B protocol 89803. J Clin Oncol 27: 1814–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonsong A, Curran S, McKay JA, Cassidy J, Murray GI, McLeod HL (2002) Topoisomerase I protein expression in primary colorectal cancer and lymph node metastases. Hum Pathol 33: 1114–1119 [DOI] [PubMed] [Google Scholar]
- Braun MS, Richman SD, Quirke P, Daly C, Adlard JW, Elliott F, Barrett JH, Selby P, Meade AM, Stephens RJ, Parmar MK, Seymour MT (2008) Predictive biomarkers of chemotherapy efficacy in colorectal cancer: results from the UK MRC FOCUS trial. J Clin Oncol 26: 2690–2698 [DOI] [PubMed] [Google Scholar]
- Calabrese CR, Almassy R, Barton S, Batey MA, Calvert AH, Canan-Koch S, Durkacz BW, Hostomsky Z, Kumpf RA, Kyle S, Li J, Maegley K, Newell DR, Notarianni E, Stratford IJ, Skalitzky D, Thomas HD, Wang LZ, Webber SE, Williams KJ, Curtin NJ (2004) Anticancer chemosensitization and radiosensitization by the novel poly(ADP-ribose) polymerase-1 inhibitor AG14361. J Natl Cancer Inst 96: 56–67 [DOI] [PubMed] [Google Scholar]
- Cecchin E, Innocenti F, D’Andrea M, Corona G, De Mattia E, Biason P, Buonadonna A, Toffoli G (2009) Predictive role of the UGT1A1, UGT1A7, and UGT1A9 genetic variants and their haplotypes on the outcome of metastatic colorectal cancer patients treated with fluorouracil, leucovorin, and irinotecan. J Clin Oncol 27: 2457–2465 [DOI] [PubMed] [Google Scholar]
- Chalmers AJ, Lakshman M, Chan N, Bristow RG (2010) Poly(ADP-ribose) polymerase inhibition as a model for synthetic lethality in developing radiation oncology targets. Semin Radiat Oncol 20(4): 274–281 [DOI] [PubMed] [Google Scholar]
- Chiang SC, Carroll J, El-Khamisy SF (2010) TDP1 serine 81 promotes interaction with DNA ligase III alpha and facilitates cell survival following DNA damage. Cell Cycle 9: 588–595 [DOI] [PubMed] [Google Scholar]
- Cortes Ledesma F, El Khamisy SF, Zuma MC, Osborn K, Caldecott KW (2009) A human 5′-tyrosyl DNA phosphodiesterase that repairs topoisomerase-mediated DNA damage. Nature 461: 674–678 [DOI] [PubMed] [Google Scholar]
- Dopeso H, Mateo-Lozano S, Elez E, Landolfi S, Ramos Pascual FJ, Hernandez-Losa J, Mazzolini R, Rodrigues P, Bazzocco S, Carreras MJ, Espin E, Armengol M, Wilson AJ, Mariadason JM, Ramon YCS, Tabernero J, Schwartz Jr S, Arango D (2010) Aprataxin tumor levels predict response of colorectal cancer patients to irinotecan-based treatment. Clin Cancer Res 16: 2375–2382 [DOI] [PubMed] [Google Scholar]
- Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P (2000) Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 355: 1041–1047 [DOI] [PubMed] [Google Scholar]
- Efimova EV, Mauceri HJ, Golden DW, Labay E, Bindokas VP, Darga TE, Chakraborty C, Barreto-Andrade JC, Crawley C, Sutton HG, Kron SJ, Weichselbaum RR (2010) Poly(ADP-ribose) polymerase inhibitor induces accelerated senescence in irradiated breast cancer cells and tumors. Cancer Res 70: 6277–6282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Khamisy SF (2011) To live or to die: a matter of processing damaged DNA termini in neurons. EMBO Mol Med 3: 78–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Khamisy SF, Hartsuiker E, Caldecott KW (2007) TDP1 facilitates repair of ionizing radiation-induced DNA single-strand breaks. DNA Repair (Amst) 6: 1485–1495 [DOI] [PubMed] [Google Scholar]
- El-Khamisy SF, Katyal S, Patel P, Ju L, McKinnon PJ, Caldecott KW (2009) Synergistic decrease of DNA single-strand break repair rates in mouse neural cells lacking both Tdp1 and aprataxin. DNA Repair (Amst) 8: 760–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Khamisy SF, Saifi GM, Weinfeld M, Johansson F, Helleday T, Lupski JR, Caldecott KW (2005) Defective DNA single-strand break repair in spinocerebellar ataxia with axonal neuropathy-1. Nature 434: 108–113 [DOI] [PubMed] [Google Scholar]
- Fallik D, Borrini F, Boige V, Viguier J, Jacob S, Miquel C, Sabourin JC, Ducreux M, Praz F (2003) Microsatellite instability is a predictive factor of the tumor response to irinotecan in patients with advanced colorectal cancer. Cancer Res 63: 5738–5744 [PubMed] [Google Scholar]
- Fuchs CS, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M, Schulz J, Richards D, Soufi-Mahjoubi R, Wang B, Barrueco J (2007) Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol 25: 4779–4786 [DOI] [PubMed] [Google Scholar]
- Gellert M, Mizuuchi K, O’Dea MH, Nash HA (1976) DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci USA 73: 3872–3876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanella BC, Stehlin JS, Wall ME, Wani MC, Nicholas AW, Liu LF, Silber R, Potmesil M (1989) DNA topoisomerase I – targeted chemotherapy of human colon cancer in xenografts. Science 246: 1046–1048 [DOI] [PubMed] [Google Scholar]
- Glynne-Jones R, Falk S, Maughan TS, Meadows HM, Sebag-Montefiore D (2007) A phase I/II study of irinotecan when added to 5-fluorouracil and leucovorin and pelvic radiation in locally advanced rectal cancer: a Colorectal Clinical Oncology Group Study. Br J Cancer 96: 551–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollins S, Myint AS, Haylock B, Wise M, Saunders M, Neupane R, Essapen S, Samuel L, Dougal M, Lloyd A, Morris J, Topham C, Susnerwala S (2011) Preoperative chemoradiotherapy using concurrent capecitabine and irinotecan in magnetic resonance imaging-defined locally advanced rectal cancer: impact on long-term clinical outcomes. J Clin Oncol 29(8): 1042–1049 [DOI] [PubMed] [Google Scholar]
- Gongora C, Vezzio-Vie N, Tuduri S, Denis V, Causse A, Auzanneau C, Collod-Beroud G, Coquelle A, Pasero P, Pourquier P, Martineau P, Del Rio M (2011) New topoisomerase I mutations are associated with resistance to camptothecin. Mol Cancer 10: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochster H, Liebes L, Speyer J, Sorich J, Taubes B, Oratz R, Wernz J, Chachoua A, Blum RH, Zeleniuch-Jacquotte A (1997) Effect of prolonged topotecan infusion on topoisomerase 1 levels: a phase I and pharmacodynamic study. Clin Cancer Res 3: 1245–1252 [PubMed] [Google Scholar]
- Horisberger K, Erben P, Muessle B, Woernle C, Stroebel P, Kaehler G, Wenz F, Hochhaus A, Post S, Willeke F, Hofheinz RD (2009) Topoisomerase I expression correlates to response to neoadjuvant irinotecan-based chemoradiation in rectal cancer. Anticancer Drugs 20: 519–524 [DOI] [PubMed] [Google Scholar]
- Hoskins JM, Marcuello E, Altes A, Marsh S, Maxwell T, Van Booven DJ, Pare L, Culverhouse R, McLeod HL, Baiget M (2008) Irinotecan pharmacogenetics: influence of pharmacodynamic genes. Clin Cancer Res 14: 1788–1796 [DOI] [PubMed] [Google Scholar]
- Hoskins JM, Rosner GL, Ratain MJ, McLeod HL, Innocenti F (2009) Pharmacodynamic genes do not influence risk of neutropenia in cancer patients treated with moderately high-dose irinotecan. Pharmacogenomics 10: 1139–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiang YH, Hertzberg R, Hecht S, Liu LF (1985) Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem 260: 14873–14878 [PubMed] [Google Scholar]
- Hsiang YH, Liu LF (1988) Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Res 48: 1722–1726 [PubMed] [Google Scholar]
- Husain I, Mohler JL, Seigler HF, Besterman JM (1994) Elevation of topoisomerase I messenger RNA, protein, and catalytic activity in human tumors: demonstration of tumor-type specificity and implications for cancer chemotherapy. Cancer Res 54: 539–546 [PubMed] [Google Scholar]
- Innocenti F, Undevia SD, Iyer L, Chen PX, Das S, Kocherginsky M, Karrison T, Janisch L, Ramirez J, Rudin CM, Vokes EE, Ratain MJ (2004) Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol 22: 1382–1388 [DOI] [PubMed] [Google Scholar]
- Interthal H, Chen HJ, Kehl-Fie TE, Zotzmann J, Leppard JB, Champoux JJ (2005) SCAN1 mutant Tdp1 accumulates the enzyme – DNA intermediate and causes camptothecin hypersensitivity. EMBO J 24: 2224–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S, Aguado M, Fallik D, Praz F (2001) The role of the DNA mismatch repair system in the cytotoxicity of the topoisomerase inhibitors camptothecin and etoposide to human colorectal cancer cells. Cancer Res 61: 6555–6562 [PubMed] [Google Scholar]
- Katyal S, el-Khamisy SF, Russell HR, Li Y, Ju L, Caldecott KW, McKinnon PJ (2007) TDP1 facilitates chromosomal single-strand break repair in neurons and is neuroprotective in vivo. EMBO J 26: 4720–4731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman M, Antonini NF, Douma J, Wals J, Honkoop AH, Erdkamp FL, de Jong RS, Rodenburg CJ, Vreugdenhil G, Loosveld OJ, van Bochove A, Sinnige HA, Creemers GJ, Tesselaar ME, Slee PH, Werter MJ, Mol L, Dalesio O, Punt CJ (2007) Sequential vs combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomised controlled trial. Lancet 370(9582): 135–142 [DOI] [PubMed] [Google Scholar]
- Koopman M, Knijn N, Richman S, Seymour M, Quirke P, van Tinteren H, van Krieken JHJM, Punt CJA, Nagtegaal ID (2009) The correlation between topoisomerase-I (Topo1) expression and outcome of treatment with capecitabine and irinotecan in advanced colorectal cancer (ACC) patients (pts) treated in the CAIRO study of the Dutch Colorectal Cancer Group (DCCG). Eur J Cancer Suppl 7: 321 [Google Scholar]
- Li XG, Haluska Jr P, Hsiang YH, Bharti A, Kufe DW, Rubin EH (1996) Identification of topoisomerase I mutations affecting both DNA cleavage and interaction with camptothecin. Ann NY Acad Sci 803: 111–127 [DOI] [PubMed] [Google Scholar]
- Lima JP, dos Santos LV, Sasse EC, Lima CS, Sasse AD (2010) Camptothecins compared with etoposide in combination with platinum analog in extensive stage small cell lung cancer: systematic review with meta-analysis. J Thorac Oncol 5: 1986–1993 [DOI] [PubMed] [Google Scholar]
- Liu C, Zhou S, Begum S, Sidransky D, Westra WH, Brock M, Califano JA (2007) Increased expression and activity of repair genes TDP1 and XPF in non-small cell lung cancer. Lung Cancer 55(3): 303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariadason JM, Arango D, Shi Q, Wilson AJ, Corner GA, Nicholas C, Aranes MJ, Lesser M, Schwartz EL, Augenlicht LH (2003) Gene expression profiling-based prediction of response of colon carcinoma cells to 5-fluorouracil and camptothecin. Cancer Res 63: 8791–8812 [PubMed] [Google Scholar]
- Miknyoczki SJ, Jones-Bolin S, Pritchard S, Hunter K, Zhao H, Wan W, Ator M, Bihovsky R, Hudkins R, Chatterjee S, Klein-Szanto A, Dionne C, Ruggeri B (2003) Chemopotentiation of temozolomide, irinotecan, and cisplatin activity by CEP-6800, a poly(ADP-ribose) polymerase inhibitor. Mol Cancer Ther 2: 371–382 [PubMed] [Google Scholar]
- Moreira MC, Barbot C, Tachi N, Kozuka N, Uchida E, Gibson T, Mendonca P, Costa M, Barros J, Yanagisawa T, Watanabe M, Ikeda Y, Aoki M, Nagata T, Coutinho P, Sequeiros J, Koenig M (2001) The gene mutated in ataxia-ocular apraxia 1 encodes the new HIT/Zn-finger protein aprataxin. Nat Genet 29(2): 189–193 [DOI] [PubMed] [Google Scholar]
- Morris EJ, Geller HM (1996) Induction of neuronal apoptosis by camptothecin, an inhibitor of DNA topoisomerase-I: evidence for cell cycle-independent toxicity. J Cell Biol 134: 757–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosesso P, Piane M, Palitti F, Pepe G, Penna S, Chessa L (2005) The novel human gene aprataxin is directly involved in DNA single-strand-break repair. Cell Mol Life Sci 62: 485–491 [DOI] [PubMed] [Google Scholar]
- O’Dwyer PJ, Catalano RB (2006) Uridine diphosphate glucuronosyltransferase (UGT) 1A1 and irinotecan: practical pharmacogenomics arrives in cancer therapy. J Clin Oncol 24: 4534–4538 [DOI] [PubMed] [Google Scholar]
- Paillas S, Boissiere F, Bibeau F, Denouel A, Mollevi C, Causse A, Denis V, Vezzio-Vie N, Marzi L, Cortijo C, Ait-Arsa I, Askari N, Pourquier P, Martineau P, Del Rio M, Gongora C (2011) Targeting the p38 MAPK pathway inhibits irinotecan resistance in colon adenocarcinoma. Cancer Res 71: 1041–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, Hamilton SR, Laurent-Puig P, Gryfe R, Shepherd LE, Tu D, Redston M, Gallinger S (2003) Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 349: 247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, Elfring GL, Miller LL (2000) Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 343: 905–914 [DOI] [PubMed] [Google Scholar]
- Seymour MT, Maughan TS, Ledermann JA, Topham C, James R, Gwyther SJ, Smith DB, Shepherd S, Maraveyas A, Ferry DR, Meade AM, Thompson L, Griffiths GO, Parmar MK, Stephens RJ (2007) Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): a randomised controlled trial. Lancet 370: 143–152 [DOI] [PubMed] [Google Scholar]
- Smith LM, Willmore E, Austin CA, Curtin NJ (2005) The novel poly(ADP-Ribose) polymerase inhibitor, AG14361, sensitizes cells to topoisomerase I poisons by increasing the persistence of DNA strand breaks. Clin Cancer Res 11: 8449–8457 [DOI] [PubMed] [Google Scholar]
- Snipstad K, Fenton CG, Kjaeve J, Cui G, Anderssen E, Paulssen RH (2010) New specific molecular targets for radio-chemotherapy of rectal cancer. Mol Oncol 4(1): 52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheij M, Vens C, van Triest B (2010) Novel therapeutics in combination with radiotherapy to improve cancer treatment: rationale, mechanisms of action and clinical perspective. Drug Resist Updat 13: 29–43 [DOI] [PubMed] [Google Scholar]
- Wang JC (1971) Interaction between DNA and an Escherichia coli protein omega. J Mol Biol 55: 523–533 [DOI] [PubMed] [Google Scholar]
- Willeke F, Horisberger K, Kraus-Tiefenbacher U, Wenz F, Leitner A, Hochhaus A, Grobholz R, Willer A, Kahler G, Post S, Hofheinz RD (2007) A phase II study of capecitabine and irinotecan in combination with concurrent pelvic radiotherapy (CapIri-RT) as neoadjuvant treatment of locally advanced rectal cancer. Br J Cancer 96: 912–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SW, Burgin Jr AB, Huizenga BN, Robertson CA, Yao KC, Nash HA (1996) A eukaryotic enzyme that can disjoin dead-end covalent complexes between DNA and type I topoisomerases. Proc Natl Acad Sci USA 93(21): 11534–11539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Miller R, Zhang W, Sharma M, Holtschlag V, Watson MA, McLeod HL (2008) Copy-number analysis of topoisomerase and thymidylate synthase genes in frozen and FFPE DNAs of colorectal cancers. Pharmacogenomics 9: 1459–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Shannon WD, Watson MA, McLeod HL (2005) Gene expression profiling of the irinotecan pathway in colorectal cancer. Clin Cancer Res 11: 2053–2062 [DOI] [PubMed] [Google Scholar]
- Zhang YW, Regairaz M, Seiler JA, Agama KK, Doroshow JH, Pommier Y (2011) Poly(ADP-ribose) polymerase and XPF-ERCC1 participate in distinct pathways for the repair of topoisomerase I-induced DNA damage in mammalian cells. Nucleic Acids Res 39(9): 3607–3620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Akopiants K, Mohapatra S, Lin PS, Valerie K, Ramsden DA, Lees-Miller SP, Povirk LF (2009) Tyrosyl-DNA phosphodiesterase and the repair of 3′-phosphoglycolate-terminated DNA double-strand breaks. DNA Repair (Amst) 8: 901–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Lee JW, Tatavarthi H, Lupski JR, Valerie K, Povirk LF (2005) Deficiency in 3′-phosphoglycolate processing in human cells with a hereditary mutation in tyrosyl-DNA phosphodiesterase (TDP1). Nucleic Acids Res 33: 289–297 [DOI] [PMC free article] [PubMed] [Google Scholar]