Abstract
Multiple sclerosis (MS) is associated with both genetic and environmental factors that influence disease susceptibility. Exposure to cigarette smoke is emerging as a viable environmental risk factor for MS that contributes to both increased disease susceptibility and more rapid disease advancement. The relative risk for MS development is approximately 1.5 for smokers compared with nonsmokers. Furthermore, there may be important interactions between smoking, an individual’s genetic background, and other environmental risk exposures. This review summarizes the current evidence supporting the association of smoking with MS risk and disease course, with additional comments on causation.
Keywords: causality, disease progression, multiple sclerosis, smoking, risk factors
Introduction
Multiple sclerosis (MS) is a central nervous system inflammatory demyelinating disease with complex etiological mechanisms [Compston and Coles, 2008]. Putatively autoimmune in origin, MS has both genetic and environmental underpinnings. For example, an international consortium recently used a very large dataset to identify 29 disease susceptibility genes, almost all of which have a direct or indirect influence on the immune system [International Multiple Sclerosis Genetics Consortium and Wellcome Trust Case Control Consortium 2, 2011]. Leading environmental risk factor candidates include infections (especially early-life Epstein–Barr virus infection) and ultraviolet light exposure and/or vitamin D status, though many others have been explored [van der Mei et al. 2011]. Most of these factors likely influence MS risk via complex gene–environment interactions acting at an individual level.
Cigarette smoking is a relatively modern habit that spread worldwide throughout the 20th century, though it began much earlier in some regions. Global consumption of cigarettes rose more than 3-fold between 1950 and 2000 [World Health Organization Tobacco Free Initiative, 2002a], a period during which MS incidence, particularly in females, has been observed to increase in most [Maghzi et al. 2010; Celius and Smestad, 2009; Orton et al. 2006b], but not all [Simpson et al. 2011], places where it has been reliably studied. Cigarette smoking prevalence varies geographically and between men and women; rates for men are generally higher except in some nations where MS is highly prevalent, including Norway, Sweden, and New Zealand, where male and female cigarette consumption rates are roughly equal [World Health Organization Tobacco Free Initiative, 2002b]. To some degree, dose and duration of exposure can be quantified for an individual. Moreover, the toxic components of tobacco smoke and its adverse health effects have been subjects of intense study for decades. Therefore, not only is smoking of interest as an environmental influence on MS, but also its status as a risk factor is supported by much more epidemiological data than are available for most other investigated risk factors. Knowledge about the biological effects of smoking could also provide insight into disease mechanism pathways in MS.
In this review we summarize the current evidence that supports an association between smoking and the risk of developing MS. It will also explore the links between smoking and MS disease course, such as the influence of smoking on measures of disease activity [i.e. clinical relapses and magnetic resonance imaging (MRI) changes], the risk of converting from a relapsing disease course to one of inexorable progression, and the rate of disability advancement. Finally, the status of smoking toward fulfilling detailed criteria for causation of MS will be analyzed.
Association of smoking with MS susceptibility
Some of the case–control studies and surveys reported in the 1960s first detected associations between smoking and MS risk or MS disease activity. A population-based case–control study from Israel revealed that a greater proportion of MS patients had smoked before clinical MS onset (44%) compared with the control group (36%; p = 0.02), although the amount smoked did not differ between patients and controls and relatively low numbers of subjects smoked more than 21 cigarettes daily [Antonovsky et al. 1965]. However, other studies, including a 1966 British survey that utilized national smoking survey data for the control sample, found no overall association in smoking between MS patients and controls sample [Simpson et al. 1966].
In the 1990s, a pair of prospective cohort studies suggested possible associations between smoking and MS risk, although the studies were designed for other purposes. The Oxford Family Planning Association (Oxford-FPA) study of contraception, in which 17,000 married women aged 25–39 years were recruited at 17 family planning clinics between 1968 and 1974, investigated the relationships of parity, oral contraceptive use, and other epidemiological and lifestyle factors with MS risk [Villard-Mackintosh and Vessey, 1993]. Cigarette smoking status was one of the variables found to be significantly associated with MS. Thorogood and colleagues reported results from another prospective British study, the Royal College of General Practitioners' Oral Contraception Study, which initially recruited over 46,000 women during the late 1960s [Thorogood and Hannaford, 1998]. As with the Oxford-FPA study, the evaluation of smoking-related outcomes was not the primary goal of the study. However, cigarette smoking was assessed as a covariate potentially affecting the influence of oral contraceptive use; smoking status was recorded as none, 1–14 cigarettes daily or ≥15 cigarettes daily at the time of recruitment. Analysis of 1996 outcome data, with 51% of the total potential woman-years of follow up available from the original cohort (based on the number of women enrolled and total follow-up duration), showed that there was no significant influence of smoking on MS risk, but there were hints of a dose–response effect. Compared with women who did not smoke at recruitment, relative risks associated with future MS development were 1.2 [95% confidence interval (CI) 0.9–2.2] for smokers of 1–14 cigarettes/day and 1.4 (95% CI 0.8–1.8) for smokers of ≥15 cigarettes/day. Notably, this study evaluated incident MS cases on the basis of the date of diagnosis (always after recruitment) and data were not obtained for smoking history following recruitment.
The past decade has seen a marked increase in the number of published investigations assessing the link between smoking and MS susceptibility and almost all have detected a significant detrimental effect. For example, a 2001 Canadian case–control study detected a marginal overall increase in MS risk in ever smokers compared with never smokers [odds ratio (OR) 1.6, 95% CI 1.0–2.4], but it clearly demonstrated a dose–response effect, with the following odds ratios: 0.7 (95% CI 0.3–1.5) for 0–10 cigarettes/day, 1.4 (95% CI 0.8–2.4) for 10–20 cigarettes/day, 1.9 (95% CI 1.2–3.2) for 20–40 cigarettes/day, and 5.5 (95% CI 1.7–17.8) for >40 cigarettes/day [Ghadirian et al. 2001]. Data collected on smoking and other lifestyle-related variables referred to the year prior to the patient’s MS diagnosis, making it susceptible to recall bias as well as raising questions about temporality, specifically whether the smoking exposure estimate accurately reflected true smoking status prior to the first clinical symptoms of MS.
Some of the methodological issues noted above were addressed by an evaluation of two cohort studies of US women, the Nurses’ Health Study (121,700 women aged 30–55 years at baseline in 1976) and the Nurses’ Health Study II (116,671 women aged 25–42 years at baseline in 1989) [Hernan et al. 2001]. Smoking history was assessed at baseline and updated with biennial questionnaires; 315 definite or probable cases of MS were documented by mid-1994 (first cohort) and mid-1995 (second cohort). Compared with that for women who never smoked, the relative incidence rate was 1.6 (95% CI 1.2–2.1) among current smokers and 1.2 (95% CI 0.9–1.6) among past smokers after adjustment for potential confounders such as age, geographic latitude, and ancestry. The relative rate increased significantly with cumulative exposure to smoking (p for trend <0.05), from 1.1 (95% CI 0.8–1.6) for 1–9 pack-years to 1.5 (95% CI 1.2–2.1) for 10–24 pack-years, and 1.7 (95% CI 1.2–2.4) for ≥25 pack-years. This prospective cohort therefore confirmed an association, with the power of a very large dataset, prospective cohort design, reassessment of exposure at different time points, addressing the temporal relationship of exposure to diagnosis (but not necessarily clinical disease onset), and a dose–response effect.
In 2009, a large, multinational European case–control study consistently noted that cigarette smoking was associated with MS risk, with the following odds ratios: Sweden, 1.5 (95% CI 1.3–1.8); Norway, 1.8 (95% CI 1.1–2.9); UK, 1.3 (95% CI 1.0–1.9) [Hedstrom et al. 2009]. This study also found that chronic use (≥15 years) of moist snuff was associated with lower MS risk (OR 0.3, 95% CI 0.1–0.8), suggesting that factors inherent in smoked tobacco, but not nicotine, could be implicated in MS risk. These findings were largely replicated by a cohort study of Swedish construction workers that assessed the relationships of smoking and moist tobacco use with MS and other inflammatory diseases, such as rheumatoid arthritis, Crohn’s disease, ulcerative colitis, and sarcoidosis [Carlens et al. 2010]. Ever smoking was associated with an increased risk for all diseases studied except sarcoidosis (lower risk) and the relative risk associated with MS was 1.9 (95% CI 1.4–2.6). In contrast, ever use of moist snuff, adjusted for smoking, was not associated with any of the diseases (relative risk for MS 1.0, 95% CI 0.8–1.4). The authors concluded that inhaled non-nicotinic components of cigarette smoke are more important than nicotine itself in the etiology of these diseases.
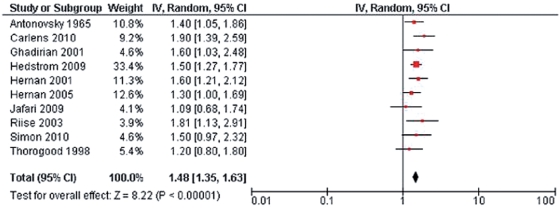
Several other smaller case–control or cohort studies have been published in the past decade; overall, most [Rodriguez Regal et al. 2009; Pekmezovic et al. 2006; Hernan et al. 2005; Riise et al. 2003] but not all [Simon et al. 2010; Silva et al. 2009; Russo et al. 2008] studies showed that smoking is associated with increased MS susceptibility. Handel and colleagues updated a meta-analysis of published data concerning smoking and MS risk [Handel et al. 2009]. Fourteen studies containing data on 3,052 MS patients and 457,619 controls were examined using both a conservative analysis (limited to 10 studies in which smoking behavior was described prior to MS onset) and a nonconservative analysis (all 14 studies, regardless of whether smoking behavior was current or occurred prior to MS onset). Smoking was associated with increased MS susceptibility in both model analyses (Figure 1; conservative, risk ratio 1.48, 95% CI 1.35–1.63, p < 10−15; nonconservative: risk ratio 1.52, 95% CI 1.39–1.66, p < 10−19). There were no significant influences on the results when adjusted for median geographic latitude or sex ratio. Of the 14 studies, 11 used a case–control design (meta-analysis relative risk 1.49, 95% CI 1.35–1.66), whereas three were cohort studies (meta-analysis relative risk 1.58, 95% CI 1.25–2.00) with similar relative risk estimates (p = 0.65).
Figure 1.
Forest plot of 10 studies included in conservative model meta-analysis of smoking and multiple sclerosis risk [Handel et al. 2011].
Some studies included in the meta-analysis suggested a dose–response effect of smoking on MS risk (Figure 2); however, methods of ascertainment were not consistent (e.g. some studies evaluated pack-years and others cigarettes per day). In a study using previously banked blood samples, Sundstrom and colleagues demonstrated that cotinine levels, which are a measure of smoking exposure, were increased in MS patients compared with controls [Sundstrom et al. 2008]. Objective markers of smoking exposure, especially in a longitudinal prospective dataset, would enhance the understanding of the timing and dose–response relationship between smoking and MS susceptibility.
Figure 2.
Forest plot of four studies included in a meta-analysis of smoking and the risk of secondary progressive multiple sclerosis [Handel et al. 2011].
The timing of smoking risk pertinent to MS is not fully understood. Maternal smoking during pregnancy was found not to increase risk in exposed children in one study [Montgomery et al. 2008], but in another parental smoking was noted as a risk for subsequent development of MS in offspring [Mikaeloff et al. 2007]. A recent population-based case–control study demonstrated that MS risk was increased among never smokers who had been exposed to passive smoking (OR 1.3, 95% CI 1.1–1.6) compared with never smokers who had never been exposed [Hedstrom et al. 2011]. Moreover, MS risk increased with increasing duration of exposure. Because epidemiological data, especially migration studies, suggest that some environmental influences on MS risk probably occur early in life (prior to age 15 years), future research must address the timing of both passive and active cigarette smoke exposure in addition to dose–response effects and interactions with other putative risk factors.
Interactions between smoking, other environmental factors, and genetics
The risk of MS results from both genetic and environmental factors. It seems likely that individual environmental factors not only independently influence MS risk but also interact with both other environmental influences and an individual’s genetic background, perhaps through several different direct or epigenetic mechanisms [Handel et al. 2009; van der Mei et al. 2011]. Leading examples of environmental risk factors include infection (especially with Epstein–Barr virus) and vitamin D status (or exposure to ultraviolet radiation) [Wingerchuk, 2011]. Effects of relative vitamin D deficiency are likely to have an impact early in life, Epstein–Barr virus infection probably influences risk during adolescence or early adulthood, primary smoking exposure can occur from late childhood through adulthood, and passive exposure to cigarette smoke can occur in utero and any time thereafter [Handel et al. 2010a]. Genetic susceptibility is increased by human leukocyte antigen (HLA) DR15*1501. The influence of each risk factor may be relatively small, requiring detection with larger and more powerful clinical, epidemiological, and genetic datasets (as well as genetic science and technology) than existed until very recently. Whereas most case–control studies discussed in the previous section adjusted analyses for potential confounders such as age and geographic latitude, newer studies are focusing intensely on determining the existence, magnitude, and mechanisms of interactions relevant to these leading factors.
There are several recent examples of such multifactorial and hypothesis-directed mechanistic investigations. Recent studies evaluating the possible role of vitamin D in regulating HLA-DRB1 expression showed that smoking enhanced the association between high anti-Epstein–Barr virus nuclear antigen (EBNA) titer and increased MS risk but did not affect the association between HLA-DR15 and MS risk [Handunnetthi et al. 2010; Simon et al. 2010]. In a population-based Swedish case–control study, there was a significant interaction between two genetic risk factors (presence of HLA-DRB1*15 and absence of HLA-A*02) among smokers but not among nonsmokers [Hedstrom et al. 2011]. Compared with nonsmokers with neither of the studied genetic risk factors, the odds ratio for MS was 13.5 (95% CI 8.1–2.6) for smokers with both genetic risk factors. The odds ratio for smokers without genetic risk was 1.4 (95% CI 0.9–2.1) and the odds ratio for nonsmokers with both genetic risk factors was 4.9 (95% CI 3.6–6.6). Among those with both genetic risk factors, smoking increased MS risk by a factor of 2.8 in those with both genetic risk factors compared with a factor of 1.4 among those without either genetic risk factor. Despite these results, others have found that smoking may not influence genetically loaded risk within families [Jafari et al. 2009]. Efforts to use data from genome-wide association studies to integrate weighted genetic risk scores for MS susceptibility with environmental factors have emerged [De Jager et al. 2009]. There may be interactions between environmental risk factors as well; for example, current smoking and cumulative tobacco consumption were associated with higher levels of Epstein–Barr virus antibodies [Nielsen et al. 2007].
Summary
In aggregate, current epidemiological data suggest that cigarette smoking, but not other forms of moist tobacco use, is associated with increased MS susceptibility. A dose–response effect, with higher consumption of cigarettes resulting in greater risk, may also exist. There are likely important interactions between smoking and other environmental and genetic risk factors for MS development.
Association of smoking with MS disease course
Observational studies suggest that cigarette smoking may exert an influence on the course of established MS. Outcomes that have been linked to smoking effects include measures of disease activity (clinical relapses and development of new lesions visible on MRI), more rapid conversion from a first ever demyelinating clinical event [also known as a clinically isolated syndrome (CIS), typically optic neuritis or partial myelitis] to confirmed MS, the rate of conversion from relapsing–remitting to secondary progressive MS, and the rate of neurological deterioration once progressive MS has been established. Evaluation of the magnitude of the effect of smoking on the course of established MS may shed light on underlying disease mechanisms and is important because more than half of MS patients smoke at some time [Marrie et al. 2009].
An association between cigarette smoking and acute MS exacerbations was observed in the 1960s [Courville et al. 1964]. Smoking was also linked to transient deterioration in motor performance of ∼10 minutes duration in 21 MS patients compared with 11 healthy controls in whom steady improvement was observed [Emre and de Decker, 1992]. These data have not been effectively duplicated.
Di Pauli and colleagues studied 129 CIS patients with brain MRI white matter lesions and the presence of cerebrospinal fluid oligoclonal bands, both indicating high risk for future development of confirmed MS [Di Pauli et al. 2008]. After follow up of 36 months, 75% of smokers but only 51% of nonsmokers developed clinically definite MS. Smokers had a significantly shorter interval to their first relapse.The hazard ratio for progression to clinically definite MS was 1.8 (95% CI, 1.2–2.8) for smokers compared with nonsmokers (p = 0.008). The authors concluded that smoking speeds conversion from CIS to confirmed MS.
Studies that evaluated the association of smoking and MS progression or disability have also typically, though not always, detected a significant relationship. For example, a cross-sectional Dutch survey showed no effect of smoking on disease progression or disability [Koch et al. 2007]. However, Hernan and colleagues found that smoking increased the risk of conversion from relapsing–remitting to secondary progressive disease (hazard ratio 3.6, 95% CI 1.3–9.9, for ever smokers compared with never smokers) [Hernan et al. 2005]. A Swedish study of self-report data from 122 newly diagnosed MS patients found that, after a median of 6 years, ever smokers were more likely to have progressive disease compared with never smokers (p < 0.01) [Sundstrom and Nystrom, 2008]. Moreover, the effects on the rate of conversion to progressive MS and earlier age of progression onset were greatest in those who began smoking before age 15 years. Starting smoking early was also associated with a higher rate of primary progressive MS when compared with never smokers (p = 0.012).
These findings were largely replicated by a larger (n = 1465) study of MS patients studied using a cross-sectional survey and a subsequent prospective follow up for a mean of 3.29 years [Healy et al. 2009]. More than half (n = 780; 53.2%) were never smokers, 428 (29.2%) were ex-smokers, and 257 (17.5%) were current smokers. Current smokers had significantly worse baseline function than never smokers in terms of Expanded Disability Status Scale (EDSS) score (adjusted p < 0.001), Multiple Sclerosis Severity Score (MSSS; adjusted p < 0.001), and brain parenchymal fraction (adjusted p = 0.004). In addition, current smokers were significantly more likely to have primary progressive MS (adjusted OR 2.41, 95% CI 1.09–5.34). At follow up, progression to secondary progressive disease occurred faster in smokers than in never smokers (hazard ratio for current smokers versus never smokers 2.50, 95% CI 1.42–4.41). In addition, brain MRI T2-weighted lesion volume increased faster (p = 0.02) and brain parenchymal fraction decreased faster (p = 0.02) in smokers than in nonsmokers. Another prospective study from Tasmania followed 203 MS patients for a median of 909 days, finding a dose–response effect (measured using cumulative pack-years) of smoking on disability [Pittas et al. 2009]. Greater consumption was associated with an increase in longitudinal MSSS (p < 0.001); similar findings were noted when the EDSS was used as the disability outcome. Smoking during the cohort period was not associated with relapse (cumulative pack years smoked after cohort entry: hazard ratio 0.94 per pack year, 95% CI 0.69–1.26).
A meta-analysis of four studies concluded that there was significant between-study heterogeneity and a trend towards smoking increasing the risk of secondary progressive MS (relative risk 1.88, 95% CI 0.98–3.61, p = 0.06) [Handel et al. 2011].
Little is known about the effects of smoking on response to disease-modifying MS therapies. A small study of 31 MS patients using subcutaneous interferon-β revealed that smokers had a much greater risk of developing neutralizing antibodies that abrogate the effectiveness of the drug [Sena et al. 2010].
Summary
The epidemiological evidence reported to date suggests that smoking is probably associated with a greater risk of conversion from relapsing–remitting to secondary progressive MS. Early and heavier cigarette consumption may increase the risk of primary progressive MS compared with a relapsing–remitting onset. Smoking may increase the rate of conversion from CIS to confirmed MS and increase the rate of accumulation of disability in established progressive forms of MS. The effects of smoking on the response to disease-modifying therapy are not known.
Smoking and MS: association and causation
Observational epidemiological studies, regardless of design, can determine associations between exposures and outcomes but cannot prove causation. However, the following criteria may be examined to judge whether causation is reasonable.
-
1.
Strength of the association. The magnitude of the effect of smoking on MS susceptibility is small (relative risk ∼1.5) but significant. The magnitude of the effect of smoking on disease course is also small and was not significant in a meta-analysis. These findings do not exclude causative effects of smoking but indicate that it either influences a small biological effect or an effect that is mitigated by other causative influences.
-
2.
Consistency. Although the individual studies of MS susceptibility did not consistently demonstrate significant effects on MS susceptibility, many of the studies were likely insufficiently sensitive (underpowered) to detect a small effect with precision. Examination of the point estimates of relative risk shows that they range from 1.09 to 1.90 but, notably, no study has demonstrated a protective effect of smoking. Moreover, the results are similar in different populations, on different continents, and using different study methods. Therefore, the data are consistent in the direction of the effect of smoking on MS risk. It remains possible, however, that unknown confounders and biases inherent to observational research could result in consistent, though misleading, results. The selection of the control group is also important in this regard. For example, overrepresentation of control patients with autoimmune diseases, which are also associated with smoking, would influence the association between smoking and MS.
-
3.
Specificity. The effects of smoking are not specific to MS risk as there are many other known detrimental health effects. This serves to highlight the problem of numerous confounders associated with smoking behavior, including baseline personality traits (risk-taking behavior, addiction, and co-addictions) [Hawkes, 2005], co-morbid illnesses (e.g. a greater risk of upper respiratory tract infections, which are associated with increased risk of MS relapse) [Sibley et al. 1985], and other factors. An interesting example of this complexity is the reported inverse link between MS and lung cancer [Handel et al. 2011].
-
4.
Temporal relationship. A critical issue in judging causation is whether the risk exposure occurs prior to the outcome in question. The methods of ascertainment of smoking exposure vary widely between studies, some using cross-sectional surveys for a one-time assessment and others using multiple prospective inquiries. This is particularly challenging for the question of MS risk because the biological onset of the disease and the factors that result in its clinical expression are generally unknown.
-
5.
Biological gradient. Demonstration of a dose–response effect, whereby increased smoking exposure results in a higher risk of MS, strengthens a causal relationship; this has not been firmly established but several individual studies do show such a gradient. Some of the methodological issues that affect the evaluation of temporality also threaten the validity of biological gradient data. Prospective studies that use objective markers of smoking exposure could enhance the data supporting this criterion.
-
6.
Biological plausibility and (7) coherence. Smoking markedly increased throughout the 20th century in many parts of the world and is one of the factors that could have influenced the increase in MS incidence and prevalence. Smoking in women, perhaps through other genetic or environmental factors, could also have influenced the increasing female:male sex ratio that has recently been detected [Orton et al. 2006]. Smoking is also associated with several other putative autoimmune diseases, such as systemic lupus erythematosus, rheumatoid arthritis, and Graves’ disease [Kallberg et al. 2011; Harel-Meir et al. 2007; Prummel and Wiersinga, 1993]. In this regard, there is some degree of plausibility and coherence in a smoking–MS link. There is also reasonable, albeit largely indirect, biological plausibility supporting the hypotheses that smoking could increase MS risk and accelerate disease progression. This will undoubtedly evolve with advances in understanding of the early pathobiology of MS and the mechanisms of progressive disease, especially axonal degeneration and failure of remyelination and neural repair. Components of cigarette smoke have demonstrable immunological effects; for example, nicotine results in T cell immunosuppression [Sopori and Kozak, 1998]. However, snuff use does not appear to increase MS risk (and may actually lower it), suggesting that nicotine alone is unlikely to explain the increased MS risk [Carlens et al. 2010; Hedstrom et al. 2009]. A role for smoked tobacco appears more plausible; at least one study detected an influence of passive smoke exposure on MS risk [Sundstrom et al. 2008]. Cigarette smoke also affects numerous immune functions important in MS, such as T cell, B cell, and natural killer cell functions [Fusby et al. 2010; Motz et al. 2010a, 2010b]. It also has anti-estrogenic effects, and female sex hormones may have favorable effects on the course of MS [Gold and Voskuhl, 2009; Michnovicz et al. 1986]. Furthermore, tobacco smoke contains nitric oxide and cyanide, both of which may have important roles in axonal degeneration and demyelination.
-
8.
Experimentation and (9) analogy. We will not have randomized, controlled trials of smoking and MS but experimental studies of animal models of central nervous system demyelinating disease would enhance our assessment of biological plausibility. Although we can use data from the effects of smoking on other diseases (e.g. other autoimmune diseases) to draw analogies with MS, this is speculative and a weak criterion for causation. Note that it would be of great interest to understand the biological effects and environmental–genetic interactions in MS and contrast them with the apparent protective effect of smoking for Parkinson disease, a neurodegenerative disease with genetic and environmental influences [Chen et al. 2010].
Summary
More observational and mechanistic research is needed, but the available evidence supports cigarette smoking as an independent risk factor for MS susceptibility (relative risk ∼1.5) and associates smoking with a greater chance of developing progressive disease and accruing more rapid disability. The mechanisms by which smoking might influence the risk of MS and its clinical course are unclear. Prospective studies that include genetic analysis, account for potential confounders, and use more objective measures of smoking exposure are needed to enhance the case that smoking plays a causative role in the risk and course of MS. In the meantime, the evidence provides MS patients with one more reason to quit smoking.
Footnotes
No specific grant from any funding agency in the public, commercial, or not-for-profit sectors was received for this work.
The authors declare no conflicts of interest in preparing this article.
References
- Antonovsky A., Leibowitz U., Smith H.A., Medalie J.M., Balogh M., Kats R., et al. (1965) Epidemiologic study of multiple sclerosis in Israel. I. An overall review of methods and findings. Arch Neurol 13: 183–193 [DOI] [PubMed] [Google Scholar]
- Carlens C., Hergens M.P., Grunewald J., Ekbom A., Eklund A., Hoglund C.O., et al. (2010) Smoking, use of moist snuff, and risk of chronic inflammatory diseases. Am J Respir Crit Care Med 181: 1217–1222 [DOI] [PubMed] [Google Scholar]
- Celius E.G., Smestad C. (2009) Change in sex ratio, disease course and age at diagnosis in Oslo MS patients through seven decades. Acta Neurol Scand Suppl 189: 27–29 [DOI] [PubMed] [Google Scholar]
- Chen H., Huang X., Guo X., Mailman R.B., Park Y., Kamel F., et al. (2010) Smoking duration, intensity, and risk of Parkinson disease. Neurology 74: 878–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston A., Coles A. (2008) Multiple sclerosis. Lancet 372: 1502–1517 [DOI] [PubMed] [Google Scholar]
- Courville C.B., Maschmeyer J.E., Delay C.P. (1964) Effects of smoking on the acute exacerbations of multiple sclerosis. Bull Los Angel Neurol Soc 29: 1–6 [PubMed] [Google Scholar]
- De Jager P.L., Chibnik L.B., Cui J., Reischl J., Lehr S., Simon K.C., et al. (2009) Integration of genetic risk factors into a clinical algorithm for multiple sclerosis susceptibility: a weighted genetic risk score. Lancet Neurol 8: 1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pauli F., Reindl M., Ehling R., Schautzer F., Gneiss C., Lutterotti A., et al. (2008) Smoking is a risk factor for early conversion to clinically definite multiple sclerosis. Mult Scler 14: 1026–1030 [DOI] [PubMed] [Google Scholar]
- Emre M., de Decker C. (1992) Effects of cigarette smoking on motor functions in patients with multiple sclerosis. Arch Neurol 49: 1243–1247 [DOI] [PubMed] [Google Scholar]
- Fusby J.S., Kassmeier M.D., Palmer V.L., Perry G.A., Anderson D.K., Hackfort B.T., et al. (2010) Cigarette smoke-induced effects on bone marrow B-cell subsets and CD4+:CD8+ T-cell ratios are reversed by smoking cessation: influence of bone mass on immune cell response to and recovery from smoke exposure. Inhal Toxicol 22: 785–796 [DOI] [PubMed] [Google Scholar]
- Ghadirian P., Dadgostar B., Azani R., Maisonneuve P. (2001) A case-control study of the association between socio-demographic, lifestyle and medical history factors and multiple sclerosis. Can J Public Health 92: 281–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold S.M., Voskuhl R.R. (2009) Estrogen treatment in multiple sclerosis. J Neurol Sci 286: 99–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel A.E., Ebers G.C., Ramagopalan S.V. (2009) Epigenetics: molecular mechanisms and implications for disease. Trends Mol Med 16: 7–16 [DOI] [PubMed] [Google Scholar]
- Handel A.E., Giovannoni G., Ebers G.C., Ramagopalan S.V. 2010a. Environmental factors and their timing in adult-onset multiple sclerosis. Nat Rev Neurosci 6: 156–166 [DOI] [PubMed] [Google Scholar]
- Handel A.E., Joseph A., Ramagopalan S.V. 2010b. Multiple sclerosis and lung cancer: an unexpected inverse association. QJM 103: 625–626 [DOI] [PubMed] [Google Scholar]
- Handel A.E., Williamson A.J., Disanto G., Dobson R., Giovannoni G., Ramagopalan S.V. (2011) Smoking and multiple sclerosis: an updated meta-analysis. PLoS ONE 6: e16149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handunnetthi L., Ramagopalan S.V., Ebers G.C., Handunnetthi L., Ramagopalan S.V., Ebers G.C. (2010) Multiple sclerosis, vitamin D, and HLA-DRB1*15. Neurology 74: 1905–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel-Meir M., Sherer Y., Shoenfeld Y. (2007) Tobacco smoking and autoimmune rheumatic diseases. Nat Clin Pract Rheumatol 3: 707–715 [DOI] [PubMed] [Google Scholar]
- Hawkes C.H. (2005) Are multiple sclerosis patients risk-takers?. QJM 98: 895–911 [DOI] [PubMed] [Google Scholar]
- Healy B.C., Ali E.N., Guttmann C.R., Chitnis T., Glanz B.I., Buckle G., et al. (2009) Smoking and disease progression in multiple sclerosis. Arch Neurol 66: 858–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom A.K., Baarnhielm M., Olsson T., Alfredsson L. (2009) Tobacco smoking, but not Swedish snuff use, increases the risk of multiple sclerosis. Neurology 73: 696–701 [DOI] [PubMed] [Google Scholar]
- Hedstrom A.K., Baarnhielm M., Olsson T., Alfredsson L. (2011) Exposure to environmental tobacco smoke is associated with increased risk for multiple sclerosis. Mult Scler 17: 788–793 [DOI] [PubMed] [Google Scholar]
- Hedstrom A.K., Sundqvist E., Baarnhielm M., Nordin N., Hillert J., Kockum I., et al. (2011) Smoking and two human leukocyte antigen genes interact to increase the risk for multiple sclerosis. Brain 134: 653–664 [DOI] [PubMed] [Google Scholar]
- Hernan M.A., Jick S.S., Logroscino G., Olek M.J., Ascherio A., Jick H., et al. (2005) Cigarette smoking and the progression of multiple sclerosis. Brain 128: 1461–1465 [DOI] [PubMed] [Google Scholar]
- Hernan M.A., Olek M.J., Ascherio A. (2001) Cigarette smoking and incidence of multiple sclerosis. Am J Epidemiol 154: 69–74 [DOI] [PubMed] [Google Scholar]
- International Multiple Sclerosis Genetics Consortium and Wellcome Trust Case Control Consortium 2 (2011) Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476: 214–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari N., Hoppenbrouwers I.A., Hop W.C., Breteler M.M., Hintzen R.Q., Jafari N., et al. (2009) Cigarette smoking and risk of MS in multiplex families. Mult Scler 15: 1363–1367 [DOI] [PubMed] [Google Scholar]
- Kallberg H., Ding B., Padyukov L., Bengtsson C., Ronnelid J., Klareskog L., et al. (2011) Smoking is a major preventable risk factor for rheumatoid arthritis: estimations of risks after various exposures to cigarette smoke. Ann Rheum Dis 70: 508–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M., van Harten A., Uyttenboogaart M., De Keyser J. (2007) Cigarette smoking and progression in multiple sclerosis. Neurology 69: 1515–1520 [DOI] [PubMed] [Google Scholar]
- Maghzi A.H., Ghazavi H., Ahsan M., Etemadifar M., Mousavi S., Khorvash F., et al. (2010) Increasing female preponderance of multiple sclerosis in Isfahan, Iran: a population-based study. Mult Scler 16: 359–361 [DOI] [PubMed] [Google Scholar]
- Marrie R.A., Cutter G., Tyry T., Campagnolo D., Vollmer T., Marrie R.A., et al. (2009) Smoking status over two years in patients with multiple sclerosis. Neuroepidemiology 32: 72–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michnovicz J.J., Hershcopf R.J., Naganuma H., Bradlow H.L., Fishman J. (1986) Increased 2-hydroxylation of estradiol as a possible mechanism for the anti-estrogenic effect of cigarette smoking. N Engl J Med 315: 1305–1309 [DOI] [PubMed] [Google Scholar]
- Mikaeloff Y., Caridade G., Tardieu M., Suissa S. the KIDSEP study group (2007) Parental smoking at home and the risk of childhood-onset multiple sclerosis in children. Brain 130: 2589–2595 [DOI] [PubMed] [Google Scholar]
- Montgomery S.M., Bahmanyar S., Hillert J., Ekbom A., Olsson T. (2008) Maternal smoking during pregnancy and multiple sclerosis amongst offspring. Eur J Neurol 15: 1395–1399 [DOI] [PubMed] [Google Scholar]
- Motz G.T., Eppert B.L., Wesselkamper S.C., Flury J.L., Borchers M.T. 2010a. Chronic cigarette smoke exposure generates pathogenic T cells capable of driving COPD-like disease in Rag2-/- mice. Am J Respir Crit Care Med 181: 1223–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motz G.T., Eppert B.L., Wortham B.W., Amos-Kroohs R.M., Flury J.L., Wesselkamper S.C., et al. 2010b. Chronic cigarette smoke exposure primes NK cell activation in a mouse model of chronic obstructive pulmonary disease. J Immunol 184: 4460–4469 [DOI] [PubMed] [Google Scholar]
- Nielsen T.R., Pedersen M., Rostgaard K., Frisch M., Hjalgrim H. (2007) Correlations between Epstein-Barr virus antibody levels and risk factors for multiple sclerosis in healthy individuals. Mult Scler 13: 420–423 [DOI] [PubMed] [Google Scholar]
- Orton S.M., Herrera B.M., Yee I.M., Valdar W., Ramagopalan S.V., Sadovnick A.D., et al. (2006) Sex ratio of multiple sclerosis in Canada: a longitudinal study. Lancet Neurol 5: 932–936 [DOI] [PubMed] [Google Scholar]
- Pekmezovic T., Drulovic J., Milenkovic M., Jarebinski M., Stojsavljevic N., Mesaros S., et al. (2006) Lifestyle factors and multiple sclerosis: a case-control study in Belgrade. Neuroepidemiology 27: 212–216 [DOI] [PubMed] [Google Scholar]
- Pittas F., Ponsonby A.L., van der Mei I.A., Taylor B.V., Blizzard L., Groom P., et al. (2009) Smoking is associated with progressive disease course and increased progression in clinical disability in a prospective cohort of people with multiple sclerosis. J Neurol 256: 577–585 [DOI] [PubMed] [Google Scholar]
- Prummel M.F., Wiersinga W.M. (1993) Smoking and risk of Graves' disease. JAMA 269: 479–482 [PubMed] [Google Scholar]
- Riise T., Nortvedt M.W., Ascherio A. (2003) Smoking is a risk factor for multiple sclerosis. Neurology 61: 1122–1124 [DOI] [PubMed] [Google Scholar]
- Rodriguez Regal A., del Campo Amigo M., Paz-Esquete J., Martinez Feijoo A., Cebrian E., Suarez Gil P., et al. (2009) [A case-control study of the influence of the smoking behaviour in multiple sclerosis]. Neurologia 24: 177–180 [PubMed] [Google Scholar]
- Russo C., Morabito F., Luise F., Piromalli A., Battaglia L., Vinci A., et al. (2008) Hyperhomocysteinemia is associated with cognitive impairment in multiple sclerosis. J Neurol 255: 64–69 [DOI] [PubMed] [Google Scholar]
- Sena A., Bendtzen K., Cascais M.J., Pedrosa R., Ferret-Sena V., Campos E., et al. (2010) Influence of apolipoprotein E plasma levels and tobacco smoking on the induction of neutralising antibodies to interferon-beta. J Neurol 257: 1703–1707 [DOI] [PubMed] [Google Scholar]
- Sibley W.A., Bamford C.R., Clark K. (1985) Clinical viral infections and multiple sclerosis. Lancet 1(8441): 1313–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva K.R., Alvarenga R.M., Fernandez Y.F.O., Alvarenga H., Thuler L.C. (2009) Potential risk factors for multiple sclerosis in Rio de Janeiro: a case-control study. Arq Neuropsiquiatr 67: 229–234 [DOI] [PubMed] [Google Scholar]
- Simon K.C., van der Mei I.A., Munger K.L., Ponsonby A., Dickinson J., Dwyer T., et al. (2010) Combined effects of smoking, anti-EBNA antibodies, and HLA-DRB1*1501 on multiple sclerosis risk. Neurology 74: 1365–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson C.A., Newell D.J., Schapira K. (1966) Smoking and multiple sclerosis. Neurology 16: 1041–1043 [DOI] [PubMed] [Google Scholar]
- Simpson S., Jr, Pittas F., van der Mei I., Blizzard L., Ponsonby A.L., Taylor B. (2011) Trends in the epidemiology of multiple sclerosis in Greater Hobart, Tasmania: 1951 to 2009. J Neurol Neurosurg Psychiatry 82: 180–187 [DOI] [PubMed] [Google Scholar]
- Sopori M.L., Kozak W. (1998) Immunomodulatory effects of cigarette smoke. J Neuroimmunol 83: 148–156 [DOI] [PubMed] [Google Scholar]
- Sundstrom P., Nystrom L. (2008) Smoking worsens the prognosis in multiple sclerosis. Mult Scler 14: 1031–1035 [DOI] [PubMed] [Google Scholar]
- Sundstrom P., Nystrom L., Hallmans G. (2008) Smoke exposure increases the risk for multiple sclerosis. Eur J Neurol 15: 579–583 [DOI] [PubMed] [Google Scholar]
- Thorogood M., Hannaford P.C. (1998) The influence of oral contraceptives on the risk of multiple sclerosis. Br J Obstet Gynaecol 105: 1296–1299 [DOI] [PubMed] [Google Scholar]
- van der Mei I.A., Simpson S., Jr, Stankovich J., Taylor B.V., van der Mei I.A.F. (2011) Individual and joint action of environmental factors and risk of MS. Neurol Clin 29: 233–255 [DOI] [PubMed] [Google Scholar]
- Villard-Mackintosh L., Vessey M.P. (1993) Oral contraceptives and reproductive factors in multiple sclerosis incidence. Contraception 47: 161–168 [DOI] [PubMed] [Google Scholar]
- Wingerchuk D.M. (2011) Environmental factors in multiple sclerosis: Epstein-Barr virus, vitamin D, and cigarette smoking. Mt Sinai J Med 78: 221–230 [DOI] [PubMed] [Google Scholar]
- World Health Organization Tobacco Free Initiative (2002a) Cigarette consumption. http://www.who.int/tobacco/en/atlas8.pdf Accessed 13 August 2011
- World Health Organization Tobacco Free Initiative (2002b) Female smoking. http://www.who.int/tobacco/en/atlas6.pdf Accessed 13 August 2011