Abstract
Objective: The objective of this study was to assess the effect of treatment with interferon (IFN) β-1a, 44 µg subcutaneously (sc) three times weekly (tiw), on clinical and magnetic resonance imaging (MRI) outcomes in patients with relapsing multiple sclerosis (MS) following mitoxantrone therapy.
Methods: This was an open-label, randomized, multicentre, rater-blinded, 96-week observational study conducted in Germany. Clinically stable patients with relapsing forms of MS, who had discontinued mitoxantrone treatment 1–6 months before study entry, were randomized to IFN β-1a sc 44 µg tiw, or no treatment. The primary endpoint was time to first relapse. Secondary endpoints included the number of relapse-free patients, disease activity assessed by MRI and time to 3-month confirmed Expanded Disability Status Scale (EDSS) progression, all at week 96.
Results: A total of 30 patients were randomized (intent-to-treat population: 14 IFN β-1a, 15 untreated; one patient from the safety population discontinued the study after 25 days owing to an adverse event and without providing any postbaseline efficacy data, and was thus excluded from the intent-to-treat population). Overall, 71.4% (10/14) of patients in the IFN β-1a group remained relapse free over 96 weeks, versus 46.7% (7/15) in the untreated group (p = 0.26). IFN β-1a delayed the time to first relapse versus no treatment (p = 0.14); time to first relapse (25th percentile) was 95.4 (IFN β-1a) versus 46.0 weeks (no treatment). Confirmed EDSS progression was observed in five patients in each treatment group. Mean change in EDSS score was 0.3 in both groups (p = 0.79). Changes in the number or volume of T1 and T2 lesions at week 96 were not significantly different between treatment groups (p > 0.05). There were no new or unexpected adverse events related to IFN β-1a treatment.
Conclusions: Several endpoints appeared to show a benefit of IFN β-1a treatment, but no significant differences could be detected owing to the small sample. Therefore, these data only permit, at best, tentative conclusions about the disease course in patients with MS after de-escalation from mitoxantrone and continuation with or without IFN β-1a. Larger confirmatory studies are required.
Keywords: advanced disease, de-escalation therapy, mitoxantrone, relapsing multiple sclerosis, subcutaneous interferon β-1a
Introduction
First-line disease-modifying treatments for relapsing forms of multiple sclerosis (MS) are now available in many countries. However, currently available immunomodulatory drugs are only partially effective and patients may still encounter relapses or disease progression. Patients with MS who do not respond to first-line immunomodulatory treatments, such as interferon beta (IFN β) and glatiramer acetate, can undergo treatment escalation to second-line therapy, which includes the immunosuppressant mitoxantrone [Karussis et al. 2008; Cohen et al. 2004; Rieckmann et al. 2008]. Mitoxantrone was originally approved for the treatment of acute myeloid leukaemia and is used in the treatment of other malignancies [US Food and Drug Administration, 2010], but has subsequently also been used effectively in patients with early, active aggressive forms of MS [Edan et al. 2007; Hartung et al. 2002; Le Page et al. 2011). Based on the results of a French and UK controlled trial [Edan et al. 1997] and the Mitoxantrone in MS (MIMS) trial [Hartung et al. 2002], the drug was licensed in the US for worsening forms of MS. However, cardiotoxicity is a serious safety concern for all patients treated with mitoxantrone and in the USA, clinical recommendations for mitoxantrone use in MS stipulate that left ventricular ejection fraction measurements should be assessed before every mitoxantrone infusion in patients with a cumulative dose of 100 mg/m2 [US Food and Drug Administration, 2010]. Further, the cumulative lifetime dose of mitoxantrone has been limited to 140 mg/m2 body surface area [Cartwright et al. 2007], which translates into a maximum treatment duration of 2–3 years [Goodin et al. 2003]. Once this cumulative dose has been reached, other treatment options, including ‘de-escalation’ to an immunomodulatory drug such as IFN β, should be considered.
There are currently no established guidelines or standardized protocols for de-escalating therapy in patients who have reached the maximum cumulative dose of mitoxantrone or who wish to discontinue mitoxantrone to save doses for future treatment cycles. To date, the beneficial effects of IFN β and glatiramer acetate on disease progression in MS following de-escalation from mitoxantrone therapy have been demonstrated only in a limited number of studies [Ramtahal et al. 2006; Vollmer et al. 2008]. It is therefore important that more data are generated to support options for de-escalation to immunomodulatory treatments in patients with MS.
The aim of the REMAIN (REbif® compared with no treatment in the therapy of relapsing Multiple Sclerosis After mItoxaNtrone) study was to assess the effect of IFN β-1a, 44 µg administered subcutaneously (sc) three times weekly (tiw), compared with no treatment, on disease stability as assessed by clinical and magnetic resonance imaging (MRI) measures in patients with relapsing MS following mitoxantrone therapy.
Methods
This was a phase IV, open-label, rater-blinded, randomized, multicentre, parallel-group study [ClinicalTrials.gov identifier: NCT00283140], planned to be conducted in Germany and Switzerland. The study was carried out in accordance with the provisions of the German Medicines Act, Good Clinical Practice (GCP) guidelines, and ethical principles based on the Declaration of Helsinki 2002. Prior to study inclusion, patients agreed to participate in the study via a signed consent form. The informed consent process was in accordance with the International Conference on Harmonisation–GCP 1997, the Declaration of Helsinki and local regulatory requirements.
Study inclusion and exclusion criteria
Eligible patients were aged 18–60 years, had relapsing–remitting MS or secondary progressive MS with superimposed relapses and an Expanded Disability Status Scale (EDSS) score of 1–6, were free from relapse within 6 months prior to screening, and had no confirmed 1-point disability progression on the EDSS within 9 months prior to screening (0.5 point for EDSS score >5.5). Patients had to have received mitoxantrone for 9–36 months (total cumulative dose, 40–120 mg/m2) and had to have received their last mitoxantrone dose between 1 and 6 months prior to the screening visit. Female patients of childbearing age could not be pregnant or breastfeeding and were required to be either surgically sterile or to be using effective contraception. Study exclusion criteria included cytokine or anticytokine therapy within 3 months prior to randomization, escalation to mitoxantrone due to EDSS progression only (without any relapse or MRI activity during the last year prior to mitoxantrone), immunomodulatory therapy other than IFN β or glatiramer acetate prior to mitoxantrone, oral or systemic corticosteroids or adrenocorticotropic hormone within 30 days prior to day 1, intravenous immunoglobulins or plasmapheresis within 6 months prior to day 1, and immunomodulatory or immunosuppressive therapy other than mitoxantrone within 12 months prior to day 1.
Study design and treatment
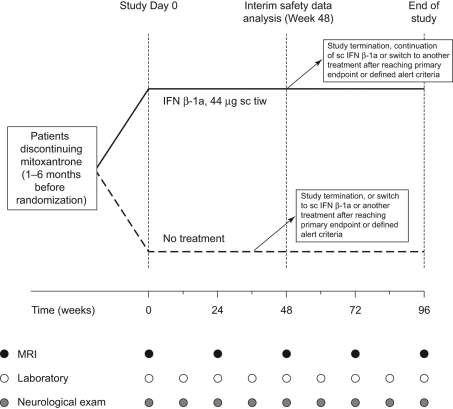
The recruitment period was from October 2005 to November 2009. Patients were assigned randomly 1:1 to IFN β-1a, 44 µg sc tiw, or no treatment (‘untreated’ group), for 96 weeks (Figure 1). The following dose titration for IFN β-1a was recommended: 11 μg sc tiw during the first 2 weeks, 22 μg sc tiw during weeks 3 and 4, and 44 μg sc tiw from week 5 onwards. All patients were advised to administer IFN β-1a at the same time each day on the same 3 days (e.g. Monday, Wednesday and Friday) at least 48 hours apart. All patients were also advised to cover potential injection sites during neurological examinations to ensure that the examining neurologist and evaluating neuroradiologist were blinded to treatment.
Figure 1.
Study design.
Deviations of ±3 days were permitted for Weeks 4 and 12, and deviations of ±7 days were permitted at Weeks 24, 36, 48, 60, 72, 84 and 96.
IFN, interferon; MRI, magnetic resonance imaging; sc, subcutaneous; tiw, three times weekly.
Efficacy and safety assessments
The recording of MS relapses was planned for each visit from baseline (Figure 1). EDSS score was assessed at each visit except week 4; additional assessments could be carried out at unscheduled visits if indicated by the disease course. MRI assessments were carried out at study day 1 and weeks 24, 48, 72 and 96 (Figure 1). Patients who discontinued the study early underwent all assessments at the final Week 96 visit. Adverse events (AEs) that were observed by the treating physician or reported by patients were documented. Other safety assessments were performed using physical examinations and laboratory measurements.
Study endpoints
The primary efficacy endpoint was the time from randomization to the first MS relapse. Secondary efficacy endpoints were the number of patients who were free from relapse at 96 weeks; absolute changes from baseline to week 96 in EDSS score; time to confirmed EDSS progression (defined as an increase of ≥1 point from baseline for EDSS scores ≤5.5 or 0.5 points for scores >5.5, confirmed at 3 months); and absolute changes from baseline to week 96 in the number and volume of T1, T1 gadolinium-enhanced (T1-Gd+), and T2 MRI lesions.
Study discontinuation
Patients had the option of discontinuing the study or switching to another therapy (including, for the untreated patients, switching to IFN β-1a, 44 µg sc tiw) upon agreement with the treating physician if any of the following conditions were satisfied: relapse, confirmed EDSS progression, more than six new T2 lesions and one T1-Gd+ lesion, more than three new T2 lesions and two T1-Gd+ lesions, or more than two new T1-Gd+ lesions. A qualifying relapse was defined as a new or worsening neurological symptom, in the absence of fever, lasting for ≥48 hours, and accompanied by an objective change in symptomatic Kurtzke Functional Systems. Study discontinuation or switching of therapy was also permitted at any time if it was in the patient’s best interest according to the investigator. Withdrawal from study medication was mandatory in the case of pregnancy, administration of excluded concomitant medication, or unremitting AEs as defined by the Common Terminology Criteria for Adverse Events version 3.0 [National Cancer Institute, 2003].
Statistical analysis
Sample size estimation was carried out for the null hypothesis that the time to relapse (primary efficacy endpoint) is equal for both treatment groups. It was calculated that 45 evaluable patients per treatment group would yield sufficient statistical power (90%; β = 0.10) to detect treatment differences, and so it was planned to randomize 100 patients in order to enrol 90 eligible patients. A two-sided log-rank test with α = 0.05 was planned as the appropriate nonparametric test. Based on results from the PRISMS (Prevention of Relapses and disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) study [Li and Paty, 1999; PRISMS Study Group, 1998; PRISMS Study Group and University of British Columbia MS/MRI Analysis Group, 2001], a median time to relapse of 4.5 months was expected for the no-treatment group, and of 9.6 months for the IFN β-1a group. A maximum study duration of 96 weeks per patient yielded a hazard ratio of 2.13.
The number of relapse-free patients was compared between groups by means of Fisher’s exact test. The log-rank test was used to test the time to 3-month confirmed EDSS progression. A last observation carried forward (LOCF) approach was adopted for statistical testing by calculating the changes between baseline and the last value prior to treatment switch or premature discontinuation, whichever happened first. LOCF variables were used for statistical testing, and the Wilcoxon–Mann–Whitney test was applied.
Statistical analyses were performed using the SAS® package (version 9.2; SAS Institute Inc., Cary, NC, USA) and nQuery Advisor software (version 5.0; Statistical Solutions, Saugus, MA, USA).
Results
Patients
Recruitment was lower than anticipated and was discontinued after 30 patients (15 per group) were randomized across 9 study centres. No patients were recruited from centres in Switzerland during this time. The safety population comprised all 30 patients. One patient from the safety population discontinued the study after 25 days owing to an AE and without providing any postbaseline efficacy data, and was thus excluded from the intent-to-treat (ITT) population. Hence, the ITT population comprised 29 patients (IFN β-1a, n = 14; untreated, n = 15). Baseline demographics and clinical characteristics were similar among the treated and untreated groups. A total of 70% (21/30) of patients were women, and the mean (standard deviation [SD]) age was 44.3 (6.7) years (Table 1). The mean (SD) time between the last mitoxantrone dose and the screening visit was 79.9 (48.4) days in the IFN β-1a group and 84.4 (62.2) days in the untreated group.
Table 1.
Baseline demographic and disease characteristics of the safety population.
| Characteristic | IFN β-1a, 44 mcg sc tiw (n = 15) | No treatment (n = 15) | Total (n = 30) |
|---|---|---|---|
| Women, n (%) | 12 (80.0) | 9 (60.0) | 21 (70.0) |
| Age, years | |||
| Mean (SD) | 44.3 (7.0) | 44.3 (6.5) | 44.3 (6.7) |
| Median (range) | 43 (34–56) | 45 (31–53) | 44 (31–56) |
| Form of MS, n (%) | |||
| Relapsing–remitting | 6 (40.0) | 7 (46.7) | 13 (43.3) |
| Secondary progressive | 9 (60.0) | 8 (53.3) | 17 (56.7) |
| Time since MS onset, yearsa | |||
| Mean (SD) | 11.1 (6.7) | 13.3 (7.7) | 12.3 (7.2) |
| Median (range) | 11.3 (2.1–23.6) | 13.1 (3.4–31.2) | 12.1 (2.1–31.2) |
| Relapses in the 12 months prior to informed consent, n (%) | |||
| 0 | 14 (93.3) | 12 (80.0) | 26 (86.7) |
| 1 | 1 (6.7) | 2 (13.3) | 3 (10.0) |
| 2 | 0 | 1 (6.7) | 1 (3.3) |
| EDSS score (median/range) | 4.3 (2–6) | 4.0 (3–6) | − |
| Duration of mitoxantrone treatment, monthsb | |||
| Mean (SD) | 19.6 (6.1) | 24.9 (8.2) | 22.3 (7.6) |
| Median (range) | 18.9 (11.9–34.9) | 22.8 (12.9–40.8) | 22.3 (11.9–40.8) |
| Total mitoxantrone dose, mg/m2 body surface areac | |||
| Mean (SD) | 71.5 (18.6) | 71.4 (15.8) | 71.4 (17.0) |
| Median (range) | 67.0 (38.5–101.0) | 66.0 (53.0–104.0) | 66.5 (38.5–104.0) |
| Reason for mitoxantrone discontinuation at last treatment cycle, n (%) | |||
| Reached planned/maximum dose | 12 (80.0) | 9 (60.0) | 21 (70.0) |
| Lack of efficacy | 0 | 0 | 0 |
| Adverse event | 1 (6.7)d | 0 | 1 (3.3) |
| De-escalation | 2 (13.3) | 5 (33.3) | 7 (23.3) |
| Data missing | 0 | 1 (6.7) | 1 (3.3) |
EDSS, Expanded Disability Status Scale; IFN, interferon; MS, multiple sclerosis; sc, subcutaneous; SD, standard deviation; tiw, three times weekly.
Data missing for one patient in IFN β-1a group.
Calculated as date of last infusion minus date of first infusion.
Calculated as sum of total doses between first and last infusion.
Leucopenia.
Overall, five patients withdrew from the study. Two patients in the IFN β-1a group discontinued due to AEs: one at week 4 (AE unknown) and one at week 86 (convulsion, status epilepticus). Another patient in the IFN β-1a group withdrew consent at week 39 (reason unspecified). In the untreated group, one patient discontinued because of an AE after 49 weeks (depression) and one patient withdrew consent at week 24 (‘wanted to be treated with sc IFN β-1a’).
Treatment exposure
Overall, 12/14 patients (85.7%) in the IFN β-1a group continued with 44 µg sc tiw dosing until their final visit; of these, one patient had their treatment dose reduced to 22 µg sc tiw following a relapse. One patient in the IFN β-1a group switched to mitoxantrone therapy at week 84 owing to an alert criterion that was based on incorrect transfer of MRI data.
A total of 7/15 patients (46.7%) in the untreated group received no active treatment until their final visit; of these, one patient had a qualifying relapse and one patient reached MRI alert criteria for study discontinuation/switch but neither patient started ‘rescue’ therapy with sc IFN β-1a. Five patients in the untreated group started IFN β-1a treatment after meeting criteria for study discontinuation/switch (one patient each at weeks 4, 36, 48, 60 and 84), and the remaining three patients in the untreated group started first with sc IFN β-1a treatment and then switched to another therapy (mitoxantrone, n = 2; natalizumab, n = 1).
Clinical outcomes
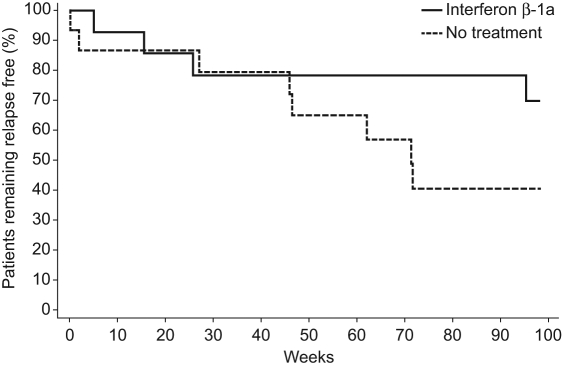
In total, four patients (28.6%) in the IFN β-1a group and eight (53.3%) in the untreated group had one or more relapses (p = 0.26, Fisher’s exact test) during the study. There was no significant difference between treatment groups in the time to first relapse (p = 0.14, two-sided log-rank test; Figure 2); time to first relapse (25th percentile) was 95.4 versus 46.0 weeks for IFN β-1a versus no treatment. In patients who had relapses, the mean (SD) time to first relapse was 35.5 (40.8) weeks for the IFN β-1a group and 40.9 (28.7) weeks for the untreated group (descriptive analyses). Median (range) time to first relapse was 20.7 (5.1–95.4) weeks in the IFN β-1a group and 46.3 (0.1–71.7) weeks in the untreated group.
Figure 2.
Kaplan–Meier curves showing time between randomization and first multiple sclerosis relapse.
Mean change in EDSS score was 0.3 in both groups (p = 0.79). Confirmed EDSS progression was observed in five patients in each of the IFN β-1a (35.7%) and untreated (33.3%) groups. There was no significant difference in the time to EDSS progression between treated and untreated groups (p = 0.90, two-sided log-rank test).
MRI activity
From baseline to week 96, there were no significant differences between groups in changes in the number or volume of T1 and T2 MRI lesions (Table 2). New T1-Gd+ lesions were observed in three patients in the IFN β-1a group and eight patients in the untreated group.
Table 2.
Change from baseline in MRI parameters (intent-to-treat population).
| Mean (SD) MRI parameters | IFN β-1a 44 µg sc tiw (n= 14) | No treatment (n = 15) | p-valuea |
|---|---|---|---|
| Number of T1 lesions | |||
| Day 1 | 16.4 (9.2) | 29.3 (17.4) | 0.9303 |
| Last visit | 16.9 (14.7) | 26.9 (15.5) | |
| Change | 0.4 (8.4) | −2.3 (10.3) | |
| T1 lesion volume (mm3) | |||
| Day 1 | 5138 (4755) | 6573 (5171) | 0.5557 |
| Last visit | 5563 (5436) | 6872 (5173) | |
| Change | 426 (2210) | 300 (1658) | |
| Number of T2 lesions | |||
| Day 1 | 34.6 (21.8) | 48.3 (28.2) | 0.2930 |
| Last visit | 30.8 (17.3) | 49.8 (32.9) | |
| Change | −3.9 (8.0) | 1.5 (13.9) | |
| T2 lesion volume (mm3) | |||
| Day 1 | 11 779 (8492) | 15 737 (10198) | 0.3261 |
| Last visit | 11 285 (8114) | 16 701 (11256) | |
| Change | −494 (1910) | 964 (5117) | |
IFN, interferon; MRI, magnetic resonance imaging; sc, subcutaneously; SD, standard deviation; tiw, three times weekly.
Testing for difference between treatment groups (Wilcoxon–Mann–Whitney test).
Safety assessments
The most common treatment-associated AEs were ‘flu-like’ symptoms, which were reported by 20.0% (3/15) of patients in the IFN β-1a group and by 25.0% (2/8) of untreated patients after they had subsequently switched to IFN β-1a treatment. Injection-site reactions were the next most common treatment-associated AEs, found in 13.3% (2/15) of patients in the IFN β-1a group and in 12.5% (1/8) of untreated patients after they had switched to IFN β-1a treatment.
No new or unexpected AEs relating to active treatment were found. Overall, in the IFN β-1a group there were 111 cases of AEs reported by 15 patients; AEs were mild or moderate in severity in 97.3% (108/111) of cases. Eight patients in the untreated group who switched to sc IFN β-1a treatment experienced 26 AEs, all of which were mild or moderate in severity. There were 36 cases of AEs reported by seven patients who remained untreated; AEs were mild or moderate in severity in all cases. One patient in the untreated group had a serious AE (depression, classified as being moderate in severity and unrelated to treatment). Five patients in the IFN β-1a group experienced serious AEs (convulsion, status epilepticus, cholecystitis chronic cholelithiasis, angle closure glaucoma, haemorrhoid operation, gastroenteritis salmonella). Of these serious AEs, only convulsion and status epilepticus (both in one patient) were listed as being possibly related to study treatment.
Discussion
In many patients with relapsing MS, disease activity and progression can be controlled with first-line immunomodulatory therapies. However, some patients still experience breakthrough disease. Traditionally, mitoxantrone was the main treatment option for escalation therapy in patients with MS who did not respond to immunomodulatory therapies [Rieckmann et al. 2004] before the introduction of natalizumab, but owing to its cardiotoxic adverse effects treatment duration is limited to a cumulative lifetime dose of 140 mg/m2 body surface area [Cartwright et al. 2007]. There is currently no standardized option for de-escalation therapy after mitoxantrone treatment. To the best of the authors’ knowledge, this is the first study to investigate the effects of de-escalation to sc IFN β-1a in patients with MS after successful stabilization of disease activity with mitoxantrone. The results for several efficacy endpoints (time to first relapse, time to 3-month confirmed EDSS progression and number and volume of T1 and T2 MRI lesions) appear to favour IFN β-1a, 44 µg sc tiw over no treatment; however, owing to a lower than expected recruitment rate due to the introduction of natalizumab as a treatment option for MS, the study was underpowered to detect a treatment effect and these differences did not reach statistical significance.
Results from small-scale studies [Ramtahal et al. 2006; Vollmer et al. 2008] have suggested that glatiramer acetate and IFN β-1b may be effective maintenance therapies following mitoxantrone treatment; however, there have been no large randomized, controlled trials with these agents.
No unexpected AEs were seen during this study: all recorded AEs were within the established tolerability profile for IFN β-1a therapy and most were mild or moderate.
The main limitation of this study was the failure to meet the recruitment target of 100 patients: recruitment was discontinued after 30 patients had been enrolled. Of note, the period of patient recruitment (October 2005 to November 2009) coincided with the return to market of natalizumab (June 2006), a second-line therapy that had previously been withdrawn from the market owing to two reports of progressive multifocal leukoencephalopathy (PML), a fatal demyelinating disease that affects the central nervous system of individuals who are immunosuppressed [Brown, 2009]. At the time, natalizumab was perceived to have a greater benefit-to-risk ratio than mitoxantrone, and may have led to fewer patients being prescribed mitoxantrone, thus decreasing the number of eligible patients for the current study and affecting patient enrolment. Further, there have been increasingly more reports of PML cases with natalizumab in recent years, and an apparent significantly increased risk following the use of immunosuppressants, including mitoxantrone [Berger, 2010]. As of early July 2011, 145 cases of PML among patients with MS treated with natalizumab have been reported worldwide and the risk of developing PML has been estimated at 1 in 330 beyond 2 years of treatment [Multiple Sclerosis Resource Centre, 2011]. Indeed, this high risk of PML with natalizumab therapy may lead to an increase in the proportion of patients who are prescribed mitoxantrone for advanced MS in the future and enable larger studies to be performed.
In addition, this was an open-label, observational study and we cannot exclude the possibility that the presence of unmeasured variables may have confounded results. These design limitations undoubtedly affect extrapolation of results to a wider setting. Since these patients are responders to treatment escalation with mitoxantrone, regardless of their disease progression on first-line therapy, they may be regarded as a selected population. Still, it is worth noting that the patients in the treated and untreated groups were well matched at baseline. In order to fully capture the effect of treatment and to better reflect real-life clinical practice, it would be interesting in future studies to assess disease stability via use of a composite measure combining clinical and MRI outcomes, as has been done in a post hoc analysis of natalizumab in relapsing MS [Havrdova et al. 2009].
Although mitoxantrone is an effective second-line therapy for highly active MS, it is associated with serious AEs that include symptomatic left ventricular ejection fraction reduction under 50%, amenorrhoea and leukaemia [Le Page et al. 2008]. Further, there is the important limitation of the maximum length of treatment owing to cardiotoxicity [Goodin et al. 2003], which requires mitoxantrone discontinuation after 2–3 years on treatment. IFN β-1a may provide one attractive option for de-escalation and maintenance therapy owing to its well-established long-term safety profile and well-characterized short- and long-term benefit on efficacy outcomes [Kappos et al. 2006]. In addition, from what is known about the mechanism of action of IFN β-1a, it can be speculated that there are unlikely to be mechanistic concerns when this drug is given sequentially following mitoxantrone treatment.
In summary, treatment with sc IFN β-1a after discontinuing mitoxantrone therapy was generally well tolerated in this sample of patients with relapsing–remitting or secondary progressive MS, with no new or unexpected AEs. The lack of statistically significant differences in efficacy outcomes between active and untreated groups likely reflected that patient recruitment was lower than anticipated; the study was underpowered to detect a statistically significant difference. These findings should therefore be considered preliminary and should be tested further in a more rigorously designed study with a larger patient population, which may help to clarify if de-escalation to sc IFN β-1a can improve disease stability in patients with advanced MS following mitoxantrone therapy.
Acknowledgments
The authors thank Professor Bernd Kieseier, Department of Neurology, Heinrich-Heine University, Düsseldorf for IFN β antibody measurement and analysis, and Dr Dieter Schremmer and Dr Ilona Mohr of GKM Gesellschaft für Therapieforschung GmbH, Munich, for statistical analysis and study coordination, respectively. The authors also thank Clare McNulty and Jaina Mistry of Caudex Medical Ltd, Oxford, UK (supported by Merck Serono S.A. – Geneva, Switzerland, an affiliate of Merck KGaA, Darmstadt, Germany), for assistance in preparing the initial draft of the manuscript, collating the comments of authors and other named contributors, and assembling tables and figures; and Joanne Tang of Caudex Medical for assistance with editing for English, formatting the manuscript to meet journal guidelines, and coordinating submission requirements.
The REMAIN study group comprised: D. Anders (Giessen); A. Bayas (Augsburg); M. Buttmann, P. Rieckmann (Würzburg/Vancouver/Bamberg); R. Gold, A. Chan (Bochum); N. Goebels (Zurich); F. Hoffmann (Halle); J. Haas (Berlin); H.-P. Hartung, B. Kieseier (Düsseldorf); F. Heidenreich (Hannover); R. Hohlfeld (München); N. König, W. Poellmann (Berg); S. Menck (Seesen); D. Pöhlau (Asbach); M. Sailer (Magdeburg); M. Schroeter (Köln); M. Saettler (Göttingen); U. Zettl (Rostock)
Footnotes
Fedor Heidenreich has received speaker honoraria and consultancy fees from Biogen Idec, Merck Serono, Novartis, Sanofi and Teva Neuroscience.
Michael Sailer has received research support and speaker honoraria from Bayer Healthcare, Biogen Idec, Merck Serono, Novartis and Teva Neuroscience.
Uwe Zettl has received research support from Bayer Healthcare, Biogen Idec, Merck Serono and Teva Neuroscience.
Norbert Zessack is a salaried employee of Merck Serono GmbH.
Hans-Peter Hartung has received personal compensation, with approval of the Rector of Heinrich-Heine-University, for activities with Bayer Healthcare, Biogen Idec, Merck Serono, Novartis, Teva and Sanofi, as a consultant/ member of steering committees.
Ralf Gold has received personal compensation for activities with Bayer Health Care, Biogen Idec, Merck Serono and Teva Neuroscience; personal compensation in an editorial capacity (Editor-in-Chief) from Therapeutic Advances in Neurological Disorders; patent payments from Biogen Idec; research support from Bayer Health Care, Biogen Idec, Merck Serono, Teva Neuroscience and Novartis.
This work was supported by Merck Serono GmbH, an affiliate of Merck KGaA, Darmstadt, Germany. The sponsor played no role in the design, execution or analysis of the trial, but reviewed the manuscript for scientific accuracy as well as provided funding for editorial support in the development of the manuscript.
References
- Berger J.R. (2010) Progressive multifocal leukoencephalopathy and newer biological agents. Drug Saf 33: 969–983 [DOI] [PubMed] [Google Scholar]
- Brown B.A. (2009) Natalizumab in the treatment of multiple sclerosis. Ther Clin Risk Manag 5: 585–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright M.S., Jeffery D.R., Lewis Z.T., Koty P.P., Stewart W.T., Molnar I. (2007) Mitoxantrone for multiple sclerosis causing acute lymphoblastic leukemia. Neurology 68: 1630–1631 [DOI] [PubMed] [Google Scholar]
- Cohen B.A., Khan O., Jeffery D.R., Bashir K., Rizvi S.A., Fox E.J., et al. (2004) Identifying and treating patients with suboptimal responses. Neurology 63(12 Suppl. 6): S33-S40 [DOI] [PubMed] [Google Scholar]
- Edan G., Comi G., Lebrun C., Brassat D., Lubetzki C., Stankoff B., et al. (2007) The French-Italian mitoxantrone-interferon-beta trial: a 3-year randomised study. Mult Scler 13(Suppl. 2): S22 (abstract 74). [Google Scholar]
- Edan G., Miller D., Clanet M., Confavreux C., Lyon-Caen O., Lubetzki C., et al. (1997) Therapeutic effect of mitoxantrone combined with methylprednisolone in multiple sclerosis: a randomised multicentre study of active disease using MRI and clinical criteria. J Neurol Neurosurg Psychiatry 62: 112–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodin D.S., Arnason B.G., Coyle P.K., Frohman E.M., Paty D.W. (2003) The use of mitoxantrone (Novantrone) for the treatment of multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 61: 1332–1338 [DOI] [PubMed] [Google Scholar]
- Hartung H.P., Gonsette R., Konig N., Kwiecinski H., Guseo A., Morrissey S.P., et al. (2002) Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet 360: 2018–2025 [DOI] [PubMed] [Google Scholar]
- Havrdova E., Galetta S., Hutchinson M., Stefoski D., Bates D., Polman C.H., et al. (2009) Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing–Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol 8: 254–260 [DOI] [PubMed] [Google Scholar]
- Kappos L., Traboulsee A., Constantinescu C., Eralinna J.P., Forrestal F., Jongen P., et al. (2006) Long-term subcutaneous interferon beta-1a therapy in patients with relapsing–remitting MS. Neurology 67: 944–953 [DOI] [PubMed] [Google Scholar]
- Karussis D., Biermann L.D., Bohlega S., Boiko A., Chofflon M., Fazekas F., et al. (2006) A recommended treatment algorithm in relapsing multiple sclerosis: report of an international consensus meeting. Eur J Neurol 13: 61–71 [DOI] [PubMed] [Google Scholar]
- Le Page E., Leray E., Edan G. (2011) Long-term safety profile of mitoxantrone in a French cohort of 802 multiple sclerosis patients: a 5-year prospective study. Mult Scler, in press [DOI] [PubMed] [Google Scholar]
- Le Page E., Leray E., Taurin G., Coustans M., Chaperon J., Morrissey S.P., et al. (2008) Mitoxantrone as induction treatment in aggressive relapsing remitting multiple sclerosis: treatment response factors in a 5 year follow-up observational study of 100 consecutive patients. J Neurol Neurosurg Psychiatry 79: 52–56 [DOI] [PubMed] [Google Scholar]
- Li D.K., Paty D.W., for the UBC MS/MRI Analysis Research Group and PRISMS Study Group (1999) Magnetic resonance imaging results of the PRISMS trial: a randomized, double-blind, placebo-controlled study of interferon-beta1a in relapsing–remitting multiple sclerosis. Ann Neurol 46: 197–206 [DOI] [PubMed] [Google Scholar]
- Multiple Sclerosis Resource Centre (2011) 10 more PML infections, 4 more deaths in Tysabri MS patients. http://www.msrc.co.uk/index.cfm/fuseaction/show/pageid/1905 (accessed 4 May 2011).
- National Cancer Institute (2003) Common Terminology Criteria for Adverse Events; version 3.0. http://safetyprofiler-ctep.nci.nih.gov/Utilities/WebHelp/adverse_event_reporting_resources/common_terminology_criteria_for_adverse_events_ctcae_v3.htm (accessed 28 January 2011).
- PRISMS Study Group (1998) Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet 352: 1498–1504 [PubMed] [Google Scholar]
- PRISMS Study Group and University of British Columbia MS/MRI Analysis Group (2001) PRISMS-4: long-term efficacy of interferon-beta-1a in relapsing MS. Neurology 56: 1628–1636 [DOI] [PubMed] [Google Scholar]
- Ramtahal J., Jacob A., Das K., Boggild M. (2006) Sequential maintenance treatment with glatiramer acetate after mitoxantrone is safe and can limit exposure to immunosuppression in very active, relapsing remitting multiple sclerosis. J Neurol 253: 1160–1164 [DOI] [PubMed] [Google Scholar]
- Rieckmann P., Toyka K.V., Bassetti C., Beer K., Beer S., Buettner U., et al. (2004) Escalating immunotherapy of multiple sclerosis– new aspects and practical application. J Neurol 251: 1329–1339 [DOI] [PubMed] [Google Scholar]
- Rieckmann P., Traboulsee A., Devonshire V., Oger J. (2008) Escalating immunotherapy of multiple sclerosis. Ther Adv Neurol Disord 1: 181–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Food and Drug Administration (2010) Mitoxantrone Hydrochloride (marketed as Novantrone and generics). http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm126445.htm
- Vollmer T., Panitch H., Bar-Or A., Dunn J., Freedman M.S., Gazda S.K., et al. (2008) Glatiramer acetate after induction therapy with mitoxantrone in relapsing multiple sclerosis. Mult Scler 14: 663–670 [DOI] [PubMed] [Google Scholar]