Abstract
Background
Vasoconstrictor therapy has been advocated as treatment for hepatorenal syndrome (HRS). Our aim was to explore whether across all tested vasoconstrictors, achievement of a substantial rise in arterial blood pressure is associated with recovery of kidney function in HRS.
Study Design
Pooled analysis of published studies identified by electronic database search.
Setting & Population
Data pooled across 501 subjects from 21 studies.
Selection Criteria for Studies
Human studies evaluating the efficacy of a vasoconstrictor administered for ≥ 72 hours in adults with HRS Type 1 or 2.
Intervention
Vasoconstrictor therapy.
Outcomes & Measurements
Cohorts’ mean arterial pressure (MAP), serum creatinine, urinary output and plasma renin activity (PRA) at baseline and at subsequent time points during treatment. Linear regression models were constructed to estimate the mean daily change in MAP, serum creatinine, urinary output and PRA for each study subgroup. Correlations were used to assess for association between variables.
Results
An increase in MAP is strongly associated with a decline in serum creatinine but not associated with an increase in urinary output. The associations were stronger when analyses were restricted to randomized clinical trials and were not limited to cohorts with either lower baseline MAP or lower baseline serum creatinine. The majority of the studies tested terlipressin as vasoconstrictor, whereas fewer studies tested ornipressin, midodrine, octreotide or norepinephrine. Excluding cohorts of subjects treated with terlipressin or ornipressin did not eliminate the association. Furthermore, a fall in PRA correlated with improvement in kidney function.
Limitations
Studies were not originally designed to test our question. We lacked access to individual patient data.
Conclusions
A rise in MAP during vasoconstrictor therapy in HRS is associated with improvement in kidney function, across the spectrum of drugs tested to date. These results support consideration for a goal-directed approach to the treatment of HRS.
Hepatorenal syndrome (HRS), an ominous complication of liver cirrhosis and fulminant liver failure, is characterized by acute dysfunction of the kidneys, typically in the setting of portal hypertension 1-3, and is associated with poor clinical outcomes and increased mortality. Various pharmacological agents have been evaluated in HRS as attempts to reverse the condition. Based on the presumed pathogenic mechanism of inappropriate pooling of blood in the splanchnic circulation due to arterial vasodilatation, several systemic vasoconstrictors have been tested as therapeutic options4,5. Among those agents, terlipressin, a vasopressin receptor agonist, has gained popularity in Europe, where it is used to treat HRS by virtue of its splanchnic vasoconstrictive effect. Terlipressin has a vasopressin receptor 1A to vasopressin receptor 2 affinity ratio of 2.2, making it slightly more selective for the vasopressin 1A receptor than is vasopressin6. Several small prospective studies have suggested a beneficial effect of terlipressin on reversal of HRS. However, the largest randomized controlled trial that has evaluated the efficacy of terlipressin in treatment of HRS failed to demonstrate a benefit on a stringent primary endpoint 7. Furthermore, since terlipressin is not approved by the US Food and Drug Administration, it is not available for clinical use in the United States. Thus, because of lack of a better alternative, clinicians in the US are forced to utilize other non-conventional treatments, such as the combination of midodrine and octreotide, a modality that lacks solid supportive evidence.
Although the purpose of vasoconstrictor therapy in HRS is to specifically optimize renal hemodynamics, this effect is typically achieved with a concomitant increase in systemic blood pressure. One study reported that individuals with HRS who experience a significant rise in mean arterial pressure (MAP) during treatment with terlipressin have a higher probability of recovering kidney function 8. However, the same predictive value of increased MAP was not found by another group 9. Nevertheless, the latter study reported that the gain in MAP at the end of treatment was significantly greater among treatment “responders” compared to “non-responders” 9. Moreover, our anecdotal clinical experience is that individuals with HRS who respond to vasoconstrictor therapy (with midodrine / octreotide, norepinephrine, or vasopressin) do so in the context of a clinically significant rise in systemic blood pressure. Therefore, we hypothesize that, independently of the pharmacological agent used, achievement of a substantial rise in arterial blood pressure during vasoconstrictor therapy correlates with recovery of kidney function in HRS. To test our hypothesis, we reviewed the published literature on treatment of HRS and performed a pooled statistical analysis to determine the relationship between the change in MAP during vasoconstrictor therapy and change in kidney function, as reflected by serum creatinine concentration and/or urinary output.
METHODS
Review Strategy and Study Selection
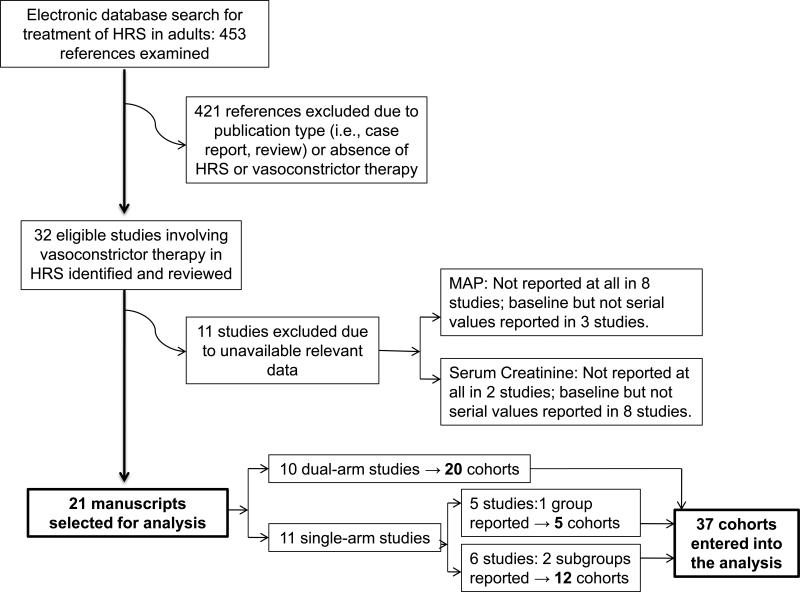
The electronic databases of PubMed, The Cochrane Library, Web of Science and LILACS were searched for publications between 1966 and January of 2011 that evaluated the efficacy of vasoconstrictor therapy for the reversal of HRS Type 1 or 2. We searched for manuscripts with the keywords: “hepatorenal syndrome”, and cross-referenced them with “treatment”, “vasoconstrictor therapy”, “reversal”, “liver cirrhosis”, “terlipressin”, “ornipressin”, “vasopressin”, “midodrine”, “octreotide”, “dopamine”, “noradrenaline”, “norepinephrine”, and “blood pressure”; limiting the search to human studies in adult subjects. Associated references were searched manually. Neither unpublished data nor abstracts were incorporated into the pool. The search generated a list of 453 publications. Criteria for eligibility for study selection were: (1) involvement of human subjects with diagnosis of HRS, either Type 1 or 2, according to the definition by the International Club of Ascites 10,11; (2) evaluation of a vasoconstrictor for the treatment of HRS, administered for at least 72 hours; (3) documentation of the effect of treatment on kidney function by serial measurement of serum creatinine; and (4) documentation of baseline and post-treatment values of MAP. Studies conducted on recurrence of HRS were excluded12. Three highly cited studies were excluded because of short duration of therapy13-15. When needed, authors of potentially eligible studies were contacted by electronic mail to collect missing data, but only 3 of 13 responded and provided the requested data. The flow chart of the study selection process is depicted in Figure 1. Eleven of 32 identified studies were excluded because of unavailable relevant data 16-26. Thus, we selected 21 publications deemed eligible for analysis, including 18 prospective and 3 retrospective studies 8,27,28. Prospective studies consisted of 6 randomized controlled 7,29-33, 1 randomized crossover 34, 2 non-randomized controlled35,36 and 9 uncontrolled trials37-45. Terlipressin was tested in 15 trials, norepinephrine in 3, octreotide in 3, midodrine in 2, ornipressin in 2, and dopamine in 1. Data collected from the original publications included: MAP, serum creatinine, urinary output, score of severity of liver disease [either Child-Pugh score or model for end-stage liver disease (MELD)] and plasma renin activity (PRA). It is important to note that our area of investigation, i.e., the association between change in MAP and change in serum creatinine, was not the primary focus of any of the trials included in our pooled analysis. Thus, we did not deem necessary to establish whether publication bias was present. Since individual study associations were not available, heterogeneity across studies was not assessed.
Figure 1.
Flow chart of study selection process
Data Abstraction and Statistical Analysis
Statistical analyses were conducted using SAS v9.2 (Cary, NC). Since primary data from the original studies were not uniformly available, analyses relied on published summary statistics that were identified for the study populations’ MAP, serum creatinine, urinary output and PRA measurements documented at baseline and at varying subsequent time points during treatment (range, 3 to 14 days). When possible, values specific to study subgroups (e.g. “responders” vs. “non-responders”, active treatment vs. placebo or comparator) were abstracted. Ultimately, summary data from 37 individual cohorts (pooled from 21 studies) were analyzed. Because published measurements of the variables of interest were available at multiple time points within each of the studies, and since the time points were not consistent across studies, we constructed regression models in order to estimate each of the subgroup's mean changes per day (and per 7 days). This process standardized the changes for comparisons across study subgroups. Associations between changes in MAP and changes in serum creatinine and urinary output, as well as changes in PRA and changes in serum creatinine, were then assessed using weighted Pearson's correlations. For the primary correlational analyses, each subgroup was weighted by its sample size, which is analogous to inverse variance weighting for meta-analyses. We conducted sensitivity analyses by first omitting the weighting scheme, and then by restricting our analyses to randomized controlled trials in order to account for study design quality. In addition, since 17 of the 21 studies involved the use of a vasopressin receptor agonist, we assessed for similar associations among cohorts where neither ornipressin nor terlipressin were tested. To determine the influence of baseline measurements of MAP and serum creatinine on the results, we performed analyses stratifying the data in tertiles of the corresponding baseline values. Furthermore, we estimated the mean increase in MAP necessary to achieve a variety of desired goals based on a linear regression model involving cohorts’ estimated changes in MAP and serum creatinine.
RESULTS
Study Characteristics
Table 1 lists general characteristics of the included studies. The series comprised publications between 1998 and 2010. Only 1 study was conducted, partially, in the US 7. Two were from India 32,33, 1 from Mexico 44, 2 from Canada 34,43, and the remainder from Europe. Ten studies were conducted under a dual-arm design, whereas 11 studies had a single treatment arm. Since 6 of the single-arm studies reported their results in 2 subgroups based on either “responder” status or treatment duration, a total of 37 cohorts (or subgroups) were available for analysis. The study population consisted of 501 subjects pooled from those cohorts. The weighted mean (± SD) age was 54.2 ± 11.1, and overall 70% of the subjects were men. The studies displayed a relatively uniform degree of severity of liver disease, with a median Child-Pugh score of 11.1 (range, 9.5 to 12.6) and a median MELD score of 31.2 (range, 26 to 33). The most common cause of liver cirrhosis was alcoholic (48% of subjects). Intravenous albumin was administered as a colloid solution in all but 4 cohorts (92.4% of the subjects).
Table 1.
General characteristics of the selected studies on vasoconstrictor pharmacotherapy in HRS
| Study (Year, Country) |
HRS Type |
Study design | Age; sexz |
Drug tested | Colloid used (dose) |
Cohort as reported and entered in the analysis |
No. per arm |
Baseline MAP (mm Hg)b |
Baseline SCr (mg/dL)b |
Duration of therapy (d) |
Child- Pugh; MELD |
Alcoholism as cause of liver failure (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Guevara41 (1998, ES) | 1 | Prospective, uncontrolled | 53 [27-72] y; 75% M | Ornipressin | Alb (1 g/kg; 20-60 g/d)a | 3-d vs 15-d course | 8 vs 8 | 72 ± 4 vs 69 ± 3 | 2.9 ± 0.5 vs 3.0 ± 0.5 | 3 vs 15 | NA; NA | 38 |
| Gulberg42 (1999, DE) | 1 | Prospective, uncontrolled | 50 ± 5 y; 67% M | Ornipressin | Alb (6-8 ascites) | Responders vs nonresponders | 5 vs 4 | 70 ± 2 vs 78 ± 4 | 4.6 ± 0.9 vs 2.1 ± 0.2 | 14c vs 14c | 12; NA | 56 |
| Angeli36 (1999, IT) | 1 | Prospective, nonrandomized, controlled | 61 ± 3 y; NA | Dopamine; midodrine + octreotide | Alb (40 g/d) | Dopamine- vs midodrine- + octreotide-treated | 8 vs 5 | 79 ± 4 vs 76 ± 3 | 3.6 ± 0.6 vs 5.0 ± 0.8 | 10 vs 10 | Cy; NA | 40 |
| Uriz38 (2000, ES) | 1,2 | Prospective, uncontrolled | 54 [42-75] y; 67% M | Terlipressin | Alb (1 g/kg; 20-40 g/d)a | Terlipressin-treated | 9 | 68 ± 2 | 3.9 ± 0.7 | 9c | NA; NA | 44 |
| Mulkay37 (2001, BE) | 1 | Prospective, uncontrolled | 54 [45-60] y; 83% M | Terlipressin | Alb (60 g/d) | Terlipressin-treated | 12 | 76 [68-83] | 3.4 [2.5-4.0] | 14 | 10; NA | 75 |
| Ortega (2002, ES) | 1,2 | Pospective, nonrandomized, controlled | 57 ± 2 y; 67% M | Terlipressin; terlipressin + albumin | Alb (1 g/kg; 20-40 g/d)a | Terlipressin-vs terlipressin- + albumin-treated | 8 vs 13 | 64 ± 4 vs 70 ± 2 | 3.4 ± 0.3 vs 3.6 ± 0.5 | 7c vs 7c | 10.6; NA | 43 |
| Duvoux (2002, FR) | 1 | Prospective, uncontrolled | 54 ± 5 y; 58% M | Norepinephrine | Alb (22 g/d) | Norepinephrine-treated | 12 | 65 ± 7 | 4.0 ± 1.8 | 10c | 11.3; NA | 67 |
| Halimi (2002, FR) | 1,2 | Retrospective, uncontrolled | 60 ± 2 y; 61% M | Terlipressin | NA | Responders vs nonresponders | 13 vs 5 | 84 ± 4 vs 76 ± 6 | 2.7 ± 1.0 vs 2.9 ± 0.8 | 5 vs 5 | 11.2; NA | 78 |
| Colle (2002, FR) | 1 | Retrospective, uncontrolled | 47 ± 2 y; 94% M | Terlipressin | Alb (20 g/d) in 13/18 cases | Responders vs nonresponders | 11 vs 7 | 74 ± 6 vs 79 ± 6 | 3.1 ± 0.5 vs 3.3 ± 1.0 | 9c vs 9c | 12.6; NA | 72 |
| Alessandria (2004. IT) | 2 | Prospective, uncontrolled | 59 ± 2; 82% M | Terlipressin | Alb (NA) | Terlipressin-treated | 11 | 91 ± 15 | 2.4 ± 0.9 | 7 | NA; NA | 64 |
| Solanki (2003, IN) | 1 | Prospective, randomized, controlled | 52 ± 5 y; 70% M | Terlipressin; placebo | Alb (40 g/d) + FFP | Terlipressin- vs placebo-treated | 12 vs 12 | 76 ± 1 vs 74 ± 1 | 2.9 ± 0.1 vs 2.2 ± 0.2 | 8 vs 8 | NA; NA | 33 |
| Pomier-Layrargues (2003, CA) | 1,2 | Prospective, randomized, crossover | 52 ± 3 y; 75% M | Octreotide; placebo | Alb (50 g/d) | Octreotide- vs placebo-treated | 7 vs 9 | 76 ± 3 vs 83 ± 5 | 2.3 ± 0.2 vs 2.4 ± 0.3 | 4 vs 4 | 12.2; NA | NA |
| Wong (2004, CA) | 1 | Prospective, uncontrolled | 55 ± 3 y; 79% M | Midodrine + octreotide | Alb (50 g/d) | Responders vs nonresponders | 10 vs 4 | 81 ± 5 vs 79 ± 4 | 2.6 ± 0.3 vs 3.9 ± 1.3 | 14c vs 14c | 9.9; NA | 100 |
| Saner (2004, DE) | 1,2 | Prospective, uncontrolled | NA | Terlipressin | GPS | Terlipressin-treated | 7 | 58 ± 4 | 3.9 ± 0.4 | 7 | NA; NA | 14 |
| Alessandria (2007, IT) | 1,2 | Prospective, randomized, controlled | 55 ± 2 y; 73% M | Norepinephrine; terlipressin | Alb (35-75 g/d) | Norepinephrine- vs terlipressin-treated | 10 vs 12 | 71 ± 2 vs 74 ± 3 | 2.3 ± 0.2 vs 2.5 ± 0.3 | 14 vs 14 | 10.5; 26 | 27 |
| Sharma (2008, IN) | 1 | Prospective, randomized, controlled | 48 ± 2 y; 85% M | Norepinephrine; terlipressin | Alb (20-40 g/d) | Norepinephrine- vs terlipressin-treated | 20 vs 20 | 81 ± 2 vs 78 ± 1 | 3.0 ± 0.5 vs 3.3 ± 1.3 | 15 vs 15 | 10.8; 30.6 | 65 |
| Sanyal (2008, US & RU) | 1 | Prospective, randomized, controlled | 52 ± 1 y; 71% M | Terlipressin; placebo | Alb (100 g; 25 g/d)a | Terlipressin- vs placebo-treated | 56 vs 56 | 76 ± 1 vs 77 ± 2 | 3.9 ± 2.1 vs 3.8 ± 1.1 | 6c vs 6c | NA; 33 | 52 |
| Martin-Llahi (2008, ES) | 1,2 | Prospective, randomized, controlled | 57 ± 2 y; 62% M | Terlipressin + Alb; Alb | Alb (1 g/kg; 40 g/d)a | Responders vs nonresponders | 10d vs 13d | 75 ± 4 vs 68 ± 3 | 2.9 ± 0.8 vs 4.0 ± 2.1 | 7c vs 7c | 10.5; 30 | 73 |
| Neri (2008, IT) | 1 | Prospective, randomized, controlled | 60 ± 4 y; 40% M | Terlipressin + Alb; Alb | Alb (1 g/kg; 20-40 g/d)a | Terlipressin + Alb- vs Alb-treated | 26 vs 26 | 68 ± 3 vs 72 ± 2 | 2.9 ± 1.2 vs 2.8 ± 1.1 | 14 vs 14 | 11.4; NA | 13 |
| Munoz (2009, MX) | 1 | Prospective, uncontrolled | 55 ± 6 y; 92% M | Terlipressin | Alb (30-80 g/d) | Responders vs nonresponders | 8 vs 5 | 70 ± 3 vs 69 ± 3 | 3.0 ± 1.7 vs 3.9 ± 1.5 | 12c vs 5c | 11.2; 30 | 31 |
| Nazar (2010, ES) | 1 | Retrospective, uncontrolled | 56 ± 1 y; 74% M | Terlipressin | Alb (1 g/kg; 40 g/d)a | Responders vs nonresponders | 18 vs 21 | 75 ± 3 vs 79 ± 2 | 3.5 ± 1.4 vs 3.9 ± 1.4 | 15 vs 15 | 11; 28 | 41 |
Conversion factor for serum creatinine in mg/dL to μmol/L, X 88.4.
Abbreviations: HRS = hepatorenal syndrome; MAP = mean arterial pressure; MELD = model for end-stage liver disease; Alb = albumin; GPS, gelatin polysuccinate; NA = not available; M, male; SCr, serum creatinine; FFP, fresh-frozen plasma; DE, Germany; ES, Spain; RU, Russia; US, United States; FR, France; BE, Belgium; CA, Canada; MX, Mexico; IT, Italy; IN, India.
Age is expressed as mean ± standard error or mean [range].
Class C corresponds to Child-Pugh scores of 10-15 points.
Values expressed as mean ± standard error or median [range].
Dose given as: (1st day; thereafter)
Values represent median or mean duration of therapy.
Values reported only for terlipressin + Alb arm, not provided for Alb-only arm.
Primary analyses
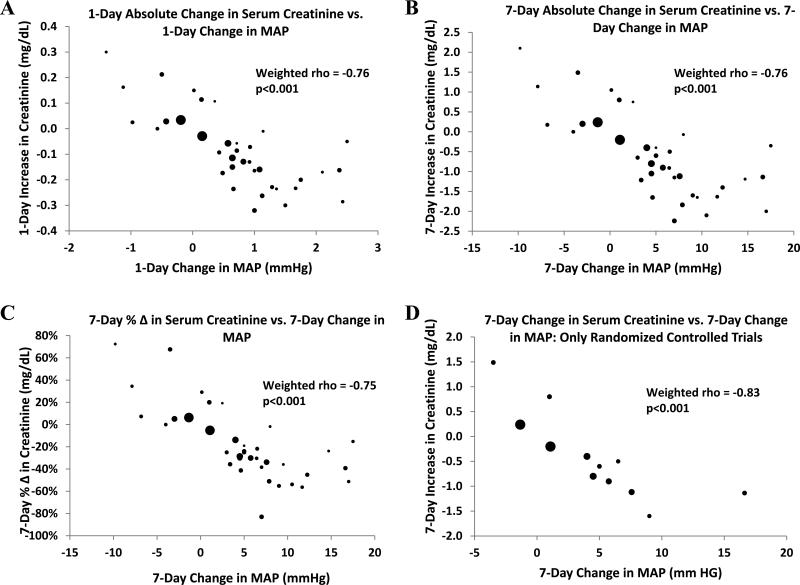
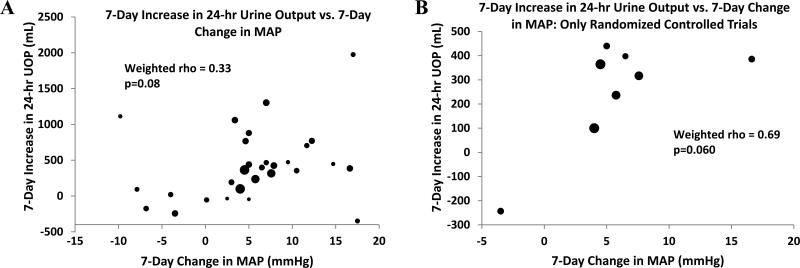
Our primary analysis revealed that an increase in MAP is strongly associated with a decline in serum creatinine (rho = -0.76, p < 0.001) (Fig 2). HRS type did not influence the association [rho = -0.80 (p < 0.001) and -0.70 (p = 0.008) for cohorts with Type 1 only and Type 1 and/or Type 2, respectively]. There was no significant association between change in MAP and change in urinary output (rho = 0.33, p = 0.08) (Fig 3). Our findings also suggest that on average, for every 1-mm Hg increase in MAP, a 0.12 mg/dl decline in serum creatinine is expected; and similarly, every 8.6-mm Hg increase in MAP is associated with a 1.0 mg/dl decline in serum creatinine. Table 2 illustrates the estimated increases in MAP that would be necessary to achieve a clinically significant gain in kidney function, using a serum creatinine of 1.5 mg/dl as a hypothetical treatment target based on the standard definition of HRS reversal4,46.
Figure 2.
Correlation between rise in MAP and change in kidney function. Diameter of each data point reflects relative sample size of cohort. In panels A-D: MAP = mean arterial pressure. In panel C axis legend and chart title: % Δ = percentage change.
Figure 3.
Correlation between rise in MAP and change in urinary output. Diameter of each data point reflects relative sample size of cohort. MAP = mean arterial pressure; UOP = urinary output.
Table 2.
Relationship between MAP and kidney function in HRS
| Baseline SCr | SCr reduction required to achieve goalb (%) | Predicted increase in MAP to achieve desired SCr goal (mm Hg)a |
|---|---|---|
| 2.0 mg/dl | 25.0 | 3.7 (2.5 to 4.9) |
| 2.5 mg/dl | 40.0 | 6.1 (4.7 to 7.5) |
| 3.0 mg/dl | 50.0 | 8.5 (6.7 to 10.3) |
| 3.5 mg/dl | 57.1 | 10.9 (8.5 to 13.3) |
| 4.0 mg/dl | 62.5 | 13.3 (10.3 to 16.3) |
SCr goal is 1.5 mg/dL
values given as mean (95% confidence interval)
Abbreviations: SCr, serum creatinine; HRS = hepatorenal syndrome, MAP = mean arterial pressure.
In 14 cohorts obtained from 7 studies, abstracted data were categorized as “responders” or “non-responders”. The weighted mean 7-day increase in MAP from baseline among “responders” was 7.0 ± 10.4 mmHg, whereas “non-responders” exhibited a significantly lower mean rise in MAP of -1.4 ± 15.5 (p = 0.04). In the remainder of the cohorts, data were abstracted from the values corresponding to a treatment arm. Twenty-five cohorts included patients who received a vasopressin receptor agonist, either terlipressin (in 21 cohorts from 15 studies) or ornipressin (in 4 cohorts from 2 studies). Among them, the weighted mean 7-day increase in MAP from baseline was 5.4 ± 5.9 mmHg. Similarly, among subjects from 3 cohorts who received norepinephrine, the corresponding mean rise in MAP was 6.5 ± 1.5. When we combined α1-adrenergic agonists, i.e., norepinephrine and midodrine (n = 6 cohorts), the mean 7-day increase in MAP was 6.5 ± 10.8 mmHg. Although the correlation between rise in MAP and decrease in serum creatinine among cohorts treated with α1-adrenergic agonists (rho = -0.32) was somewhat lower compared with cohorts treated with vasopressin receptor agonists (rho = -0.81), the limited number of α1-adrenergic agonists cohorts precludes a formal comparison. On the other hand, among cohorts that included placebo-treated subjects, MAP fell by 0.7 ± 4.5 mmHg.
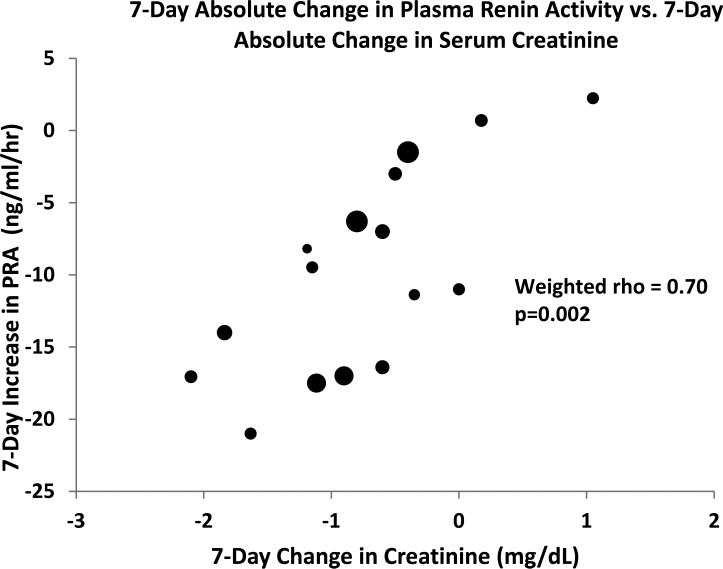
In view of the role of activation of the renin-angiotensin system in HRS, we determined the relationship between changes in PRA during vasoconstrictor therapy and kidney recovery among 15 cohorts from 8 studies that reported PRA values. Two studies that only reported plasma renin concentration were not included. We found that reduction in PRA correlates strongly with decrease in serum creatinine (rho = 0.70, p = 0.001) (Fig 4).
Figure 4.
Correlation between change in PRA and change in kidney function. Diameter of each data point reflects relative sample size of cohort. PRA = plasma renin activity
Sensitivity analyses
Weighting data did not influence our findings, since unweighted analyses yielded correlation coefficients similar to those derived from weighted analyses (i.e., change in MAP vs. change in serum creatinine: rho = -0.75, p < 0.001; change in MAP vs. change in urinary output: rho = 0.28, p = 0.1; and change in serum creatinine vs. change in PRA: rho = 0.73, p = 0.001). Analyses restricted to randomized clinical trials revealed that the correlations were stronger in randomized controlled trials than those observed in the full analysis (MAP vs. serum creatinine: rho = -0.83, p < 0.001; MAP vs. urinary output: rho = 0.69, p = 0.06; PRA vs. serum creatinine: rho = 0.91, p = 0.01) (Figs 2D and 3B). Therefore, the observed associations did not appear to be driven by clinical trials with study design of inferior quality. Furthermore, when the analyses were restricted to the 12 cohorts that did not involve the use of either terlipressin or ornipressin, the correlation coefficient between change in MAP and serum creatinine changed from -0.76 to -0.65, and the p-value increased from < 0.001 to 0.02, but remained statistically significant. Similarly, the magnitude of the weighted correlations between change in MAP and urinary output (rho = 0.31, p = 0.3) and between serum creatinine and PRA (rho = 0.78, p = 0.04) remained essentially unchanged.
Secondary analyses
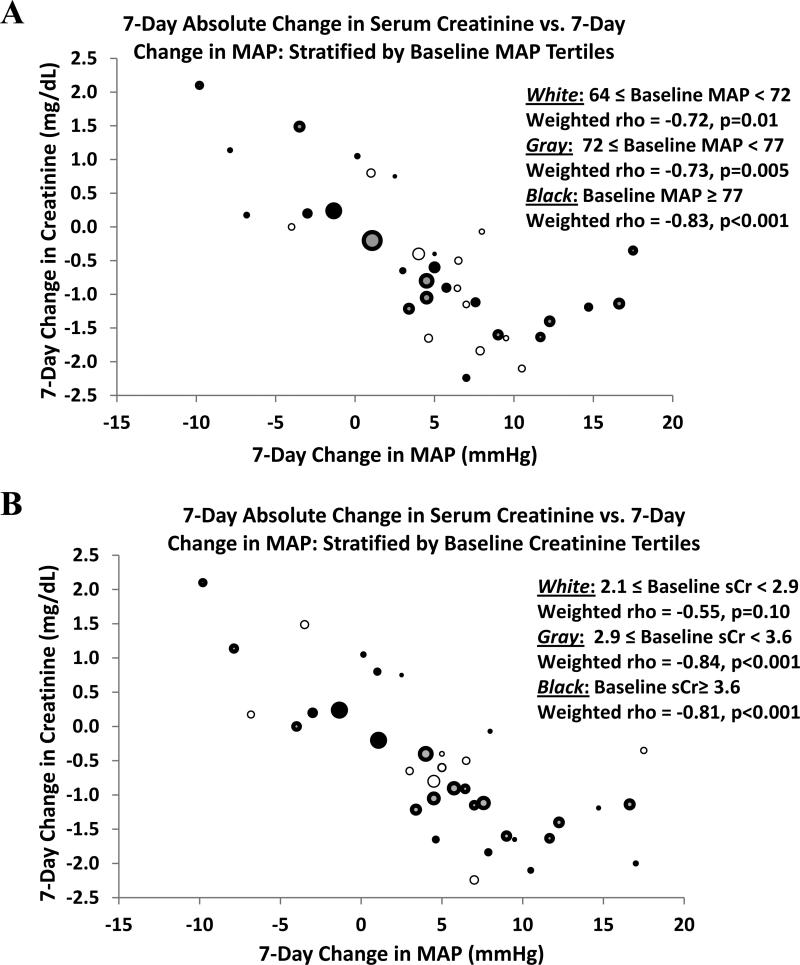
The magnitude of the weighted correlation between change in MAP and change in serum creatinine was not limited to cohorts with either a lower baseline MAP (rho = -0.72 for baseline MAP between 64 and 72 mmHg, p = 0.01; rho = -0.73 for values between 72 and 77 mmHg, p = 0.005; and rho = -0.83 for values above 77 mmHg, p < 0.001) or a specific threshold of lower baseline serum creatinine (rho = -0.55 for baseline serum creatinine between 2.1 and 2.8 mg/dl, p = 0.1; rho = -0.84 for values between 2.9 and 3.6 mg/dl, p < 0.001; and rho = -0.81 for values above 3.6 mg/dl, p <0.001) (Fig 5).
Figure 5.
Effect of baseline variables on estimated correlations: A) stratified by tertiles of baseline MAP; B) stratified by tertiles of baseline serum creatinine. Diameter of each data point reflects relative sample size of cohort.
DISCUSSION
Our analysis demonstrates a strong correlation between the increase in MAP during vasoconstrictor therapy in HRS and the therapeutic response. Improvement in kidney function tightly correlates with the magnitude of the rise in MAP. Although these findings seem intuitive, no previous study was designed to specifically explore this relationship. Of note, several studies individually reported a similar association between improvement in systemic hemodynamics and HRS reversal8,27,29-31,33,35,36,38-41,45. In contrast, a few other studies did not find such association7,28,32,34,37,42-44. The strength of our study is that it demonstrates the presence of this association in a large number of patients, quantifies the relationship between the parameters analyzed, and allows performing the analyses at different levels of arterial blood pressure. Previous pooled analyses have focused on the effectiveness of individual drugs rather than blood pressure per se 47,48. While some studies of HRS treatment assessed the predictive value of baseline MAP on kidney outcome, they did not measure the association between changes in MAP during treatment and the concomitant change in kidney function 28,30,32, except for 1 study8. Notably, in agreement with our results, that study reported that a rise in MAP of at least 5 mmHg at Day 3 of therapy with terlipressin provided an odds ratio of 9.49 (95% CI, 1.01 – 89.32) for reversal of HRS 8. In addition, a decrease in MAP from a baseline value of 83 ± 9 mmHg during the non-azotemic state to 75 ± 7 mmHg at the time of diagnosis of HRS has been described49, suggesting that development of HRS is associated with a fall in blood pressure, even though MAP remains within normal limits. Thus, it seems reasonable that resolution of HRS should be accompanied by recovery of MAP to pre-HRS levels.
The observed correlation, however, does not prove causality. It is unclear whether a rise in MAP during HRS treatment causes a decrease in serum creatinine or vice versa. Thus, the statistical association and its clinical applicability must be interpreted with caution. Notwithstanding, it is difficult to conceive of a scenario where improvement in kidney function leads to a rise in MAP. Moreover, recovery from other causes of acute kidney injury does not typically lead to a rise in systemic blood pressure. On the other hand, restoration of systemic blood pressure from hemodynamic shock limits the ischemic insult and promotes organ perfusion 50, possibly enhancing kidney recovery. Therefore, it seems more plausible that a rise in MAP induced by vasoconstrictor therapy facilitates improvement in kidney function. Inappropriate pooling of blood by splanchnic vasodilatation reduces systemic blood pressure and diminishes renal blood flow in HRS51. Consequently, the observed rise in MAP may optimize kidney perfusion by resetting the renal perfusion pressure back to the autoregulatory range. Importantly, in almost all included studies, intravenous albumin was the colloid of choice for optimization of volemia, before and during vasoconstrictor therapy, based on its potential to prevent HRS52.
Our findings were not restricted to cohorts with low MAP at baseline. Even an increase in MAP from normal to “supranormal” levels is associated with improvement in kidney function. The mammalian kidney starts to lose its blood flow autoregulation below a threshold MAP of about 75 to 80 mmHg 53. Therefore, it could be hypothesized that kidney perfusion is optimized when MAP is risen to at least 80 mmHg. Nonetheless, the traditional goal of MAP during vasopressor therapy in hemodynamic shock is 65 mmHg or higher 54. Achieving a MAP of 85 mmHg was not associated with improvement in kidney function in septic shock 55,56. However, those studies only evaluated short-term infusion of vasopressors, and may have been underpowered. Moreover, HRS is a “functional” state57, therefore, conceivably more responsive to hemodynamic manipulation than states of established tubular injury. In addition, it has been postulated that activation of the renal sympathetic system in HRS shifts the renal autoregulatory curve to the right 51. As a result, a MAP of about 90 mmHg might be required for maximal renal blood flow optimization in HRS. Alternatively, a “supranormal” MAP may merely represent a surrogate indicator of effective splanchnic vasoconstriction.
Consistent with the pronounced depletion of effective circulatory volume characteristic of HRS 4, PRA was found to be elevated at baseline in all studies where it was measured, denoting activation of the renin-angiotensin system. More importantly, we observed a remarkable correlation between a decrease in PRA during vasoconstrictor therapy and improvement in kidney function, supporting the notion that correction of systemic hemodynamics is critical for renal recovery in HRS. However, since the fall in PRA and rise in MAP occurred simultaneously, we cannot ascertain whether the improvement in kidney function follows an improvement in kidney perfusion pressure or relates to diminished angiotensin II-mediated reduction in ultrafiltration coefficient. Despite the strong correlation between rise in MAP and improvement in kidney function, the change in urinary output did not correlate with the hemodynamic upsurge. Since liver cirrhosis is a state of avid sodium retention58, discordant effects of improved hemodynamics on glomerular filtration rate and natriuresis are plausible. Moreover, vasoconstrictor therapy potentially leads to tubular effects that may limit diuresis, i.e., antidiuresis by vasopressin receptor agonists6 and sodium reabsorption by α1-adrenergic agonists59.
Our study has limitations. First, it is a pooled analysis, not a prospective randomized controlled trial designed to test our hypothesis a priori. The original studies included in the analysis were not designed to test our hypothesis. Besides, we did not have access to individual patient data. It has been pointed out that excessive use of meta-analyses in clinical nephrology ought to be discouraged 60. Criticism is linked to the inherent limitations of the methodology of meta-analyses, including publication bias, inconsistent study quality, heterogeneity among studies and arbitrary outcome selection. Such limitations may be applicable to this study. However, it is also acknowledged that meta-analyses are justified in areas where there is lack of irrefutable evidence favoring a specific treatment, which happens to be the case of vasoconstrictor therapy in HRS. Although vasoconstrictors have consistently shown to improve kidney function in HRS, efficacy rates average around 50%. Besides, meta-analyses offer the benefit of increasing the power of individual studies when their sample sizes are small. Indeed, studies on HRS treatment have generally been small, so data pooling in this setting seems reasonable.
The bulk of our results are driven by studies that tested terlipressin as vasoconstrictor, although the observed associations were not limited to those studies. Other drugs such as midodrine or norepinephrine have only been tested in a few studies. From the mechanistic standpoint, because norepinephrine induces afferent arteriole vasoconstriction through α1 adrenergic receptor stimulation, its role in HRS treatment may appear as counterintuitive. Practitioners have been resistant to adopt its use because of fear of aggravating renal hypoperfusion. Indeed, norepinephrine was shown not to improve renal hemodynamics in HRS after 2-hour administration 61. However, norepinephrine was found to offer similar clinical benefit compared to terlipressin in 2 head-to-head studies 29,32. Moreover, norepinephrine improved renal blood flow in several studies in the setting of septic shock 53,62,63. This beneficial effect of norepinephrine on kidney perfusion could arise from increasing MAP through its α1-mediated effect on systemic vascular resistance and its β1-mediated inotropic effect. Thus, we speculate that the effect of norepinephrine on systemic and splanchnic circulation overcomes its local effect on renal vasculature. Besides, prolonged infusion, i.e., at least 72 hours, may be necessary to induce a significant enhancement of renal hemodynamics in HRS.
In conclusion, our analysis indicates that an increase in MAP during vasoconstrictor therapy in patients with HRS is associated with a decrease in serum creatinine and a trend to increase in urinary output, irrespective of baseline MAP. These results are hypothesis-generating and support consideration for a goal-directed approach to the treatment of HRS. Perhaps, independent of which vasoconstrictor is chosen, targeting a systematic rise in MAP of ~10-15 mmHg during vasoconstrictor therapy may lead to more favorable kidney outcomes. However, a prospective study testing the safety and effectiveness of such approach would be necessary before it could be widely advocated.
Acknowledgements
A preliminary version of this study appeared in abstract form at the annual meeting of the American Society of Nephrology; November of 2010; Denver, CO.
We thank Dr. Michael E. Ullian for his critical review of this manuscript.
Support: This project was supported by the South Carolina Clinical & Translational Research Institute, Medical University of South Carolina's CTSA, NIH/NCRR grant UL1RR029882. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCRR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no relevant financial interests.
References
- 1.Epstein M, et al. Renal failure in the patient with cirrhosis. The role of active vasoconstriction. Am J Med. 1970;49:175–185. doi: 10.1016/s0002-9343(70)80073-0. [DOI] [PubMed] [Google Scholar]
- 2.Gines P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361:1279–1290. doi: 10.1056/NEJMra0809139. [DOI] [PubMed] [Google Scholar]
- 3.Ring-Larsen H. Renal blood flow in cirrhosis: relation to systemic and portal haemodynamics and liver function. Scand J Clin Lab Invest. 1977;37:635–642. doi: 10.3109/00365517709100657. [DOI] [PubMed] [Google Scholar]
- 4.Arroyo V, Terra C, Gines P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J Hepatol. 2007;46:935–946. doi: 10.1016/j.jhep.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Moreau R, Lebrec D. Acute kidney injury: new concepts. Hepatorenal syndrome: the role of vasopressors. Nephron Physiol. 2008;109:p73–79. doi: 10.1159/000142939. [DOI] [PubMed] [Google Scholar]
- 6.Pesaturo AB, Jennings HR, Voils SA. Terlipressin: vasopressin analog and novel drug for septic shock. Ann Pharmacother. 2006;40:2170–2177. doi: 10.1345/aph.1H373. [DOI] [PubMed] [Google Scholar]
- 7.Sanyal AJ, et al. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology. 2008;134:1360–1368. doi: 10.1053/j.gastro.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nazar A, et al. Predictors of response to therapy with terlipressin and albumin in patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology. 51:219–226. doi: 10.1002/hep.23283. [DOI] [PubMed] [Google Scholar]
- 9.Boyer TD, et al. Predictors of response to terlipressin plus albumin in hepatorenal syndrome (HRS) type 1: relationship of serum creatinine to hemodynamics. J Hepatol. doi: 10.1016/j.jhep.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arroyo V, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23:164–176. doi: 10.1002/hep.510230122. [DOI] [PubMed] [Google Scholar]
- 11.Salerno F, Gerbes A, Gines P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310–1318. doi: 10.1136/gut.2006.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alessandria C, et al. Midodrine in the prevention of hepatorenal syndrome type 2 recurrence: a case-control study. Dig Liver Dis. 2009;41:298–302. doi: 10.1016/j.dld.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Hadengue A, et al. Beneficial effects of the 2-day administration of terlipressin in patients with cirrhosis and hepatorenal syndrome. J Hepatol. 1998;29:565–570. doi: 10.1016/s0168-8278(98)80151-7. [DOI] [PubMed] [Google Scholar]
- 14.Lenz K, et al. Beneficial effect of 8-ornithin vasopressin on renal dysfunction in decompensated cirrhosis. Gut. 1989;30:90–96. doi: 10.1136/gut.30.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lenz K, et al. Ornipressin in the treatment of functional renal failure in decompensated liver cirrhosis. Effects on renal hemodynamics and atrial natriuretic factor. Gastroenterology. 1991;101:1060–1067. doi: 10.1016/0016-5085(91)90734-3. [DOI] [PubMed] [Google Scholar]
- 16.Danalioglu A, et al. Terlipressin and albumin combination treatment in hepatorenal syndrome. Hepatogastroenterology. 2003;50(Suppl 2):ccciii–cccv. [PubMed] [Google Scholar]
- 17.Eisenman A, Armali Z, Enat R, Bankir L, Baruch Y. Low-dose vasopressin restores diuresis both in patients with hepatorenal syndrome and in anuric patients with end-stage heart failure. J Intern Med. 1999;246:183–190. doi: 10.1046/j.1365-2796.1999.00556.x. [DOI] [PubMed] [Google Scholar]
- 18.Esrailian E, Pantangco ER, Kyulo NL, Hu KQ, Runyon BA. Octreotide/Midodrine therapy significantly improves renal function and 30-day survival in patients with type 1 hepatorenal syndrome. Dig Dis Sci. 2007;52:742–748. doi: 10.1007/s10620-006-9312-0. [DOI] [PubMed] [Google Scholar]
- 19.Kaffy F, et al. Octreotide in the treatment of the hepatorenal syndrome in cirrhotic patients. J Hepatol. 1999;30:174. doi: 10.1016/s0168-8278(99)80025-7. [DOI] [PubMed] [Google Scholar]
- 20.Kalil JR, et al. Poor outcomes with treatment of hepatorenal syndrome type 1 with splanchnic vasoconstrictors and albumin: report of seven cases and review of the literature. Arq Gastroenterol. 2009;46:214–218. doi: 10.1590/s0004-28032009000300014. [DOI] [PubMed] [Google Scholar]
- 21.Kiser TH, et al. Vasopressin, not octreotide, may be beneficial in the treatment of hepatorenal syndrome: a retrospective study. Nephrol Dial Transplant. 2005;20:1813–1820. doi: 10.1093/ndt/gfh930. [DOI] [PubMed] [Google Scholar]
- 22.Moreau R, et al. Terlipressin in patients with cirrhosis and type 1 hepatorenal syndrome: a retrospective multicenter study. Gastroenterology. 2002;122:923–930. doi: 10.1053/gast.2002.32364. [DOI] [PubMed] [Google Scholar]
- 23.Skagen C, Einstein M, Lucey MR, Said A. Combination treatment with octreotide, midodrine, and albumin improves survival in patients with type 1 and type 2 hepatorenal syndrome. J Clin Gastroenterol. 2009;43:680–685. doi: 10.1097/MCG.0b013e318188947c. [DOI] [PubMed] [Google Scholar]
- 24.Triantos CK, et al. Terlipressin therapy for renal failure in cirrhosis. Eur J Gastroenterol Hepatol. 22:481–486. doi: 10.1097/MEG.0b013e3283345524. [DOI] [PubMed] [Google Scholar]
- 25.von Kalckreuth V, Glowa F, Geibler M, Lohse AW, Denzer UW. Terlipressin in 30 patients with hepatorenal syndrome: results of a retrospective study. Z Gastroenterol. 2009;47:21–26. doi: 10.1055/s-0028-1109084. [DOI] [PubMed] [Google Scholar]
- 26.Duhamel C, Mauillon J, Berkelmans I, Bourienne A, Tranvouez JL. Hepatorenal syndrome in cirrhotic patients: terlipressine is a safe and efficient treatment; propranolol and digitalic treatments: precipitating and preventing factors? Am J Gastroenterol. 2000;95:2984–2985. doi: 10.1111/j.1572-0241.2000.03214.x. [DOI] [PubMed] [Google Scholar]
- 27.Colle I, et al. Clinical course, predictive factors and prognosis in patients with cirrhosis and type 1 hepatorenal syndrome treated with Terlipressin: a retrospective analysis. J Gastroenterol Hepatol. 2002;17:882–888. doi: 10.1046/j.1440-1746.2002.02816.x. [DOI] [PubMed] [Google Scholar]
- 28.Halimi C, et al. Effect of terlipressin (Glypressin) on hepatorenal syndrome in cirrhotic patients: results of a multicentre pilot study. Eur J Gastroenterol Hepatol. 2002;14:153–158. doi: 10.1097/00042737-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Alessandria C, et al. Noradrenalin vs terlipressin in patients with hepatorenal syndrome: a prospective, randomized, unblinded, pilot study. J Hepatol. 2007;47:499–505. doi: 10.1016/j.jhep.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Martin-Llahi M, et al. Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology. 2008;134:1352–1359. doi: 10.1053/j.gastro.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 31.Neri S, et al. Terlipressin and albumin in patients with cirrhosis and type I hepatorenal syndrome. Dig Dis Sci. 2008;53:830–835. doi: 10.1007/s10620-007-9919-9. [DOI] [PubMed] [Google Scholar]
- 32.Sharma P, Kumar A, Shrama BC, Sarin SK. An open label, pilot, randomized controlled trial of noradrenaline versus terlipressin in the treatment of type 1 hepatorenal syndrome and predictors of response. Am J Gastroenterol. 2008;103:1689–1697. doi: 10.1111/j.1572-0241.2008.01828.x. [DOI] [PubMed] [Google Scholar]
- 33.Solanki P, et al. Beneficial effects of terlipressin in hepatorenal syndrome: a prospective, randomized placebo-controlled clinical trial. J Gastroenterol Hepatol. 2003;18:152–156. doi: 10.1046/j.1440-1746.2003.02934.x. [DOI] [PubMed] [Google Scholar]
- 34.Pomier-Layrargues G, Paquin SC, Hassoun Z, Lafortune M, Tran A. Octreotide in hepatorenal syndrome: a randomized, double-blind, placebo-controlled, crossover study. Hepatology. 2003;38:238–243. doi: 10.1053/jhep.2003.50276. [DOI] [PubMed] [Google Scholar]
- 35.Ortega R, et al. Terlipressin therapy with and without albumin for patients with hepatorenal syndrome: results of a prospective, nonrandomized study. Hepatology. 2002;36:941–948. doi: 10.1053/jhep.2002.35819. [DOI] [PubMed] [Google Scholar]
- 36.Angeli P, et al. Reversal of type 1 hepatorenal syndrome with the administration of midodrine and octreotide. Hepatology. 1999;29:1690–1697. doi: 10.1002/hep.510290629. [DOI] [PubMed] [Google Scholar]
- 37.Mulkay JP, et al. Long-term terlipressin administration improves renal function in cirrhotic patients with type 1 hepatorenal syndrome: a pilot study. Acta Gastroenterol Belg. 2001;64:15–19. [PubMed] [Google Scholar]
- 38.Uriz J, et al. Terlipressin plus albumin infusion: an effective and safe therapy of hepatorenal syndrome. J Hepatol. 2000;33:43–48. doi: 10.1016/s0168-8278(00)80158-0. [DOI] [PubMed] [Google Scholar]
- 39.Saner F, et al. Terlipressin and gelafundin: safe therapy of hepatorenal syndrome. Eur J Med Res. 2004;9:78–82. [PubMed] [Google Scholar]
- 40.Duvoux C, et al. Effects of noradrenalin and albumin in patients with type I hepatorenal syndrome: a pilot study. Hepatology. 2002;36:374–380. doi: 10.1053/jhep.2002.34343. [DOI] [PubMed] [Google Scholar]
- 41.Guevara M, et al. Reversibility of hepatorenal syndrome by prolonged administration of ornipressin and plasma volume expansion. Hepatology. 1998;27:35–41. doi: 10.1002/hep.510270107. [DOI] [PubMed] [Google Scholar]
- 42.Gulberg V, Luppa P, Pauletzki J, Paumgartner G, Gerbes AL. [Successful conservative therapy of hepatorenal syndrome with vasopressin-1-receptor antagonist ornipressin]. Z Gastroenterol. 1998;36:1053–1058. [PubMed] [Google Scholar]
- 43.Wong F, Pantea L, Sniderman K. Midodrine, octreotide, albumin, and TIPS in selected patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology. 2004;40:55–64. doi: 10.1002/hep.20262. [DOI] [PubMed] [Google Scholar]
- 44.Munoz LE, et al. Reversal of hepatorenal syndrome in cirrhotic patients with terlipressin plus albumin. First experience in Mexico. Ann Hepatol. 2009;8:207–211. [PubMed] [Google Scholar]
- 45.Alessandria C, et al. Renal failure in cirrhotic patients: role of terlipressin in clinical approach to hepatorenal syndrome type 2. Eur J Gastroenterol Hepatol. 2002;14:1363–1368. doi: 10.1097/00042737-200212000-00013. [DOI] [PubMed] [Google Scholar]
- 46.Mackelaite L, Alsauskas ZC, Ranganna K. Renal failure in patients with cirrhosis. Med Clin North Am. 2009;93:855–869, viii. doi: 10.1016/j.mcna.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Gluud LL, Christensen K, Christensen E, Krag A. Systematic review of randomized trials on vasoconstrictor drugs for hepatorenal syndrome. Hepatology. 51:576–584. doi: 10.1002/hep.23286. [DOI] [PubMed] [Google Scholar]
- 48.Sagi SV, Mittal S, Kasturi KS, Sood GK. Terlipressin therapy for reversal of type 1 hepatorenal syndrome: a meta-analysis of randomized controlled trials. J Gastroenterol Hepatol. 25:880–885. doi: 10.1111/j.1440-1746.2009.06132.x. [DOI] [PubMed] [Google Scholar]
- 49.Ruiz-del-Arbol L, et al. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology. 2005;42:439–447. doi: 10.1002/hep.20766. [DOI] [PubMed] [Google Scholar]
- 50.Ince C, Sinaasappel M. Microcirculatory oxygenation and shunting in sepsis and shock. Crit Care Med. 1999;27:1369–1377. doi: 10.1097/00003246-199907000-00031. [DOI] [PubMed] [Google Scholar]
- 51.Stadlbauer V, et al. Relationship between activation of the sympathetic nervous system and renal blood flow autoregulation in cirrhosis. Gastroenterology. 2008;134:111–119. doi: 10.1053/j.gastro.2007.10.055. [DOI] [PubMed] [Google Scholar]
- 52.Sort P, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403–409. doi: 10.1056/NEJM199908053410603. [DOI] [PubMed] [Google Scholar]
- 53.Bellomo R, Giantomasso DD. Noradrenaline and the kidney: friends or foes? Crit Care. 2001;5:294–298. doi: 10.1186/cc1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herget-Rosenthal S, Saner F, Chawla LS. Approach to hemodynamic shock and vasopressors. Clin J Am Soc Nephrol. 2008;3:546–553. doi: 10.2215/CJN.01820407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bourgoin A, et al. Increasing mean arterial pressure in patients with septic shock: effects on oxygen variables and renal function. Crit Care Med. 2005;33:780–786. doi: 10.1097/01.ccm.0000157788.20591.23. [DOI] [PubMed] [Google Scholar]
- 56.LeDoux D, Astiz ME, Carpati CM, Rackow EC. Effects of perfusion pressure on tissue perfusion in septic shock. Crit Care Med. 2000;28:2729–2732. doi: 10.1097/00003246-200008000-00007. [DOI] [PubMed] [Google Scholar]
- 57.Koppel MH, et al. Transplantation of cadaveric kidneys from patients with hepatorenal syndrome. Evidence for the functionalnature of renal failure in advanced liver disease. N Engl J Med. 1969;280:1367–1371. doi: 10.1056/NEJM196906192802501. [DOI] [PubMed] [Google Scholar]
- 58.Schrier RW, et al. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151–1157. doi: 10.1002/hep.1840080532. [DOI] [PubMed] [Google Scholar]
- 59.Hesse IF, Johns EJ. The role of alpha-adrenoceptors in the regulation of renal tubular sodium reabsorption and renin secretion in the rabbit. Br J Pharmacol. 1985;84:715–724. doi: 10.1111/j.1476-5381.1985.tb16154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mohan S, Radhakrishnan J. Do meta-analyses in nephrology change the way we treat patients? Kidney Int. 78:1080–1087. doi: 10.1038/ki.2010.323. [DOI] [PubMed] [Google Scholar]
- 61.Salo J, et al. Renal and neurohormonal changes following simultaneous administration of systemic vasoconstrictors and dopamine or prostacyclin in cirrhotic patients with hepatorenal syndrome. J Hepatol. 1996;25:916–923. doi: 10.1016/s0168-8278(96)80297-2. [DOI] [PubMed] [Google Scholar]
- 62.Albanese J, et al. Renal effects of norepinephrine in septic and nonseptic patients. Chest. 2004;126:534–539. doi: 10.1378/chest.126.2.534. [DOI] [PubMed] [Google Scholar]
- 63.Martin C, Papazian L, Perrin G, Saux P, Gouin F. Norepinephrine or dopamine for the treatment of hyperdynamic septic shock? Chest. 1993;103:1826–1831. doi: 10.1378/chest.103.6.1826. [DOI] [PubMed] [Google Scholar]