Abstract
Placental transfer of maternal IgG antibodies to the fetus is an important mechanism that provides protection to the infant while his/her humoral response is inefficient. IgG is the only antibody class that significantly crosses the human placenta. This crossing is mediated by FcRn expressed on syncytiotrophoblast cells. There is evidence that IgG transfer depends on the following: (i) maternal levels of total IgG and specific antibodies, (ii) gestational age, (iii) placental integrity, (iv) IgG subclass, and (v) nature of antigen, being more intense for thymus-dependent ones. These features represent the basis for maternal immunization strategies aimed at protecting newborns against neonatal and infantile infectious diseases. In some situations, such as mothers with primary immunodeficiencies, exogenous IgG acquired by intravenous immunoglobulin therapy crosses the placenta in similar patterns to endogenous immunoglobulins and may also protect the offspring from infections in early life. Inversely, harmful autoantibodies may cross the placenta and cause transitory autoimmune disease in the neonate.
1. Introduction
Anti-infectious fetal protection is provided by several factors acting together. The uterine cavity contains innate immune detection and effector systems that maintain sterility, detect infection and, under conditions of substantial microbial invasion, induce expression of mediators that could accelerate lung maturation and induce a preterm labor to deliver the fetus from a threatening environment [1]. The vaginal tract, which is normally colonized with multiple microorganisms, is separated from the normally sterile intrauterine compartment by the cervical plug, which contains several antimicrobial proteins and peptides (APPs), including lactoferrin and α-defensins. Inside the uterine cavity, the amniotic fluid contains acute phase proteins, such as soluble CD14 and lipopolysaccharide- (LPS-) binding protein (LBP), which modulates the endotoxic activity of LPS and cationic membrane-active APPs, such as lactoferrin, bactericidal/permeability-increasing protein, histones, and defensins [2]. In preterm labor, increased concentrations of group II phospholipase A2 are found, and this enzyme has been associated with a remarkable potency against Gram-positive bacteria [3, 4].
At birth, the neonate presents an increased susceptibility to infectious agents due to functional immaturity of his/her immune system. Some functions are particularly immature, whereas other aspects are functional at birth even in extremely preterm newborn infants. Neutrophils have a small storage pool at birth, and this cell lineage is less responsive to chemoattractants than later in development. Monocytes/macrophages are reported to be functionally adequate but have limitations in chemotactic responsiveness. Infant blood monocytes produce less IFN-α, IFN-γ, and IL-12 subunit p70 (IL-12 p70) than cells obtained from adults. However, production of these cytokines rapidly increases between birth and 1 or 2 years of age. In contrast, infant cells show a greater capacity to produce IL-10 and to induce IL-17-producing helper T cells (Th17 cells) in response to Toll-like receptor (TLR) stimulation by producing IL-6 and IL-23 [2]. Furthermore, individual infant cells are less able than adult cells to produce multiple cytokines simultaneously in response to TLR agonists; that is, infant cells are less polyfunctional [5]. The predominance of a Th17-like pattern combined with considerable IL-10 production may contribute to diminished T helper type 1 (Th1) responses, resulting in greater susceptibility to intracellular infections and diminished vaccine responses during infancy [6].
Neonatal T CD4+ cells present an intrinsic immaturity with a diminished capacity to generate memory cells and reduced Th1 effector functions such as the production of less IFN-γ and lower CD40L expression. These deficiencies seem mainly to be related to the fact that the cells are still naive, having met few antigens [7]. Thymic recent emigrants (TRECs), which are T cells recently migrated from the thymus, are present in a large proportion in the periphery of human infants, and these TRECs are impaired in their acquisition of Th1 function [8]. CD4+ T cell responses, but not CD8+ T cell responses, develop more slowly in infants than in adults after primary infection with cytomegalovirus or herpes simplex virus [9]. In addition, responses to some vaccines, such as vaccines for hepatitis B virus and oral poliovirus vaccine, result in less Th1 activity and a bias toward Th2 function [10]. The ability of proinflammatory cytokines to induce spontaneous abortion is likely to be an important reason for the strong bias of the maternal and fetal immune systems of multiple mammalian species towards Th2-cell-polarizing cytokines [1, 11]. The Th2 locus is hypomethylated in both human and mouse infants, contributing to the expression of these cytokine genes, which corresponds to the propensity for Th2-polarizing cytokine responses in infants [12, 13]. Thus, infants have a dominant anti-inflammatory cytokine profile that seems to be induced during fetal life [7]. It has been demonstrated that in the in utero environment, CD4+CD25hiFoxp3+ regulatory T cells dominate the fetal circulation, suppressing reactivity to noninherited maternal antigens [14] and possibly promoting a generally suppressive environment.
Regarding neonatal antibody responses, several studies have shown a delayed onset, lower peak levels, a shorter duration, differences in the distribution of IgG isotypes (with infants showing lower IgG2 than adults), and lower affinity and reduced heterogeneity. Antibody responses to thymus-independent type 2 antigens (including bacterial polysaccharides) are also deficient [15]. There is no transplacental transfer of complement system elements, and neonates have relatively low levels of some components [16]. Furthermore, neonatal and infantile B cells have low expression of CD21 (complement receptor 2), which explains the inadequate response to polysaccharides [17]. Interestingly, the increase in CD21 levels that occurs during development coincides with the response to polysaccharides [18].
Considering that after exposure to each new microbe it takes time to develop each specific protective immune response, the placental transfer of maternal immunoglobulins to the fetus is a specific adaptative mechanism that, to some extent, minimizes the deficiencies in antibody production and confers short-term passive immunity. Moreover, additional immune response support is given by the mother through breast milk, which contains functional nutrients and IgA antibodies that provide efficient protection directly after birth by preventing adherence of infectious agents on the mucosal membranes and ultimately their entrance into tissues.
2. IgG Placental Transfer Is Mediated by FcRn
In humans, substances that pass from maternal blood to fetal blood must traverse the histological barrier, which consists of two cell layers: the multinucleated syncytiotrophoblasts (STBs) and endothelial cells of the fetal capillaries. Furthermore, fibroblasts and Hofbauer cells (i.e., placental macrophages) are found in the villous stroma and are presumably involved in the binding and trapping of immune complexes [19].
Although this barrier separates the blood in maternal and fetal circulation, it is not a simple physical barrier. A wide range of substances, including nutrients and solutes, are efficiently transferred actively or passively through the placenta to the fetus, and this mechanism is essential for normal fetal growth and development. Most low molecular mass compounds (<500 Da) simply diffuse through the placental tissue interposed between the maternal and fetal circulation. Some low molecular weight substances, such as ions and amino acids, show unidirectional transfer across the placenta. Substances of very high molecular weight do not usually traverse the placenta, but there are a few exceptions, such as immunoglobulin G (IgG), which has a molecular mass of approximately 160 kDa.
Of the five antibody classes, only significant amounts of IgG are transferred across the placenta. On the basis of the observation that whole IgG molecules or Fc fragments of IgG pass into the fetal circulation more readily than F(ab′)2 fragments [20], it was hypothesized that IgG Fc receptors (FcγRs) on placental cells may be involved in IgG transfer across placenta. Later, it was established that this specific transport of IgG is carried out by the neonatal Fc receptor (FcRn) [21, 22]. This has been demonstrated unequivocally in ex vivo perfused placenta by comparing the transport of a recombinant, humanized IgG1 antibody with that of a mutated variant that does not bind to FcRn [23]. FcRn is composed of an integral membrane glycoprotein with an apparent molecular weight of 40–45 kDa for the α-chain, which is noncovalently associated with β2-microglobulin (β2 m) [24]. Thus, while the major ligands of FcRn are IgG and albumin, FcRn is most closely structurally related to major histocompatibility complex (MHC) class I molecules, with which it shares 22%–29% sequence homology. In contrast to other Fcγ-receptors, FcRn exhibits a characteristic pH-dependency of IgG binding, demonstrating a high affinity for IgG at pH 6.0, but 100-times lower affinity at physiological pH (7.4) [25]. Thus, FcRn is unable to bind IgG at the apical side of STB facing the maternal blood.
It is, therefore, assumed that IgG present in high concentrations in the maternal circulation (10–20 mg/mL) is taken up by fluid-phase endocytosis by STB and then binds to the FcRn in the acidic environment of endosomes [26]. Bound IgG may then be transcytosed to the basolateral side, where it is released upon exposure to neutral pH (7.4). The FcRn molecule may then be recycled to the maternal membrane to perform additional rounds of transcytosis, as observed in other systems [27]. Therefore, pH-dependent binding of IgG to FcRn allows for IgG transport through a cell layer and down a concentration gradient of IgG [28–30] (Figure 1).
Figure 1.
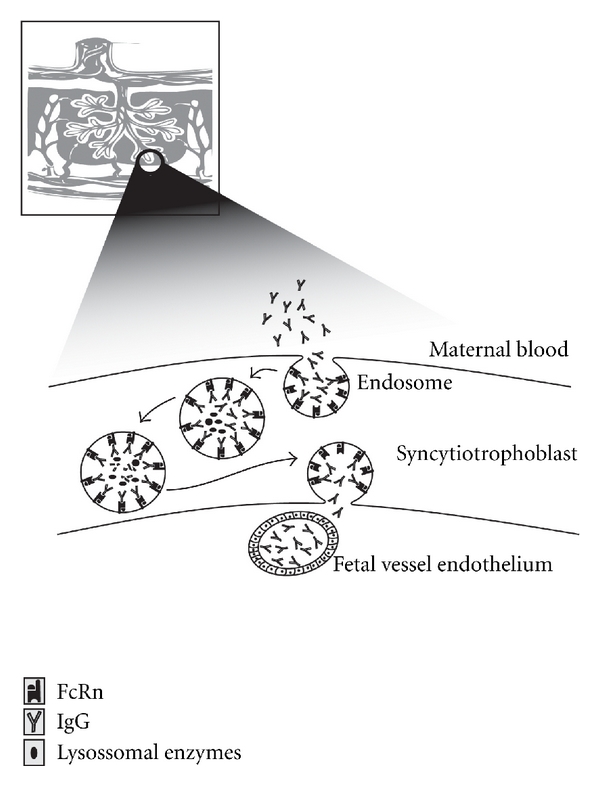
IgG transfer from the mother to the fetus occurs during pregnancy across the syncytiotrophoblasts of the placenta. Syncytiotrophoblasts are bathed in maternal blood and internalize maternal IgG in endosomes. FcRn is expressed on the internal surfaces of the endosome. Upon acidification in the endosome, maternal IgG bound to FcRn is protected from degradation by lysosomal enzymes and then is transcytosed. The endosomes fuse with the membrane on the fetal side of the syncytiotrophoblast, where the physiological pH promotes the dissociation of IgG from FcRn to the fetal circulation. High levels of IgG antibodies cause IgG degradation due to the saturation of FcRn receptors.
Transcytosed IgG may or may not pass through the stroma before reaching the fetal blood vessels. It remains controversial as to whether FcRn is also expressed in the fetal vessel endothelium, where greater evidence exists for the action of alternative Fc receptors in further movement of IgG [31, 32]. This IgG transport model is supported by the in situ localization of IgG and FcRn subunits and by studies investigating IgG transport in ex vivo perfused placentae [33]. The FcRn α-chain has been found to be localized mainly in intracellular vesicles and to a minor extent at the apical membrane of STB of first trimester and term placentae [26, 33, 34].
The function of FcRn also extends to many other sites within the body, where it plays an important role in modulating lifelong humoral and cell-mediated immune responses. It is also expressed in both endothelial and bone marrow-derived cells and plays an integral role in protecting IgG from catabolism, which allows IgG to be recycled to the cell surface and back into the bloodstream, extending its half-life in the serum of adults [35]. FcRn is also expressed in many other tissues in the adult animal, including barrier sites such as the blood-brain interface, the glomerular filter in the kidneys and the intestinal epithelium, where its function of modulating IgG transport to promote host defense or to control immune-complex deposition is still speculative [36].
To be transferred through human placenta, maternal IgG must cross the STBs, the stroma of the intravillous space, and the fetal vessel endothelium. These tissues express unique patterns of various types of Fc receptors of IgG including FcγRI, FcγRII, and FcγRIII. In the placenta, FcγRI has been found in the loose connective tissue, mononuclear phagocytes, and the Hofbauer cells, which are morphologically defined as macrophages due to their ability to perform phagocytosis and to interact with IgG. Trophoblast cells in term placentae express both FcγRIII and FcRn. Placental FcγRIII is a membrane-spanning FcγRIIIa isoform, which is predominantly expressed by Natural Killer (NK) cells. The binding of FcγRIII (also called CD16) on NK cells to immune complexes or IgG on target cells, or treatment with an anti-CD16 monoclonal antibody to crosslink membrane spanning FcγRIII induces NK cell activation. This activation leads to upregulation of the transcripts for cytokines such as IFN-γ and TNF-α [37, 38]. These observations indicate that FcγRIIIa on trophoblasts may bind immune complexes or antibody-coated particles in the maternal circulation and may induce the transcription of cytokines or trigger cell-mediated immunity.
Fetal endothelial cells in placenta express FcγRII and FcRn although data regarding FcRn expression in the endothelium are still conflicting [39].
3. Placental Transport of IgG Depends on Maternal Levels
The newborn IgG antibodies' levels usually correlate with maternal ones (Figure 2); however, the IgG binding to FcRn receptor can be saturated. Thus, the amount of IgG transmitted depends on the amount of cell surface receptors, because unbound IgG molecules are digested by lysosomal enzymes inside the vesicles [40]. This has been reported in several works performed in certain regions of Africa showing lower cord/maternal placental transfer ratios of total IgG, indicating that this limitation of active placental transfer of antibodies is related to the higher maternal IgG levels common in Africa [41–43]. It was reported by Michaux et al. [44] that total IgG concentrations in cord sera tend to be lower than in their mothers when total IgG levels in maternal serum reached 15 g/L. This is in agreement with other works that have demonstrated significant negative correlations between maternal levels of IgG and placental transfer ratios to the neonate for both total IgG and, interestingly, IgG specific to measles, LPS and other antigens [41, 45, 46].
Figure 2.
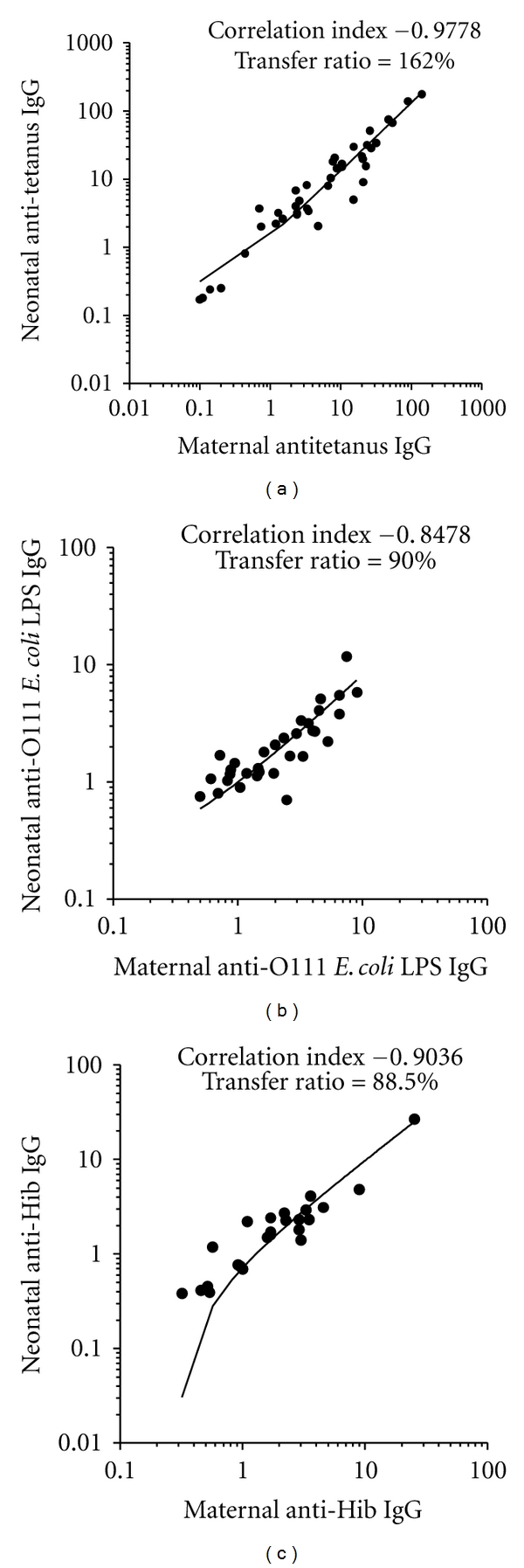
Correlation indexes and placental transfer ratios of maternal and term cord blood IgG levels reactive with tetanus toxoid, O111 LPS from enteropathogenic E. coli and Hib polysaccharide. Correlation indexes and placental transfer is higher to thymus-dependent antigens, as tetanus toxoid than to thymus-independent antigens type I and II, as LPS and polysaccharides, respectively.
Since the 1970s, Mäntyjärvi et al. [47] have demonstrated that neonatal anti-influenza A2 IgG levels on average tends to exceed that of the mother if the maternal level is low or normal. When the mother has a high content of total IgG or of a specific antibody, the neonatal value usually remained below the maternal one. This inverse relationship between the efficiency of placental transfer to the respective maternal level was also demonstrated for herpes simplex virus, tetanus toxoid, streptolysin O, and S. pneumoniae [48]. This is an interesting observation, because it is known that placental transport is mediated by the interaction between the Fc portion of IgG and the FcRn receptor, in which the Fab portion of this immunoglobulin is not involved. However, this phenomenon suggests an involvement of antigenic specificity of the antibody for this transport, but further studies are needed to investigate the mechanism involved.
4. IgG Transport Depends on the Subclass
It is not clear why some antibody specificities exhibit different transfer impairments in different studies [49]. A plausible explanation may lie in variation in the IgG subclass responses to different antigens and the different affinities of these subclasses to the IgG-transporting FcRn receptors [50, 51]. Preferential transport occurs for IgG1, followed by IgG4, IgG3, and IgG2, for which the FcRn receptors have the lowest affinity [52] (Figure 3). This has been clearly demonstrated in studies on the transfer pattern of different types of specific IgG antibodies showing peculiarities in this transmission. IgG1 and IgG3 are transferred more efficiently across the placenta than IgG2. Furthermore, the transfer of antibodies against viral proteins and antitoxins of the IgG1 subclass occurs more readily. However, antibodies against encapsulated bacteria (Haemophilus influenzae, Neisseria meningitidis, and Streptococcus pneumoniae) in which IgG2 prevails, at least after natural exposure, are transferred less efficiently [53, 54], and an effective transplacental transmission of IgG antibodies reactive with LPS involving the IgG1 and IgG2 subclasses was confirmed in our previous studies [55].
Figure 3.

Percentages of placental transfer ratios of IgG subclasses delivered to preterm and term newborns in different gestational weeks. IgG1 and IgG3 transfer ratios rose with increasing gestational age, with IgG1 showing a peak transfer ratio at 37 weeks of pregnancy. IgG2 transfer ratios are always lower than the other IgG subclasses [52].
In addition, it has been demonstrated that in term neonates with a low birth weight, all IgG subclasses were transferred with reduced efficiency, but IgG1 and IgG2 subclasses were transferred with significantly less efficiency than IgG3 and IgG4. These results demonstrate that low birth weight is associated with impaired placental transfer of IgG1 and IgG2 subclasses.
Overall, at term, IgG in cord blood has a good correlation with maternal levels, and placental transfer is systematically higher to thymus-dependent antigens (proteins), as tetanus toxoid than to thymus-independent antigens, both type I and II, as LPS and polysaccharides, respectively [56] (Figure 2).
5. IgG Transport Depends on Gestational Age
IgG transfer from mother to fetus begins as early as 13 weeks of gestation, and transport happens in a linear fashion as the pregnancy progresses, with the largest amount transferred in the third trimester [39]. Malek and colleagues [57] demonstrated a continuous rise in IgG levels in the fetal circulation between 17 and 41 weeks of gestation. Fetal IgG concentrations were only 5%–10% of the maternal levels at weeks 17–22 but reached 50% of the maternal concentrations at weeks 28–32. The majority of IgG is acquired by the fetus during the last 4 weeks of pregnancy, and fetal IgG concentrations usually exceed maternal ones by 20%–30% at full term [39]. Interestingly, a sharp increase in cord blood levels occurs after the 36th week of gestation.
At term, dependent on the immunological experience of the mother, placental transfer allows the newborn to acquire different specificities of IgG antibodies, resulting in an identical recognition pattern of antigens between the mother and her offspring. As shown in Figure 4, an immunoblotting assay demonstrates identical patterns of enterohemorrhagic E. coli (EHEC) antigen recognition between paired mother and term cord sera, thus confirming abundant transfer of the maternal antibody repertoire to the newborn at least for protein antigens.
Figure 4.

Immunoblotting of anti-EHEC O157:H7 IgG antibodies in the paired maternal and cord samples. Bacterial proteins were separated by 12.5% SDS-PAGE. Paired maternal and cord serum samples are identified numerically. M: maternal serum; C: cord serum; NHS: pool of healthy adult serum samples (normal human serum). The immunoblots were developed with antihuman IgG conjugate. Molecular weight standards are on the left for samples 1–8 and on the right for the pool of normal human serum. It was observed that there is almost complete identity between the antigens recognized by maternal and umbilical cord sera [58].
Maternal age, weight, parity, and type of delivery do not influence placental antibody transfer [59].
Total IgG concentrations in newborns, therefore, are directly related to length of gestation, and infants born at less than 33 weeks of gestation have substantially lower IgG levels than full-term babies (Figure 5). As the expression of FcRn receptor is dependent on gestational age and seems to be more highly expressed in the third trimester of human pregnancy, a reduced placental transfer of antibodies is observed at early stages of gestation. This fact results in a reduced transfer of IgG subclasses, especially IgG1 and IgG2, in preterm compared with full-term babies [56].
Figure 5.
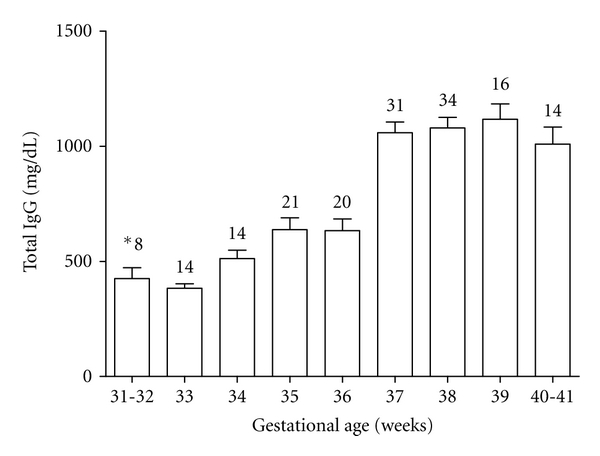
Total IgG concentrations in cord serum samples from newborns in different gestational weeks [46, 60]. *Number of samples in each period.
Accordingly, in a recent study, van den Berg et al. [54] found significantly lower transplacental transmission of IgG in preterm infants (<32 weeks) than in full-term infants for antibodies against diphtheria, tetanus, pertussis, Haemophilus influenza type b (Hib), and Neisseria meningitides serogroup C. In agreement with these data, Silveira Lessa et al. [46] evaluated the placental transfer ratios of IgG antibodies reactive with Klebsiella, Pseudomonas, and E. coli O111, O26, and O6 lipopolysaccharides and showed lower anti-LPS IgG transfer ratios in preterm groups (<33 weeks and >33 weeks) as compared with term ones (>37 weeks).
6. The Influence of Maternal Immunization
A main focus of the study of IgG transport across the placenta is maternal vaccination. Currently, several routine vaccines are recommended for pregnant women, such as tetanus toxoid vaccine and inactivated influenza virus vaccine. Others are used in special circumstances, including polysaccharide vaccines, such as pneumococcal polysaccharide vaccine and meningococcal polysaccharide vaccine, and inactivated viral vaccines, such as hepatitis A and B, rabies virus, or inactivated poliovirus vaccines [61]. All of these vaccines are given to protect women from serious diseases during pregnancy and the postpartum period while potentially providing benefits to the fetus and neonate due to placental transmission of those maternal antibodies.
However, many factors may limit the placental transfer efficacy after maternal vaccination, such as the timing between immunization at the pregnancy and delivery, the gestational age of the fetus at birth, total maternal IgG levels and the maternal vaccine-specific IgG and IgG subclasses concentrations [62, 63].
Several randomized studies have been conducted aiming to study the effectiveness of maternal vaccination, targeting important pathogens in early childhood [64, 65]. Prospective studies have demonstrated higher cord antibody levels to influenza in babies born to mothers immunized during pregnancy [66]. Infants of vaccinated mothers were 45%–48% less likely to have influenza hospitalizations than infants of unvaccinated mothers [67]. Maternal influenza vaccination effectiveness for both mother and newborn was also demonstrated in developing countries [68, 69].
Recently, it was showed that Tdap (tetanus and diphtheria toxoids and acellular pertussis antigens) vaccination in pregnancy was safe and significantly increased antibody titers against those antigens. These data reinforced that maternal Tdap vaccination in the second trimester may prevent neonatal pertussis disease in the first 5-6 months of life until infants receive active vaccinations with Tdap at 2, 4, and 6 months of age and establish active immunity [70].
In clinical studies, several factors can affect the transport of antibodies specific to vaccine antigens, particularly the type of vaccine administered. Vaccines with the ability to induce higher maternal levels of IgG and specifically IgG1, such as Hib polysaccharide- (PRP-) conjugate vaccines result in increased concentrations of IgG1 delivered to the fetus [61] and significantly more PRP-specific IgG antibodies during at least the first 2 months of life in diverse populations [71–75]. The same was observed for type III capsular polysaccharide of Group B Streptococcus (GBS) conjugated to tetanus toxoid vaccine, demonstrating an efficient transport of GBS-specific IgG antibodies to the neonate [76].
Reports on maternal-fetal transfer of antibodies against the capsular polysaccharides of S. pneumoniae have demonstrated that even term infants generally receive only a fraction (50%–85%) of either naturally acquired [77] or polysaccharide vaccine-induced antibodies from their mothers [63, 78–82]. Although higher IgG antibody levels are found in offspring of immunized compared to unimmunized women, these titers are not maintained for a long time after birth, they likely increase protection from invasive pneumococcal disease until around 120 days after birth, when disease rates are very high [78]. In contrast to the polysaccharide vaccine, maternal immunization schedules including a conjugate pneumococcal vaccine have the potential advantage of stimulating a larger quantity of antibodies of the IgG1 rather than the IgG2 subclass [83].
Another point that merits discussion is that higher doses of passively acquired antibodies may suppress antibody responses to active immunization in early infancy. Several studies have also reported that maternal antibodies can inhibit infant responses to measles, tetanus, whole cell pertussis, and Hib vaccines; this effect varies considerably between different vaccines and studies [84–87]. Regarding toxoids, it was observed that infants who had considerable levels of pre-existing antibodies exhibited lower responses after active immunization to diphtheria toxoid after the second dose, but after 12 months of life, antibody titers do not differ between those infants whose mothers had low titers. For the conjugate PRP-T vaccine, the anti-Hib antibody response was not affected by high maternal antitoxin titers; however, the infants' response to tetanus toxoid was dampened by these high titers. Despite this, all infants achieved protective levels of tetanus antitoxin-IgG after the booster dose with PRP-T. Regarding polysaccharide vaccines, studies have shown no difference in immune response of infants whose mothers received the vaccine or not during pregnancy when they are given the doses at 6–8 months of life. This observation was made with both meningococcal polysaccharide and Hib vaccines [88].
The mechanisms through which maternal antibodies inhibit infant responses to vaccination are not fully understood. However, some plausible explanations are as follows: (i) neutralization of live viral vaccines, (ii) vaccine antigen immune complexes inhibiting infant B cell activation mediated by FcγRIIb receptor, (iii) effective elimination of vaccine antigen coated with maternal IgG antibodies via Fc-dependent phagocytosis, and (iv) vaccine antigenic epitopes being masked or hidden by maternal antibodies, preventing binding by infant B cells [84, 89]. Although persistence of maternal antibodies may limit infant antibody responses, induction of T-cell responses remain largely unaffected by these passively transferred antibodies, because the administration of repeated vaccine doses, as routinely performed for diphtheria-tetanus-pertussis-polio and Hib vaccines, is often sufficient to overcome inhibition by maternal antibodies [90].
7. Placental Transport of IgG in Infectious Diseases
It is well known that antibody transport during pregnancy can be affected by a number of factors and clinical conditions, including placental abnormalities, total IgG concentration in maternal blood, the gestational age of the fetus at birth, and maternal pathologies, such as hypergammaglobulinemia, HIV infection, and placental malaria [91–93]. In addition, preterm labors and intrauterine growth retardation are associated with a number of pathologies, such as chronic hypertensive disease or hypertensive disease during pregnancy, preeclampsia, gestational diabetes, and infections whose influence in maternal antibody levels is still unknown [94].
In cases of maternal HIV infection or placental injuries, like malaria, a great decrease in antibody transfer has been reported [48, 75, 95–97]. A multivariate regression analysis study determined that placental malaria or maternal HIV infection, independent of maternal hypergammaglobulinemia, are conditions that affect placental transfer of antibodies, and if the mother also has high IgG serum levels, placental transfer is even more impaired [92].
It has been recently demonstrated that HIV-exposed but uninfected infants have reduced transplacental transfer of Hib-, pertussis-, pneumococcus-, and tetanus-specific antibodies than their non-HIV exposed peers. These findings were consistent with two other studies in HIV-infected women from Kenya, indicating that maternal HIV is associated with lower tetanus and measles-specific antibodies in cord blood and also with reduced placental antibody transfer [98, 99]. However, although prenatal HIV exposure was associated with lower specific antibody levels in exposed uninfected infants compared with unexposed infants at birth, after 16 weeks of life, robust and significantly higher antibody responses to pertussis and pneumococcus following routine vaccination were observed in the group of exposed uninfected infants compared with control infants. Therefore, HIV exposure is associated with a greater change in antibody levels between birth and 16 weeks [100].
8. Placental Transfer in Mothers with Primary Immunodeficiencies
Women with humoral deficiencies are dependent on exogenous administration of lgG to prevent recurrent infections with possible severe morbidity and even mortality. In addition, in the absence of the intravenous immunoglobulin (IVIG) therapy, their fetuses may also have an increased risk of infection during intrauterine life and during the first few months after birth because of reduced transplacental transfer of immunoglobulins from those mothers to their offspring [101].
Common variable immunodeficiency (CVID) is not an extremely rare disorder, and currently, many patients reach childbearing age in reasonably good health and become pregnant. CVID represents a heterogeneous group of immunologic disorders, characterized by reduced serum immunoglobulin levels and impaired antibody responses, with variable T cell numbers and function [102]. Its genetic heterogeneity has been studied in the last few years, with the identification of underlying defects in the following genes: ICOS (inducible costimulator), BAFF-R (B-cell-activating factor receptor), TACI (transmembrane activator and calcium-modulator and cyclophilin ligand interactor), CD19, and, more recently, CD20 and CD81 deficiencies [103].
There are only a few reports on total immunoglobulin placental transfer in those cases [104–106], but it was recently shown that CVID mothers under IVIG therapy efficiently transferred exogenous IgG through the placenta in similar patterns as endogenous immunoglobulins, as demonstrated by the following: (i) cord blood IgG levels in term babies were even greater than in the mothers, (ii) a preferential transfer of IgG1, IgG3 and IgG4 compared with IgG2, (iii) antiprotein IgG antibody levels equivalent to or higher than maternal ones in cord serum and good transfer of antipolysaccharide IgG antibodies, and (iv) similar anti-S. pneumoniae avidity indexes between mothers and their respective neonates (Table 1) [107]. Thus, CVID patients must be informed about the relevance of regular IVIG administration during pregnancy not only for their own health but also for the immunity of their immature offspring.
Table 1.
Serum immunoglobulin levels, specific antibody concentrations and avidity indexes in the maternal and cord serum and cord/maternal serum ratios for IgG antibodies from a mother with CVID.
| Maternal | Cord | Placental Transfer ratio (%) | |
|---|---|---|---|
| Total Immunoglobulin Concentrations | |||
|
| |||
| IgG (mg/dL) | 473.0 | 912.0 | 190 |
| IgM (mg/dL) | <6.0 | 12.0 | – |
| IgA (mg/dL) | <3.0 | <3.0 | – |
| IgG1 (mg/dL) | 362.0 | 752.0 | 210 |
| IgG2 (mg/dL) | 249.0 | 192.0 | 80 |
| IgG3 (mg/dL) | 10.0 | 20.0 | 200 |
| IgG4 (mg/dL) | 6.0 | 21.0 | 350 |
|
| |||
| Specific IgG Antibodies Levels | |||
|
| |||
| IgG anti-tetanus toxoid (UI/mL) | 1.6 | 3.1 | 190 |
|
| |||
| IgG anti-Hib PRP* (mg/L) | 3.8 | 3.7 | 100 |
|
| |||
| IgG anti-PS§1 (mg/L)/avidity (M)+ | 2.3/>3.0 | 2.6/>3.0 | 110 |
| IgG anti-PS3 (mg/L)/avidity (M) | 2.4/>3.0 | 2.8/>3.0 | 120 |
| IgG anti-PS5 (mg/L)/avidity (M) | 7.3/2.7 | 8.0/2.7 | 110 |
| IgG anti-PS6 (mg/L)/avidity (M) | 6.9/2.7 | 6.6/>3.0 | 100 |
| IgG anti-PS9 (mg/L)/avidity (M) | 4.4/2.9 | 4.7/>3.0 | 110 |
| IgG anti-PS14 (mg/L)/avidity (M) | 12.8/2.5 | 15.0/2.8 | 120 |
–IgM and IgA maternal/cord blood ratios were not performed;
*PRP—polyribosyl–ribitolphosphate polymers;
§Anti-PS—Anti-Streptococcus pneumoniae polysaccharide;
+(M) —Avidity index in molarity.
9. Placental Transfer in Mothers with Autoimmune Diseases
There are circumstances in which placental transmission of antibodies is detrimental to the neonate. Neonatal lupus erythematosus (NLE) is a rare disease considered to be the exemplary prototypic model of passively acquired systemic autoimmune disease [108]. Maternal IgG autoantibodies against Ro/SSA and/or La/SSB or, less commonly, to U1-ribonucleoprotein (U1-RNP), are transported through the placenta and harm the fetus by causing injury to the skin (cutaneous rash). One of the strongest clinical associations is the development of congenital heart block, which is most often of third-degree severity in a structurally normal heart. This abnormality is an alarming prospect facing 2% of mothers with these autoantibodies [109]. The risk of having a second baby with NLE among women who have already had a baby with NLE increases to 15% [110].
Sera of patients with autoimmune disorders contain an active idiotypic-anti-idiotypic network, which can also be induced in experimental animals following immunization with B-cell epitopes of autoantigens. It has been shown that sera of pregnant women with anti-La/SSB autoantibodies who carry a healthy baby have significantly higher levels of anti-idiotypic antibodies to anti-La/SSB, suggesting that these may serve as protective antibodies for the development of congenital heart block [111]. Therefore, the presence of anti-idiotypic antibodies to autoantibodies against La/SSB may protect the fetus by blocking pathogenic maternal autoantibodies.
The transference of autoantibodies was also reported in neonatal pemphigus, which is characterized as a rare transitory autoimmune blistering disease caused by transfer of maternal IgG autoantibodies specific for desmoglein 3 to the neonate when the mother is affected with pemphigus [112]. This disease is clinically characterized by transient flaccid blisters and erosions on the skin and rarely the mucosa. However, by 3 months, IgG antidesmoglein levels in the neonate are within normal limits [113]. Transient neonatal autoimmune diseases have also been reported for myasthenia gravis and antiphospholipid syndrome, and recently, a case was reported of a newborn with transient epidermolysis bullosa acquisita, a chronic, autoimmune bullous dermatosis due to the passive transfer of maternal autoantibodies against the noncollagenous terminus of the α chain of type VII collagen [114–116].
In autoimmune diseases in which pathogenic or excess IgG antibodies are the etiological agents, such as myasthenia gravis, bullous pemphigoid, idiopathic thrombocytopenic purpura (ITP), and systemic lupus erythematosus (SLE), it is sometimes advantageous to reduce endogenous serum IgG levels by interfering with FcRn function. One possible way to interfere with the function of FcRn is to overload it with “innocuous” IgG. As FcRn functions as the IgG homeostatic receptor, the level of FcRn expression determines the serum concentration of IgG. Administering large quantities of exogenous IgG raises the serum concentration above this equilibrium set point and saturates FcRn [117]. As a result, the excess IgG that does not bind to FcRn enters the degradative pathway. This results in a shortening of the serum IgG half-life. High-dose IVIG treatment is thought to exert an immunomodulatory effect by numerous mechanisms, including engagement of the inhibitory FcγRIIb receptor [118] and by FcRn saturation [117].
In mouse models of bullous pemphigoid, ITP and autoimmune arthritis, IVIG treatment results in the dilution of pathogenic antibodies to levels beneath the disease-causing threshold [30, 119, 120]. The fact that a therapeutic effect for IVIG is maintained in FcγRIIb-deficient mice and is attenuated in FcRn-deficient mice is strong evidence that an important mechanism of action of IVIG is its ability to compromise FcRn function [30, 121]. This approach provides a valuable tool to prevent neonatal autoimmune disease by exploiting the saturation of FcRn by high doses of IVIG [122–125].
Finally, one interesting point that has been well explored in murine models but not yet in humans is that placental-derived IgG antibodies exert long-life immunoregulatory functions, including imprinting the fetal immune network [126]. Thus, by crossing the placenta, maternal IgG, in addition to providing anti-infectious protection to the infant, could have other active immunoregulatory long-term effects. This mechanism of transplacental antibodies transfer could also be involved in the recognition of allergens and priming of small populations of allergen-specific T cells in the newborn during intrauterine life, which could represent a normal stimulatory signal [127].
10. Conclusions and Perspectives
The maternal IgG antibody transfer varies as a result of total and specific maternal IgG levels, IgG subclass (and thus, the nature of antigen), gestational age, and placental integrity. Knowledge of the features of placental transmission of IgG antibodies is crucial to exploit and manipulate this mechanism to benefit the newborn. The finding that mothers respond well to vaccination and are able to transfer their entire antibody repertoire to their infants is encouraging, raising the possibility of providing protection until the time when the infant is vaccinated. Overall, the employment of IVIG therapy promises to be an area of active research with applications in mothers with primary immunodeficiencies to promote maternal and newborn protection against infections and in the treatment of various antibody-mediated autoimmune diseases, modulating transfer of harmful autoantibodies.
Acknowledgments
Financial support: this work was supported by FAPESP 2008/58238-4 Grant. The authors are grateful to Rafael Noel Ruaro for his valuable help with Figure 1.
References
- 1.Maródi L. Innate cellular immune responses in newborns. Clinical Immunology. 2006;118(2-3):137–144. doi: 10.1016/j.clim.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nature Reviews Immunology. 2007;7(5):379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 3.Weinrauch Y, Abad C, Liang NS, Lowry SF, Weiss J. Mobilization of potent plasma bactericidal activity during systemic bacterial challenge: role of group IIa phospholipase A2 . Journal of Clinical Investigation. 1998;102(3):633–638. doi: 10.1172/JCI3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koyama M, Ito S, Nakajima A, et al. Elevations of group II phospholipase A2 concentrations in serum and amniotic fluid in association with preterm labor. American Journal of Obstetrics and Gynecology. 2000;183(6):1537–1543. doi: 10.1067/mob.2000.107789. [DOI] [PubMed] [Google Scholar]
- 5.Kollmann TR, Crabtree J, Rein-Weston A, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. Journal of Immunology. 2009;183(11):7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.PrabhuDas M, Adkins B, Gans H, et al. Challenges in infant immunity: implications for responses to infection and vaccines. Nature Immunology. 2011;12(3):189–194. doi: 10.1038/ni0311-189. [DOI] [PubMed] [Google Scholar]
- 7.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nature Reviews Immunology. 2004;4(7):553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 8.Haines CJ, Giffon TD, Lu LS, et al. Human CD4+ T cell recent thymic emigrants are identified by protein tyrosine kinase 7 and have reduced immune function. Journal of Experimental Medicine. 2009;206(2):275–285. doi: 10.1084/jem.20080996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson CB, Kollmann TR. Induction of antigen-specific immunity in human neonates and infants. Nestle Nutrition Workshop Series. 2008;61:183–193. doi: 10.1159/000113493. [DOI] [PubMed] [Google Scholar]
- 10.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nature Reviews Immunology. 2004;4(7):553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 11.Firth MA, Shewen PE, Hodgins DC. Passive and active components of neonatal innate immune defenses. Animal Health Research Reviews. 2005;6(2):143–158. doi: 10.1079/ahr2005107. [DOI] [PubMed] [Google Scholar]
- 12.Webster RB, Rodriguez Y, Klimecki WT, Vercelli D. The human IL-13 locus in neonatal CD4+ T cells is refractory to the acquisition of a repressive chromatin architecture. Journal of Biological Chemistry. 2007;282(1):700–709. doi: 10.1074/jbc.M609501200. [DOI] [PubMed] [Google Scholar]
- 13.Rose S, Lichtenheld M, Foote MR, Adkins B. Murine neonatal CD4+ cells are poised for rapid Th2 effector-like function. Journal of Immunology. 2007;178(5):2667–2678. doi: 10.4049/jimmunol.178.5.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mold JE, Michaelsson J, Burt TD, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322(5907):1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marshall-Clarke S, Reen D, Tasker L, Hassan J. Neonatal immunity: how well has it grown up? Immunology Today. 2000;21(1):35–41. doi: 10.1016/s0167-5699(99)01548-0. [DOI] [PubMed] [Google Scholar]
- 16.Schelonka RL, Infante AJ. Neonatal immunology. Seminars in Perinatology. 1998;22(1):2–14. doi: 10.1016/s0146-0005(98)80003-7. [DOI] [PubMed] [Google Scholar]
- 17.Griffioen AW, Franklin SW, Zegers BJ, Rijkers GT. Expression and functional characteristics of the complement receptor type 2 on adult and neonatal B lymphocytes. Clinical Immunology and Immunopathology. 1993;69(1):1–8. doi: 10.1006/clin.1993.1142. [DOI] [PubMed] [Google Scholar]
- 18.Klein Klouwenberg P, Bont L. Neonatal and infantile immune responses to encapsulated bacteria and conjugate vaccines. Clinical and Developmental Immunology. 2008;2008 doi: 10.1155/2008/628963. Article ID 628963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simister NE. Human placental Fc receptors and the trapping of immune complexes. Vaccine. 1998;16(14-15):1451–1455. doi: 10.1016/s0264-410x(98)00107-8. [DOI] [PubMed] [Google Scholar]
- 20.Brambell FW, Hemmings WA, Oakley CL, Porter RR. The relative transmission of the fractions of papain hydrolyzed homologous gamma-globulin from the uterine cavity to the foetal circulation in the rabbit. Proceedings of the Royal Society of London B. 1960;151:478–482. doi: 10.1098/rspb.1960.0011. [DOI] [PubMed] [Google Scholar]
- 21.Simister NE, Rees AR. Isolation and characterization of an Fc receptor from neonatal rat small intestine. European Journal of Immunology. 1985;15(7):733–738. doi: 10.1002/eji.1830150718. [DOI] [PubMed] [Google Scholar]
- 22.Simister NE, Mostov KE. An Fc receptor structurally related to MHC class I antigens. Nature. 1989;337(6203):184–187. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- 23.Firan M, Bawdon R, Radu C, et al. The MHC class I-related receptor, FcRn, plays an essential role in the maternofetal transfer of γ-globulin in humans. International Immunology. 2001;13(8):993–1002. doi: 10.1093/intimm/13.8.993. [DOI] [PubMed] [Google Scholar]
- 24.Israel EJ, Wilsker DF, Hayes KC, Schoenfeld D, Simister NE. Increased clearance of IgG in mice that lack β2-microglobulin: possible protective role of FcRn. Immunology. 1996;89(4):573–578. doi: 10.1046/j.1365-2567.1996.d01-775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaughn DE, Bjorkman PJ. Structural basis of pH-dependent antibody binding by the neonatal Fc receptor. Structure. 1998;6(1):63–73. doi: 10.1016/s0969-2126(98)00008-2. [DOI] [PubMed] [Google Scholar]
- 26.Kristoffersen BK. Human placental Fc γ-binding proteins in the maternofetal transfer of IgG. Acta Pathologica, Microbiologica et Immunologica Scandinavica, Supplement. 1996;104(64):5–36. doi: 10.1111/j.1600-0463.1996.tb05583.x. [DOI] [PubMed] [Google Scholar]
- 27.Ober RJ, Martinez C, Lai X, Zhou J, Ward ES. Exocytosis of IgG as mediated by the receptor, FcRn: an analysis at the single-molecule level. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(30):11076–11081. doi: 10.1073/pnas.0402970101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simister NE, Story CM, Chen HL, Hunt JS. An IgG-transporting Fc receptor expressed in the syncytiotrophoblast of human placenta. European Journal of Immunology. 1996;26(7):1527–1531. doi: 10.1002/eji.1830260718. [DOI] [PubMed] [Google Scholar]
- 29.Radulescu L, Antohe F, Jinga V, Ghetie V, Simionescu M. Neonatal Fc receptors discriminates and monitors the pathway of native and modified immunoglobulin G in placental endothelial cells. Human Immunology. 2004;65(6):578–585. doi: 10.1016/j.humimm.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 30.Akilesh S, Petkova S, Sproule TJ, et al. The MHC class I-like Fc receptor promotes humorally mediated autoimmune disease. Journal of Clinical Investigation. 2004;113(9):1328–1333. doi: 10.1172/JCI18838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antohe F, Rǎdulescu L, Gafencu A, Gheţie V, Simionescu M. Expression of functionally active FcRn and the differentiated bidirectional transport of IgG in human placental endothelial cells. Human Immunology. 2001;62(2):93–105. doi: 10.1016/s0198-8859(00)00244-5. [DOI] [PubMed] [Google Scholar]
- 32.Simister NE. Placental transport of immunoglobulin G. Vaccine. 2003;21(24):3365–3369. doi: 10.1016/s0264-410x(03)00334-7. [DOI] [PubMed] [Google Scholar]
- 33.Leach L, Eaton BM, Firth JA, Contractor SF. Uptake and intracellular routing of peroxidase-conjugated immunoglobulin-G by the perfused human placenta. Cell and Tissue Research. 1990;261(2):383–388. doi: 10.1007/BF00318681. [DOI] [PubMed] [Google Scholar]
- 34.Fuchs R, Ellinger I. Endocytic and transcytotic processes in villous syncytiotrophoblast: role in nutrient transport to the human fetus. Traffic. 2004;5(10):725–738. doi: 10.1111/j.1600-0854.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- 35.Kane SV, Acquah LA. Placental transport of immunoglobulins: a clinical review for gastroenterologists who prescribe therapeutic monoclonal antibodies to women during conception and pregnancy. American Journal of Gastroenterology. 2009;104(1):228–233. doi: 10.1038/ajg.2008.71. [DOI] [PubMed] [Google Scholar]
- 36.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nature Reviews Immunology. 2007;7(9):715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 37.Anegon I, Cuturi MC, Trinchieri G, Perussia B. Interaction of Fc receptor (CD16) ligands induces transcription of interleukin 2 receptor (CD25) and lymphokine genes and expression of their products in human natural killer cells. Journal of Experimental Medicine. 1988;167(2):452–472. doi: 10.1084/jem.167.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cassatella MA, Anegon I, Cuturi MC, Griskey P, Trinchieri G, Perussia B. FcγR(CD16) interaction with ligand induces Ca2+ mobilization and phosphoinositide turnover in human natural killer cells. Role of Ca2+ in FcγR(CD16)-induced transcription and expression of lymphokine genes. Journal of Experimental Medicine. 1989;169(2):549–567. doi: 10.1084/jem.169.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saji F, Samejima Y, Kamiura S, Koyama M. Dynamics of immunoglobulins at the feto-maternal interface. Reviews of Reproduction. 1999;4(2):81–89. doi: 10.1530/ror.0.0040081. [DOI] [PubMed] [Google Scholar]
- 40.Saji F, Koyama M, Matsuzaki N. Current topic: human placental Fc receptors. Placenta. 1994;15(5):453–466. doi: 10.1016/s0143-4004(05)80415-1. [DOI] [PubMed] [Google Scholar]
- 41.Hartter HK, Oyedele OI, Dietz K, et al. Placental transfer and decay of maternally acquired antimeasles antibodies in Nigerian children. Pediatric Infectious Disease Journal. 2000;19(7):635–641. doi: 10.1097/00006454-200007000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Englund JA. The influence of maternal immunization on infant immune responses. Journal of Comparative Pathology. 2007;137(supplement 1):S16–S19. doi: 10.1016/j.jcpa.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Okoko BJ, Wesuperuma LH, Ota MO, et al. Influence of placental malaria infection and maternal hypergammaglobulinaemia on materno-foetal transfer of measles and tetanus antibodies in a rural west African population. Journal of Health, Population, and Nutrition. 2001;19(2):59–65. [PubMed] [Google Scholar]
- 44.Michaux JL, Heremans JF, Hitzig WH. Immunoglobulin levels in cord-blood serum of negroes and Caucasians. Tropical and Geographical Medicine. 1966;18(1):10–14. [PubMed] [Google Scholar]
- 45.Gonçalves G, Cutts FT, Hills M, et al. Transplacental transfer of measles and total IgG. Epidemiology and Infection. 1999;122(2):273–279. doi: 10.1017/s0950268899002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silveira Lessa AL, Krebs VL, Brasil TB, et al. Preterm and term neonates transplacentally acquire IgG antibodies specific to LPS from Klebsiella pneumoniae, Escherichia coli and Pseudomonas aeruginosa . FEMS Immunology and Medical Microbiology. 2011;62(2):236–243. doi: 10.1111/j.1574-695X.2011.00807.x. [DOI] [PubMed] [Google Scholar]
- 47.Mäntyjärvi R, Hirvonen T, Toivanen P. Maternal antibodies in human neonatal sera. Immunology. 1970;18(3):449–451. [PMC free article] [PubMed] [Google Scholar]
- 48.de Moraes-Pinto MI, Almeida AC, Kenj G, et al. Placental transfer and maternally acquired neonatal IgG immunity in human immunodeficiency virus infection. Journal of Infectious Diseases. 1996;173(5):1077–1084. doi: 10.1093/infdis/173.5.1077. [DOI] [PubMed] [Google Scholar]
- 49.Wesumperuma HL, Perera AJ, Pharoah PO, Hart CA. The influence of prematurity and low birthweight on transplacental antibody transfer in Sri Lanka. Annals of Tropical Medicine and Parasitology. 1999;93(2):169–177. doi: 10.1080/00034989958654. [DOI] [PubMed] [Google Scholar]
- 50.Ferrante A, Beard LJ, Feldman RG. IgG subclass distribution of antibodies to bacterial and viral antigens. Pediatric Infectious Disease Journal. 1990;9(supplement 8):S16–S24. [PubMed] [Google Scholar]
- 51.Kameda T, Koyama M, Matsuzaki N, Taniguchi T, Saji F, Tanizawa O. Localization of three subtypes of Fcγ receptors in human placenta by immunohistochemical analysis. Placenta. 1991;12(1):15–26. doi: 10.1016/0143-4004(91)90506-b. [DOI] [PubMed] [Google Scholar]
- 52.Costa-Carvalho BT, Vieira HM, Dimantas RB, et al. Transfer of IgG subclasses across placenta in term and preterm newborns. Brazilian Journal of Medical and Biological Research. 1996;29(2):201–204. [PubMed] [Google Scholar]
- 53.Nagao AT, Costa-Carvalho BT, Arslanian C, et al. Placental transfer of IgG antibodies against Haemophilus influenzae type b capsular polysaccharide in Brazilian term and preterm newborns. Journal of Tropical Pediatrics. 1999;45(3):171–173. doi: 10.1093/tropej/45.3.171. [DOI] [PubMed] [Google Scholar]
- 54.van den Berg JP, Westerbeek EA, Berbers GA, et al. Transplacental transport of IgG antibodies specific for pertussis, diphtheria, tetanus, Haemophilus influenzae type b, and neisseria meningitidis serogroup C is lower in preterm compared with term infants. Pediatric Infectious Disease Journal. 2010;29(9):801–805. doi: 10.1097/inf.0b013e3181dc4f77. [DOI] [PubMed] [Google Scholar]
- 55.Nagao AT, Friedlander-Del Nero D, Arslanian C, Carneiro-Sampaio MM. Elevated levels and different repertoire profile of colostral anti-LPS antibodies may have a significant role in compensating newborn immunity. Scandinavian Journal of Immunology. 2001;53(6):602–609. doi: 10.1046/j.1365-3083.2001.00921.x. [DOI] [PubMed] [Google Scholar]
- 56.Okoko BJ, Wesumperuma HL, Fern J, Yamuah LK, Hart CA. The transplacental transfer of IgC subclasses: influence of prematurity and low birthweight in the Gambian population. Annals of Tropical Paediatrics. 2002;22(4):325–332. doi: 10.1179/027249302125001985. [DOI] [PubMed] [Google Scholar]
- 57.Malek A, Sager R, Kuhn P, Nicolaides KH, Schneider H. Evolution of maternofetal transport of immunoglobulins during human pregnancy. American Journal of Reproductive Immunology. 1996;36(5):248–255. doi: 10.1111/j.1600-0897.1996.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 58.Palmeira P, Yu Ito L, Arslanian C, Carneiro-Sampaio MM. Passive immunity acquisition of maternal anti-enterohemorrhagic Escherichia coli (EHEC) O157:H7 IgG antibodies by the newborn. European Journal of Pediatrics. 2007;166(5):413–419. doi: 10.1007/s00431-006-0250-9. [DOI] [PubMed] [Google Scholar]
- 59.Doroudchi M, Samsami Dehaghani A, Emad K, Ghaderi A. Placental transfer of rubella-specific IgG in fullterm and preterm newborns. International Journal of Gynecology and Obstetrics. 2003;81(2):157–162. doi: 10.1016/s0020-7292(02)00442-3. [DOI] [PubMed] [Google Scholar]
- 60.Costa-Carvalho BT, Vieira HMS, Carbonare SB, et al. Niveles de inmunoglonulinas y lisozimas en sangre de cordon umbilical en recien nacidos de diversas edades gestacionales. Revista Latinoamericana de Perinatologia. 1988;8(3):98–105. [Google Scholar]
- 61.Englund JA. Maternal immunization with inactivated influenza vaccine: rationale and experience. Vaccine. 2003;21(24):3460–3464. doi: 10.1016/s0264-410x(03)00351-7. [DOI] [PubMed] [Google Scholar]
- 62.Munoz FM, Englund JA. A step ahead: infant protection through maternal immunization. Pediatric Clinics of North America. 2000;47(2):449–463. doi: 10.1016/s0031-3955(05)70217-0. [DOI] [PubMed] [Google Scholar]
- 63.Munoz FM, Englund JA. Vaccines in pregnancy. Infectious Disease Clinics of North America. 2001;15(1):253–271. doi: 10.1016/s0891-5520(05)70278-6. [DOI] [PubMed] [Google Scholar]
- 64.Englund JA, Mbawuike IN, Hammill H, et al. Maternal immunization with influenza or tetanus toxoid vaccine for passive antibody protection in young infants. Journal of Infectious Diseases. 1993;168(3):647–656. doi: 10.1093/infdis/168.3.647. [DOI] [PubMed] [Google Scholar]
- 65.Yeager DP, Toy EC, Baker B. Influenza vaccination in pregnancy. American Journal of Perinatology. 1999;16(6):283–286. doi: 10.1055/s-2007-993873. [DOI] [PubMed] [Google Scholar]
- 66.Benowitz I, Esposito DB, Gracey KD, Shapiro ED, Vazquez M. Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants. Clinical Infectious Diseases. 2010;51(12):1355–1361. doi: 10.1086/657309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poehling KA, Szilagyi PG, Staat MA, et al. Impact of maternal immunization on influenza hospitalizations in infants. American Journal of Obstetrics and Gynecology. 2011;204(supplement 6):S141–S148. doi: 10.1016/j.ajog.2011.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eick AA, Uyeki TM, Klimov A, et al. Maternal influenza vaccination and effect on influenza virus infection in young infants. Archives of Pediatrics and Adolescent Medicine. 2011;165(2):104–111. doi: 10.1001/archpediatrics.2010.192. [DOI] [PubMed] [Google Scholar]
- 69.Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. New England Journal of Medicine. 2008;359(15):1555–1564. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 70.Gall SA, Myers J, Pichichero M. Maternal immunization with tetanusdiphtheriapertussis vaccine: effect on maternal and neonatal serum antibody levels. American Journal of Obstetrics and Gynecology. 2011;204(4):334.e1–334.e5. doi: 10.1016/j.ajog.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 71.Nahm MH, Glezen P, Englund J. The influence of maternal immunization on light chain response to Haemophilus influenzae type b vaccine. Vaccine. 2003;21(24):3393–3397. doi: 10.1016/s0264-410x(03)00340-2. [DOI] [PubMed] [Google Scholar]
- 72.Glezen WP, Englund JA, Siber GR, et al. Maternal immunization with the capsular polysaccharide vaccine for Haemophilus influenzae type b. Journal of Infectious Diseases. 1992;165(supplement 1):S134–S136. doi: 10.1093/infdis/165-supplement_1-s134. [DOI] [PubMed] [Google Scholar]
- 73.Englund JA, Glezen WP, Turner C, et al. Transplacental antibody transfer following maternal immunization with polysaccharide and conjugate Haemophilus influenzae type b vaccines. Journal of Infectious Diseases. 1995;171(1):99–105. doi: 10.1093/infdis/171.1.99. [DOI] [PubMed] [Google Scholar]
- 74.Englund JA, Glezen WP, Thompson C, et al. Haemophilus influenzae type b-specific antibody in infants after maternal immunization. Pediatric Infectious Disease Journal. 1997;16(12):1122–1130. doi: 10.1097/00006454-199712000-00005. [DOI] [PubMed] [Google Scholar]
- 75.Mulholland K, Suara RO, Siber G, et al. Maternal immunization with Haemophilus influenzae type b polysaccharide-tetanus protein conjugate vaccine in The Gambia. Journal of the American Medical Association. 1996;275(15):1182–1188. [PubMed] [Google Scholar]
- 76.Baker CJ, Rench MA, McInnes P. Immunization of pregnant women with group B streptococcal type III capsular polysaccharide-tetanus toxoid conjugate vaccine. Vaccine. 2003;21(24):3468–3472. doi: 10.1016/s0264-410x(03)00353-0. [DOI] [PubMed] [Google Scholar]
- 77.Baril L, Briles DE, Crozier P, et al. Natural materno-fetal transfer of antibodies to PspA and to PsaA. Clinical and Experimental Immunology. 2004;135(3):474–477. doi: 10.1111/j.1365-2249.2003.02357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lehmann D, Pomat WS, Riley ID, Alpers MP. Studies of maternal immunisation with pneumococcal polysaccharide vaccine in Papua New Guinea. Vaccine. 2003;21(24):3446–3450. doi: 10.1016/s0264-410x(03)00348-7. [DOI] [PubMed] [Google Scholar]
- 79.Quiambao BP, Nohynek HM, Käyhty H, et al. Immunogenicity and reactogenicity of 23-valent pneumococcal polysaccharide vaccine among pregnant Filipino women and placental transfer of antibodies. Vaccine. 2007;25(22):4470–4477. doi: 10.1016/j.vaccine.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 80.Quiambao BP, Nohynek H, Käyhty H, et al. Maternal immunization with pneumococcal polysaccharide vaccine in the Philippines. Vaccine. 2003;21(24):3451–3454. doi: 10.1016/s0264-410x(03)00349-9. [DOI] [PubMed] [Google Scholar]
- 81.Almeida VDC, Mussi-Pinhata MM, De Souza CB, et al. Immunogenicity of 23-valent pneumococcal polysaccharide vaccine in HIV-infected pregnant women and kinetics of passively acquired antibodies in young infants. Vaccine. 2009;27(29):3856–3861. doi: 10.1016/j.vaccine.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 82.Munoz FM, Englund JA, Cheesman CC, et al. Maternal immunization with pneumococcal polysaccharide vaccine in the third trimester of gestation. Vaccine. 2001;20(5-6):826–837. doi: 10.1016/s0264-410x(01)00397-8. [DOI] [PubMed] [Google Scholar]
- 83.Vidarsson G, Sigurdardottir ST, Gudnason T, et al. Isotypes and opsonophagocytosis of pneumococcus type 6B antibodies elicited in infants and adults by an experimental pneumococcus type 6B- tetanus toxoid vaccine. Infection and Immunity. 1998;66(6):2866–2870. doi: 10.1128/iai.66.6.2866-2870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Siegrist CA. Mechanisms by which maternal antibodies influence infant vaccine responses: review of hypotheses and definition of main determinants. Vaccine. 2003;21(24):3406–3412. doi: 10.1016/s0264-410x(03)00342-6. [DOI] [PubMed] [Google Scholar]
- 85.Gans H, DeHovitz R, Forghani B, et al. Measles and mumps vaccination as a model to investigate the developing immune system: passive and active immunity during the first year of life. Vaccine. 2003;21(24):3398–3405. doi: 10.1016/s0264-410x(03)00341-4. [DOI] [PubMed] [Google Scholar]
- 86.Gans H, Yasukawa L, Rinki M, et al. Immune responses to measles and mumps vaccination of infants at 6, 9, and 12 months. Journal of Infectious Diseases. 2001;184(7):817–826. doi: 10.1086/323346. [DOI] [PubMed] [Google Scholar]
- 87.Gans HA, Arvin AM, Galinus J, et al. Deficiency of the humoral immune response to measles vaccine in infants immunized at age 6 months. Journal of the American Medical Association. 1998;280(6):527–532. doi: 10.1001/jama.280.6.527. [DOI] [PubMed] [Google Scholar]
- 88.Glezen WP. Effect of maternal antibodies on the infant immune response. Vaccine. 2003;21(24):3389–3392. doi: 10.1016/s0264-410x(03)00339-6. [DOI] [PubMed] [Google Scholar]
- 89.Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nature Reviews Immunology. 2009;9(3):185–194. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- 90.Bonhoeffer J, Siegrist CA, Heath PT. Immunisation of premature infants. Archives of Disease in Childhood. 2006;91(11):929–935. doi: 10.1136/adc.2005.086306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bulmer JN, Rasheed FN, Morrison L, Francis N, Greenwood BM. Placental malaria. II. A semi-quantitative investigation of the pathological features. Histopathology. 1993;22(3):219–225. doi: 10.1111/j.1365-2559.1993.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 92.de Moraes-Pinto MI, Verhoeff F, Chimsuku L, et al. Placental antibody transfer: influence of maternal HIV infection and placental malaria. Archives of Disease in Childhood. 1998;79(3):F202–F205. doi: 10.1136/fn.79.3.f202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fried M, Muga RO, Misore AO, Duffy PE. Malaria elicits type 1 cytokines in the human placenta: IFN-γ and TNF-α associated with pregnancy outcomes. Journal of Immunology. 1998;160(5):2523–2530. [PubMed] [Google Scholar]
- 94.Beebe LA, Cowan LD, Altshuler G. The epidemiology of placental features: associations with gestational age and neonatal outcome. Obstetrics and Gynecology. 1996;87(5, part 1):771–778. doi: 10.1016/0029-7844(95)00483-1. [DOI] [PubMed] [Google Scholar]
- 95.Brair ME, Brabin BJ, Milligan P, Maxwell S, Hart CA. Reduced transfer of tetanus antibodies with placental malaria. The Lancet. 1994;343(8891):208–209. doi: 10.1016/s0140-6736(94)90991-1. [DOI] [PubMed] [Google Scholar]
- 96.de Moraes-Pinto MI, Farhat CK, Carbonare SB, et al. Maternally acquired immunity in newborns from women infected by the human immunodeficiency virus. Acta Paediatrica, International Journal of Paediatrics. 1993;82(12):1034–1038. doi: 10.1111/j.1651-2227.1993.tb12805.x. [DOI] [PubMed] [Google Scholar]
- 97.Farquhar C, Nduati R, Haigwood N, et al. High maternal HIV-1 viral load during pregnancy is associated with reduced placental transfer of measles IgG antibody. Journal of Acquired Immune Deficiency Syndromes. 2005;40(4):494–497. doi: 10.1097/01.qai.0000168179.68781.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cumberland P, Shulman CE, Maple PA, et al. Maternal HIV infection and placental malaria reduce transplacental antibody transfer and tetanus antibody levels in newborns in Kenya. Journal of Infectious Diseases. 2007;196(4):550–557. doi: 10.1086/519845. [DOI] [PubMed] [Google Scholar]
- 99.Scott S, Cumberland P, Shulman CE, et al. Neonatal measles immunity in rural Kenya: the influence of HIV and placental malaria infections on placental transfer of antibodies and levels of antibody in maternal and cord serum samples. Journal of Infectious Diseases. 2005;191(11):1854–1860. doi: 10.1086/429963. [DOI] [PubMed] [Google Scholar]
- 100.Jones CE, Naidoo S, De Beer C, et al. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. Journal of the American Medical Association. 2011;305(6):576–584. doi: 10.1001/jama.2011.100. [DOI] [PubMed] [Google Scholar]
- 101.Berger M, Cupps TR, Fauci AS. High-dose immunoglobulin replacement therapy by slow subcutaneous infusion during pregnancy. Journal of the American Medical Association. 1982;247(20):2824–2825. [PubMed] [Google Scholar]
- 102.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clinical Immunology. 1999;92(1):34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 103.Eibel H, Salzer U, Warnatz K. Common variable immunodeficiency at the end of a prospering decade: towards novel gene defects and beyond. Current Opinion in Allergy and Clinical Immunology. 2010;10(6):526–533. doi: 10.1097/ACI.0b013e32833fea1c. [DOI] [PubMed] [Google Scholar]
- 104.Vitoratos N, Bakas P, Kalampani H, Creatsas G. Maternal common variable immunodeficiency and pregnancy. Journal of Obstetrics and Gynaecology. 1999;19(6):654–655. doi: 10.1080/01443619963987. [DOI] [PubMed] [Google Scholar]
- 105.Gardulf A, Anderson E, Lindqvist M, Hansen S, Gustafson R. Rapid subcutaneous IgG replacement therapy at home for pregnant immunodeficient women. Journal of Clinical Immunology. 2001;21(2):150–154. doi: 10.1023/a:1011051704960. [DOI] [PubMed] [Google Scholar]
- 106.Olatunbosun OA. Hypogammaglobulinemia associated with pregnancy. International Journal of Gynecology and Obstetrics. 1993;40(2):162–163. doi: 10.1016/0020-7292(93)90379-b. [DOI] [PubMed] [Google Scholar]
- 107.Palmeira P, Costa-Carvalho BT, Arslanian C, et al. Transfer of antibodies across the placenta and in breast milk from mothers on intravenous immunoglobulin. Pediatric Allergy and Immunology. 2009;20(6):528–535. doi: 10.1111/j.1399-3038.2008.00828.x. [DOI] [PubMed] [Google Scholar]
- 108.Kobayashi R, Mii S, Nakano T, Harada H, Eto H. Neonatal lupus erythematosus in Japan: a review of the literature. Autoimmunity Reviews. 2009;8(6):462–466. doi: 10.1016/j.autrev.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 109.Friedman DM, Kim MY, Copel JA, et al. Utility of cardiac monitoring in fetuses at risk for congenital heart block : the PR interval and dexamethasone evaluation (PRIDE) prospective study. Circulation. 2008;117(4):485–493. doi: 10.1161/CIRCULATIONAHA.107.707661. [DOI] [PubMed] [Google Scholar]
- 110.Escobar MC, Gómez-Puerta JA, Albert D, Ferrer Q, Girona J. Recurrent congenital heart block in neonatal lupus. Clinical Rheumatology. 2007;26(7):1161–1163. doi: 10.1007/s10067-006-0282-6. [DOI] [PubMed] [Google Scholar]
- 111.Stea EA, Routsias JG, Clancy RM, et al. Anti-La/SSB antiidiotypic antibodies in maternal serum: a marker of low risk for neonatal lupus in an offspring. Arthritis and Rheumatism. 2006;54(7):2228–2234. doi: 10.1002/art.21954. [DOI] [PubMed] [Google Scholar]
- 112.Parlowsky T, Welzel J, Amagai M, Zillikens D, Wygold T. Neonatal pemphigus vulgaris: IgG4 autoantibodies to desmoglein 3 induce skin blisters in newborns. Journal of the American Academy of Dermatology. 2003;48(4):623–625. doi: 10.1067/mjd.2003.170. [DOI] [PubMed] [Google Scholar]
- 113.Campo-Voegeli A, Muñiz F, Mascaró JM, et al. Neonatal pemphigus vulgaris with extensive mucocutaneous lesions from a mother with oral pemphigus vulgaris. British Journal of Dermatology. 2002;147(4):801–805. doi: 10.1046/j.1365-2133.2002.04969.x. [DOI] [PubMed] [Google Scholar]
- 114.Saint-Faust M, Perelman S, Dupont D, Velin P, Chatel M. Transient neonatal myasthenia gravis revealing a myasthenia gravis and a systemic lupus erythematosus in the mother: case report and review of the literature. American Journal of Perinatology. 2010;27(2):107–110. doi: 10.1055/s-0029-1224873. [DOI] [PubMed] [Google Scholar]
- 115.Tincani A, Rebaioli CB, Andreoli L, Lojacono A, Motta M. Neonatal effects of maternal antiphospholipid syndrome. Current Rheumatology Reports. 2009;11(1):70–76. doi: 10.1007/s11926-009-0010-8. [DOI] [PubMed] [Google Scholar]
- 116.Abrams ML, Smidt A, Benjamin L, et al. Congenital epidermolysis bullosa acquisita: vertical transfer of maternal autoantibody from mother to infant. Archives of Dermatology. 2011;147(3):337–341. doi: 10.1001/archdermatol.2010.317. [DOI] [PubMed] [Google Scholar]
- 117.Yu Z, Lennon VA. Mechanism of intravenous immune globulin therapy in antibody-mediated autoimmune diseases. New England Journal of Medicine. 1999;340(3):227–228. doi: 10.1056/NEJM199901213400311. [DOI] [PubMed] [Google Scholar]
- 118.Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291(5503):484–486. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 119.Li N, Zhao M, Hilario-Vargas J, et al. Complete FcRn dependence for intravenous Ig therapy in autoimmune skin blistering diseases. Journal of Clinical Investigation. 2005;115(12):3440–3450. doi: 10.1172/JCI24394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hansen RJ, Balthasar JP. Intravenous immunoglobulin mediates an increase in anti-platelet antibody clearance via the FcRn receptor. Thrombosis and Haemostasis. 2002;88(6):898–899. [PubMed] [Google Scholar]
- 121.Li N, Zhao M, Hilario-Vargas J, et al. Complete FcRn dependence for intravenous Ig therapy in autoimmune skin blistering diseases. Journal of Clinical Investigation. 2005;115(12):3440–3450. doi: 10.1172/JCI24394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kaaja R, Julkunen H. Prevention of recurrence of congenital heart block with intravenous immunoglobulin and corticosteroid therapy: comment on the editorial by Buyon et al. Arthritis and Rheumatism. 2003;48(1):280–282. doi: 10.1002/art.10716. [DOI] [PubMed] [Google Scholar]
- 123.Tran HB, Cavill D, Buyon JP, Gordon TP. Intravenous immunoglobulin and placental transport of anti-Ro/La antibodies: comment on the letter by Kaaja and Julkunen. Arthritis and Rheumatism. 2004;50(1):337–338. doi: 10.1002/art.11498. [DOI] [PubMed] [Google Scholar]
- 124.Branch DW, Peaceman AM, Druzin M, et al. A multicenter, placebo-controlled pilot study of intravenous immune globulin treatment of antiphospholipid syndrome during pregnancy. American Journal of Obstetrics and Gynecology. 2000;182(1, part 1):122–127. doi: 10.1016/s0002-9378(00)70500-x. [DOI] [PubMed] [Google Scholar]
- 125.Berkowitz RL, Lesser ML, McFarland JG, et al. Antepartum treatment without early cordocentesis for standard-risk alloimmune thrombocytopenia: a randomized controlled trial. Obstetrics and Gynecology. 2007;110(2, part 1):249–255. doi: 10.1097/01.AOG.0000270302.80336.dd. [DOI] [PubMed] [Google Scholar]
- 126.Lemke H, Coutinho A, Lange H. Lamarckian inheritance by somatically acquired maternal IgG phenotypes. Trends in Immunology. 2004;25(4):180–186. doi: 10.1016/j.it.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 127.Casas R, Björkstén B. Detection of Fel d 1-immunoglobulin G immune complexes in cord blood and sera from allergic and non-allergic mothers. Pediatric Allergy and Immunology. 2001;12(2):59–64. doi: 10.1034/j.1399-3038.2001.012002059.x. [DOI] [PubMed] [Google Scholar]


