Abstract
Context:
It is unclear whether cardiovascular disease is present in primary hyperparathyroidism (PHPT).
Objective:
Aortic valve structure and function were compared in PHPT patients and population-based controls.
Design:
This is a case-control study.
Setting:
The study was conducted in a university hospital metabolic bone disease unit.
Participants:
We studied 51 patients with PHPT and 49 controls.
Outcome Measures:
We measured the aortic valve calcification area and the transaortic pressure gradient.
Results:
Aortic valve calcification area was significantly higher in PHPT (0.24 ± 0.02 vs. 0.17 ± 0.02 cm2, p<0.01), although there was no difference in the peak transaortic pressure gradient, a functional measure of valvular calcification (5.6 ± 0.3 vs. 6.0 ± 0.3 mm Hg, P = 0.39). Aortic valve calcification area was positively associated with PTH (r = 0.34; P < 0.05) but not with serum calcium, phosphorus, or 25-hydroxyvitamin D levels or with calcium-phosphate product. Serum PTH level remained an independent predictor of aortic valve calcification area after adjustment for age, sex, body mass index, smoking status, history of hypercholesterolemia and hypertension, and estimated glomerular filtration rate.
Conclusions:
Mild PHPT is associated with subclinical aortic valve calcification. PTH, but not serum calcium concentration, predicted aortic valve calcification. PTH was a more important predictor of aortic valve calcification than well-accepted cardiovascular risk factors.
Classical primary hyperparathyroidism (PHPT) was a symptomatic disease with increased cardiovascular morbidity and mortality (1, 2). Left ventricular mass was increased and regressed after parathyroidectomy, and valvular and myocardial calcifications were common (3, 4). Today, most patients with PHPT have mild hypercalcemia and few, if any, symptoms. As the clinical and biochemical features of PHPT have become more subtle over time, the investigation of cardiovascular manifestations of the disease has turned to less clinically overt abnormalities.
Aortic valve calcification represents an early atherosclerotic change of the aortic valve and can lead to aortic valve stenosis (5). Although this independent risk factor for cardiovascular mortality and morbidity (6) was common in classical severe PHPT, the cohorts described had significantly higher calcium levels (range 10.9–13.1 mg/dl) than do typical PHPT patients today, making it difficult to extrapolate their data to the majority of patients with PHPT.
In a cohort with mild PHPT, we have previously reported subclinical carotid abnormalities but no increase in left ventricular mass, diastolic dysfunction, or mitral or myocardial calcifications (7, 8). Recent data in secondary hyperparathyroidism due to chronic renal failure suggest that progression of aortic valve stenosis is related to PTH level (9). Given these findings and newly available echocardiographic techniques to assess aortic valve calcification quantitatively, we extend our investigation in this cohort to determine whether aortic valve calcification is increased in mild PHPT.
Subjects and Methods
This is a case-control study comparing aortic valve structure and function in patients with PHPT with normal controls. The current study represents further echocardiographic analysis of the same cohort in whom we have previously reported carotid and cardiac findings (7, 8). As previously described (7, 8), participants represent consecutive PHPT patients between the ages of 45–75 yr who agreed to participate in the study between October 2005 and September 2008. PHPT was diagnosed by the presence of hypercalcemia (calcium >10.2 but <12.0 mg/dl to study the presence of aortic valve findings in those with mild hypercalcemia) and an elevated or inappropriately normal serum PTH level. Fractional excretion of calcium was measured to exclude familial hypocalciuric hypercalcemia, and none had drug-induced hyperparathyroidism. Exclusion criteria included reported use of bisphosphonates within 2 months and initiation or changes in cholesterol-lowering medications within 2 yr of entry to the study (by patient report) (7, 8). Controls were age-, gender-, and race/ethnicity-matched participants in the Northern Manhattan Study (a population-based prospective cohort study designed to investigate cardiovascular risk factors in 3298 community subjects; recruitment and enrollment as described) (10, 11), who had undergone transthoracic echocardiography using the Sonos 5500 (Philips Medical Systems, Andover, MA). All patients gave written, informed consent. This study was approved by the Institutional Review Board of Columbia University Medical Center.
Cardiovascular risk factors
Demographic data, cardiac risk factors, and medical history were obtained from participants. These included race/ethnicity by self-identification, coronary artery disease (history of myocardial infarction, angina, angioplasty or coronary artery bypass surgery), hypercholesterolemia (a physician's report of elevated lipid levels or being on a lipid-lowering medication), hypertension (systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or self-report of hypertension or antihypertensive medication use), diabetes mellitus (fasting blood glucose level >126 mg/dl, self-report of diabetes, or use of insulin or other hypoglycemic medications), and cigarette smoking (categorized as nonsmoker or ever smoked).
Biochemical evaluation
Fasting samples for serum calcium, phosphorus, creatinine, and cholesterol were measured by an automated chemistry analyzer. PTH was measured by immunochemiluminometric assay for intact PTH (Scantibodies Laboratories, Inc., Santee, CA), which detects PTH (1–84) and PTH (7–84). Serum 25-hydroxyvitamin D was measured by RIA (Diasorin, Stillwater, MN).
Transthoracic echocardiography and definition of calcification area
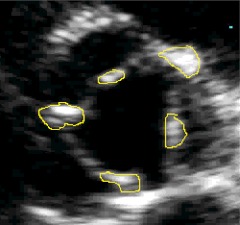
Transthoracic echocardiography was performed using a commercially available system (Sonos 5500; Philips) by a single trained, registered sonographer according to a standardized protocol. Left ventricular end-diastolic diameter, interventricular septum thickness, and posterior wall thickness were measured from a parasternal long-axis view according to the recommendations of the American Society of Echocardiography (12). Peak aortic jet flow was measured on continuous-wave Doppler echocardiograms. Aortic valve calcification was defined as bright dense echoes of more than 1 mm on one or more cusps (13). The extent of aortic valve calcification was visually assessed according to the standard visual score method as follows: 1, no calcification; 2, mildly calcified (small isolated spots); 3, moderately calcified (multiple larger spots); and 4, heavily calcified (extensive thickening and calcification of all cusps) (14). Calcification area for each aortic valve leaflet was also measured by a single investigator who was blinded to group status (Fig. 1). A total valve score (aortic valve calcification area) was calculated as the sum of individual leaflet scores. The mean aortic valve gradient, a functional measure of the severity of aortic valve calcification, was obtained by tracing the continuous wave flow velocity signal across the aortic valve.
Fig. 1.
Representative two-dimensional transthoracic echocardiography (short-axis view) image of the aortic valve (in a patient with primary hyperparathyroidism) indicating the assessment of aortic valve calcification area. Areas of calcification are surrounded by solid lines.
Statistical analysis
Statistical analysis was performed using SPSS version 11.5J (Chicago, IL). Categorical variables are described by number and percentages. Continuous variables are expressed as mean value ± se. Differences between groups were assessed with the χ2 test for categorical variables, and comparisons of continuous variables were made using unpaired t test. Correlation coefficients between aortic valve calcification area and potential markers of calcification (calcium, PTH, phosphorus, calcium-phosphorus product, and 25-hydroxyvitamin D) were determined by linear regression analysis. Multiple regression analysis was carried out to determine whether PTH level is an independent predictor of aortic valve calcification area after controlling for the known risk factors for aortic valve calcification area in the general population [age, sex, body mass index, smoking status, history of hypercholesterolemia and hypertension, and estimated glomerular filtration rate (calculated by the Modification of Diet in Renal Disease formula)] (15–19). Standardized β-coefficients (β-coefficient/sd; change in the outcome per sd increase in the predictor) were calculated to determine which predictor (measured in different units) had the greatest effect on the outcome. A P value <0.05 was considered statistically significant.
Results
Clinical and biochemical data
Consistent with the known demographics of PHPT, cases were predominantly female and had biochemical evidence typical of mild PHPT [serum calcium = 10.4 ± 0.1 mg/dl (normal range = 8.7–10.2 mg/dl); PTH = 85 ± 5 pg/ml (normal range = 10–66 pg/ml)]. Control subjects had more cardiovascular risk factors than PHPT subjects, with higher systolic and diastolic blood pressure and body mass index as well as low-density lipoprotein levels (Table 1). Although PTH and 25-hydroxyvitamin D were not available on Northern Manhattan Study controls, serum calcium levels were normal and significantly lower than in PHPT (9.4 ± 0.1 mg/dl; P < 0.001).
Table 1.
Participant characteristics and cardiovascular risk factors
| Variable | PHPT (n = 51) | Controls (n = 49) | P value |
|---|---|---|---|
| Age (yr) | 61.4 ± 1.0 | 63.4 ± 0.8 | 0.13 |
| Race/ethnicity | |||
| Caucasian (%) | 100 | 96 | 0.24 |
| Hispanic (%) | 12 | 7 | 0.49 |
| African-American (%) | 0 | 4 | 0.24 |
| Male (%) | 20 | 24 | 0.56 |
| Coronary artery disease (%) | 8 | 4 | 0.43 |
| Hypercholesterolemia (%) | 41 | 45 | 0.71 |
| Hypertension (%) | 29 | 22 | 0.43 |
| Diabetes mellitus (%) | 2 | 4 | 0.53 |
| Ever smoke | 49 | 51 | 0.84 |
| Body mass index (kg/m2) | 25 ± 0.6 | 27 ± 0.9 | <0.05 |
| Systolic blood pressure (mm Hg) | 124 ± 2.6 | 138 ± 3.1 | <0.01 |
| Diastolic blood pressure (mm Hg) | 75 ± 1.6 | 81 ± 1.7 | <0.05 |
| Total cholesterol (mg/dl) | 211 ± 4.9 | 218 ± 5.7 | 0.35 |
| Triglycerides (mg/dl) | 98 ± 7.8 | 70 ± 7.4 | <0.05 |
| High-density lipoprotein (mg/dl) | 69 ± 2.5 | 66 ± 2.5 | 0.41 |
| Low-density lipoprotein (mg/dl) | 122 ± 4.4 | 138 ± 5.2 | <0.05 |
| Glomerular filtration rate (ml/min · 1.73 m2) | 76 ± 2.6 | 73 ± 2.1 | 0.37 |
Data are expressed as mean ± se or as a percentage.
Cardiac structure and function
All participants (PHPT and control) had mild aortic valve calcification (calcification score = 2, defined as small isolated spots of calcification or a single larger spot). Although the calcification was mild using the visual calcification score, when the quantitative technique was used, the area of calcification was 41% greater in the PHPT patients compared with controls (adjusted for age, sex, body mass index, smoking status, history of hypercholesterolemia and hypertension, and estimated glomerular filtration rate; Table 2). Despite the increase in calcification area, peak and mean transaortic pressure gradient, functional measures of the severity of aortic valve stenosis, were not different between the groups. Other measures of cardiac structure that can be affected by aortic valve stenosis, including left ventricular end-diastolic and end-systolic dimension, ventricular septal thickness, and posterior left ventricular wall thickness were normal and also did not differ between groups (Table 2).
Table 2.
Echocardiographic data
| Variable | PHPT (n = 51) | Controls (n = 49) | Normal range | P value |
|---|---|---|---|---|
| Left ventricle end-diastolic dimension, (mm) | 45.0 ± 0.6 | 44.4 ± 0.6 | 39–54 | 0.51 |
| Left ventricle end-systolic dimension, (mm) | 27.6 ± 0.6 | 28.0 ± 0.6 | 0.62 | |
| Ventricular septum, (mm) | 10.9 ± 0.2 | 11.0 ± 0.2 | 6–10 | 0.74 |
| Posterior left ventricle wall, (mm) | 10.6 ± 0.2 | 10.9 ± 0.2 | 6–10 | 0.26 |
| Mean transaortic pressure gradient, (mm Hg) | 2.8 ± 0.2 | 3.2 ± 0.2 | 0.16 | |
| Peak transaortic pressure gradient, (mm Hg) | 5.6 ± 0.3 | 6.0 ± 0.3 | <25.0 | 0.39 |
| Aortic valve calcification area (cm2) | 0.24 ± 0.02 | 0.17 ± 0.02 | <0.01 |
Data are expressed as mean ± se. Data are adjusted for age, sex, body mass index, smoking status, estimated glomerular filtration, and history of hypercholesterolemia and hypertension.
Relationship between indices of mineral metabolism and aortic valve calcification area
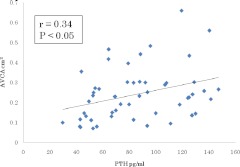
There was no linear association between serum calcium, phosphorus, calcium-phosphorus product, or 25-hydroxyvitamin D level and aortic valve calcification area in PHPT patients (Table 3). However, serum PTH level positively correlated with aortic valve calcification area (r = 0.34; P < 0.05; Fig. 2). When multiple regression analysis was carried out to evaluate the influence of PTH and cardiovascular risk factors on aortic valve calcification area, only serum PTH level remained a significant predictor of valve calcification [model coefficient of determination (R2) = 0.306; P < 0.05; Table 4].
Table 3.
Univariate correlates of aortic valve calcification area in patients with primary hyperparathyroidism
| Variable | Aortic valve calcification area |
|
|---|---|---|
| r | P | |
| Calcium | 0.03 | 0.81 |
| Phosphorus | 0.01 | 0.51 |
| Calcium-phosphorus product | 0.11 | 0.43 |
| 25-Hydroxyvitamin D | 0.04 | 0.80 |
| PTH | 0.34 | <0.05 |
Fig. 2.
Relationship between PTH level and aortic valve calcification area (AVCA) in patients with primary hyperparathyroidism.
Table 4.
Multiple regression model of aortic valve calcification area in patients with primary hyperparathyroidism
| Variable | β | se | P value | Model R2 |
|---|---|---|---|---|
| PTH | 0.318 | 0.001 | 0.046 | R2 = 0.306 |
| Male | 0.214 | 0.049 | 0.162 | P = 0.037 |
| Ever smoke | −0.225 | 0.036 | 0.110 | |
| Age | 0.153 | 0.003 | 0.300 | |
| Body mass index | −0.254 | 0.005 | 0.127 | |
| Hypertension | 0.130 | 0.045 | 0.417 | |
| Hypercholesterolemia | 0.177 | 0.043 | 0.278 | |
| Glomerular filtration rate | −0.084 | 0.001 | 0.544 |
R2, Coefficient of determination.
Discussion
Aortic valve calcification was commonplace in PHPT cohorts with significantly higher serum calcium and PTH levels than are typically observed today (4, 20, 21). In a cohort in whom we have previously reported normal left ventricular mass index and diastolic function (8), this report demonstrates that aortic valve calcification is also a finding in mild PHPT and is associated with PTH levels, independent of serum calcium, vitamin D, and cardiovascular risk factors.
It is unclear whether the calcification observed in our cohort portends an increased risk of cardiovascular events. Aortic valve calcification without any resultant outflow obstruction, as observed in this study, is a subclinical abnormality that is common in the elderly (16, 22). Once aortic valve calcification has occurred, it causes less effective stress sharing between valve leaflets and accelerates injury to endothelial cells on those leaflets (23). In general, this is an irreversible process that leads to severe calcification and clinically significant stenosis in 10% of subjects (4, 24). A recent study in non-PHPT patients with mild to moderate asymptomatic aortic stenosis, found that the degree of aortic calcification was the most powerful predictor of clinical outcome (24, 25).
The finding that aortic valve calcification is associated with PTH levels in PHPT may provide insight into the pathogenesis of aortic valve calcification, which is not completely understood. Despite associations with various cardiovascular risks (15–19), factors other than aging and atherosclerosis must play a role in the development of aortic calcification, because only 50% of patients with severe aortic stenosis have significant coronary artery disease (26). PTH is a key hormonal regulator of mineral metabolism, essential for both bone formation and osteoblast activity. Similar to vascular calcification, aortic valve calcification is an active process resembling osteogenesis (27), which involves osteoblast transformation of valvular tissue (28, 29). Although the specific role of PTH in this process is incompletely known, PTH levels are associated with aortic valve calcification in secondary hyperparathyroidism due to renal failure in both animal models and humans (9, 30). Recent work also indicates that a PTH gene single-nucleotide polymorphism is associated with severe calcific aortic stenosis (31). Our results support the hypothesis that PTH may play a role in mediating aortic valve calcification in PHPT patients. The finding that mild calcification was present in both PHPT and controls but those with PHPT had greater calcification area suggests that elevated PTH may not initiate the calcification process but may exacerbate it once underway.
We have previously reported that this PHPT cohort did not have increased mitral annular calcification (8). This difference may be more apparent than real, because the methodology used in this study is more sensitive, assessing calcification quantitatively (calcification area) as opposed to just qualitatively (presence or absence of mitral valve calcification as in our previous study). However, aortic valvular calcification is more common than mitral annular calcification in non-PHPT individuals, due to greater mechanical stress from differences in flow dynamics on the aortic compared with the mitral valve (22, 32–34). Alternatively, the aortic valve may be more sensitive to PTH or respond differently to the effects of PHPT.
This study has several limitations. First, although the control group was a random sample of community individuals, they had more cardiovascular risk factors than PHPT patients. Although we adjusted for cardiovascular risk factors known to be associated with aortic valve calcification in our analysis, the presence of increased cardiovascular risks in the control population may have biased against our finding a greater increase in aortic valve calcification in PHPT. Second, the study represents a convenience sample of patients with PHPT, including those who met as well as those who did not meet surgical criteria for parathyroidectomy, and the sample size does not allow us to conclude whether findings have different clinical significance in these subgroups. Finally, we did not have vitamin D or PTH data available on our control population, making it impossible to determine whether the association between aortic valve calcification area and vitamin D status or PTH is different in PHPT and non-PHPT populations.
Despite these limitations, the study has important strengths. We characterized the effect of PHPT on aortic valve structure and function in a homogeneous group of patients with biochemically mild disease. Furthermore, valve calcification area was determined by a single individual blinded to disease status, and we were able to adjust our analyses for known cardiovascular risk factors that may have confounded previous studies. Finally, this study affords some mechanistic insight into aortic valve calcification in PHPT by providing the first data on an association with PTH levels and suggests that future studies be designed to further examine the role of PTH in the physiology of aortic valve calcification in PHPT and in non-hyperparathyroid populations.
We conclude that mild PHPT does affect the aortic valve and that aortic valve calcification area in PHPT is increased and independently associated with the degree of PTH elevation. It will be of interest to determine whether surgical cure of PHPT diminishes the progression of aortic valve calcification, which could significantly impact management of this important and common endocrine disorder.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 DK066329, K24 DK074457, and R37 NS29993.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- PHPT
- Primary hyperparathyroidism.
References
- 1. Palmér M, Adami HO, Bergström R, Akerström G, Ljunghall S. 1987. Mortality after surgery for primary hyperparathyroidism: a follow-up of 441 patients operated on from 1956 to 1979. Surgery 102:1–7 [PubMed] [Google Scholar]
- 2. Hedback G, Oden A, Tisell LE. 1991. The influence of surgery on the risk of death in patients with primary hyperparathyroidism. World J Surg 15:399–405; discussion 406–397 [DOI] [PubMed] [Google Scholar]
- 3. Stefenelli T, Abela C, Frank H, Koller-Strametz J, Globits S, Bergler-Klein J, Niederle B. 1997. Cardiac abnormalities in patients with primary hyperparathyroidism: implications for follow-up. J Clin Endocrinol Metab 82:106–112 [DOI] [PubMed] [Google Scholar]
- 4. Stefenelli T, Mayr H, Bergler-Klein J, Globits S, Woloszczuk W, Niederle B. 1993. Primary hyperparathyroidism: incidence of cardiac abnormalities and partial reversibility after successful parathyroidectomy. Am J Med 95:197–202 [DOI] [PubMed] [Google Scholar]
- 5. Cosmi JE, Kort S, Tunick PA, Rosenzweig BP, Freedberg RS, Katz ES, Applebaum RM, Kronzon I. 2002. The risk of the development of aortic stenosis in patients with ‘benign’ aortic valve thickening. Arch Intern Med 162:2345–2347 [DOI] [PubMed] [Google Scholar]
- 6. Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. 1999. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med 341:142–147 [DOI] [PubMed] [Google Scholar]
- 7. Walker MD, Fleischer J, Rundek T, McMahon DJ, Homma S, Sacco R, Silverberg SJ. 2009. Carotid vascular abnormalities in primary hyperparathyroidism. J Clin Endocrinol Metab 94:3849–3856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walker MD, Fleischer JB, Di Tullio MR, Homma S, Rundek T, Stein EM, Zhang C, Taggart T, McMahon DJ, Silverberg SJ. 2010. Cardiac structure and diastolic function in mild primary hyperparathyroidism. J Clin Endocrinol Metab 95:2172–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iwata S, Hyodo E, Yanagi S, Hayashi Y, Nishiyama H, Kamimori K, Ota T, Matsumura Y, Homma S, Yoshiyama M. 24 June 2011. Parathyroid hormone and systolic blood pressure accelerate the progression of aortic valve stenosis in chronic hemodialysis patients. Int J Cardiol 10.1016/j.ijcard.2011.06.025 [DOI] [PubMed] [Google Scholar]
- 10. Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, Shea S, Paik MC, Hauser WA. 1998. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol 147:259–268 [DOI] [PubMed] [Google Scholar]
- 11. White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, Sacco RL. 2005. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation 111:1327–1331 [DOI] [PubMed] [Google Scholar]
- 12. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. 2005. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18:1440–1463 [DOI] [PubMed] [Google Scholar]
- 13. Wang AY, Woo J, Wang M, Sea MM, Ip R, Li PK, Lui SF, Sanderson JE. 2001. Association of inflammation and malnutrition with cardiac valve calcification in continuous ambulatory peritoneal dialysis patients. J Am Soc Nephrol 12:1927–1936 [DOI] [PubMed] [Google Scholar]
- 14. Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, Maurer G, Baumgartner H. 2000. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 343:611–617 [DOI] [PubMed] [Google Scholar]
- 15. Boon A, Cheriex E, Lodder J, Kessels F. 1997. Cardiac valve calcification: characteristics of patients with calcification of the mitral annulus or aortic valve. Heart 78:472–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. 1997. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 29:630–634 [DOI] [PubMed] [Google Scholar]
- 17. Lindroos M, Kupari M, Valvanne J, Strandberg T, Heikkilä J, Tilvis R. 1994. Factors associated with calcific aortic valve degeneration in the elderly. Eur Heart J 15:865–870 [DOI] [PubMed] [Google Scholar]
- 18. Fox CS, Larson MG, Vasan RS, Guo CY, Parise H, Levy D, Leip EP, O'Donnell C J, D'Agostino RB, Sr., Benjamin EJ. 2006. Cross-sectional association of kidney function with valvular and annular calcification: the Framingham heart study. J Am Soc Nephrol 17:521–527 [DOI] [PubMed] [Google Scholar]
- 19. JH, Shlipak MG, Katz R, Budoff MJ, Shavelle DM, Probstfield JL, Takasu J, Detrano R, O'Brien KD. 2007. Kidney function and aortic valve and mitral annular calcification in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis 50:412–420 [DOI] [PubMed] [Google Scholar]
- 20. Längle F, Abela C, Koller-Strametz J, Mittelböck M, Bergler-Klein J, Stefenelli T, Woloszczuk W, Niederle B. 1994. Primary hyperparathyroidism and the heart: cardiac abnormalities correlated to clinical and biochemical data. World J Surg 18:619–624 [DOI] [PubMed] [Google Scholar]
- 21. Niederle B, Stefenelli T, Glogar D, Woloszczuk W, Roka R, Mayr H. 1990. Cardiac calcific deposits in patients with primary hyperparathyroidism: preliminary results of a prospective echocardiographic study. Surgery 108:1052–1056; discussion 1056–1057 [PubMed] [Google Scholar]
- 22. Lindroos M, Kupari M, Heikkila J, Tilvis R. 1993. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol 21:1220–1225 [DOI] [PubMed] [Google Scholar]
- 23. Deck JD. 1986. Endothelial cell orientation on aortic valve leaflets. Cardiovasc Res 20:760–767 [DOI] [PubMed] [Google Scholar]
- 24. Faggiano P, Antonini-Canterin F, Erlicher A, Romeo C, Cervesato E, Pavan D, Piazza R, Huang G, Nicolosi GL. 2003. Progression of aortic valve sclerosis to aortic stenosis. Am J Cardiol 91:99–101 [DOI] [PubMed] [Google Scholar]
- 25. Rosenhek R, Klaar U, Schemper M, Scholten C, Heger M, Gabriel H, Binder T, Maurer G, Baumgartner H. 2004. Mild and moderate aortic stenosis. Natural history and risk stratification by echocardiography. Eur Heart J 25:199–205 [DOI] [PubMed] [Google Scholar]
- 26. Mazzone A, Venneri L, Berti S. 2007. Aortic valve stenosis and coronary artery disease: pathophysiological and clinical links. J Cardiovasc Med (Hagerstown) 8:983–989 [DOI] [PubMed] [Google Scholar]
- 27. Demer LL, Tintut Y. 2008. Vascular calcification: pathobiology of a multifaceted disease. Circulation 117:2938–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, Spelsberg T. 2003. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation 107:2181–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aikawa E, Nahrendorf M, Sosnovik D, Lok VM, Jaffer FA, Aikawa M, Weissleder R. 2007. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation 115:377–386 [DOI] [PubMed] [Google Scholar]
- 30. Shuvy M, Abedat S, Beeri R, Danenberg HD, Planer D, Ben-Dov IZ, Meir K, Sosna J, Lotan C. 2008. Uraemic hyperparathyroidism causes a reversible inflammatory process of aortic valve calcification in rats. Cardiovasc Res 79:492–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schmitz F, Ewering S, Zerres K, Klomfass S, Hoffmann R, Ortlepp JR. 2009. Parathyroid hormone gene variant and calcific aortic stenosis. J Heart Valve Dis 18:262–267 [PubMed] [Google Scholar]
- 32. Fox CS, Parise H, Vasan RS, Levy D, O'Donnell CJ, D'Agostino RB, Plehn JF, Benjamin EJ. 2004. Mitral annular calcification is a predictor for incident atrial fibrillation. Atherosclerosis 173:291–294 [DOI] [PubMed] [Google Scholar]
- 33. Fox E, Harkins D, Taylor H, McMullan M, Han H, Samdarshi T, Garrison R, Skelton T. 2004. Epidemiology of mitral annular calcification and its predictive value for coronary events in African Americans: the Jackson Cohort of the Atherosclerotic Risk in Communities Study. Am Heart J 148:979–984 [DOI] [PubMed] [Google Scholar]
- 34. Allison MA, Cheung P, Criqui MH, Langer RD, Wright CM. 2006. Mitral and aortic annular calcification are highly associated with systemic calcified atherosclerosis. Circulation 113:861–866 [DOI] [PubMed] [Google Scholar]