Abstract
Context:
Endometriosis affects approximately 10% of women in the United States and causes pain and infertility. Decidualization of endometrial stromal cells from women with endometriosis is aberrant.
Objective:
The objective of this study was to investigate a potential mechanism for the inadequate decidual response in stromal cells from ovarian endometriomas.
Design:
Stromal cells of the endometrium from women without endometriosis (HSC) or from ovarian endometriomas (OsisSC) were grown in culture and treated with 10 μm LY294002 or 250 nm MK2206, 100 nm medroxyprogesterone acetate (M), and 0.5 mm dibutyryl cAMP (A) or infection with 100 multiplicity of infection adenoviral constructs containing wild-type Forkhead box O1 or triple-mutant FOXO1. Real-time PCR was used to measure the expression of FOXO1, IGF binding protein-1 (IGFBP1), and prolactin (PRL) mRNA, and Western blot and immunohistochemical staining were used to detect the levels of progesterone receptor (PR), FOXO1, AKT, and p(Ser473)-AKT protein in vitro or in vivo.
Results:
Expression of the decidua-specific genes, IGFBP1 and PRL, were significantly lower in OsisSC compared with normal HSC in response to M+A treatment. Basal expression levels of PRA, PRB, and FOXO1 proteins were dramatically lower in OsisSC. Overexpression of triple-mutant FOXO1 increased mRNA levels of IGFBP1 and PRL in OsisSC in the presence of M+A, whereas the overexpression of wild-type FOXO1 had no effect. AKT was highly phosphorylated in OsisSC compared with HSC and inhibition of phosphatidylinositol 3-kinase, with LY294002, increased levels of FOXO1 protein as well as IGFBP1 mRNA in the presence of M+A. Moreover, inhibition of AKT with MK2206, an allosteric AKT inhibitor, dramatically increased the accumulation of nuclear FOXO1 as well as expression of IGFBP1. Finally, immunohistochemical staining demonstrated higher p(Ser473)-AKT and lower FOXO1 levels in endometriosis tissues, compared with normal endometrial tissues.
Conclusions:
In endometriotic stromal cells, overactivation of the phosphatidylinositol 3-kinase/AKT signaling pathway contributes to the reduced expression of the decidua-specific gene, IGFBP1, potentially through reduced levels of nuclear FOXO1.
Endometriosis is a common gynecological disorder with a prevalence of up to 10% of the female population in the United States. This disorder is estrogen dependent and characterized by the presence and growth of the endometrial glands and stroma outside of the uterus, most commonly in the pelvic peritoneum, ovaries, and the pouch of Douglas (1, 2). The main clinical complications of this disease include chronic pelvic pain and infertility as well as increased susceptibility to developing cancers (1). The most widely accepted hypothesis regarding the pathogenesis of endometriosis is the implantation and growth of disseminated endometrial fragments and viable cells after retrograde flow of menses during menstruation (3). To date, the therapeutic strategies for this disease are limited by the unacceptable side effects and frequent recurrence. Progestins have been used to treat pain, most likely by suppressing or attenuating ovulation; however, its direct effects on endometriotic lesions have not been studied in any detail.
One of the remodeling events of the endometrium that occurs in response to progesterone is the process of decidualization. Specifically, endometrial stroma differentiates to a dense decidua to prepare the endometrium for embryo implantation (4). Decidualized stromal cells possess characteristics of secretory cells and abundantly secrete the proteins, prolactin (PRL) and IGF binding protein-1 (IGFBP1). Although it is established that progesterone is a key hormone involved in initiating and prolonging the decidualization process, it has been demonstrated at the molecular level that other pathways, such as the protein kinase A pathway, as well as other transcription factors are involved, amplifying the progesterone-mediated decidual response (5–9). FOXO1, a member of forkhead-box O family, is a nuclear transcription factor involved in cell cycle arrest, differentiation, and apoptosis. It has been demonstrated that cAMP and progesterone pathways are essential in initiating and maintaining decidualization. Specifically, it has been shown that cAMP and progestins decrease phosphorylated AKT levels and increase nuclear FOXO1 levels in endometrial stromal cells (10, 11). Furthermore, FOXO1 can physically associate with the progesterone receptor to modulate expression of decidua-specific genes (9, 11, 12). Gene profiling studies revealed that approximately 15% of genes significantly regulated during decidualization were under the influence of FOXO1 and that a subset of these genes was also dependent on the progesterone receptor (PR) (11).
In this study, we demonstrate that expression of IGFBP1 and PRL in primary endometriotic stromal cells (OsisSC) is dramatically lower in response to the decidual stimuli, medroxyprogesterone acetate and dibutyryl cAMP (M+A). Levels of FOXO1 and PR are lower in endometriosis. We also found that levels of p(Ser473)-AKT were consistently higher in endometriotic stromal cells compared with normal endometrial stromal cells. Upon inhibition of both phosphatidylinositol 3-kinase (PI3K) and AKT, levels of nuclear FOXO1 increased and expression of IGFBP1 significantly increased in response to M+A. Our data show that down-regulation of FOXO1 due to overactive PI3K/AKT pathway contributes to the reduced expression of the decidual gene, IGFBP1, in endometriosis.
Materials and Methods
Tissue collection and primary cell culture
Normal endometrial tissues were obtained from premenopausal women undergoing hysterectomy for uterine leiomyoma or pelvic organ prolapse. Ectopic endometriotic lesions were obtained from ovarian endometriomas from patients undergoing ovarian cystectomy or oophorectomy. Tissues were obtained from both the proliferative and secretory phases of the menstrual cycle. None of the subjects received any preoperative hormonal therapy within 6 months of surgery. Brown or dark red lesions inside the ovary chocolate cysts were collected and diagnosis of ovary endometriomas was confirmed by pathological reports. Tissues for immunohistochemical staining for PR, FOXO1, and p(Ser473)-AKT were obtained from the Prentice Women's Hospital at Northwestern University (Chicago, IL). Written informed consents were obtained from all subjects before surgery. Stromal cells were isolated from normal endometrium and ovary endometriomas as previously described (13). Cells were cultured in DMEM/F12 (1:1) (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS) and penicillin (100 U/ml) and streptomycin (100 U/ml) at 37 C in humidified atmosphere with 5% CO2. Culture medium was changed every 3 d. All portions of the study were approved by the Northwestern Institutional Review Board for Human Research.
Hormonal treatments
Medroxyprogesterone acetate and dibutyryl-cAMP (Sigma, St. Louis, MO) were dissolved in ethanol or water at stock concentrations of 5 and 200 mm, respectively, and stored at −20 C. At 80% confluence, cells were treated with final concentrations of 100 nm medroxyprogesterone acetate and 0.5 mm dibutyryl-cAMP in 2% charcoal-stripped FBS for 48 h (9). We referred to this hormonal treatment as M+A. For inhibitor treatments, cells were pretreated with 20 μm MG132 (Calbiochem, San Diego, CA), 10 μm LY294002 (Sigma), 10 μm U0126 (Promega, Madison, WI), or 250 nm MK2206 (Merck, Whitehouse Station, NJ) and then treated with M+A in the presence of inhibitors and incubated for 48 h.
RNA isolation and real-time PCR
Cells were lysed using Trizol reagent (Sigma), and total RNA was extracted following the standard manufacturer's protocol. Five hundred nanograms of total RNA were reverse transcribed in a final volume of 20 μl to generate cDNA with the Superscript III first-strand synthesis system (Invitrogen). Real-time PCR analysis was performed using the ABI 7000 sequence detection system and the ABI Power Sybr Green gene expression detection system (Applied Biosystems Inc., Foster City, CA) to quantify IGFBP1, PRL, and FOXO1 mRNA levels. Ribosomal protein, large, P0 was used as an internal control. All PCR were run for 40 cycles (95 C for 15 sec, 60 C for 1 min) after 10 min incubation at 95 C. The fold change in expression of each gene was calculated using the change in cycle threshold value method (ΔΔ Ct) (14).
Western blot analysis
For total cell extracts, cells were lysed with M-PER mammalian protein extraction reagent (Thermo scientific, Rockford, IL) supplemented with protease and phosphatase inhibitors (Sigma) to recuperate total proteins. Cytoplasmic and nuclear fractions were isolated using the commercially available NE-PER nuclear and cytoplasmic extraction reagents (Thermo Scientific). Protein concentrations were determined by the Micro bicinchoninic assay protein assay kit (Thermo Scientific). Forty micrograms of total protein extract boiled with 5× loading buffer were run on 8% polyacrylamide gel and then transferred to a polyvinyl difluoride membrane. The membranes were blocked with 5% milk in Tris-buffered saline with 0.1% Tween 20 (TBST) for 1 h at room temperature and then incubated overnight at 4 C with antibodies against PR (Dako, Fort Collins, CO), FOXO1 (Bethyl Laboratories, Montgomery, TX), p(Ser473)-AKT, total AKT, phospho-44/42MAPK(ERK1/2) (Thr202/Tyr204), total ERK (Cell Signaling, Danvers, MA), and β-actin (Sigma). After washes with TBST, the blots were incubated with horseradish peroxidase (HRP)-conjugated goat antirabbit or mouse secondary antibodies (1:10,000) for 1 h and then detected with a chemiluminescent detection kit (Thermo Scientific).
Adenovirus infection
At 80% confluence, primary OsisSC and stromal cells of the endometrium from women without endometriosis (HSC) were infected with the adenovirus constructs expressing the wild-type FOXO1 (WT-FOXO1) or triple-mutant FOXO1 (Tm-FOXO1). The Tm-FOXO1 construct has mutations at Thr-24, Ser-256, and Ser-319, which cannot be phosphorylated and thus remains constitutively active in the nucleus. Cells were infected for 24 h with a multiplicity of infection of 100 plaque-forming units per cell. Infection of adenovirus containing empty vector was used as the control. After infection, cells were washed and treated with or without M+A for 48 h in media containing 2% charcoal-stripped FBS and antibiotics.
Immunofluorescent staining
Cells were fixed with 4% paraformaldehyde (Sigma), and coverslips were then washed with PBS and permeabilized with 0.1% Triton X-100-0.1% deoxycholate (Sigma). Cells were blocked with 5% BSA (Sigma) made in PBS. Primary antibody to FOXO1 (Cell Signaling) was added and incubated overnight at 4 C. A secondary Alexa Fluor 488 goat antirabbit IgG (Invitrogen) was used. Cells were then mounted with mounting media (Invitrogen) for fluorescence on glass slides and visualized using a fluorescent inverted microscope, Axiovert 200 (Zeiss, Thornwood, NY).
Immunohistochemical staining
Fresh tissues were fixed in buffered formalin and embedded in paraffin. Embedded tissues were cut into 4-μm sections and then mounted onto glass slides. Tissue sections were deparaffinized and rehydrated, and antigen retrieval was performed by heating sections for 40 min in citrate buffer at pH 6.0. Endogenous peroxidase activity was blocked by incubating sections with 3% H2O2 for 10 min. Sections were blocked with protein block for 10 min (Dako) followed by rinsing with TBST and then incubated with primary antibodies to FOXO1 (Bethyl Laboratories) and p(Ser473)-AKT (Cell Signaling) overnight at 4 C in a humidified chamber. Slides were rinsed with TBST and HRP-conjugated secondary antibodies were applied for 30 min. HRP activity was detected using diaminobenzidine tetrahydrochloride as substrate for 7 min and then counterstained with hematoxylin. Sections incubated with dilution buffer without primary antibody were used as the negative control. For quantification of staining, slides were first scanned with TissueGnostics (TissueFax version 2.0.41.47; Vienna, Austria), and the immunointensity and percentage of immunopositive stromal cells were measured with HistoQuest software (TissueGnostics USA Ltd., Los Angeles, CA; version 2.0.2.0252) at the Cell Imaging Facility Core at Northwestern University. A two-score system for immunointensity and immunopositivity was used for quantifying the immunoreactivity for each marker. The immunointensity was scored on a scale of: 0, negative; 1, weak; 2, moderate; 3, strong. The percentage of immunopositive cells was scored on a scale of: 1, 1–10%; 2, 11–50%; 3, 51–80%; 4, greater than 80%. The combination score for the two-score system was calculated by multiplying the scores of immunointensity by the scores of immunopositivity (immunointensity × immunopositivity). Three different regions of each section were assessed under the same parameters.
Statistical analysis
All values were presented as mean ± sem. All fold changes were first log transformed and statistical significance between samples was analyzed using the paired Student t test. Values of P ≤ 0.05 (*) were considered statistically significant.
Results
Decidua-specific gene expression is lower in HSC and OsisSC in response to hormonal stimuli in vitro
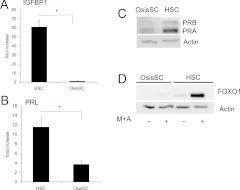
It has been well established that primary HSC undergo decidualization in vitro in response to M+A. Here we measured two decidua-specific genes, IGFBP1 and PRL, in OsisSC. As shown in Fig. 1, A and B, mRNA levels of IGFBP1 and PRL significantly increased in HSC upon M+A treatment. OsisSC isolated from ovarian endometriomas, however, expressed dramatically lower levels of IGFBP1 and PRL mRNA in response to M+A. Levels of PR and FOXO1, previously shown to be key mediators in decidualization (9), were dramatically lower in OsisSC compared with HSC (Fig. 1, C and D). In addition, although treatment with M+A increased FOXO1 protein levels in HSC, a similar increase did not occur in OsisSC (Fig. 1D). The decrease in these transcription factors could be contributing to the blunted expression of IGFBP1 and PRL upon M+A treatment.
Fig. 1.
Decidual gene expression in HSC and OsisSC in response to hormonal treatment. At 80% confluence, primary HSC and OsisSC cells were treated with 100 nm medroxyprogesterone acetate and 0.5 mm cAMP for 48 h in 2% charcoal-stripped FBS. Expression of decidua-specific genes, IGFBP1 (A) and PRL (B), were analyzed by real-time PCR. All values were normalized with internal control ribosomal protein, large, P0, and data are expressed as fold changes compared with its own vehicle control and are the mean ± sem of three independent experiments. PR (C) and FOXO1 (D) protein levels were detected by Western blot, and actin was used as the loading control. *, P ≤ 0.05. Data are representative of three independent experiments.
Overexpression of nuclear FOXO1 protein increased expression of IGFBP1 and PRL in OsisSC
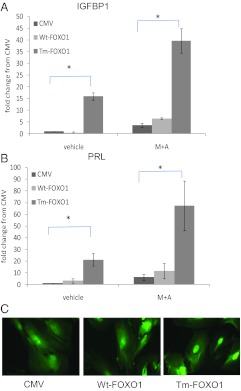
Because it has previously been shown that PR levels are lower in endometriotic tissues and cells (15, 16), we focused on the role of FOXO1 in OsisSC in decidualization. As shown in Fig. 2, overexpression of the constitutively active Tm-FOXO1 in OsisSC increased both IGFBP1 and PRL expression. Expression of these genes was further increased upon treatment with M+A. Contrary to this, WT-FOXO1 overexpression was unable to influence expression of IGFBP1 or PRL (Fig. 2) in the presence or absence of M+A treatment. These results strongly suggest that WT-FOXO1 is not being retained in the nucleus and thus incapable of having transcriptional function.
Fig. 2.
Effect of FOXO1 overexpression on decidual gene expression in OsisSC. OsisSC were infected with 100 multiplicity of infection adenovirus vectors expressing WT-FOXO1 or Tm-FOXO1 for 24 h and subsequently treated with M+A for 48 h. Real-time PCR was used to measure mRNA levels of IGFBP1 (A) and PRL (B). C, Immunofluorescent staining of FOXO1 in OsisSC infected with empty CMV vector, WT-FOXO1, or Tm-FOXO1 was done. Data are expressed as fold changes compared with vehicle CMV control and are the mean ± sem of five independent experiments. *, P ≤ 0.05.
OsisSC exhibit increased AKT activity
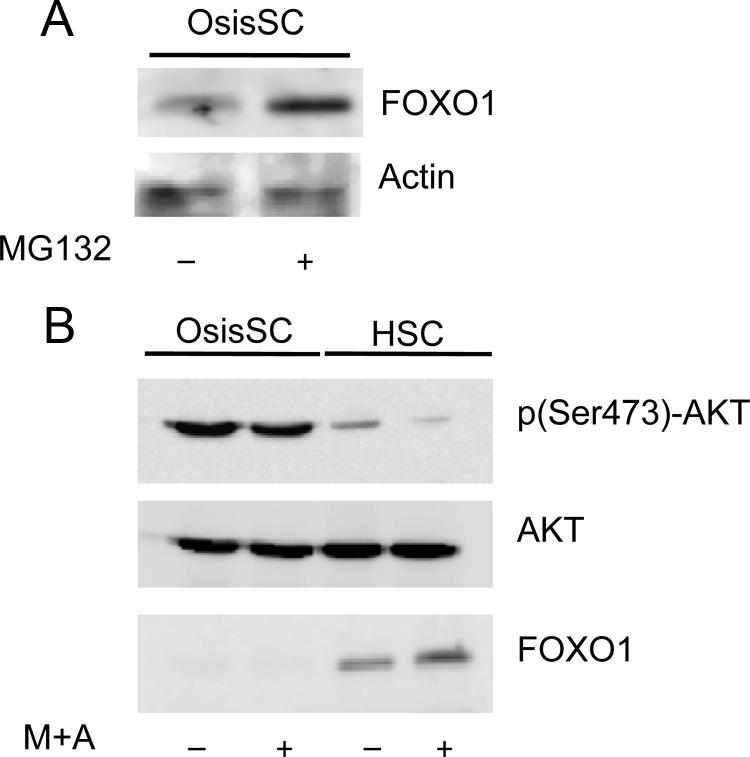
Previously we demonstrated that FOXO1 protein levels in endometrial adenocarcinoma were significantly lower compared with normal endometrial tissues and that FOXO1 degradation could contribute to this decrease (17). To determine whether the lower levels of FOXO1 in OsisSC were due to protein degradation, we treated OsisSC with a general proteasome inhibitor, MG132. A significant increase of FOXO1 protein levels was observed upon MG132 treatment (Fig. 3A). It has been shown that phosphorylation of FOXO1 at Ser256 residue targets it for ubiquitination-mediated protein degradation and AKT is one of the major kinases involved in phosphorylating FOXO1 at this site. Thus, levels of p(Ser473)-AKT were measured in OsisSC and HSC in the presence and absence of M+A. Western blot analysis showed that OsisSC exhibited higher p(Ser473)-AKT levels compared with HSC (Fig. 3B). When treated with M+A, the expression levels of p(Ser473)-AKT decreased in HSC; however, this decrease was not as dramatic in OsisSC. Furthermore, the protein levels of FOXO1 were higher in HSC and lower in OsisSC.
Fig. 3.
Levels of p(Ser473)-AKT and FOXO1 in OsisSC and HSC. A, OsisSC were treated with vehicle or 20 μm MG132, and then total proteins were isolated for analysis of FOXO1 expression. B, OsisSC and HSC were treated with vehicle or M+A. Western blots were performed to detect p(Ser473)-AKT, total AKT, and FOXO1 protein levels. Actin was used as the loading control. Blots are representative of three independent experiments.
Inhibition of the PI3K/AKT pathway increased FOXO1 and IGFBP1 expression in OsisSC
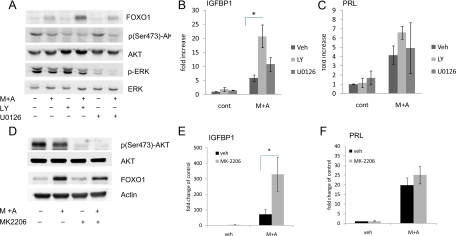
To demonstrate that increased AKT activity is contributing to the decreased decidual response to M+A in OsisSC, a PI3K inhibitor, LY294002, was used. Upon inhibition of PI3K, levels of p(Ser473)-AKT decreased (Fig. 4A). A corresponding increase in FOXO1 protein was observed in OsisSC treated with M+A (Fig. 4A). As a comparison, cells were also treated with the ERK inhibitor, U0126, and as expected, U0126 significantly decreased phosphorylated ERK protein at basal levels as well as response to hormonal treatment. Contrary to LY294002 treatment, U0126 did not significantly alter the levels of FOXO1 protein. These data indicate that the PI3K/AKT pathway is the predominant signaling pathway that leads to dramatically lower expression of FOXO1 in OsisSC. We next determined whether IGFBP1 and PRL expression was modulated upon inhibition of either PI3K or ERK in response to M+A treatment. IGFBP1 levels increased significantly when treated with LY294002 and M+A treatment compared with M+A alone in OsisSC (Fig. 4B). Although the addition of U0126 with M+A also increased IGFBP1 mRNA expression, this increase was not statistically different from the M+A treatment alone. Finally, these inhibitors did not change basal levels of IGFBP1 mRNA in OsisSC. Interestingly, although the PRL levels increased after treatment with M+A alone, the differences in expression with either LY294002 or U0126 were not statistically significant.
Fig. 4.
Effect of PI3K and AKT inhibition on FOXO1 levels and decidual gene expression in OsisSC. OsisSC cells were preincubated with 20 μm LY294002 or 20 μm U0126 (A) or 250 nm MK-2206 (D) followed by incubation with M+A or vehicle (Veh) for 48 h. Total FOXO1, p(Ser473)-AKT, AKT, phosphorylated ERK, and ERK proteins were measured with Western blot analyses, and representative blots are shown. Total mRNA were isolated from the OsisSC treated as previously described, and real-time PCR was done to measure the mRNA levels of IGFBP1 (B and E) and PRL (C and F). Data are expressed as fold changes compared with vehicle control and are the mean ± sem of five independent experiments. *, P ≤ 0.05.
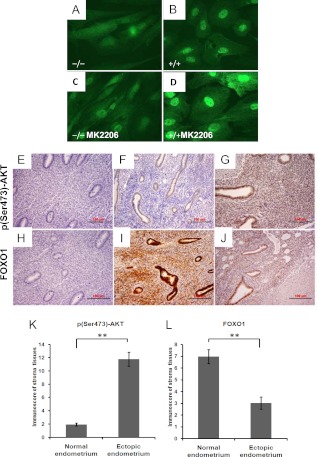
To confirm that the PI3K/AKT pathway plays a role in the decreased FOXO1 protein and blunted decidual response, a novel allosteric AKT inhibitor, MK-2206, was tested in the presence and absence of M+A. As expected, MK-2206 lowered the levels of p(Ser473)-AKT, whereas the total levels of AKT remained similar (Fig. 4D). Surprisingly, MK-2206 did not affect the total protein levels of FOXO1 upon M+A treatment (Fig. 4D), whereas it significantly increased IGFBP1 expression (Fig. 4E). Again, there was no effect on PRL expression in response to MK2206 with M+A (Fig. 4F). In light of this, FOXO1 localization was monitored by immunofluorescent staining (Fig. 5, A–D). Vehicle-treated OsisSC exhibited weak staining of FOXO1 protein throughout the cytoplasm, with no obvious nuclear staining. Upon M+A treatment, FOXO1 protein was increased in the nucleus. Cells treated with MK-2206 and M+A exhibited the highest nuclear FOXO1 staining (Fig. 5D). These data demonstrate that inhibition of AKT increases the accumulation of nuclear FOXO1 protein, which can then increase expression of IGFBP1 in response to M+A.
Fig. 5.
Expression of FOXO1 in endometriosis. A–D, OsisSC were pretreated with vehicle (A and B) or 250 nm MK-2206 (C and D) followed by incubation with vehicle (A and C) or M+A (B and D). Immunohistochemical staining for FOXO1 was done. Magnification, ×630. E–J, Immunohistochemical staining was done in normal endometrium (F and I) and endometriotic tissues from adnexa (G and J). Immunostaining for p(Ser473)-AKT (F and G) and FOXO1 (I and J) were done. E and H, Negative controls without primary antibody were done. Quantification of immunoreactivity for p(Ser473)-AKT (K) and FOXO1 (L) in the stroma of normal endometrium (n = 6) and ectopic endometriotic tissues (n = 6) were done. **, P ≤ 0.01. Magnification, ×200.
FOXO1 and p(Ser473)-AKT expression in normal endometrium and ectopic endometriotic tissues in vivo
Levels of FOXO1 and p(Ser473)-AKT were measured by immunohistochemical staining of normal human endometrium and adnexal lesions of endometriosis. As shown in Fig. 5, positive staining for p(Ser473)-AKT occurred in the epithelial glands of normal endometrium (Fig. 5F). By contrast, p(Ser473)-AKT staining in endometriotic tissues was stronger, especially in the stroma (Fig. 5G). Levels of FOXO1 were high in the epithelial glands and in stroma in normal endometrial tissues (Fig. 5I). However, in endometriotic lesions, levels of FOXO1 were dramatically lower, especially in stroma, compared with normal endometrial tissues (Fig. 5J). Furthermore, our quantification of immunoreactivity for these two proteins in the stroma confirmed that the level of p(Ser473)-AKT was significantly higher in endometriotic tissues compared with normal endometrium (Fig. 5K; P ≤ 0.01) and that the FOXO1 levels were significantly lower in the endometriotic tissues vs. the normal endometrium (Fig. 5L; P ≤ 0.01).
Discussion
In this study, we investigated the potential role of the PI3K/AKT/FOXO1 pathway for the suboptimal decidual response observed in OsisSC in vitro. We demonstrated that: 1) OsisSC and endometriotic tissues exhibited dramatically lower expression of FOXO1 and increased levels p(Ser473)-AKT; 2) overexpression of nuclear FOXO1 increased expression of decidua-specific genes, IGFBP1 and PRL, in OsisSC; and 3) inhibition of PI3K and AKT increased nuclear FOXO1 levels as well as IGFBP1 gene expression in response to M+A. We conclude that lower levels of FOXO1 protein resulting from hyperactivation of the PI3K/AKT pathway contribute to the decreased IGFBP1 gene expression in OsisSC.
The blunted decidual response in endometriosis has been observed in cultured primary endometriotic stromal cells from peritoneal, ovarian, and deeply infiltrating endometriosis (18). Furthermore, the decreased decidual response was shown in eutopic endometriotic stromal cells from women with endometriosis accompanied by aberrant activation of the MAPK pathway (19, 20). Consistent with these reports, our study confirmed that OsisSC exhibited an attenuated decidual response with dramatically lower expressions of IGFBP1 and PRL mRNA in response to M+A treatment. The reasons for this decreased decidual response are multifactorial. We and others (15, 16) have observed that PR levels in endometriotic cells are lower compared with endometrial stromal cells from women without endometriosis. Because PR can interact with other nuclear transcriptional factors to synergistically regulate decidua-specific genes (9, 21, 22), its down-regulation in endometriotic stromal cells undoubtedly contributes to the decrease in decidualization observed in these cells.
FOXO1 is a transcription factor that has also been shown to play an important role in decidualization (9, 11, 12, 23, 24). In a study that examined endometrial biopsied tissues from 209 women with endometriosis, levels of FOXO1 mRNA were found to be decreased compared with endometrium from disease-free women at the onset of the secretory phase (25). Microarray analysis of the eutopic endometrium from women with and without moderate/severe-stage endometriosis across various phases of the menstrual cycle revealed a decrease in FOXO1 expression during the secretory phase as well (26). Another group compared gene expression of ectopic tissues to eutopic endometrium from women with endometriosis and found a decrease in FOXA2 expression in the ectopic tissues (27). In our study, we have shown a decrease in FOXO1 protein in stromal cells from ovarian endometriomas as well as in other ectopic lesions. The decrease in FOXO1 levels appears to occur at the posttranslational level. One mechanism by which FOXO1 levels decrease is through ubiquitination and subsequent proteasomal degradation that occurs upon phosphorylation (28). In our study, overexpression of the constitutively active FOXO1, in which the three phosphorylation sites, Thr-24, Ser-256, and Ser-319, are mutated, caused an increase in the expression of IGFBP1 and PRL, whereas overexpression of the WT-FOXO1 did not. Furthermore, inhibition of PI3K resulted in increased endogenous FOXO1 proteins. These data strongly suggest that increased activation of this pathway in OsisSC promotes translocation of FOXO1 to the cytoplasm and undergoes modifications for degradation. Aside from these posttranslational modifications of FOXO1, another mechanism involving microRNA could be playing a role in decreasing FOXO1 levels in endometriosis. Recently miRNA targeting FOXO1, miR-9, miR-27, miR-96, miR-153, miR-182, miR-183, and miR-186 have been identified and found to be increased in endometrial adenocarcinomas (29). Whether these miRNA are found in ectopic lesions in women with endometriosis and are responsible for the decreased FOXO1 levels requires further investigation.
Interestingly, although overexpression of Tm-FOXO1 in OsisSC increased levels of both IGFBP1 and PRL in the presence and absence of M+A, inhibition of PI3K or AKT significantly increased levels of IGFBP1 only in response to M+A. Although expression of PRL did increase, it did not reach statistical significance, indicating that perhaps the increase in nuclear FOXO1 levels with the inhibitors was insufficient to enhance PRL expression or that other key regulatory mechanisms affected by the PI3K/AKT pathway are at play for PRL. Transcription factors that have been shown to regulate the prolactin promoter include FOXO1, signal transducer and activator of transcription 5, CCAAT/enhancer-binding protein C/EBPβ, p53, and PR (22, 24, 30). Recently it has been shown that Akt and signal transducer and activator of transcription 5 are functionally interconnected to regulate mammary epithelial differentiation and lactation (31). AKT-mediated phosphorylation of p300/cAMP response element-binding protein can disrupt its binding to CCAAT/enhancer-binding protein-β and influence its transcriptional activation of IGFBP1 in liver cells (32). Akt can promote degradation of p53 through the activation of Mdm2 (33). Thus, overactive AKT may differentially affect the expression of decidua-specific genes.
It has been demonstrated that eutopic endometrium from women with endometriosis exhibited increased activation of the MAPK pathway (20), which could be due to decreased expression of genes associated with inactivating this pathway (26). For instance, ERBB receptor feedback inhibitor 1, a negative regulator of MAPK signaling, was found to be decreased. Velarde et al. (20) showed that increased ERK1/2 activity in the eutopic endometrial stromal cells from women with endometriosis inhibited cAMP-mediated down-regulation of cyclin D1. Interestingly, inhibiting of ERK1/2 activity by U0126 did not restore cAMP-induced IGFBP1 expression in endometriotic cells in their study, suggesting that the AKT pathway may be responsible. Indeed, our data support this because inhibition of AKT potentiated cAMP-induction of IGFBP1, but inhibition of ERK1/2 did not. In our study, a modest increase in IGFBP1 expression upon ERK1/2 inhibition was observed but did not reach statistical significance. FOXO1 can be phosphorylated by ERK and p38 (34) as well as other kinases such as dual-specificity tyrosine-(Y)-phosphorylation regulated kinase 1A (35), casein kinase 1 (36), and serum/glucocorticoid-regulated kinase 1 (37). These kinases may play a role in regulating IGFBP1 in OsisSC. Our data show, however, that the PI3K/AKT pathway is the predominant pathway that attenuates short-term M+A-induced IGFBP1 expression.
The reasons for the hyperactivation of the PI3K/AKT pathway in endometriosis are unclear. Gene expression analysis studies revealed an increase in expression of important mediators and regulators of the AKT pathway, including AKT1 and 4E-binding protein 1 in the eutopic endometrium of women with endometriosis (38) and insulin-like growth factor 2; actinin, alpha 4; AXL receptor tyrosine kinase; and SHC (Src homology 2 domain containing) transforming protein 1 in epithelial cells from ovarian endometrioma specimens (39). Phosphatase and tensin homolog (PTEN), which is a negative regulator of the AKT pathway, was demonstrated to be down-regulated in both the eutopic and ectopic endometrial epithelial cells from endometriosis due to transcriptional and posttranscriptional regulation of the gene (40). Their proposed model described that in the eutopic and ectopic endometriotic epithelial cells, estradiol activates the PI3K/AKT, MAPK/ERK, and nuclear factor-κB (NFκB) pathways, which then enhances NFκB binding to suppress PTEN transcription and expression, which thereby facilities the activity of PI3K/AKT. This then creates a positive feedback loop, in which the activated PI3K/AKT pathway can further promote suppression of PTEN through NFκB. Finally, numerous receptors to growth factors, cytokines, and even hormones can activate the AKT pathway. Banu et al. (41) demonstrated the activation of ERK1/2, AKT, NFκB, and β-catenin pathways by prostaglandins in endometriotic cell lines. Given that endometriosis is an estrogen-dependent disease that is associated with inflammation, there is an abundance of ligands that could potentially activate the AKT pathway through its receptors.
The question of whether resistance to decidualization of ectopic lesions promotes their proliferation and/or survival remains to be answered. Would sensitization of ectopic lesions to decidualization promote the clearance or eradication of the ectopic tissues? Further experimentation is needed to address these questions. It is well known, however, that the PI3K/AKT pathway promotes survival and proliferation. It is unclear as to why activities of the MAPK and AKT pathways are heighted in endometriosis or whether endometriotic cells are dependent on this increased kinase state for survival. Future treatments for endometriosis should consider not only hormonal therapies but small molecule inhibitors that target these overactive pathways. Numerous inhibitors of kinases that target the PI3K/AKT pathways are currently being explored for the treatment of many malignant conditions and are being tested in clinical trials (42–50). Additional studies are underway to test the efficacy of AKT inhibitors in promoting the decidualization and apoptosis of endometriotic cells in vitro and in vivo.
Acknowledgments
We are grateful to Denise Scholtens for helping with the statistical analysis, Jennifer Eaton for helping with tissue collection, the Imaging and Pathology Core facilities at the Robert Lurie Cancer Center at Northwestern University, and Cancer Therapy Evaluation Program for providing the Merck compound, MK-2206.
This work was supported by National Institutes of Health/ Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant HD044715.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- FBS
- Fetal bovine serum
- FOXO1
- forkhead-box O1
- HRP
- horseradish peroxidase
- HSC
- stromal cells of the endometrium from women without endometriosis
- IGFBP1
- IGF binding protein-1
- M+A
- medroxyprogesterone acetate and dibutyryl cAMP
- NFκB
- nuclear factor-κB
- OsisSC
- primary endometriotic stromal cells
- PI3K
- phosphatidylinositol 3-kinase
- PR
- progesterone receptor
- PRL
- prolactin
- PTEN
- phosphatase and tensin homolog
- TBST
- Tris-buffered saline with Tween 20
- Tm-FOXO1
- triple-mutant FOXO1
- WT-FOXO1
- wild-type FOXO1.
References
- 1. Bulun SE. 2009. Endometriosis. N Engl J Med 360:268–279 [DOI] [PubMed] [Google Scholar]
- 2. Giudice LC, Kao LC. 2004. Endometriosis. Lancet 364:1789–1799 [DOI] [PubMed] [Google Scholar]
- 3. Sampson J. 1927. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol 14:422–469 [Google Scholar]
- 4. Wynn RM. 1974. Ultrastructural development of the human decidua. Am J Obstet Gynecol 118:652–670 [DOI] [PubMed] [Google Scholar]
- 5. Brar AK, Frank GR, Kessler CA, Cedars MI, Handwerger S. 1997. Progesterone-dependent decidualization of the human endometrium is mediated by cAMP. Endocrine 6:301–307 [DOI] [PubMed] [Google Scholar]
- 6. Brosens JJ, Hayashi N, White JO. 1999. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology 140:4809–4820 [DOI] [PubMed] [Google Scholar]
- 7. Telgmann R, Maronde E, Taskén K, Gellersen B. 1997. Activated protein kinase A is required for differentiation-dependent transcription of the decidual prolactin gene in human endometrial stromal cells. Endocrinology 138:929–937 [DOI] [PubMed] [Google Scholar]
- 8. Tierney EP, Tulac S, Huang ST, Giudice LC. 2003. Activation of the protein kinase A pathway in human endometrial stromal cells reveals sequential categorical gene regulation. Physiol Genomics 16:47–66 [DOI] [PubMed] [Google Scholar]
- 9. Takano M, Lu Z, Goto T, Fusi L, Higham J, Francis J, Withey A, Hardt J, Cloke B, Stavropoulou AV, Ishihara O, Lam EW, Unterman TG, Brosens JJ, Kim JJ. 2007. Transcriptional cross talk between the forkhead transcription factor forkhead box O1A and the progesterone receptor coordinates cell cycle regulation and differentiation in human endometrial stromal cells. Mol Endocrinol 21:2334–2349 [DOI] [PubMed] [Google Scholar]
- 10. Feroze-Zaidi F, Fusi L, Takano M, Higham J, Salker MS, Goto T, Edassery S, Klingel K, Boini KM, Palmada M, Kamps R, Groothuis PG, Lam EW, Smith SK, Lang F, Sharkey AM, Brosens JJ. 2007. Role and regulation of the serum- and glucocorticoid-regulated kinase 1 in fertile and infertile human endometrium. Endocrinology 148:5020–5029 [DOI] [PubMed] [Google Scholar]
- 11. Labied S, Kajihara T, Madureira PA, Fusi L, Jones MC, Higham JM, Varshochi R, Francis JM, Zoumpoulidou G, Essafi A, Fernandez de Mattos S, Lam EW, Brosens JJ. 2006. Progestins regulate the expression and activity of the forkhead transcription factor FOXO1 in differentiating human endometrium. Mol Endocrinol 20:35–44 [DOI] [PubMed] [Google Scholar]
- 12. Kim JJ, Buzzio OL, Li S, Lu Z. 2005. Role of FOXO1A in the regulation of insulin-like growth factor-binding protein-1 in human endometrial cells: interaction with progesterone receptor. Biol Reprod 73:833–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ryan IP, Schriock ED, Taylor RN. 1994. Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J Clin Endocrinol Metab 78:642–649 [DOI] [PubMed] [Google Scholar]
- 14. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2[−ΔΔC(T)] method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 15. Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. 2000. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab 85:2897–2902 [DOI] [PubMed] [Google Scholar]
- 16. Tamaya T, Motoyama T, Ohono Y, Ide N, Tsurusaki T, Okada H. 1979. Steroid receptor levels and histology of endometriosis and adenomyosis. Fertil Steril 31:396–400 [DOI] [PubMed] [Google Scholar]
- 17. Ward EC, Hoekstra AV, Blok LJ, Hanifi-Moghaddam P, Lurain JR, Singh DK, Buttin BM, Schink JC, Kim JJ. 2008. The regulation and function of the forkhead transcription factor, Forkhead box O1, is dependent on the progesterone receptor in endometrial carcinoma. Endocrinology 149:1942–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ. 2006. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril 85:564–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aghajanova L, Hamilton A, Kwintkiewicz J, Vo KC, Giudice LC. 2009. Steroidogenic enzyme and key decidualization marker dysregulation in endometrial stromal cells from women with versus without endometriosis. Biol Reprod 80:105–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Velarde MC, Aghajanova L, Nezhat CR, Giudice LC. 2009. Increased mitogen-activated protein kinase kinase/extracellularly regulated kinase activity in human endometrial stromal fibroblasts of women with endometriosis reduces 3′,5′-cyclic adenosine 5′-monophosphate inhibition of cyclin D1. Endocrinology 150:4701–4712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Christian M, Pohnke Y, Kempf R, Gellersen B, Brosens JJ. 2002. Functional association of PR and CCAAT/enhancer-binding protein β isoforms: promoter-dependent cooperation between PR-B and liver-enriched inhibitory protein, or liver-enriched activatory protein and PR-A in human endometrial stromal cells. Mol Endocrinol 16:141–154 [DOI] [PubMed] [Google Scholar]
- 22. Gellersen B, Brosens IA, Brosens JJ. 2007. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med 25:445–453 [DOI] [PubMed] [Google Scholar]
- 23. Buzzio OL, Lu Z, Miller CD, Unterman TG, Kim JJ. 2006. FOXO1A differentially regulates genes of decidualization. Endocrinology 147:3870–3876 [DOI] [PubMed] [Google Scholar]
- 24. Gellersen B, Brosens J. 2003. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol 178:357–372 [DOI] [PubMed] [Google Scholar]
- 25. Shazand K, Baban S, Privé C, Malette B, Croteau P, Lagacé M, Racine JB, Hugo P. 2004. FOXO1 and c-jun transcription factors mRNA are modulated in endometriosis. Mol Hum Reprod 10:871–877 [DOI] [PubMed] [Google Scholar]
- 26. Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. 2007. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 148:3814–3826 [DOI] [PubMed] [Google Scholar]
- 27. Eyster KM, Klinkova O, Kennedy V, Hansen KA. 2007. Whole genome deoxyribonucleic acid microarray analysis of gene expression in ectopic versus eutopic endometrium. Fertil Steril 88:1505–1533 [DOI] [PubMed] [Google Scholar]
- 28. Huang H, Tindall DJ. 2011. Regulation of FOXO protein stability via ubiquitination and proteasome degradation. Biochim Biophys Acta 1813:1961–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Myatt SS, Wang J, Monteiro LJ, Christian M, Ho KK, Fusi L, Dina RE, Brosens JJ, Ghaem-Maghami S, Lam EW. 2010. Definition of microRNAs that repress expression of the tumor suppressor gene FOXO1 in endometrial cancer. Cancer Res 70:367–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schneider-Merck T, Pohnke Y, Kempf R, Christian M, Brosens JJ, Gellersen B. 2006. Physical interaction and mutual transrepression between CCAAT/enhancer-binding protein beta and the p53 tumor suppressor. J Biol Chem 281:269–278 [DOI] [PubMed] [Google Scholar]
- 31. Chen CC, Boxer RB, Stairs DB, Portocarrero CP, Horton RH, Alvarez JV, Birnbaum MJ, Chodosh LA. 2010. Akt is required for Stat5 activation and mammary differentiation. Breast Cancer Res 12:R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guo S, Cichy SB, He X, Yang Q, Ragland M, Ghosh AK, Johnson PF, Unterman TG. 2001. Insulin suppresses transactivation by CAAT/enhancer-binding proteins β (C/EBPβ). Signaling to p300/CREB-binding protein by protein kinase B disrupts interaction with the major activation domain of C/EBPβ. J Biol Chem 276:8516–8523 [DOI] [PubMed] [Google Scholar]
- 33. Ogawara Y, Kishishita S, Obata T, Isazawa Y, Suzuki T, Tanaka K, Masuyama N, Gotoh Y. 2002. Akt enhances Mdm2-mediated ubiquitination and degradation of p53. J Biol Chem 277:21843–21850 [DOI] [PubMed] [Google Scholar]
- 34. Asada S, Daitoku H, Matsuzaki H, Saito T, Sudo T, Mukai H, Iwashita S, Kako K, Kishi T, Kasuya Y, Fukamizu A. 2007. Mitogen-activated protein kinases, Erk and p38, phosphorylate and regulate Foxo1. Cell Signal 19:519–527 [DOI] [PubMed] [Google Scholar]
- 35. Woods YL, Rena G, Morrice N, Barthel A, Becker W, Guo S, Unterman TG, Cohen P. 2001. The kinase DYRK1A phosphorylates the transcription factor FKHR at Ser329 in vitro, a novel in vivo phosphorylation site. Biochem J 355:597–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rena G, Bain J, Elliott M, Cohen P. 2004. D4476, a cell-permeant inhibitor of CK1, suppresses the site-specific phosphorylation and nuclear exclusion of FOXO1a. EMBO Rep 5:60–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. 2001. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol Cell Biol 21:952–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Laudanski P, Szamatowicz J, Kowalczuk O, Kuźmicki M, Grabowicz M, Chyczewski L. 2009. Expression of selected tumor suppressor and oncogenes in endometrium of women with endometriosis. Hum Reprod 24:1880–1890 [DOI] [PubMed] [Google Scholar]
- 39. Honda H, Barrueto FF, Gogusev J, Im DD, Morin PJ. 2008. Serial analysis of gene expression reveals differential expression between endometriosis and normal endometrium. Possible roles for AXL and SHC1 in the pathogenesis of endometriosis. Reprod Biol Endocrinol 6:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang H, Zhao X, Liu S, Li J, Wen Z, Li M. 2010. 17βE2 promotes cell proliferation in endometriosis by decreasing PTEN via NFκB-dependent pathway. Mol Cell Endocrinol 317:31–43 [DOI] [PubMed] [Google Scholar]
- 41. Banu SK, Lee J, Speights VO, Jr, Starzinski-Powitz A, Arosh JA. 2009. Selective inhibition of prostaglandin E2 receptors EP2 and EP4 induces apoptosis of human endometriotic cells through suppression of ERK1/2, AKT, NFκB, and β-catenin pathways and activation of intrinsic apoptotic mechanisms. Mol Endocrinol 23:1291–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Steelman LS, Stadelman KM, Chappell WH, Horn S, Bäsecke J, Cervello M, Nicoletti F, Libra M, Stivala F, Martelli AM, McCubrey JA. 2008. Akt as a therapeutic target in cancer. Expert Opin Ther Targets 12:1139–1165 [DOI] [PubMed] [Google Scholar]
- 43. Kim LC, Song L, Haura EB. 2009. Src kinases as therapeutic targets for cancer. Nat Rev Clin Oncol 6:587–595 [DOI] [PubMed] [Google Scholar]
- 44. McCubrey JA, Steelman LS, Abrams SL, Chappell WH, Russo S, Ove R, Milella M, Tafuri A, Lunghi P, Bonati A, Stivala F, Nicoletti F, Libra M, Martelli AM, Montalto G, Cervello M. 2009. Emerging Raf inhibitors. Expert Opin Emerg Drugs 14:633–648 [DOI] [PubMed] [Google Scholar]
- 45. McCubrey JA, Steelman LS, Franklin RA, Abrams SL, Chappell WH, Wong EW, Lehmann BD, Terrian DM, Basecke J, Stivala F, Libra M, Evangelisti C, Martelli AM. 2007. Targeting the RAF/MEK/ERK, PI3K/AKT and p53 pathways in hematopoietic drug resistance. Adv Enzyme Regul 47:64–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Druker BJ. 2002. STI571 (Gleevec) as a paradigm for cancer therapy. Trends Mol Med 8:S14–S18 [DOI] [PubMed] [Google Scholar]
- 47. Becker J. 2004. Signal transduction inhibitors—a work in progress. Nat Biotechnol 22:15–18 [DOI] [PubMed] [Google Scholar]
- 48. Carter CA, Kelly RJ, Giaccone G. 2009. Small-molecule inhibitors of the human epidermal receptor family. Expert Opin Investig Drugs 18:1829–1842 [DOI] [PubMed] [Google Scholar]
- 49. Castaneda CA, Gomez HL. 2009. Pazopanib: an antiangiogenic drug in perspective. Future Oncol 5:1335–1348 [DOI] [PubMed] [Google Scholar]
- 50. Lane HA, Breuleux M. 2009. Optimal targeting of the mTORC1 kinase in human cancer. Curr Opin Cell Biol 21:219–229 [DOI] [PubMed] [Google Scholar]