Abstract
Objective:
Our objective was to determine whether exenatide exerts an antiinflammatory effect.
Research Design and Methods:
Twenty-four patients were prospectively randomized to be injected sc with either exenatide 10 μg twice daily [n = 12; mean age = 56 ± 3 yr; mean body mass index = 39.8 ± 2 kg/m2; mean glycosylated hemoglobin (HbA1c) = 8.6 ± 0.4%] or placebo twice daily (n = 12; mean age = 54 ± 4 yr; mean body mass index = 39.1 ± 1.6 kg/m2; mean HbA1c = 8.5 ± 0.3%) for 12 wk. Fasting blood samples were obtained at 0, 3, 6, and 12 wk. Blood samples were also collected for up to 6 h after a single dose of exenatide (5 μg) or placebo.
Results:
Fasting blood glucose fell from 139 ± 17 to 110 ± 9 mg/dl, HbA1c from 8.6 ± 0.4 to 7.4 ± 0.5% (P < 0.05), and free fatty acids by 21 ± 5% from baseline (P < 0.05) with exenatide. There was no weight loss. There was a significant reduction in reactive oxygen species generation and nuclear factor-κB binding by 22 ± 9 and 26 ± 7%, respectively, and the mRNA expression of TNFα, IL-1β, JNK-1, TLR-2, TLR-4, and SOCS-3 in mononuclear cells by 31 ± 12, 22 ± 10, 20 ± 11, 22 ± 9, 16 ± 7, and 31 ± 10%, respectively (P < 0.05 for all) after 12 wk of exenatide. After a single injection of exenatide, there was a reduction by 20 ± 7% in free fatty acids, 19 ± 7% in reactive oxygen species generation, 39 ± 11% in nuclear factor-κB binding, 18 ± 9% in TNFα expression, 26 ± 7% in IL-1β expression, 18 ± 7% in JNK-1 expression, 24 ± 12% in TLR-4 expression, and 23 ± 11% in SOCS-3 expression (P < 0.05 for all). The plasma concentrations of monocyte chemoattractant protein-1, matrix metalloproteinase-9, serum amyloid A, and IL-6 were suppressed after 12 wk exenatide treatment by 15 ± 7, 20 ± 11, 16 ± 7, and 22 ± 12%, respectively (P < 0.05 for all).
Conclusions:
Exenatide exerts a rapid antiinflammatory effect at the cellular and molecular level. This may contribute to a potentially beneficial antiatherogenic effect. This effect was independent of weight loss.
Exenatide is a stabilized derivative of exendin-4 and is a glucagon-like peptide-1 (GLP-1) receptor agonist (1, 2). It thus mimics the biological actions of GLP-1. It induces the release of insulin from the β-cells while mediating the suppression of glucagon release from the α-cells of the pancreatic islet (3, 4). In addition, it crosses the blood-brain barrier and suppresses appetite, food intake, and body weight (5, 6). It also slows gastric emptying. In view of these properties and the consistent ability to predictably reduce blood glucose concentrations, its use in the treatment of type 2 diabetes is now established.
In 2007, we demonstrated for the first time that exenatide had two nonmetabolic but important actions: the reduction in plasma C-reactive protein (CRP) concentrations and systolic blood pressure (7). These actions were further confirmed by us in a longer-term study, in which it was also demonstrated that these effects were durable and that the cessation of exenatide treatment led to the reversal of these effects (8). The suppression of CRP concentration and the reduction in systolic blood pressure by exenatide have now been confirmed by other studies (9, 10). Exendin-4 was also shown to increase regulatory T cell numbers and the production of the antiinflammatory cytokine IL-10 in NOD mice with type 1 diabetes (11). More recently, exenatide has been shown to suppress oxidative stress and inflammatory mediators including isoprostane, CRP, and monocyte chemoattractant protein (MCP)-1 in type 2 diabetics (12) GLP-1 agonists have also been shown to suppress atherogenesis, improve endothelial function, and have cardioprotective effects (13–16). Because these actions are of potential benefit in reducing cardiovascular risk, an important issue in patients with type 2 diabetes, they require further investigation. Because atherosclerosis is a chronic inflammation of the arterial wall (17), we have now hypothesized that exenatide exerts a comprehensive antiinflammatory effect as reflected in the suppression of inflammatory indices in the peripheral blood mononuclear cells (MNC) and in plasma.
Subjects and Methods
Subjects
This is a single-center, randomized, placebo-control, single-blinded (patient) prospective study. Twenty-four obese patients with type 2 diabetes with a glycosylated hemoglobin (HbA1c) between 7.5 and 9% participated in this study. None of the subjects had any microvascular or macrovascular complications of diabetes. All patients were on stable doses of oral antidiabetic medications and insulin and had stable weight for 4 wk before the study. All patients were on metformin (1–2 g/d), and 14 patients were on sulfonylureas (glyburide or glipizide 5–10 mg/d). Ten subjects (five each in placebo and exenatide group) were on basal insulin (glargine) once daily, two in placebo group on Novolog Mix 70/30 twice daily, and 12 (five placebo and seven exenatide group) on basal (glargine/detimir) and bolus (lispro or aspart) regimen. The dose of statins and angiotensin converting enzyme inhibitors were not changed during the study. None of the subjects were on thiazolidinediones, antioxidants, or nonsteroidal antiinflammatory drugs. Patients' demographic data are summarized in Table 1. All patients met with the dietitian and certified diabetes educator on d 0. General dietary recommendations as per American Diabetes Association guidelines were made to all subjects. They were randomized to receive either exenatide 10 μg or placebo (supplied by Amylin, San Diego, CA) 30 min before breakfast and dinner for 12 wk. However, the dose of exenatide was started at 5 μg twice daily for 1 wk to ensure tolerability. Blood samples were collected before (d 0) and at 3, 6, and 12 wk after start of treatment after an overnight fast. For the single-dose effect study, blood samples were also collected at 0, 2, 4, and 6 h after a single dose of exenatide (5 μg) or placebo on d 0 of the 12-wk study while subjects were fasting. Insulin doses were titrated downward by 20% to prevent hypoglycemia after the initiation of study treatment in subjects with HbA1c below 8%. After initiation of study treatment, insulin doses were titrated on wk 3 and 6 to target fasting glucose of less than 100 mg/dl and 2-h postprandial glucose of less than 160 mg/dl. Titration of insulin was done by a blinded investigator. The protocol was approved by the Human Research Committee of the State University of New York at Buffalo. An informed consent was signed by all subjects. The trial was registered with www.clinicaltrials.gov NCT number 01154933.
Table 1.
Patients' demographic data at baseline and after 12 wk of 10 μg exenatide or placebo twice-daily therapy
| Placebo |
Exenatide |
|||
|---|---|---|---|---|
| Baseline | At 12 wk | Baseline | At 12 wk | |
| Age (yr) | 54 ± 4 | 56 ± 3 | ||
| Weight (lbs.) | 231 ± 13 | 234 ± 18 | 251 ± 18 | 251 ± 20 |
| Body mass index (kg/m2) | 39.1 ± 1.6 | 39.1 ± 1.7 | 39.8 ± 2.0 | 39.2 ± 1.8 |
| HbA1c | 8.5 ± 0.3 | 8.0 ± 0.3 | 8.6 ± 0.4 | 7.4 ± 0.5a |
| Diabetes duration (yr) | 12 ± 2 | 12 ± 2 | ||
| Fasting glucose (mg/dl) | 128 ± 13 | 139 ± 33 | 139 ± 17 | 110 ± 9a |
| Fasting insulin (μU/ml) | 13.1 ± 3.1 | 13.9 ± 5.9 | 12.7 ± 2.8 | 16.4 ± 3.2a |
| FFA (mm) | 0.64 ± 0.08 | 0.61 ± 0.09 | 0.69 ± 0.07 | 0.50 ± 0.03a |
| Systolic blood pressure (mm Hg) | 128 ± 5 | 130 ± 6.0 | 134 ± 6 | 127 ± 5 |
| Diastolic blood pressure (mm Hg) | 78 ± 2 | 76 ± 4 | 82 ± 2 | 77 ± 3 |
| Insulin dose (U) | 82 ± 13 | 88 ± 13 | 105 ± 30 | 105 ± 31 |
Data are presented as mean ± se.
P < 0.05, paired t test compared with baseline.
MNC isolation
Blood samples were collected in Na-EDTA and carefully layered on Lympholyte medium (Cedarlane Laboratories, Hornby, Ontario, Canada). Samples were centrifuged and two bands separate out at the top of the red blood cell pellet. The MNC band was harvested and washed twice with Hanks' balanced salt solution. This method provides yields greater than 95% MNC in the preparation.
Reactive oxygen species (ROS) generation measurement by chemiluminescence
Five hundred microliters of MNC (2 × 105 cells) were delivered into a Chronolog Lumi-aggregometer cuvette. Luminol was then added, followed by 1.0 μl of 10 mm formylmethionyl leucinyl phenylalanine. In this assay system, the release of superoxide radical, as measured by chemiluminescence, has been shown to be linearly correlated with that measured by the ferricytochrome C method (18).
Nuclear factor-κB (NFκB) DNA-binding activity
Nuclear NFκB and Oct-1 DNA-binding activity was measured by EMSA. Nuclear extracts were prepared from MNC and by high-salt extraction as previously described (19, 20). The specificity of the bands was confirmed by supershifting these bands with specific antibodies against Rel-A (p65), p50, and Oct-1 or with nonspecific antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). NFκB binding was normalized to that from Oct-1and is presented as NFκB to Oct-1 ratio.
Quantification of JNK-1, TLR-2, TLR-4, TNFα, and SOCS-3 expression
The mRNA expression of JNK-1, TLR-2, TLR-4, TNFα, SOCS-3, IL-1β, and IL-10 was measured in MNC by RT-PCR. Total RNA was isolated using commercially available RNAqueous-4 PCR Kit (Ambion, Austin, TX). Real-time RT-PCR was performed using Stratagene (La Jolla, CA) Mx3000P QPCR system, Sybr Green Master Mix (QIAGEN, Valencia, CA) and gene-specific primers (Life Technologies, Rockville, MD). The expression of JNK-1, TLR-2, TLR-4, TNFα, SOCS-3, IL-1β, and IL-10 were easily detectable with an average cycle threshold numbers of 26.8, 25.3, 25.5, 29.8, 27.2, 28.1, and 31.8, respectively. All values were normalized to the expression of a group of housekeeping genes including actin, ubiquitin C, and cyclophilin A.
Western blotting
MNC total cell lysates were prepared, and electrophoresis and immunoblotting were carried out as described before (21). Monoclonal antibodies against c-Jun N-terminal kinase (JNK)-1, toll-like receptor (TLR)-2, TLR-4, TNFα, suppressor of cytokine signaling (SOCS)-3 (Abcam, Cambridge, MA), and actin (Santa Cruz Biotechnology) were used, and all values were corrected for loading to actin.
Plasma measurements
Glucose concentrations were measured in plasma by YSI (Yellow Springs, OH) 2300 STAT Plus glucose analyzer. ELISA was used to measure plasma concentrations of insulin (Diagnostic Systems Laboratories Inc., Webster, TX), IL-6, MCP-1, matrix metalloproteinase (MMP)-9 (R&D Systems), and serum amyloid A (SAA) (Invitrogen, Carlsbad, CA).
Statistical analysis
Statistical analysis was conducted using SigmaStat software (SPSS Inc., Chicago, IL). All data are represented as mean ± se. Baseline measurements were normalized to 100%, and changes from baseline were calculated as percent change from baseline. Statistical analysis was carried out using one-way repeated-measures ANOVA (RMANOVA) with Holm-Sidak post hoc test. Two-factor RMANOVA followed by Dunnett's post hoc was used for multiple comparisons between different treatments. Paired t test and Student's t test were used where appropriate. Multivariate analysis of changes in inflammatory mediators from baseline with changes in free fatty acids (FFA), insulin, glucose, percent HbA1c, body mass index, and systolic and diastolic blood pressure was performed using multiple linear regression.
Results
Change in glucose homeostasis and body weight after exenatide treatment
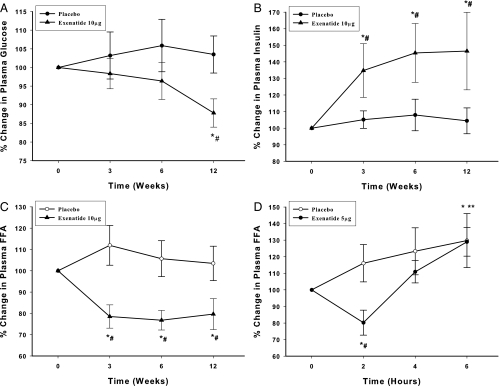
Fasting blood glucose and HbA1c fell, whereas insulin increased (P < 0.05; Fig. 1, A and B, and Table 1) in the exenatide group, whereas it did not change significantly in the placebo group. Additionally, FFA fell after exenatide (Fig. 1C and Table 1; P < 0.05). There was no significant change in body weight in either group (Table 1). None of the subjects had any major hypoglycemic episodes. A single dose of exenatide (5 μg) caused a significant reduction in FFA by 20 ± 7% (from 0.69 ± 0.07 to 0.53 ± 0.05 mm, P < 0.05) at 2 h, but FFA increased above the baseline by 6 h in both groups (Fig. 1D).
Fig. 1.
Percent change in fasting blood glucose (A), insulin (B), and FFA (C) after placebo and exenatide 10 μg twice daily for 12 wk and change in FFA (D) after a single dose of 5 μg exenatide or placebo in type 2 diabetic subjects. Data are presented as mean ± se; n = 12 each. * and **, P < 0.05 by RMANOVA (compared with baseline) in exenatide and placebo groups, respectively; #, P < 0.05 by two-way RMANOVA compared with control groups.
Effect of exenatide on ROS generation in MNC
There was a significant reduction by 22 ± 9% below the baseline in ROS generation by MNC at 6 wk exenatide treatment. This ROS-suppressive effect continued until 12 wk of treatment. There was no significant change in ROS generation in the placebo group, and the difference between the two treatment arms was statistically significant by two-way RMANOVA (Fig. 2A, P < 0.05). ROS generation by MNC was also suppressed by 19 ± 7% at 6 h after a single dose of exenatide 5 μg (Fig. 2B; P < 0.05 by paired t test at 6 h compared with baseline).
Fig. 2.
Percent change in ROS generation by MNC after placebo and exenatide 10 μg bid for 12 wk (A) and after 6 h of a single dose of placebo or exenatide (5 μg) (B) in type 2 diabetic subjects. Data are presented as mean ± se; n = 12 each. *, P < 0.05 by RMANOVA (compared with baseline); ^, P < 0.05 by paired t test (compared with baseline); #, P < 0.05 by two-way RMANOVA compared with control groups.
Effect of exenatide on NFκB activation and TNF-α expression in MNC
Exenatide treatment significantly suppressed the relative DNA binding of NFκB (calculated as a ratio of NFκB to Oct-1) by 29 ± 8 and 26 ± 7% at 6 and 12 wk, respectively, whereas there was no significant change in NFκB binding in the placebo group (Fig. 3, A and B; P < 0.05). NFκB binding in MNC was also suppressed significantly by 44 ± 8% at 2 h after a single dose of exenatide and reverted to baseline levels by 6 h after exenatide injection (Fig. 3, A and C, P < 0.05). The change in NFκB binding was associated with a significant suppression in the mRNA expression of TNFα and IL-1β, major NFκB target genes, by 31 ± 12 and 22 ± 10%, respectively, after 12 wk of exenatide treatment and by 18 ± 9% at 2 h and 26 ± 7% at 4 h, respectively, after a single dose of exenatide (Fig. 3, D–G, P < 0.05). The mRNA expression of IL-10 was not affected by exenatide treatment.
Fig. 3.
A, Gel shift assay showing the NFκB and Oct-1 binding to the double-stranded oligonucleotide containing NFκB DNA-binding site in the exenatide group after 6 h of a single dose of placebo or exenatide 5 μg (micg) and exenatide 10 μg twice-daily treatment for 12 wk (A). B–G, Change in NFκB/Oct-1 DNA-binding activity (B and C) and mRNA expression of TNFα (D and E) and IL-1β (F and G) from baseline (100%) after placebo and exenatide 10 μg twice-daily treatment for 12 wk and after 6 h of a single dose of placebo or exenatide (5 μg) in type 2 diabetic subjects. Data are presented as mean ± se; n = 12 each. *, P < 0.05 by RMANOVA (compared with baseline); #, P < 0.05 by two-way RMANOVA compared with control groups. W12, Week 12; SS, supershift; NSA, nonspecific antibodies.
Effect of exenatide on other cellular proinflammatory mediators in MNC
The mRNA expression of JNK-1, TLR-4, TLR-2, and SOCS-3 in MNC fell by 20 ± 11, 16 ± 7, 22 ± 9, and 31 ± 10%, respectively [Supplemental Fig. 1, A, C, E, and F (published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org); P < 0.05) after 12 wk exenatide, whereas there was no significant change in these indices in the placebo group. The change in JNK-1, TLR-2, and SOCS-3 mRNA expression was observed as early as 3 wk after treatment with exenatide, whereas it fell significantly by 17 ± 7, 33 ± 10, and 26 ± 8%, respectively, below the baseline (P < 0.05). JNK-1, TLR-4, and SOCS-3 mRNA expression in MNC was also suppressed by 18 ± 7, 24 ± 12, and 23 ± 11%, respectively, at 2 h (for JNK-1 and TLR-4) and 6 h (for SOCS-3) after a single dose of exenatide (Fig. 4, B, D, and G; P < 0.05). This was associated with a significant fall in the protein expression of JNK-1, TLR-2, and SOCS-3 by 16 ± 7, 15 ± 7, and 13 ± 6%, respectively, below the baseline (Fig. 4, A–D; P < 0.05). TLR-4 protein did not change significantly after treatment with exenatide despite the significant change in its mRNA expression. There was no significant change in JNK-1, TLR-4, TLR-2, and SOCS-3 expression after a single dose of exenatide or 12 wk of placebo.
Fig. 4.
Representative Western blot (A) and densitometry analysis showing percent change in JNK-1 (B), TLR-2 (C), and SOCS-3 (D) proteins in MNC after placebo and exenatide 10 μg twice-daily treatment for 12 wk (W) in type 2 diabetic subjects. Data are presented as mean ± se; n = 12 each. *, P < 0.05 by RMANOVA (compared with baseline); #, P < 0.05 by two-way RMANOVA compared with control groups.
Effect of exenatide on circulating proinflammatory mediators
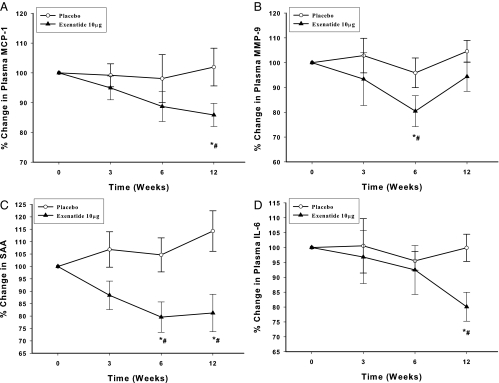
Circulating levels of MCP-1 fell significantly by 15 ± 7% (from 422 ± 33 to 367 ± 35 ng/ml at 12 wk; Fig. 5A; P < 0.05), MMP-9 fell by 20 ± 11% (from 346 ± 49 to 267 ± 44 ng/ml at 6 wk; Fig. 5B; P < 0.05), SAA fell by 16 ± 7% (from 30.9 ± 4.5 to 25.3 ± 3.8 μg/ml at 12 wk; Fig. 5C; P < 0.05), and IL-6 fell by 22 ± 12% (from 3.01 ± 0.49 to 2.07 ± 0.57 pg/ml at 12 wk; Fig. 5D; P < 0.05) after exenatide treatment, whereas there was no change in these indices in the placebo group.
Fig. 5.
Change in plasma concentrations of MCP-1 (A), MMP-9 (B), SAA (C), and IL-6 (D) after placebo and exenatide 10 μg daily for 12 wk in type 2 diabetic subjects. Data are presented as mean ± se; n = 12 each. *, P < 0.05 by RMANOVA (compared with baseline); #, P < 0.05 by two-way RMANOVA compared with control groups.
Discussion
Our data show clearly that exenatide suppresses several indices of inflammation when given over a period of 12 wk. They include ROS generation by MNC, intranuclear NFκB binding, and the expression of TNFα, JNK-1, TLR-2, TLR-4, IL-1β, and SOCS-3 in MNC. In addition, there was a fall in the plasma concentrations of MCP-1, SAA, IL-6, and MMP-9. Because there was no reduction in weight with exenatide in our study, these changes were independent of weight loss. Thus, exenatide exerts an antiinflammatory effect that is not dependent on weight reduction, consistent with our original observation with plasma CRP concentrations (7). This effect is even more impressive because it was observed in patients taking insulin, which has an antiinflammatory effect of its own (21).
NFκB is responsible for the transcription of more than 200 proinflammatory genes including TNFα (22). The suppression of JNK-1, IL-1β, TLR-2, and TLR-4 also reflects a general antiinflammatory effect because all three are inflammatory mediators. JNK-1 induces the phosphorylation of c-Jun, which is a component of the proinflammatory transcription factor activator protein-1, which regulates the transcription of MMP (23). This action of JNK-1 thus induces inflammation and increases MMP secretion. Consistent with this, exenatide also suppressed plasma concentration of MMP-9. TLR-2 binds to and mediates inflammatory signals from products of Gram-positive bacteria, whereas TLR-4 binds to and mediates inflammatory signals from endotoxin, a product of Gram-negative bacteria (24, 25). TLR-4 is also expressed in the atherosclerotic plaque, and its deletion is inhibitory to atherosclerosis (26). In addition to these actions, JNK-1, TLR-2, TLR-4, and SOCS-3 also mediate insulin resistance (27, 28). JNK-1 causes serine phosphorylation of insulin receptor substrate-1 and thus inhibits downstream insulin signal transduction. The deletion of TLR-4 and TLR-2 leads to protection from insulin resistance (29, 30). The expression of SOCS-3 is induced by TNFα, and it interferes with insulin signal transduction at the insulin receptor substrate-1 level (31). Thus, along with an antiinflammatory and antioxidant effect, it is possible that exenatide may potentially exert an insulin-sensitizing effect.
MCP-1 is a potent chemokine, and it has a nonredundant role in the pathogenesis of atherosclerosis because its deletion leads to a significant reduction in the magnitude of atherosclerosis in the ApoE-deleted mouse (32). It is generated in large quantities by the cells in the atherosclerotic plaque, and it attracts more monocytes in to the plaque. MMP-9 is a collagenase that dissolves collagen in the plaque to render it unstable and vulnerable to rupture (33). This action of MMP-9 as a collagenase also allows inflammatory cells to spread more easily in tissue spaces. SAA is produced by the liver in a fashion similar to CRP and is a marker of inflammation (34). It is carried by the high-density lipoprotein particle and can alter the biological properties of this particle by making it more proinflammatory.
In our study, there was no weight loss with exenatide, and the actions of exenatide were independent of change in weight on multivariable analysis (data not shown). An explanation for the lack of exenatide-induced weight loss could be the short duration of our study in subjects on relatively large doses of insulin. HbA1c was also reduced significantly from 8.6 to 7.4%, and the reduction in calorie loss from glycosuria could have neutralized the effect of exenatide on weight loss.
Because exenatide is known to induce weight loss, the study was prospectively designed to also test the acute effect of a single injection of 5 μg exenatide on inflammatory factors and oxidative stress before the initiation of regular doses of the drug. Indeed, we observed a significant, although transient, reduction in ROS generation, intranuclear NFκB binding, and the expression of TNFα, TLR-4, JNK-1 and SOCS-3. These effects occurred at 2 h after the injection at which time exenatide has a peak plasma concentration. This rapid effect is a remarkable observation considering that a small dose of 5 μg was injected into obese diabetics. Clearly, this drug is a potent and rapidly acting antiinflammatory agent, whose action is independent of weight loss.
In cultured adipocytes, exendin-4 (parent molecule of exenatide) has been shown to increase the expression of adiponectin and decrease that of IL-6 and MCP-1 (35). In apolipoprotein E-deficient mice, Exendin-4 has been shown to reduce monocyte adhesion to endothelial cells and suppress atherogenesis (13). These effects were associated with a suppression of TNFα, MCP-1, and NFκB in the macrophages. A single injection of exenatide has been shown to reduce postprandial triglycerides and remnant lipoprotein cholesterol and triglycerides and to improve endothelial function in humans (14). Consistent with this are our previous observations that exenatide and liraglutide treatments are associated with a reduction in plasma triglyceride concentrations by 35% (7, 8, 36). GLP-1infusion, exenatide, and liraglutide have also been shown to reduce myocardial infarct size in experimental myocardial infarction (15, 16). Thus, GLP-1 agonists have been shown to have antiinflammatory and an antioxidant effect that are direct and independent of weight loss.
Some of the changes in inflammatory mediators were seen after a single injection and at 12 wk but not at 3 and 6 wk. Inflammatory mediators were measured sequentially for 6 h after the single injection, whereas only a fasting sample was collected at 3, 6, and 12 wk. The reduction in inflammatory mediators, although significant, was transient after the single injection, coinciding with the timing of the peak concentration of exenatide. It is possible that exenatide therapy for a longer duration is required to cause changes in baseline concentrations (as measured at 3, 6, and 12 wk) of the inflammatory mediators that did not change at 3 and 6 wk but reduced after 12 wk.
Other possible mechanisms for the antiinflammatory and antioxidant effects of exenatide include the suppression of FFA, enhancement of the antiinflammatory action of insulin, the reduction in glucagon, and changes in dietary composition. In our study, there was a significant fall in FFA concentrations both with the single injection and after 12 wk of exenatide. Thus, it is possible that the fall in FFA concentrations contributes to the observed antiinflammatory and antioxidant effects of exenatide (37). It is also possible that exenatide might have enhanced the antiinflammatory effects of insulin because all our subjects were on insulin therapy (21). We did not collect samples that could be tested for glucagon. It is, however, possible that the observed effects could in part be secondary to a suppression of glucagon. It is also possible that changes in diet could have contributed to the changes in inflammatory parameters. A limitation of the study is that diet recommendations were made at baseline and dietary history was not collected at the end of the study. However, because there was no significant weight loss in the exenatide group, it is likely that subjects did not make any substantial dietary changes during the course of the study. Another limitation of the study is the small sample size. Future studies with a larger sample size need to be done to confirm our findings and to comprehensively assess the mechanisms underlying the antiinflammatory effects of exenatide.
The antiinflammatory effects of exenatide allied with its systolic blood pressure-lowering effects are likely to be atheroprotective and cardioprotective in the long term. Long-term studies are required to investigate these issues. In addition, the impressive triglyceride-lowering effect described earlier may add to these benefits.
In conclusion, exenatide when administered for 12 wk exerts a comprehensive ROS suppressive and antiinflammatory effect that in the long term could be potentially antiatherogenic. In addition, even a single dose of 5 μg exenatide leads to a transient suppression of key inflammatory mediators. On the basis of these observations, exenatide is a rapidly acting antiinflammatory agent, and these effects are independent of its effect on weight loss.
Supplementary Material
Acknowledgments
Disclosure Summary: P.D. is supported by grants from the National Institutes of Health (R01 DK069805 and RO1 DK075877) and the American Diabetes Association (708CR13) and by grants from Merck, Amylin, and Abbott Pharmaceuticals. Other authors have nothing to disclose.
Footnotes
- CRP
- C-reactive protein
- FFA
- free fatty acids
- GLP-1
- glucagon-like peptide-1
- JNK
- c-Jun N-terminal kinase
- MCP
- monocyte chemoattractant protein
- MMP
- matrix metalloproteinase
- MNC
- mononuclear cells
- NFκB
- nuclear factor-κB
- RMANOVA
- repeated-measures ANOVA
- ROS
- reactive oxygen species
- SAA
- serum amyloid A
- SOCS
- suppressor of cytokine signaling
- TLR
- toll-like receptor.
References
- 1. Edwards CM, Stanley SA, Davis R, Brynes AE, Frost GS, Seal LJ, Ghatei MA, Bloom SR. 2001. Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab 281:E155–E161 [DOI] [PubMed] [Google Scholar]
- 2. Barnett AH. 2005. Exenatide. Drugs Today (Barc) 41:563–578 [DOI] [PubMed] [Google Scholar]
- 3. MacDonald PE, El-Kholy W, Riedel MJ, Salapatek AM, Light PE, Wheeler MB. 2002. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes 51(Suppl 3):S434–S442 [DOI] [PubMed] [Google Scholar]
- 4. Holz GG, Chepurny OG. 2003. Glucagon-like peptide-1 synthetic analogs: new therapeutic agents for use in the treatment of diabetes mellitus. Curr Med Chem 10:2471–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rodriquez de Fonseca F, Navarro M, Alvarez E, Roncero I, Chowen JA, Maestre O, Gómez R, Muñoz RM, Eng J, Blázquez E. 2000. Peripheral versus central effects of glucagon-like peptide-1 receptor agonists on satiety and body weight loss in Zucker obese rats. Metabolism 49:709–717 [DOI] [PubMed] [Google Scholar]
- 6. Szayna M, Doyle ME, Betkey JA, Holloway HW, Spencer RG, Greig NH, Egan JM. 2000. Exendin-4 decelerates food intake, weight gain, and fat deposition in Zucker rats. Endocrinology 141:1936–1941 [DOI] [PubMed] [Google Scholar]
- 7. Viswanathan P, Chaudhuri A, Bhatia R, Al-Atrash F, Mohanty P, Dandona P. 2007. Exenatide therapy in obese patients with type 2 diabetes mellitus treated with insulin. Endocr Pract 13:444–450 [DOI] [PubMed] [Google Scholar]
- 8. Varanasi A, Chaudhuri A, Dhindsa S, Arora A, Lohano T, Vora MR, Dandona P. 2010. Durability of effects of exenatide treatment on glycemic control, body weight, systolic blood pressure, C-reactive protein and triglyceride concentrations. Endocr Pract 17:192–200 [DOI] [PubMed] [Google Scholar]
- 9. Bunck MC, Diamant M, Eliasson B, Cornér A, Shaginian RM, Heine RJ, Taskinen MR, Yki-Järvinen H, Smith U. 2010. Exenatide affects circulating cardiovascular risk biomarkers independently of changes in body composition. Diabetes Care 33:1734–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Derosa G, Maffioli P, Salvadeo SA, Ferrari I, Ragonesi PD, Querci F, Franzetti IG, Gadaleta G, Ciccarelli L, Piccinni MN, D'Angelo A, Cicero AF. 2010. Exenatide versus glibenclamide in patients with diabetes. Diabetes Technol Ther 12:233–240 [DOI] [PubMed] [Google Scholar]
- 11. Xue S, Wasserfall CH, Parker M, Brusko TM, McGrail S, McGrail K, Moore M, Campbell-Thompson M, Schatz DA, Atkinson MA, Haller MJ. 2008. Exendin-4 therapy in NOD mice with new-onset diabetes increases regulatory T cell frequency. Ann NY Acad Sci 1150:152–156 [DOI] [PubMed] [Google Scholar]
- 12. Wu JD, Xu XH, Zhu J, Ding B, Du TX, Gao G, Mao XM, Ye L, Lee KO, Ma JH. 2011. Effect of exenatide on inflammatory and oxidative stress markers in patients with type 2 diabetes mellitus. Diabetes Technol Ther 13:143–148 [DOI] [PubMed] [Google Scholar]
- 13. Arakawa M, Mita T, Azuma K, Ebato C, Goto H, Nomiyama T, Fujitani Y, Hirose T, Kawamori R, Watada H. 2010. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes 59:1030–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koska J, Schwartz EA, Mullin MP, Schwenke DC, Reaven PD. 2010. Improvement of postprandial endothelial function after a single dose of exenatide in individuals with impaired glucose tolerance and recent-onset type 2 diabetes. Diabetes Care 33:1028–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Noyan-Ashraf MH, Momen MA, Ban K, Sadi AM, Zhou YQ, Riazi AM, Baggio LL, Henkelman RM, Husain M, Drucker DJ. 2009. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes 58:975–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Timmers L, Henriques JP, de Kleijn DP, Devries JH, Kemperman H, Steendijk P, Verlaan CW, Kerver M, Piek JJ, Doevendans PA, Pasterkamp G, Hoefer IE. 2009. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol 53:501–510 [DOI] [PubMed] [Google Scholar]
- 17. Ross R. 1999. Atherosclerosis: an inflammatory disease. N Engl J Med 340:115–126 [DOI] [PubMed] [Google Scholar]
- 18. Dandona P, Thusu K, Cook S, Snyder B, Makowski J, Armstrong D, Nicotera T. 1996. Oxidative damage to DNA in diabetes mellitus. Lancet 347:444–445 [DOI] [PubMed] [Google Scholar]
- 19. Andrews NC, Faller DV. 1991. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res 19:2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ghanim H, Aljada A, Hofmeyer D, Syed T, Mohanty P, Dandona P. 2004. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation 110:1564–1571 [DOI] [PubMed] [Google Scholar]
- 21. Dandona P, Aljada A, Mohanty P, Ghanim H, Hamouda W, Assian E, Ahmad S. 2001. Insulin inhibits intranuclear nuclear factor κ-B and stimulates Iκ-B in mononuclear cells in obese subjects: evidence for an antiinflammatory effect? J Clin Endocrinol Metab 86:3257–3265 [DOI] [PubMed] [Google Scholar]
- 22. Barnes PJ, Karin M. 1997. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 336:1066–1071 [DOI] [PubMed] [Google Scholar]
- 23. Crowe DL, Tsang KJ, Shemirani B. 2001. Jun N-terminal kinase 1 mediates transcriptional induction of matrix metalloproteinase 9 expression. Neoplasia 3:27–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. 1999. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem 274:10689–10692 [DOI] [PubMed] [Google Scholar]
- 25. Hallman M, Rämet M, Ezekowitz RA. 2001. Toll-like receptors as sensors of pathogens. Pediatr Res 50:315–321 [DOI] [PubMed] [Google Scholar]
- 26. Li H, Sun B. 2007. Toll-like receptor 4 in atherosclerosis. J Cell Mol Med 11:88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bennett BL, Satoh Y, Lewis AJ. 2003. JNK: a new therapeutic target for diabetes. Curr Opin Pharmacol 3:420–425 [DOI] [PubMed] [Google Scholar]
- 28. Hotamisligil GS. 2006. Inflammation and metabolic disorders. Nature 444:860–867 [DOI] [PubMed] [Google Scholar]
- 29. Ehses JA, Meier DT, Wueest S, Rytka J, Boller S, Wielinga PY, Schraenen A, Lemaire K, Debray S, Van Lommel L, Pospisilik JA, Tschopp O, Schultze SM, Malipiero U, Esterbauer H, Ellingsgaard H, Rütti S, Schuit FC, Lutz TA, Böni-Schnetzler M, Konrad D, Donath MY. 2010. Toll-like receptor 2-deficient mice are protected from insulin resistance and β-cell dysfunction induced by a high-fat diet. Diabetologia 53:1795–1806 [DOI] [PubMed] [Google Scholar]
- 30. Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. 2006. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116:3015–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Emanuelli B, Peraldi P, Filloux C, Chavey C, Freidinger K, Hilton DJ, Hotamisligil GS, Van Obberghen E. 2001. SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-α in the adipose tissue of obese mice. J Biol Chem 276:47944–47949 [DOI] [PubMed] [Google Scholar]
- 32. Aiello RJ, Bourassa PA, Lindsey S, Weng W, Natoli E, Rollins BJ, Milos PM. 1999. Monocyte chemoattractant protein-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 19:1518–1525 [DOI] [PubMed] [Google Scholar]
- 33. Galis ZS, Sukhova GK, Lark MW, Libby P. 1994. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest 94:2493–2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O'Brien KD, Chait A. 2006. Serum amyloid A: the “other” inflammatory protein. Curr Atheroscler Rep 8:62–68 [DOI] [PubMed] [Google Scholar]
- 35. Kim Chung le T, Hosaka T, Yoshida M, Harada N, Sakaue H, Sakai T, Nakaya Y. 2009. Exendin-4, a GLP-1 receptor agonist, directly induces adiponectin expression through protein kinase A pathway and prevents inflammatory adipokine expression. Biochem Biophys Res Commun 390:613–618 [DOI] [PubMed] [Google Scholar]
- 36. Varanasi A, Bellini N, Rawal D, Vora M, Makdissi A, Dhindsa S, Chaudhuri A, Dandona P. 2011. Liraglutide as additional treatment for type 1 diabetes. Eur J Endocrinol 165:77–84 [DOI] [PubMed] [Google Scholar]
- 37. Tripathy D, Mohanty P, Dhindsa S, Syed T, Ghanim H, Aljada A, Dandona P. 2003. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes 52:2882–2887 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.