Abstract
Context:
Disorders of steroidogenesis have been instrumental in delineating human steroidogenic pathways. Each genetic disorder seemed to correspond to a different steroidogenic activity, helping to identify several enzymes. Beginning in 1972, several patients have been reported as having “17,20 lyase deficiency,” but there have been inconsistent genetic findings.
Objective:
This manuscript reviews the biochemistry, genetics, and clinical disorders of 17,20 lyase activity, which converts 21-carbon precursors of glucocorticoids to 19-carbon precursors of sex steroids.
Findings:
A single enzyme, cytochrome P450c17, catalyzes both 17α-hydroxylase activity and 17,20 lyase activity. The 17,20 lyase activity is especially sensitive to the activities of the accessory proteins P450 oxidoreductase and cytochrome b5. The first cases of genetically and biochemically proven 17,20 lyase deficiency were reported in 1997, in which specific P450c17 mutations were identified that lost 17,20 lyase activity but not 17α-hydroxylase activity when assayed in vitro. Subsequent work identified other P450c17 mutations and mutations in the genes encoding P450 oxidoreductase and cytochrome b5. Recently, the initially reported cases from 1972 were found to carry mutations in two aldo-keto reductases, AKR1C2 and AKR1C4. These AKR1C isozymes catalyze 3α-hydroxysteroid dehydrogenase activity in the so-called “backdoor pathway” by which the fetal testis produces dihydrotestosterone without the intermediacy of testosterone.
Conclusions:
17,20 Lyase deficiency should be considered a syndrome with multiple causes, and not a single disease. Study of this very rare disorder has substantially advanced our understanding of the pathways, mechanisms, and control of androgen synthesis. Mutations in other, as-yet unidentified genes may also cause this phenotype.
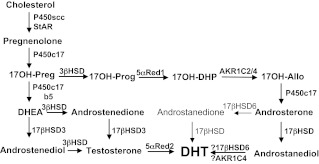
The classic pathways of adrenal and gonadal androgen synthesis have been known for over 50 yr (reviewed in Ref. 1). Cholesterol is first converted to pregnenolone. In the past, this was thought to involve three separate enzymes (20-hydroxylase, a 22-hydroxylase, and “20,22 desmolase”), but it is now clear that all three reactions are catalyzed by mitochondrial cytochrome P450scc. Pregnenolone may then be converted to other Δ5 steroids: 17α-OH-pregnenolone (17-Preg), dehydroepiandrosterone (DHEA), and androstenediol. These Δ5 steroids may be converted to their Δ4 homologs— progesterone, 17α-OH-progesterone (17OHP), androstenedione, and testosterone—by 3β-hydroxysteroid dehydrogenase (3βHSD) (Fig. 1). Both progesterone and pregnenolone readily undergo 17α-hydroxylation to 17-Preg and 17OHP, respectively; a defect in 17α-hydroxylation was first described clinically and hormonally in 1966 by Biglieri et al. (2). To produce sex steroids, 21-carbon (C21) 17-hydroxysteroids such as 17-Preg or 17OHP must then be cleaved to 19-carbon (C19) steroids; this removal of carbon atoms no. 20 and 21 is “17,20 lyase activity” (formerly called 17,20 desmolase activity in some clinical literature). The nature of the enzyme catalyzing this step was a source of confusion and controversy for many years.
Fig. 1.
The pathways of androgen biosynthesis. The classic pathway and the alternative “backdoor pathway” are shown. The classic pathway proceeds from 17OH-Preg to DHEA, to androstenedione or androstenediol to testosterone in the testis, and thence to DHT in genital skin. The backdoor pathway proceeds from 17OH-Preg to 17OH-Prog, 17OH-DHP, 17OH-Allo, androsterone, androstanediol, and thence to DHT, all in the testis. The enzymes and cofactors shown in the classic pathway are: P450scc (cholesterol side-chain cleavage enzyme), StAR (steroidogenic acute regulatory protein), P450c17 (17α-hydroxylase/17,20-lyase), 3β-HSD (3β-hydroxysteroid dehydrogenase, type 2), cytochrome b5, 17β-HSD3 (17β-hydroxysteroid dehydrogenase, type 3), and 5αR2 (5α-reductase, type 2). The alternative pathway is characterized by the presence of three additional enzymes: 5αR1 (5α-reductase, type 1), reductive 3α-HSD (AKR1C2/4), and oxidative 3α-HSD (17β-HSD6, also known as RoDH and/or AKR1C4). Steroid names include: 17OH-Preg, 17-hydroxypregnenolone; 17OH-Prog, 17-hydroxyprogesterone; 17OH-DHP, 17-hydroxydihydroprogesterone (5α-pregnan-17α-ol-3,20-dione); 17OH-Allo, 17-hydroxy-allopregnanolone (5α-pregnan-3α,17α-diol-20-one); androstenediol, androsta-5-ene-3β,17β-diol; and androstanediol, 5α-androstane-3α,17β-diol.
During the early history of molecular genetics, the discoveries that one gene encodes one enzymatic function (3) and that one gene encodes one polypeptide chain (4) were widely interpreted to mean that each enzymatic activity required a separate gene. This is true in most cases, and most enzymes are named for the single activity they catalyze (e.g. glucose-6-phosphate dehydrogenase is the name of the gene, the protein, and the enzyme activity). Thus, it was eminently logical to presume that 17α-hydroxylase and 17,20 desmolase were different proteins encoded by different genes, and many investigators also believed that there had to be different genes and enzymes involved in different tissues (adrenal, gonad). After all, in the 1970s it was estimated that the human genome had to have over 100,000 protein-coding genes, whereas a recent estimate puts this number at 22,333 (5).
Until the genes for most steroidogenic enzymes were cloned in the 1980s (1), the identification of defects in steroidogenesis was necessarily indirect. Investigators who studied patients lacking the biologically active end-products (aldosterone, cortisol, testosterone, estradiol) sought accumulations of precursor steroids in blood or their metabolites in urine, in addition to the absence of the expected product. Sometimes affected tissues could be incubated with radiolabeled precursor steroids, and the defective enzymatic activity could be demonstrated more directly. These approaches successfully delineated much of what we know about the defects in 21-hydroxylation that constitute the common forms of congenital adrenal hyperplasia and led to the discoveries of 11-hydroxylase deficiency (6), 3β-HSD deficiency (7), and 17-hydroxylase deficiency (2). This approach also led to some notably incorrect conclusions; for example, congenital lipoid adrenal hyperplasia is not the enzymatic defect “20,22-desmolase deficiency” as initially described (8), but it is actually a defect in a cholesterol transporter, the steroidogenic acute regulatory protein (9, 10). This approach also failed with so-called “17,20 desmolase deficiency,” first reported in 1972 (11), but it has taken longer to unravel this issue because the cases initially thought to represent a single disorder have been found to represent defects in at least four different genetic loci, and possibly more. Furthermore, although publications concerning 17,20 lyase deficiency have typically sought patients with apparently normal cortisol values and low C19 steroids, clinical criteria for this diagnosis have not been established, and developing such criteria will require detailed clinical, hormonal, genetic, and biochemical investigation of more patients.
P450c17 Catalyzes Both 17α-Hydroxylase and 17,20 Lyase Activities
Many steroidogenic enzymes are forms of cytochrome P450. The cytochrome P450 enzymes are heme-containing proteins of about 500 amino acids that absorb light at 450 nm in their reduced state; “P450” refers to a pigment absorbing at 450 nm. All P450 enzymes receive electrons from reduced nicotinamide adenine dinucleotide phosphate (NADPH) and mediate catalysis via the iron atom in the heme ring. Steroidogenic P450 enzymes may be found both in the endoplasmic reticulum and in mitochondria (for review, see Ref. 12). The first activity described for a P450 enzyme was steroid 21-hydroxylation (13). The demonstration that 21-hydroxylation was P450-dependent led to the determination that there are many types of cytochrome P450 that catalyzed many different reactions, and indirect evidence suggested that 17α-hydroxylation and 17,20 lyase activity were catalyzed by separate cytochrome P450 enzymes (14). This view was consistent with early clinical reports of 17,20 desmolase deficiency as a disorder unrelated to 17α-hydroxylase or its deficiency (11, 15). In view of this apparently self-evident conventional wisdom that 17α-hydroxylation and 17,20 lyase activity had to be catalyzed by separate enzymes, the reports by Nakajin and Hall (16–18) showing that both activities were catalyzed by a single microsomal P450 enzyme were questioned (19) and generally ignored. Nevertheless, Nakajin and Hall (20) later proved that a single porcine P450 enzyme catalyzed both 17α-hydroxylase and 17,20 lyase activities, although this work incorrectly concluded that there were different enzymes in the adrenal and testis. Shortly thereafter, Yanagibashi and Hall (21) showed that the degree of 17,20 lyase activity was dependent on the availability of P450 oxidoreductase (POR), the obligatory electron transfer protein required by all microsomal P450 enzymes. Residual doubts that a single P450 could catalyze both 17α-hydroxylase and 17,20 lyase activities were dispelled when Zuber et al. (22) showed that the expression of bovine P450c17 cDNA in COS-1 cells conferred both 17-hydroxylase and 17,20 lyase activities on these otherwise nonsteroidogenic cells. The notion that different enzymes would be found in the adrenal and testis was dispelled by cloning identical cDNA from human adrenal and testis (23) and by cloning the single human gene (now termed CYP17A1) for P450c17 (24). Initial work with rodent, bovine, and porcine P450c17 indicated that 17-Preg and 17OHP were equally good substrates for the 17,20 lyase activity or, in rodents, that the preferred substrate was 17OHP, in which case androgen synthesis could proceed through androstenedione without the intermediacy of DHEA. However, human P450c17 catalyzes the 17,20 lyase reaction using 17-Preg as its principal substrate (25, 26) and detailed characterization of the enzymology of human P450c17 showed that the human enzyme exhibits a 50:1 preference for 17-Preg as a substrate (27), so that conversion of 17OHP to androstenedione can be significant only when the concentrations of 17OHP are extraordinarily high, as in 21-hydroxylase deficiency.
Enzymology of P450c17
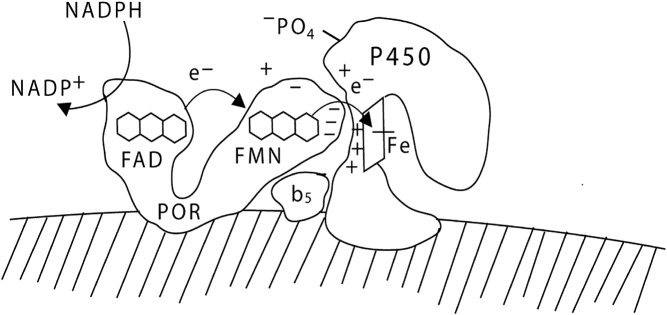
Like all microsomal P450 enzymes, P450c17 receives electrons from NADPH via POR, a membrane-bound protein that contains two flavin moieties. Crystal structures of N-terminally truncated forms of rat POR (28, 29), dynamic modeling based on nuclear magnetic resonance and small-angle x-ray scattering (30), and crystallographic characterization of conformation-altering disulfide cross-linked mutants (31) have revealed much about the structure and mode of action of POR. POR has two distinct domains: one contains a flavin adenine dinucleotide (FAD) moiety, and the other contains a flavin mononucleotide (FMN) moiety. These two domains are separated by a flexible hinge region. The FAD moiety of POR receives two electrons from NADPH; this elicits a conformational change in the hinge region, aligning the isoalloxazine rings of the FAD and the FMN, permitting the electrons to flow from the FAD to the FMN. The POR then returns to its initial, more open conformation, allowing the FMN domain to interact with the redox-partner binding site of the P450, permitting the electrons from the FMN to be transferred one at a time to the P450 (Fig. 2) (reviewed in Ref. 12).
Fig. 2.
Electron transport to P450c17. POR, bound to the endoplasmic reticulum, receives a pair of electrons (e-) from NADPH and transfers them to its FAD moiety. Receipt of the electrons elicits a conformational change in POR, permitting the isoalloxazine rings of the FAD and FMN moieties to come close together so that the electrons pass from the FAD to the FMN. The POR then returns to its original orientation, permitting the FMN domain of POR to interact with the redox-partner binding site of P450c17. The electrons then travel through the P450c17 protein to reach the heme group and mediate catalysis. The interaction of POR and P450c17 is coordinated by negatively charged acidic residues on the electron-donating surface of the FMN domain of POR and positively charged basic residues in the concave redox-partner binding site of P450c17. The interaction of P450c17 and POR is facilitated by the allosteric action of cytochrome b5, and by the serine phosphorylation of P450c17. [© W. L. Miller.]
Three factors, all governing electron transfer from POR to P450c17, regulate the ratio of 17,20 lyase activity to 17-hydroxylase activity. First, 17,20 lyase activity is favored by high molar ratios of POR to P450c17 (21, 26). Second, cytochrome b5 acts as an allosteric factor (rather than as an alternate electron donor) and promotes the interaction of POR and P450c17, favoring the 17,20 lyase reaction (27, 32, 76). Third, 17,20 lyase activity is facilitated by the phosphorylation of serine and/or threonine residues on P450c17 by a cAMP-dependent protein kinase (33–36). Thus, the availability of electrons determines whether P450c17 performs only 17α-hydroxylation or whether it also performs 17,20 bond scission. The structural data for rat POR (28–31) and computational modeling of human POR (37, 38) both show that the electron-donating surface of the FMN domain of POR is characterized by acidic residues. By contrast, computational modeling of human P450c17 shows that its redox partner binding site, which receives the electrons from POR, is characterized by basic residues (39, 40), so that P450c17 and POR interact by electrostatic interactions. Competition between P450c17 and P450c21 for available 17OHP is not important in determining whether 17OHP undergoes 21-hydroxylation or 17,20 bond scission because human P450c17 catalyzes the conversion of 17OHP to androstenedione very poorly (27). Thus, the regulation of 17,20 lyase activity, and consequently of androgen production, depends on the following factors that facilitate the flow of electrons to P450c17: high concentrations of POR, the presence of cytochrome b5, and serine phosphorylation of P450c17.
P450c17 Mutations That Selectively Lose 17,20 Lyase Activity
The most logical place to search for genetic causes of isolated 17,20 lyase deficiency is in P450c17 itself. However, this has proved to be surprisingly difficult. The first molecular genetic study searching for the cause of 17,20 lyase deficiency studied a patient reported clinically in 1982 (41). That clinical report described a patient who appeared to be different from the 1972 index case of 17,20 desmolase deficiency (11) in that the 1972 case was thought to have a partial defect in both the Δ5 and Δ4 pathways, and the 1982 case was thought to have a complete defect in the Δ4 pathway only (41). The inability of human P450c17 to catalyze 17,20 lyase activity with 17OHP as its substrate was not known in 1972 or 1982; in retrospect, the clinical findings in the 1982 paper clearly identified a typical case of complete 17α-hydroxylase deficiency. When this patient's CYP17A1 gene was sequenced, she was found to have a stop codon on one allele (Q461X) and the missense mutation R496C on the other allele; in vitro, R496C retained only 10% of wild-type 17α-hydroxylase activity and only 4% of 17,20 lyase activity, indicating that the patient had to have nearly complete 17α-hydroxylase deficiency (42). This patient was then reported to have experienced conversion from pure 17,20 desmolase to combined 17,20 desmolase/17α-hydroxylase deficiency (43), but it is now clear that she always had 17α-hydroxylase deficiency.
The experience described above suggested that isolated 17,20 lyase activity might not exist. Nevertheless, in 1997, mutations were found in the redox-partner binding site of P450c17 from two 46,XY patients who had clinical features suggesting isolated 17,20 lyase deficiency: they had normal basal (but ACTH-unresponsive) cortisol, low C19 steroids, and genital ambiguity (39). Each patient was homozygous for a P450c17 missense mutation: one patient carried the mutation R347H, and the other carried R358Q. Consistent with the crucial role of electron transfer in 17,20 lyase activity, mutations R347H and R358Q were shown to lie in the redox-partner binding site of P450c17 and to change the surface charge distribution (39). These mutations impaired the ability of P450c17 to interact with POR and selectively impaired 17,20 lyase activity, but they had minimal effect on 17α-hydroxylase activity (39). Advanced computational modeling of P450c17 has confirmed that these residues are surface-exposed in the redox-partner binding site (40). With the development of a highly reliable yeast system for studying the enzymology of P450c17 and the associated demonstration that cytochrome b5 promotes 17,20 lyase activity by allosteric action rather than as an alternative electron donor (27), it became possible to study these mutations with biochemical rigor. Those studies showed that P450c17 carrying either the R347H or R358Q mutation had the same Michaelis constant (Km) as the wild-type enzyme, but had reduced maximum velocities (Vmax) for the 17,20 lyase reaction and were subject to competitive inhibition, confirming that the mutations did not affect the active site. Furthermore, these studies showed that the impairment in 17,20 lyase activity could be partially corrected by the presence of a molar excess of cytochrome b5, providing further evidence for the role of b5 as an allosteric factor promoting the interaction of P450c17 with POR (32). A subsequent study found two additional patients with R347H and two with the similar mutation R347C, which caused an indistinguishable phenotype and also had selective loss of 17,20 lyase activity in vitro (44); another study reported four more such patients, all from The Netherlands (45). A somewhat different P450c17 mutation, E305G, affects the active site and directly impairs the ability of P450c17 to convert 17-Preg to DHEA, but it did not impair 17α-hydroxylase activity when assayed in transfected yeast or mammalian cells (46), although the affected patients had impaired cortisol responses to ACTH (47). Interestingly, this unusual mutant increased the very low capacity of P450c17 to convert 17OHP to androstenedione about 10-fold, partially ameliorating the loss of 17,20 lyase activity in the dominant Δ5 pathway; nevertheless, the 46,XY index patient had micropenis and hypospadias, further confirming that the Δ5 pathway is the predominant pathway for human androgen biosynthesis (27, 48).
A Mutation in POR That Selectively Impairs 17,20 Lyase Activity
The study of P450c17 mutations that appear to cause isolated 17,20 lyase deficiency can be daunting. Shortly after the description of the first patients with true 17,20 lyase deficiency (39), another paper reported apparent 17,20 lyase deficiency. A consanguineous 46,XY infant had micropenis and undescended testes; cortisol was only partially responsive to ACTH, but 17OHP was hyperresponsive, and C19 steroids (DHEA, androstenedione, and testosterone) were low and unresponsive to ACTH or human chorionic gonadotropin (49). Sequencing the CYP17A1 gene appeared to show compound heterozygosity for the premature stop codon V74X inherited from the mother and the missense mutation F417C inherited from the father. Expression of the F417C mutant in COS-1 cells appeared to show a reduction in 17α-hydroxylase activity to 26% of wild-type, with no measurable 17,20 lyase activity. Addition of iodosobenzine, an electron donor, appeared to result in partial restoration of 17,20 lyase activity, so the authors concluded that F417C disrupted electron transfer from POR. However, the computational model of P450c17 (40) indicated that F417 was adjacent to but not within the redox-partner binding site and that changing F417 to C would disrupt the protein's conformation. Gupta et al. (50) then investigated the enzymology of the F417C mutant in detail. In both yeast and COS cells, F417C was devoid of all activity, and spectral studies showed that this mutant could not bind the heme needed for any activity. Thus, Gupta et al. (50) showed that F417C could not cause isolated 17,20 lyase deficiency and speculated that the initial investigators had been misled by the use of gas chromatography to assay total steroids from the transfected cells, rather than using radiolabeled precursors that permit the definitive identification of precursor-product relationships. This controversy was resolved when Hershkovitz et al. (51) sequenced DNA from both the index case and three other affected members of the extended family and found that there were no mutations in CYP17A1 but instead found that all four affected individuals were homozygous for the POR mutation G539R. A previous study had found POR G539R in compound heterozygosity with a POR null allele in a patient with severe POR deficiency causing Antley-Bixler syndrome and showed that this POR mutant retained 46% of wild-type activity to support the 17α-hydroxylase activity of P450c17, but could only support 8% of the 17,20 lyase activity (37). Thus, in the homozygous state, POR G539R had sufficient activity to prevent Antley-Bixler syndrome, but, as expected from the retention of only 8% of wild-type 17,20 lyase activity, the patients had low C19 steroids and hypergonadotropic hypogonadism. Thus, this specific POR mutant caused a form of apparent 17,20 lyase deficiency.
Mutation of Cytochrome b5
With the demonstration that disruption of electron transfer from POR to P450c17 caused 17,20 lyase deficiency, either by mutations in P450c17 or POR, it was logical to search for mutations in cytochrome b5 as another potential cause of apparent 17,20 lyase deficiency. Until recently, only one case of b5 deficiency had been reported: the patient had methemoglobinemia and ambiguous genitalia, but unfortunately no studies of adrenal or gonadal steroidogenesis were reported (52, 53). Methemoglobinemia is an expected consequence of b5 deficiency because the reduction of methemoglobin is the principal physiological role of b5, and the common cause of methemoglobinemia is deficiency of cytochrome b5 reductase; this association led to the initial report in 1994 (53). In 2010, Kok et al. (54) described a consanguineous 46,XY infant homozygous for the CYB5 gene mutation W27X who had micropenis, bifid scrotum, and scrotal hypospadias. At 3 months of age, there was hypergonadotropic hypogonadism with low adrenal and gonadal C19 steroids and a normal cortisol response to ACTH; the methemoglobin level was elevated 4-fold above the upper limit of normal, but the patient did not have clinical methemoglobinemia. The reason for the presence of severe methemoglobinemia in the index case (52, 53) and the lack of clinically significant methemoglobinemia in the patient of Kok et al. (54) remains unknown. Cytochrome b5 residues E48 and E49 are required for the stimulation of 17,20 lyase activity (55), and these residues are absent in the patient with W27X; the residues required for the reduction of methemoglobin have not been mapped, but it would seem logical that this activity should require binding of heme, which requires residues H43 and H67 (56). Thus, W27X should be a wholly null mutation. Further reports of this rare disorder will be of interest.
3α-HSD Deficiency: Mutations in AKR1C2 and AKR1C4
With the discovery that many patients thought to have 17,20 desmolase deficiency did not have CYP17A1 mutations, it became of interest to reassess the initial cases. Recent analysis of the patients initially described in 1972 (11) has led to a surprising observation that has dramatically revised classical views of the mechanisms of male sexual development. The initial cases were male cousins who presented at birth with cryptorchidism and undervirilized external genitalia; their maternal “aunt” had tall stature, ambiguous genitalia, and primary amenorrhea but had a 46,XY karyotype and no uterus. Studies of urinary steroid metabolites in 1972 suggested isolated 17,20-lyase deficiency, but subsequent DNA sequencing found no mutations in CYP17A1 (57). The hormonal data appeared to be inconsistent with 17βHSD3 or 5α-reductase deficiencies, and no mutations were found in the POR, NR4A1, or NR3C4 genes encoding POR, steroidogenic factor-1, or androgen receptor (57). A novel, alternative or backdoor pathway leading to the synthesis of dihydrotestosterone (DHT) without the intermediacy of DHEA, androstenedione, or testosterone was described in marsupials in 2002 (58, 59), and some data suggest that this pathway may be relevant to the virilization of female fetuses with POR deficiency (60). Thus, mutations in the enzymes mediating androgen production through the backdoor pathway to DHT were considered.
The backdoor pathway is remarkable for having both reductive and oxidative 3α-HSD steps: the reductive reaction converts 17OH-dihydroprogesterone (17OH-DHP) to 17OH-allopregnanolone, and the oxidative reaction converts androstanediol (3α-Diol) to DHT (Fig. 1). Although all the human genes participating in the backdoor pathway have not been identified, reductive 3α-HSD activity can be catalyzed by an aldo-keto reductase called AKR1C2 (61, 62) and possibly other enzymes; the oxidative 3α-HSD activity can be catalyzed by 17βHSD6 (also known as RoDH) (63), and possibly by AKR1C4 (64, 65) (reviewed in Ref. 1).
The AKR1C2 genes in Zachmann's original patients from 1972 all carried missense mutations (57). Two affected 46,XY genetic males who had severe undervirilization and female sex assignments were compound heterozygotes for AKR1C2 mutations I79V and H90Q and for I79V and N300T. A heterozygote carrying only I79V was moderately undervirilized, and a heterozygote carrying N300T had normal male external genitalia; both fathered children, although one required in vitro fertilization. A 46,XX woman who was compound heterozygous for I79V and H90Q was phenotypically normal and bore three children. Thus, the disorder followed sex-limited recessive genetics. Genome-wide scanning of nine members of the index family with 811 microsatellite markers tested for involvement of other genes, identifying potential loci only on chromosomes 22 and 10, thus excluding RoDH (HSD17B6) (chromosome 12), cytochrome b5 (CYB5) (chromosome 18), and 5α-reductase type 1 (SRD5A1) (chromosome 5). Detailed analysis of these chromosomes with additional microsatellite markers gave a LOD score of 3.2 for markers D22S1167 and D22S1144, but no factors that appear to be involved in steroidogenesis lie in this region. By contrast, microsatellite markers D10S1713 and D10S2382 gave scores of 6.3 and 6.2, respectively, identifying the AKR1C locus. Sequencing the exons of the other four AKR1C genes in this locus from the proband identified no further mutations.
However, when assessed in vitro, the mutant AKR1C2 enzymes found in the index family retained 22–82% of wild-type activity (57), suggesting that another gene was probably involved. Analysis of AKR1C cDNA prepared from patient leukocyte RNA showed that some of the AKR1C4 mRNA was spliced incorrectly. Sequencing of the AKR1C4 cDNA and gene identified an intronic mutation 106 bases upstream from exon 2 that caused this exon to be skipped, and expression of a AKR1C4 minigene construct carrying the wild-type and mutant intron confirmed that this single base change disrupted RNA splicing (57). Thus, in this family, partial defects in both AKR1C2 and AKR1C4 (digenic inheritance) combined to impair testicular synthesis of DHT. The essential role of AKR1C2 was then proven by finding an unrelated, phenotypically female, 46,XY patient who had mutations only in AKR1C2. A complex genetic recombination resulted in a nonfunctional hybrid AKR1C1/AKR1C2 gene on one allele, whereas the other allele bore the AKR1C2 mutant H222Q, which lacked all detectable activity in vitro (57), so that all AKR1C2 activity was lost.
Analyses of the expression of AKR1C transcripts in steroidogenic tissues showed that AKR1C2 is abundantly expressed in the fetal testis but minimally expressed in the adult testis; by contrast, it was minimally expressed in the fetal adrenal but is well-expressed in the adult adrenal (57). AKR1C4 was found in fetal and adult testes at lower levels, and RoDH was found at even lower levels (57). Thus, it appears that both AKR1C2 and AKR1C4 participate in the backdoor pathway to DHT in the fetal testis, and that either their combined partial loss of function or complete loss of AKR1C2 function can cause incomplete male genital development. Because the patients with AKR1C2/4 mutations have intact P450c17, POR, and b5, they most likely have wholly normal 17,20 lyase activity. However, these patients only consented to genetic studies and refused any further genital or hormonal examinations, hence much remains to be learned about the hormonal profiles hence pubertal development in patients with AKR disorders.
The traditional view of male external genital development has been that fetal testicular testosterone is converted to DHT by 5α-reductase type 2 in genital skin, which then acts as a paracrine factor to stimulate fusion of the labioscrotal folds (66). This view is supported by the incomplete external genital development in persons with severe deficiencies of 5α-reductase type 2. The new observations concerning AKR1C2 and the backdoor pathway indicate that DHT produced in the testis via the backdoor pathway also acts as a hormone to induce labioscrotal fusion. Thus, both the classic and backdoor pathways appear to be needed, and DHT acts in male genital development both as a paracrine factor and as a hormone. This remarkable finding, which dramatically revises our understanding of the mechanisms by which male sexual differentiation occurs, illustrates the importance of detailed studies of rare patients, such as those who appear to have 17,20 lyase deficiency.
Future Directions
Apparent 17,20 lyase deficiency is a complex syndrome that can be caused by mutations in several genes. Although this disorder is very rare, its study provides an excellent example of how the detailed study of a rare disorder has substantially improved our understanding of a basic physiological process: in this case, illustrating the key roles of POR and cytochrome b5 in the synthesis of adrenal androgen precursors, and leading to the discovery of the crucial role of AKR1C2 in fetal testicular synthesis of DHT and the role of fetal testicular DHT in normal male genital development. Review of the cases described above makes it clear that 17,20 lyase deficiency is an extremely difficult diagnosis to establish, so that the contemporary endocrinologist must be skeptical about clinical case reports of this disorder that do not include a genetic diagnosis and proof that the identified mutation indeed reduces 17,20 lyase activity while preserving 17α-hydroxylase activity. It would be of substantial interest if the patients who were only reported clinically (15, 67–72) could be restudied with appropriate genetic and biochemical techniques. Similarly, one might consider other genes, which, if disordered, might cause a clinically similar syndrome. Because the phosphorylation of P450c17 stimulates 17,20 lyase activity (33–35), the as-yet unidentified kinase that catalyzes this phosphorylation is an obvious candidate. Because adrenarche represents a growth of the adrenal zona reticularis and an induction of the transcription of the gene for cytochrome b5 (73, 74), disruption of factors regulating the transcription of cytochrome b5 (75) or other unknown factors responsible for these changes should resemble adrenal-specific 17,20 lyase deficiency. Many other scenarios may also be envisioned. Thus, the regulation of androgen synthesis at this key step is likely to remain a fruitful area of research for some time.
Acknowledgments
The author thanks Drs. Christa Flück, Amit V. Pandey, and Anna Biason-Lauber, who did most of the work in the AKR1C study.
This work was supported by National Institutes of Health Grant DK37922.
Disclosure Summary: The author has nothing to declare.
Footnotes
- AKR
- Aldo-keto reductase
- DHEA
- dehydroepiandrosterone
- DHT
- dihydrotestosterone
- FAD
- flavin adenine dinucleotide
- FMN
- flavin mononucleotide
- 3βHSD
- 3β-hydroxysteroid dehydrogenase
- NADPH
- reduced nicotinamide adenine dinucleotide phosphate
- 17OH-DHP
- 17OH-dihydroprogesterone
- 17OHP
- 17α-OH-progesterone
- POR
- P450 oxidoreductase
- 17-Preg
- 17α-OH-pregnenolone.
References
- 1. Miller WL, Auchus RJ. 2011. The molecular biology, biochemistry and physiology of human steroidogenesis and its disorders. Endocr Rev 32:81–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Biglieri EG, Herron MA, Brust N. 1966. 17α-hydroxylation deficiency in man. J Clin Invest 45:1946–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beadle GW, Tatum EL. 1941. Genetic control of biochemical reactions in Neurospera. Proc Natl Acad Sci USA 27:499–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yanofsky C, Carlton BC, Guest JR, Helinski DR, Henning U. 1964. The colinearity of gene structure and protein structure. Proc Natl Acad Sci 51:266–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pertea M, Salzberg SL. 2010. Between a chicken and a grape: estimating the number of human genes. Genome Biol 11:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eberlein WR, Bongiovanni AM. 1956. Plasma and urinary corticosteroids in the hypertensive form of congenital adrenal hyperplasia. J Biol Chem 223:85–94 [PubMed] [Google Scholar]
- 7. Bongiovanni AM, Kellenbenz G. 1962. The adrenogenital syndrome with deficiency of 3β-hydroxysteroid dehydrogenase. J Clin Invest 41:2086–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prader A, Gurtner HP. 1955. [The syndrome of male pseudohermaphrodism in congenital adrenocortical hyperplasia without overproduction of androgens (adrenal male pseudohermaphrodism)] (in German). Helvetica Paediatrica Acta 10:397–412 [PubMed] [Google Scholar]
- 9. Lin D, Sugawara T, Strauss JF, 3rd, Clark BJ, Stocco DM, Saenger P, Rogol A, Miller WL. 1995. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science 267:1828–1831 [DOI] [PubMed] [Google Scholar]
- 10. Bose HS, Sugawara T, Strauss JF, 3rd, Miller WL. 1996. The pathophysiology and genetics of congenital lipoid adrenal hyperplasia. N Engl J Med 335:1870–1878 [DOI] [PubMed] [Google Scholar]
- 11. Zachmann M, Völlmin JA, Hamilton W, Prader A. 1972. Steroid 17,20 desmolase deficiency: a new cause of male pseudohermaphroditism. Clin Endocrinol (Oxf) 1:369–385 [DOI] [PubMed] [Google Scholar]
- 12. Miller WL. 2005. Regulation of steroidogenesis by electron transfer. Endocrinology 146:2544–2550 [DOI] [PubMed] [Google Scholar]
- 13. Cooper DY, Levin S, Narasimhulu S, Rosenthal O, Estabrook RW. 1965. Photochemical action spectrum of terminal oxidase of mixed function oxidase systems. Science 147:400–402 [DOI] [PubMed] [Google Scholar]
- 14. Betz G, Tsai P, Weakley R. 1976. Heterogeneity of cytochrome P-450 in rat testis microsomes. J Biol Chem 251:2839–2841 [PubMed] [Google Scholar]
- 15. Goebelsmann U, Zachmann M, Davajan V, Israel R, Mestman JH, Mishell DR. 1976. Male pseudohermaphroditism consistent with 17,20 desmolase deficiency. Gynecol Invest 7:138–156 [DOI] [PubMed] [Google Scholar]
- 16. Nakajin S, Hall PF. 1981. Microsomal cytochrome P450 from neonatal pig testis. Purification and properties of a C21 steroid side-chain cleavage system (17α-hydroxylase-C17,20 lyase). J Biol Chem 256:3871–3876 [PubMed] [Google Scholar]
- 17. Nakajin S, Shively JE, Yuan PM, Hall PF. 1981. Microsomal cytochrome P450 from neonatal pig testis: two enzymatic activities (17α-hydroxylase and C17,20-lyase) associated with one protein. Biochemistry 20:4037–4042 [DOI] [PubMed] [Google Scholar]
- 18. Nakajin S, Hall PF, Onoda M. 1981. Testicular microsomal cytochrome P450 for C21 steroid side chain cleavage. J Biol Chem 256:6134–6169 [PubMed] [Google Scholar]
- 19. Bumpus JA, Dus KM. 1982. Bovine adrenocortical microsomal hemeproteins P-45017α and P-450C-21. Isolation, partial characterization, and comparison to P-450SCC. J Biol Chem 257:12696–12704 [PubMed] [Google Scholar]
- 20. Nakajin S, Shinoda M, Haniu M, Shively JE, Hall PF. 1984. C21 steroid side-chain cleavage enzyme from porcine adrenal microsomes. Purification and characterization of the 17α-hydroxylase/C17,20 lyase cytochrome P450. J Biol Chem 259:3971–3976 [PubMed] [Google Scholar]
- 21. Yanagibashi K, Hall PF. 1986. Role of electron transport in the regulation of the lyase activity of C-21 side-chain cleavage P450 from porcine adrenal and testicular microsomes. J Biol Chem 261:8429–8433 [PubMed] [Google Scholar]
- 22. Zuber MX, Simpson ER, Waterman MR. 1986. Expression of bovine 17α-hydroxylase cytochrome P450 cDNA in non-steroidogenic (COS-1) cells. Science 234:1258–1261 [DOI] [PubMed] [Google Scholar]
- 23. Chung BC, Picado-Leonard J, Haniu M, Bienkowski M, Hall PF, Shively JE, Miller WL. 1987. Cytochrome P450c17 (steroid 17α-hydroxylase/17,20 lyase): cloning of human adrenal and testis cDNAs indicates the same gene is expressed in both tissues. Proc Natl Acad Sci USA 84:407–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Picado-Leonard J, Miller WL. 1987. Cloning and sequence of the human gene encoding P450c17 (steroid 17α-hydroxylase/17,20 lyase): similarity to the gene for P450c21. DNA 6:439–448 [DOI] [PubMed] [Google Scholar]
- 25. Lin D, Harikrishna JA, Moore CC, Jones KL, Miller WL. 1991. Missense mutation serine106→proline causes 17α-hydroxylase deficiency. J Biol Chem 266:15992–15998 [PubMed] [Google Scholar]
- 26. Lin D, Black SM, Nagahama Y, Miller WL. 1993. Steroid 17α-hydroxylase and 17,20 lyase activities of P450c17: contributions of serine106 and of P450 reductase. Endocrinology 132:2498–2506 [DOI] [PubMed] [Google Scholar]
- 27. Auchus RJ, Lee TC, Miller WL. 1998. Cytochrome b5 augments the 17,20 lyase activity of human P450c17 without direct electron transfer. J Biol Chem 273:3158–3165 [DOI] [PubMed] [Google Scholar]
- 28. Wang M, Roberts DL, Paschke R, Shea TM, Masters BS, Kim JJ. 1997. Three-dimensional structure of NADPH-cytochrome P450 reductase: prototype for FMN- and FAD-containing enzymes. Proc Natl Acad Sci USA 94:8411–8416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hubbard PA, Shen AL, Paschke R, Kasper CB, Kim JJ. 2001. NADPH-cytochrome P450 oxidoreductase. Structural basis for hydride and electron transfer. J Biol Chem 276:29163–29170 [DOI] [PubMed] [Google Scholar]
- 30. Ellis J, Gutierrez A, Barsukov IL, Huang WC, Grossmann JG, Roberts GC. 2009. Domain motion in cytochrome P450 reductase: conformational equilibria revealed by NMR and small-angle X-ray scattering. J Biol Chem 284:36628–36637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xia C, Hamdane D, Shen AL, Choi V, Kasper CB, Pearl NM, Zhang H, Im SC, Waskell L, Kim JJ. 2011. Conformational changes of NADPH-cytochrome P450 oxidoreductase are essential for catalysis and cofactor binding. J Biol Chem 286:16246–16260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Geller DH, Auchus RJ, Miller WL. 1999. P450c17 mutations R347H and R358Q selectively disrupt 17,20 lyase activity by disrupting interactions with P450 oxidoreductase and cytochrome b5. Mol Endocrinol 13:167–175 [DOI] [PubMed] [Google Scholar]
- 33. Zhang LH, Rodriguez H, Ohno S, Miller WL. 1995. Serine phosphorylation of human P450c17 increases 17,20 lyase activity: implications for adrenarche and for the polycystic ovary syndrome. Proc Natl Acad Sci USA 92:10619–10623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pandey AV, Mellon SH, Miller WL. 2003. Protein phosphatase 2A and phosphoprotein SET regulate androgen production by P450c17. J Biol Chem 278:2837–2844 [DOI] [PubMed] [Google Scholar]
- 35. Pandey AV, Miller WL. 2005. Regulation of 17,20 lyase activity by cytochrome b5 and by serine phosphorylation of P450c17. J Biol Chem 280:13265–13271 [DOI] [PubMed] [Google Scholar]
- 36. Tee MK, Dong Q, Miller WL. 2008. Pathways leading to the phosphorylation of P450c17 and to the post-translational regulation of androgen biosynthesis. Endocrinology 149:2667–2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang N, Pandey AV, Agrawal V, Reardon W, Lapunzina PD, Mowat D, Jabs EW, Van Vliet G, Sack J, Flück CE, Miller WL. 2005. Diversity and function of mutations in P450 oxidoreductase in patients with Antley-Bixler syndrome and disordered steroidogenesis. Am J Hum Genet 76:729–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Flück CE, Mullis PE, Pandey AV. 2009. Modeling of human P450 oxidoreductase structure by in silico mutagenesis and MD simulation. Mol Cell Endocrinol 313:17–22 [DOI] [PubMed] [Google Scholar]
- 39. Geller DH, Auchus RJ, Mendonça BB, Miller WL. 1997. The genetic and functional basis of isolated 17,20 lyase deficiency. Nat Genet 17:201–205 [DOI] [PubMed] [Google Scholar]
- 40. Auchus RJ, Miller WL. 1999. Molecular modeling of human P450c17 (17α-hydroxylase/17,20- lyase): insights into reaction mechanisms and effects of mutations. Mol Endocrinol 13:1169–1182 [DOI] [PubMed] [Google Scholar]
- 41. Zachmann M, Werder EA, Prader A. 1982. Two types of male pseudohermaphroditism due to 17,20 desmolase deficiency. J Clin Endocrinol Metab 55:487–490 [DOI] [PubMed] [Google Scholar]
- 42. Yanase T, Waterman MR, Zachmann M, Winter JS, Simpson ER, Kagimoto M. 1992. Molecular basis of apparent isolated 17,20 lyase deficiency: compound heterozygous mutations in the C-terminal region (Arg(496)→Cys, Gln(461)→Stop) actually cause combined 17α-hydroxylase/17,20 lyase deficiency. Biochim Biophys Acta 1139:275–279 [DOI] [PubMed] [Google Scholar]
- 43. Zachmann M, Kempken B, Manella B, Navarro E. 1992. Conversion from pure 17,20 desmolase to combined 17,20 desmolase/17α-hydroxylase deficiency with age. Acta Endocrinol 127:97–99 [DOI] [PubMed] [Google Scholar]
- 44. Van Den Akker EL, Koper JW, Boehmer AL, Themmen AP, Verhoef-Post M, Timmerman MA, Otten BJ, Drop SL, De Jong FH. 2002. Differential inhibition of 17α-hydroxylase and 17,20 lyase activities by three novel missense CYP17 mutations identified in patients with P450c17 deficiency. J Clin Endocrinol Metab 87:5714–5721 [DOI] [PubMed] [Google Scholar]
- 45. ten Kate-Booij MJ, Cobbaert C, Koper JW, de Jong FH. 2004. Deficiency of 17,20-lyase causing giant ovarian cysts in a girl and a female phenotype in her 46,XY sister: case report. Hum Reprod 19:456–459 [DOI] [PubMed] [Google Scholar]
- 46. Sherbet DP, Tiosano D, Kwist KM, Hochberg Z, Auchus RJ. 2003. CYP17 mutation E305G causes isolated 17,20 lyase deficiency by selectively altering substrate binding. J Biol Chem 278:48563–48569 [DOI] [PubMed] [Google Scholar]
- 47. Tiosano D, Knopf C, Koren I, Levanon N, Hartmann MF, Hochberg Z, Wudy SA. 2008. Metabolic evidence for impaired 17α-hydroxylase activity in a kindred bearing the E305G mutation for isolated 17,20 lyase activity. Eur J Endocrinol 158:385–392 [DOI] [PubMed] [Google Scholar]
- 48. Flück CE, Miller WL, Auchus RJ. 2003. The 17,20 lyase activity of cytochrome P450c17 from human fetal testis favors the Δ5 steroidogenic pathway. J Clin Endocrinol Metab 88:3762–3766 [DOI] [PubMed] [Google Scholar]
- 49. Biason-Lauber A, Leiberman E, Zachmann M. 1997. A single amino acid substitution in the putative redox partner-binding site of P450c17 as cause of isolated 17,20 lyase deficiency. J Clin Endocrinol Metab 82:3807–3812 [DOI] [PubMed] [Google Scholar]
- 50. Gupta MK, Geller DH, Auchus RJ. 2001. Pitfalls in characterizing P450c17 mutations associated with isolated 17,20 lyase deficiency. J Clin Endocrinol Metab 86:4416–4423 [DOI] [PubMed] [Google Scholar]
- 51. Hershkovitz E, Parvari R, Wudy SA, Hartmann MF, Gomes LG, Loewental N, Miller WL. 2008. Homozygous mutation G539R in the gene for P450 oxidoreductase in a family previously diagnosed as having 17,20 lyase deficiency. J Clin Endocrinol Metab 93:3584–3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hegesh E, Hegesh J, Kaftory A. 1986. Congenital methemoglobinemia with deficiency of cytochrome b5. N Engl J Med 314:757–761 [DOI] [PubMed] [Google Scholar]
- 53. Giordano SJ, Kaftory A, Steggles AW. 1994. A splicing mutation in the cytochrome b5 gene from a patient with congenital methemoglobinemia and pseudohermaphroditism. Hum Genet 93:568–570 [DOI] [PubMed] [Google Scholar]
- 54. Kok RC, Timmerman MA, Wolffenbuttel KP, Drop SL, de Jong FH. 2010. Isolated 17,20 lyase deficiency due to the cytochrome b5 mutation W27X. J Clin Endocrinol Metab 95:994–999 [DOI] [PubMed] [Google Scholar]
- 55. Naffin-Olivos JL, Auchus RJ. 2006. Human cytochrome b5 requires residues E48 and E49 to stimulate the 17,20-lyase activity of cytochrome P450c17. Biochemistry 45:755–762 [DOI] [PubMed] [Google Scholar]
- 56. Argos P, Mathews FS. 1975. The structure of ferrocytochrome b5 at 2.8 Å resolution. J Biol Chem 250:747–751 [PubMed] [Google Scholar]
- 57. Flück CE, Meyer-Böni M, Pandey AV, Kempná P, Miller WL, Schoenle EJ, Biason-Lauber A. 2011. Why boys will be boys: two pathways of fetal testicular androgen biosynthesis are needed for male sexual differentiation. Am J Hum Genet 89:201–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wilson JD, Auchus RJ, Leihy MW, Guryev OL, Estabrook RW, Osborn SM, Shaw G, Renfree MB. 2003. 5α-androstane-3α,17β-diol is formed in Tammar wallaby pouch young testes by a pathway involving 5α-pregnane-3α,17α-diol-20-one as a key intermediate. Endocrinology 144:575–580 [DOI] [PubMed] [Google Scholar]
- 59. Auchus RJ. 2004. The backdoor pathway to dihydrotestosterone. Trends Endocrinol Metab 15:432–438 [DOI] [PubMed] [Google Scholar]
- 60. Homma K, Hasegawa T, Nagai T, Adachi M, Horikawa R, Fujiwara I, Tajima T, Takeda R, Fukami M, Ogata T. 2006. Urine steroid hormone profile analysis in cytochrome P450 oxidoreductase deficiency: implication for the backdoor pathway to dihydrotestosterone. J Clin Endocrinol Metab 91:2643–2649 [DOI] [PubMed] [Google Scholar]
- 61. Rizner TL, Lin HK, Peehl DM, Steckelbroeck S, Bauman DR, Penning TM. 2003. Human type 3 3α-hydroxysteroid dehydrogenase (aldo-keto reductase 1C2) and androgen metabolism in prostate cells. Endocrinology 144:2922–2932 [DOI] [PubMed] [Google Scholar]
- 62. Jin Y, Penning TM. 2006. Multiple steps determine the overall rate of the reduction of 5α-dihydrotestosterone catalyzed by human type 3 3α-hydroxysteroid dehydrogenase: implications for the elimination of androgens. Biochemistry 45:13054–13063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Biswas MG, Russell DW. 1997. Expression cloning and characterization of oxidative 17β- and 3α-hydroxysteroid dehydrogenases from rat and human prostate. J Biol Chem 272:15959–15966 [DOI] [PubMed] [Google Scholar]
- 64. Dufort I, Soucy P, Labrie F, Luu-The V. 1996. Molecular cloning of human type 3 3α-hydroxysteroid dehydrogenase that differs from 20α-hydroxysteroid dehydrogenase by seven amino acids. Biochem Biophys Res Commun 228:474–479 [DOI] [PubMed] [Google Scholar]
- 65. Lin HK, Jez JM, Schlegel BP, Peehl DM, Pachter JA, Penning TM. 1997. Expression and characterization of recombinant type 2 3α-hydroxysteroid dehydrogenase (HSD) from human prostate: demonstration of bifunctional 3α/17β-HSD activity and cellular distribution. Mol Endocrinol 11:1971–1984 [DOI] [PubMed] [Google Scholar]
- 66. Wilson JD, George FW, Griffin JE. 1981. The hormonal control of sexual development. Science 211:1278–1284 [DOI] [PubMed] [Google Scholar]
- 67. Forest MG, Lecornu M, de Peretti E. 1980. Familial male pseudohermaphroditism due to 17–20-desmolase deficiency. 1. In vivo endocrine studies. J Clin Endocrinol Metab 50:826–833 [DOI] [PubMed] [Google Scholar]
- 68. Campo S, Moteagudo C, Nicolau G, Pellizzari E, Belgorosky A, Stivel M, Rivarola M. 1981. Testicular function in prepubertal male pseudohermaphroditism. Clin Endocrinol (Oxf) 14:11–22 [DOI] [PubMed] [Google Scholar]
- 69. Larrea F, Lisker R, Bañuelos R, Bermúdez JA, Herrera J, Núñez Rasilla V, Pérez-Palacios G. 1983. Hypergonadotropic hypogonadism in an XX female subject due to 17,20 steroid desmolase deficiency. Acta Endocrinol 103:400–405 [DOI] [PubMed] [Google Scholar]
- 70. Kaufman FR, Costin G, Goebelsmann U, Stanczyk FZ, Zachmann M. 1983. Male pseudohermaphroditism due to 17,20 desmolase deficiency. J Clin Endocrinol Metab 57:32–36 [DOI] [PubMed] [Google Scholar]
- 71. de Peretti E, Pradon M, Forest MG. 1984. 17,20-desmolase deficiency in a female newborn, paradoxically virilized in utero. J Steroid Biochem 20:455–458 [DOI] [PubMed] [Google Scholar]
- 72. Simsek E, Ozdemir I, Lin L, Achermann JC. 2005. Isolated 17,20 lyase (desmolase) deficiency in a 46,XX female presenting with delayed puberty. Fertil Steril 83:1548.e23–1548.e26 [DOI] [PubMed] [Google Scholar]
- 73. Miller WL. 2009. Androgen synthesis in adrenarche. Rev Endocr Metab Disord 10:3–17 [DOI] [PubMed] [Google Scholar]
- 74. Nakamura Y, Gang HX, Suzuki T, Sasano H, Rainey WE. 2009. Adrenal changes associated with adrenarche. Rev Endocr Metab Disord 10:19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Huang N, Dardis A, Miller WL. 2005. Regulation of cytochrome b5 gene transcription by Sp3, GATA6, and steroidogenic factor 1 in human adrenal NCI-H295A cells. Mol Endocrinol 19:2020–2034 [DOI] [PubMed] [Google Scholar]
- 76. Lee-Robichaud P, Akhtar ME, Akhtar M. 1999. Lysine mutagenesis identifies cationic charges of human CYP17 that interact with cytochrome b5 to promote male sex-hormone biosynthesis. Biochem J 342:309–312 [PMC free article] [PubMed] [Google Scholar]