Abstract
Context:
Aging is associated with insulin resistance and unfavorable changes in body composition including increased fat accumulation, particularly in visceral and ectopic depots. Recent studies suggest that skeletal muscle mitochondrial activity may underlie some age-associated metabolic abnormalities.
Objective:
Our objective was to measure mitochondrial capacity and coupling of the vastus lateralis muscle in elderly and young adults using novel in vivo approaches and relate mitochondrial activity to metabolic characteristics.
Design:
This was a cross-sectional study.
Participants and Intervention:
Fourteen sedentary young (seven males and seven females, 20–34 yr of age) and 15 sedentary elderly (seven males and eight females, 70–84 yr of age) nonobese subjects selected for similar body weight underwent measures of body composition by magnetic resonance imaging and dual-energy x-ray absorptiometry, oral glucose tolerance, and in vivo mitochondrial activity by 31P magnetic resonance and optical spectroscopy. Muscle biopsy was carried out in the same muscle to measure mitochondrial content, antioxidant activity, fiber type, and markers of mitochondrial biogenesis.
Results:
Elderly volunteers had reduced mitochondrial capacity (P = 0.05) and a trend for decreased coupling efficiency (P = 0.08) despite similar mitochondrial content and fiber type distribution. This was accompanied by greater whole-body oxidative stress (P = 0.007), less skeletal muscle mass (P < 0.001), more adipose tissue in all depots (P ≤ 0.002) except intramyocellular (P = 0.72), and lower glucose tolerance (P = 0.07).
Conclusions:
Elderly adults show evidence of altered mitochondrial activity along with increased adiposity, oxidative stress, and reduced glucose tolerance, independent of obesity. We propose that mild uncoupling may be induced secondary to age-associated oxidative stress as a mechanism to dissipate the proton-motive force and protect against further reactive oxygen species production and damage.
The U.S. population is aging. By 2030, more than 71 million people, or nearly 20% of the population, will be over 65 yr old (U.S. Census Bureau). As the population ages, the prevalence of several chronic health problems, including obesity, cardiovascular disease, type 2 diabetes, and some forms of cancer also rises. Commonly observed with aging and often preceding disease onset are insulin resistance and impaired glucose tolerance, thought to be key in the development of age-related disease (1). Causes for the disruption in insulin signaling are less clear but have been associated with undesirable changes in body composition with age, i.e. loss of skeletal muscle mass and increased lipid accumulation, particularly in ectopic depots such as liver, skeletal muscle, and pancreatic β-cells (2, 3).
In recent years, skeletal muscle mitochondria has gained attention in age-related insulin resistance. Along with contractile proteins, skeletal muscle mitochondria are thought to be lost with age and over time may lead to a significant decrease in muscle mass and strength known as sarcopenia (4), a primary contributor to age-associated frailty. The mitochondrial theory of aging postulates that aging is accelerated by reactive oxygen species (ROS) production by the mitochondria, which over time damages mitochondrial DNA, lipids, and proteins, eventually causing electron transport chain dysfunction, cellular senescence, and cell death (5). Several studies have indeed shown reduced mitochondrial content and function with aging. For example, Conley et al. (6) found lower mitochondrial volume density and a 30% reduction in oxidative capacity per mitochondrial volume in elderly compared with younger adults. Amara et al. (7) later reported that mitochondrial coupling was altered in aging predominantly in type II fibers (first dorsal interosseous muscle), which were 40% more uncoupled in elderly compared with younger adults. These fibers also had significantly lower cellular [ATP], leading to the theory that decreased mitochondrial content and increased uncoupling with age compromised the energy state of the cell leading to apoptotic signaling and cellular death.
More studies are showing reduced mitochondrial content and capacity with age. Furthermore, limited data support a relationship between age-associated reductions in mitochondrial capacity and metabolic abnormalities including ectopic lipid accumulation and possibly whole-body insulin resistance (8). Data are still lacking on whether mitochondria are uncoupled with aging, and if so, are associated with a more favorable or worse metabolic profile. Here we studied healthy young and elderly adults with similar body weight and body mass index (BMI) to investigate mitochondrial capacity and coupling using novel in vivo approaches combining 31P magnetic resonance and optical spectroscopy. To determine the metabolic profile, we measured body composition and ectopic fat accumulation, oxidative stress, and glucose tolerance.
Materials and Methods
Volunteers
Fourteen young (20–34 yr old; seven males and seven females) and 15 elderly (70–84 yr old; seven males and eight females) Caucasian adults were selected for similar weight and BMI. Participants were healthy, nonobese, nonsmoking, not on any medications affecting metabolism or body weight, and sedentary by self-report (<2 h exercise per week for at least 6 months). To objectively determine daily physical activity, participants wore a previously validated (9) activity monitor (SenseWear Pro3; BodyMedia, Inc., Pittsburgh, PA) for 4 continuous days including while sleeping within 1 wk of completing the study. All subjects had normal fasting glucose at screening and reported no first-degree family history of type 2 diabetes. Participants gave written informed consent, and the study was approved by the Institutional Review Board of the Pennington Biomedical Research Center.
Body composition and ectopic lipid
Total-body skeletal muscle (SM) and adipose tissue (AT) were measured by multislice magnetic resonance imaging (MRI) (3T Signa Excite; General Electric, Milwaukee, WI) (10). Adipose tissue was further categorized as sc AT (SAT), visceral AT (VAT) or intermuscular AT (IMAT) (11). MRI volume estimates were converted into mass using assumed densities (12). All scans were read by the same analyst in the Image Analysis Laboratory of the New York Obesity Research Center, and the coefficients of variation for three repeated readings of the same scan for SAT, VAT, and IMAT volumes were 0.96, 1.97, and 0.65%, respectively. A second measure of body composition was obtained by dual-energy x-ray absorptiometry (DXA) scan (Hologics, QDA 4500A; Bedford, MA) and fat-free mass (FFM) and fat mass (FM) were calculated from weight and whole-body percent fat.
Lipid content of the soleus muscle [intramyocellular lipid (IMCL)] and liver [intrahepatic lipid (IHL)] were measured by 1H magnetic resonance spectroscopy (MRS) using the PRESS box technique (13). Peak positions and areas of interest were determined by time domain fitting using jMRUI (Java-based magnetic resonance user interface) (14) and were fitted using sets of prior knowledge (15). Areas of interest were expressed in arbitrary units per voxel area relative to internal water (×100) (16). Reproducibility testing [coefficient of variation (CV)] for MRS and optical spectroscopy (see In vivo mitochondrial activity, below) scans consisted of measuring 10 subjects one time on two different days, between which they performed normal daily activities. In our hands, the CV for IMCL is ±8.3% and for IHL is ±9.9%.
Glucose tolerance
A 75-g oral glucose tolerance test was performed the day after DXA/MRI/MRS scans and after a 14-h overnight fast. Glucose, insulin, and free fatty acids (FFA) were measured in blood samples collected at baseline and at 30, 60, 90, and 120 min after consuming the glucose beverage. Leptin (immunoassay from Linco Research, St. Charles, MO) and adiponectin (RIA kit; Linco) were measured in the baseline samples. The Matsuda index (17) was used as the composite measure of glucose tolerance.
In vivo mitochondrial activity
Resting O2 uptake was measured in the vastus lateralis (VL) muscle by optical spectroscopy (Horiba Jobin Yvon, Edison, NJ) (7). Spectra were acquired during a baseline rest period and during inflation of a cuff (17 min) above the site of interest to 60 mm Hg above systolic blood pressure. The decreases in [myoglobin (Mb)-O2] and [hemoglobin (Hb)-O2] during cuff inflation were used to calculate O2 uptake by separating the O2 saturations. The Hb level ([Hb]) was determined using a mass-balance approach that used the rate of desaturation of O2 carriers to determine the relative ([Hb]/[Mb]): [Hb] = (Δ%Hb-O2/Δ%Mb-O2) × [Mb]; where %Mb-O2 and %Hb-O2 are the slope of the lines for the change in saturation of Mb and Hb during ischemia over the linear portion of the desaturation curves. Mb level was measured in the VL by gel electrophoresis. Briefly, Mb was separated from other proteins on an 18% Tris-glycine Bio-Rad (Hercules, CA) Criterion gel, stained with Coomassie blue, imaged, and quantified using NIH Image. Horse Mb was used as a standard on each run on each gel. Quantification was done in triplicate for each sample, and the mean Mb concentration was used for the calculation of the O2 consumption rates. The CV for O2 consumption is ±13.7%.
ATP turnover (ATPflux) and mitochondrial capacity (ATPmax) were measured in the VL by 31P MRS as previously described (18, 19). Briefly, for ATPflux, spectra were acquired during a baseline rest period and during cuff inflation. The breakdown of phosphocreatine (PCr) under anoxic conditions represents net ATP demand, i.e. cellular ATP use minus glycolytic ATP supply (7, 20). The free-induction decays were line-broadened with the half-height width of the resting PCr peak and Fourier-transformed into spectra. PCr, inorganic phosphate, and ATP peak areas were determined using Varian Unity INOVA software (Varian Associates, Inc., Palo Alto, CA). The CV for ATPflux is ±11.0% (21). For ATPmax, spectra were acquired every 1.5 sec during a 24-, 30-, or 36-sec ballistic exercise obtained by kicking against Velcro straps positioned tight across the leg and thigh. Exercise time and intensity was targeted to drop PCr by 33–50% of basal levels and to avoid a pH below 6.8, because lower pH inhibits oxidative phosphorylation and results in an artificially low value. ATPmax was calculated using the PCr recovery time constant (τ) and [PCr]rest: ATPmax = [PCr]rest/τ. The reproducibility of ATPmax measurements has been published (21, 22), and the CV is ±7.0%. Mitochondrial coupling was calculated as the ratio of ATP turnover to O2 uptake and was divided by two to yield P/O (i.e., number of ATPs produced per pair of electrons traveling through the ETS). The CV is ±5% (n = 4).
Muscle biopsy
Biopsy was performed at the same site as the spectroscopy measurements using the Bergstrom technique (23). Mitochondrial content was determined by maximal citrate synthase activity and by protein content of electron transport system (ETS) complexes I–V (oxidative phosphorylation, OXPHOS), measured using primary antibody cocktail (Total OXPHOS, 1:208, MS601; MitoSciences, Eugene, OR) with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Abcam, Cambridge, MA) as the loading control. Bands were visualized and densitometry values were quantified using an Odyssey 9120 Infrared Imaging System (LI-COR, Lincoln, NE).
Oxidative stress
Urinary isoprostanes, an accepted measure of oxidative stress, were measured as previously described (24). Total superoxide dismutase (SOD) activity (Cu/Zn-, Mn-, and Fe-SOD) was measured in VL muscle lysate by measuring the dismutation of superoxide radicals generated by xanthine oxidase and hypoxanthine (Cayman Chemical, Ann Arbor, MI; http://www.caymanchem.com). One unit of SOD is defined as the amount of enzyme needed to exhibit 50% dismutation of the superoxide radical.
Fiber type and IMCL
Fiber type and IMCL content of the VL was quantified by immunohistochemistry (25). Images were taken using a Leica confocal microscope (Leica SP5). Type I fibers were counted and VL IMCL content was determined by measuring the intensity of bodipy staining using Sigma Scan Pro software version 5.0.0 (SPSS Inc., Chicago, IL).
Mitochondrial biogenesis
Total AMP-activated protein kinase-α (AMPKα), phospho- (p-)AMPKαS485, peroxisome proliferator-activated receptor γ coactivator 1 α (PGC-1α), and NAD-dependent deacetylase sirtuin-1 (SIRT-1) were measured in protein extracts by Western immunoblotting by incubation with primary antibodies (anti-AMPK and anti-SIRT1 from Cell Signaling Technologies, Danvers, MA; anti-PGC-1α from Abcam, Cambridge, MA; and horseradish peroxidase-conjugated antirabbit or antimouse IgG from Santa Cruz Biotechnology, Santa Cruz, CA), and proteins of interest were visualized by enhanced chemiluminescence detection (GE Healthcare, Pittsburgh, PA). Densitometry of autoradiographs was used for determining protein abundance. GAPDH was used as the loading control.
Statistical analysis
Data are expressed as mean ± se, and the level of significance for all statistical tests was set at P < 0.05. Analyses were performed using SAS version 9.1.3 and JMP version 8 (SAS Institute, Cary, NC). Because males and females were included in the study, differences between the young and elderly groups were analyzed using two-way ANOVA, controlling for the independent effects of age group and sex (i.e. significance represents the effect of age group independent from sex and sex × age group interaction). Statistical significance for all multiple comparisons was adjusted with respect to the Tukey-Kramer method to control for type I errors. Pearson correlations were performed on normally distributed variables of interest.
Results
Participant characteristics
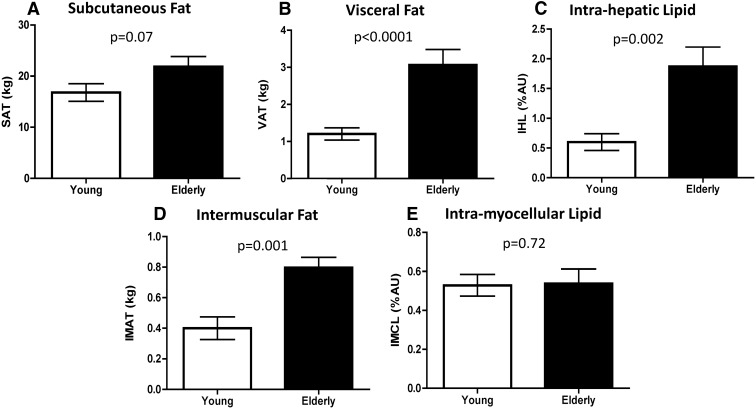
Per study design, weight and BMI were similar between groups; however, elderly subjects had less SM and greater FM and percent body fat (Table 1). Total FFM was lower in elderly subjects (50.4 ± 2.0 vs. 54.9 ± 2.3 kg, P = 0.02), and furthermore, the ratio of SM to FFM was lower (0.44 ± 0.01 vs. 0.50 ± 0.01, P < 0.001), indicating that a lesser proportion of lean mass was muscle in the elderly. Further categorizing the AT depots revealed that elderly subjects had 30% more SAT, 260% more VAT, and 217% more IHL (Table 1 and Fig. 1, A–C). Additionally, IMAT was two times higher in the elderly (Fig. 1D); however, IMCL of both the soleus (0.54 ± 0.07 vs. 0.53 ± 0.06 arbitrary units, P = 0.72, Fig. 1E) and VL (12.4 ± 1.2 vs. 14.1 ± 1.7 arbitrary units, P = 0.46) was similar between groups (young and elderly, respectively).
Table 1.
Participant characteristics
| Young | Elderly | P value | |
|---|---|---|---|
| Number (M/F) | 14 (7/7) | 15 (7/8) | |
| Age (yr) | 27.2 ± 1.0 | 75.7 ± 1.0 | |
| Anthropometry | |||
| Height (cm) | 172 ± 2.4 | 169 ± 2.3 | 0.23 |
| Weight (kg) | 75.1 ± 2.5 | 76.2 ± 1.8 | 0.66 |
| BMI (kg/m2) | 25.4 ± 0.7 | 26.8 ± 0.7 | 0.17 |
| Body composition (MRI) | |||
| SM mass (kg) | 27.6 ± 1.5 | 21.7 ± 1.3 | 0.0002 |
| Total AT (kg) | 18.4 ± 1.8 | 25.8 ± 2.0 | 0.01 |
| SAT (kg) | 16.8 ± 1.7 | 21.9 ± 2.0 | 0.07 |
| VAT (kg) | 1.2 ± 0.2 | 3.1 ± 0.4 | <0.0001 |
| IMAT (kg) | 0.40 ± 0.07 | 0.80 ± 0.07 | 0.001 |
| % body fat (DXA) | 24.8 ± 2.3 | 34.4 ± 2.5 | 0.001 |
| Physical activity | |||
| AEE (kcal/d)a | 624 ± 115 | 234 ± 38 | 0.003 |
| Physical activity duration (min/d)b | 125 ± 24 | 50 ± 8 | 0.005 |
| Average MET over 24 h | 1.50 ± 0.06 | 1.16 ± 0.04 | <0.001 |
| Steps/d | 8,537 ± 1,033 | 5,977 ± 423 | 0.03 |
| Biochemical assays | |||
| Glucose (mg/dl) | 86.7 ± 3.4 | 92.5 ± 1.5 | 0.13 |
| Insulin (μU/ml) | 9.3 ± 2.3 | 10.7 ± 1.8 | 0.64 |
| Triacylglycerol (mg/dl) | 74 ± 7 | 107 ± 11 | 0.02 |
| FFA (mmol/liter) | 0.49 ± 0.04 | 0.65 ± 0.09 | 0.13 |
| Leptin (ng/ml) | 10.5 ± 2.1 | 22.7 ± 4.4 | 0.0003 |
| Adiponectin (HMW, ng/ml) | 3564 ± 712 | 3033 ± 302 | 0.45 |
| T3 (ng/dl) | 113 ± 4 | 119 ± 6 | 0.37 |
| hsCRP (mg/liter) | 0.83 ± 0.18 | 2.55 ± 0.57 | 0.01 |
| TNF-α (pg/ml) | 4.54 ± 0.43 | 5.31 ± 0.43 | 0.19 |
Data are mean ± se. P values are calculated from two-way ANOVA and represent the effect of age group independent of sex and sex × age group interaction. F, Females; HMW, high molecular weight; hsCRP, high-sensitivity C-reactive protein; M, males.
Activity energy expenditure (AEE) estimated from the SenseWear Pro3 Monitor (BodyMedia).
Physical activity duration is defined as the amount of time (minutes per day) spent at 3.0 or more metabolic equivalents (MET).
Fig. 1.
SAT (A), VAT (B), IHL (C), IMAT, (D), and IMCL (E) in young and elderly subjects. A, B, and D were measured by MRI in 14 young and 11 elderly subjects; C and E were measured by MRS in 14 young and 14 elderly subjects.
Although all subjects were considered sedentary based on self-reported physical activity, activity monitor data showed that the elderly were significantly less active than young subjects, including lower daily activity energy expenditure, fewer minutes per day in physical activity (≥3.0 metabolic equivalents), and fewer steps per day (Table 1).
Fasting FFA were not significantly different between groups; however, elderly subjects had higher circulating triacylglycerol concentrations. Leptin was over twice as high in elderly subjects and tended to remain higher even after controlling for FM and sex by multiple regression (P = 0.06). High molecular weight adiponectin was not different between groups, nor was thyroid profile. High-sensitivity C-reactive protein, a marker of general systemic inflammation, was significantly higher in the elderly subjects whereas TNF-α was not different between groups (Table 1).
Glucose tolerance
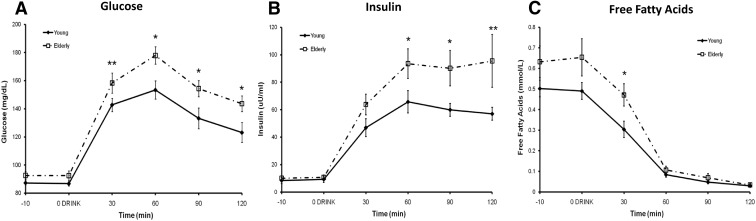
Fasting glucose and insulin concentrations were similar between groups (Table 1); however, responses to the oral glucose tolerance test (Fig. 2, A–C) revealed that elderly subjects were less glucose tolerant (Matsuda index 3.60 ± 0.50 vs. 5.05 ± 0.53, P = 0.07). Even in the absence of obesity, seven of the 15 elderly volunteers had glucose levels over 140 mg/dl at min 120, diagnostic of impaired glucose tolerance or prediabetes. The higher FFA at min 30 in the elderly likely reflected baseline concentrations rather than decreased sensitivity of AT to insulin.
Fig. 2.
Plasma glucose (A), insulin (B), and FFA (C) during a 2-h 75-g oral glucose load. Data are on all 14 young and 15 elderly participants. *, Difference between young and elderly subjects, P ≤ 0.05; **, difference between young and elderly subjects, P < 0.10.
Oxidative stress
Urinary isoprostanes concentration, an index of whole-body oxidative damage, was 38% higher in the elderly subjects (31.2 ± 2.3 vs. 22.6 ± 1.4 ng/mg Cr, P = 0.007) indicating substantially greater oxidative stress. Muscle SOD activity, a first-line cellular defense against oxidative damage, was not different between young and elderly (171.8 ± 9.0 vs. 155.2 ± 8.5 U/ml, respectively, P = 0.30).
Mitochondrial capacity and content
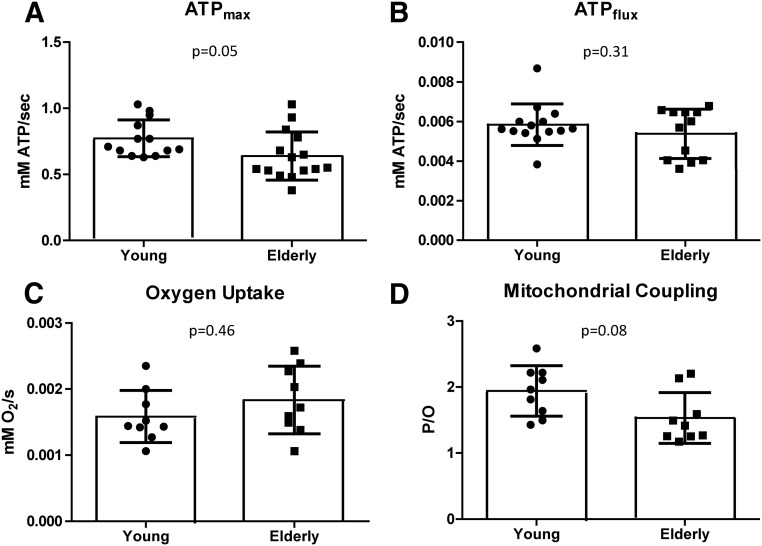
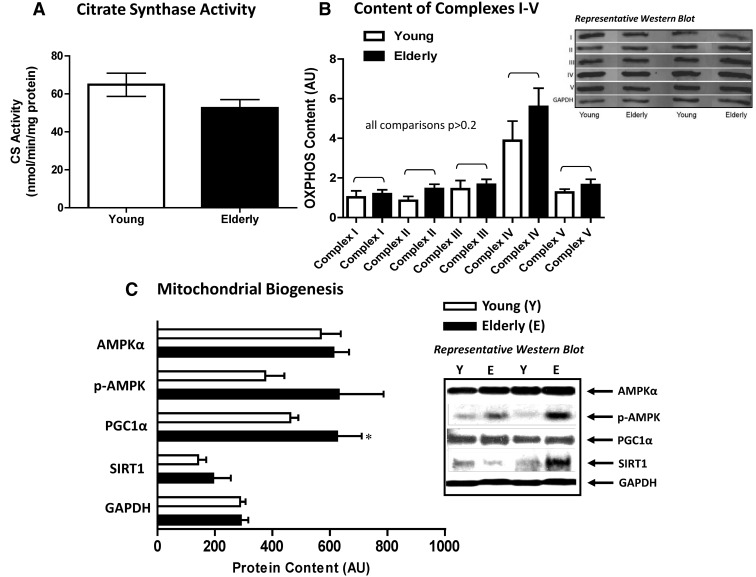
Mitochondrial capacity was 17% lower in elderly vs. young subjects (0.64 ± 0.05 vs. 0.77 ± 0.04 mm ATP/sec, P = 0.05) (Fig. 3A), whereas mitochondrial content was not significantly different by citrate synthase activity (young, 14.2 ± 1.3 and elderly, 11.5 ± 1.0 nmol/min·mg protein, P = 0.11; Fig. 4A) or ETS complexes I–V (all comparisons, P ≥ 0.21; Fig. 4B). Because ATPmax is thought to be an integrated measure of mitochondrial content and function (6, 25), we examined it in relation to other mitochondrial markers. ATPmax was related to citrate synthase activity (r2 = 0.18; P = 0.03) but not total OXPHOS content (P = 0.97) or percent type I fibers (P = 0.65). Together these results imply that mitochondrial capacity/activity may be distinct from mitochondrial content, particularly in elderly, and suggests a dysfunctional component to ATP-generating capacity with aging. Lower ATPmax was associated with greater adiposity including higher IMAT (r2 = 0.32; P = 0.004), VAT (r2 = 0.21; P = 0.02), percent fat (r2 = 0.17; P = 0.05), and higher whole-body oxidative stress (r2 = 0.26; P = 0.006) and was weakly associated with greater SAT (r2 = 0.13, P = 0.08) and lower glucose tolerance (r2 = 0.13; P = 0.06). These associations did not persist when examined within a group, indicating that the relationships were mostly due to age group.
Fig. 3.
Maximal ATP production (A), resting ATP turnover or flux (B), resting oxygen uptake (C), and resting P/O (D) measured by spectroscopy in young and elderly subjects. For A, n = 13 young and 15 elderly; for B, n = 14 young and 13 elderly; for C and D, n = 9 young and 9 elderly.
Fig. 4.
Mitochondrial content by citrate synthase activity (maximal enzyme assay, A) and OXPHOS electron transport complexes I–V (Western blot, B) and mitochondrial biogenesis (Western blot with GAPDH as loading control, C) in the VL muscle of young and elderly subjects. For A, n = 13 young and 14 elderly; for B, n = 6 young and 7 elderly; for C, n = 8 young and 8 elderly, with n = 6 young and 7 elderly for PGC-1α. *, Difference between young and elderly subjects, P < 0.10.
Mitochondrial coupling
Because ATPmax is a measure of mitochondrial capacity during recovery after exercise depletion, we also wanted to examine basal mitochondrial activity (ATPflux) at rest. ATPflux can be considered a measure of cellular ATP demand and together with O2 consumption provides a measure of mitochondrial efficiency. We found no differences in ATPflux or O2 uptake between young and elderly subjects (ATPflux, 0.0058 ± 0.0003 vs. 0.0054 ± 0.0004 mm ATP sec−1, P = 0.31; O2 uptake, 0.0016 ± 0.0001 vs. 0.0018 ± 0.0002 mm sec−1, P = 0.46; respectively; Fig. 3, B and C). When combined, P/O ratio was 21% lower in the elderly (1.53 ± 0.13 vs. 1.94 ± 0.13, P = 0.08; Fig. 3D), demonstrating a trend for aged mitochondria to be less coupled. Neither P/O ratio nor ATPflux was associated with mitochondrial content or capacity, and only weak negative relationships were found with adiposity indices (ATPflux and SAT, r2 = 0.14, P = 0.08; ATPflux and percent fat, r2 = 0.15, P = 0.07; P/O and VAT, r2 = 0.21, P = 0.08). Within age group, ATP demand was positively associated with glucose tolerance in the elderly (r2 = 0.36; P = 0.04), whereas P/O ratio was positively related to IMCL content (of the VL) in the young (r2 = 0.64; P = 0.02). Interestingly, in the entire group, we saw a positive relationship between P/O ratio and muscle SOD activity; i.e. those who had higher mitochondrial efficiency had higher muscular antioxidant activity (r2 = 0.47; P = 0.005).
Fiber type
To investigate whether differences in mitochondrial activity might be reflective of differences in skeletal muscle fiber type with aging, we measured VL fiber type. We found no differences in the percentage of type I (45 ± 2 vs. 46 ± 4%) or type II (55 ± 2 vs. 54 ± 4%, P = 0.79, in young and elderly, respectively) fibers between groups. Interestingly, young adults who had a greater percentage of type I fibers had a lower P/O ratio (r2 = 0.64; P = 0.02), a relationship that was absent in the elderly volunteers.
Mitochondrial biogenesis
Finally, we investigated the protein content of key markers of mitochondrial biogenesis. Aging has been associated with decreased AMPK-induced mitochondrial biogenesis (26); however, we found a trend for PGC-1α to be higher (35%, P = 0.08) in the elderly subjects, and although not reaching significance, mean p-AMPKS485 was 68% higher (P = 0.15; Fig. 4C). SIRT-1 was not different between groups (0.46), nor was the ratio of p-AMPKS485 to total AMPKα (P = 0.24). This indicates that at minimum, mitochondrial biogenesis was not lower in the elderly subjects.
Discussion
Conley et al. (6) previously reported a 50% reduction in oxidative capacity in elderly compared with young subjects, half of which was due to reduced mitochondrial volume and half due to reduced mitochondrial function. The greater loss of function relative to content indicated a 30% reduction in the capacity of each mitochondrion to maximally generate ATP. Consistently, we found lower mitochondrial capacity (ATPmax) in nonobese elderly subjects compared with young adults despite similar mitochondrial content, implying a dysfunctional component of elderly mitochondria. Cellular ATP demand and O2 consumption were not different between groups; however, when combined, mitochondrial efficiency tended to be lower in the elderly subjects. The altered mitochondrial activity in the elderly was accompanied by greater adiposity (sc, visceral, hepatic, and intermuscular depots), reduced glucose tolerance, and increased oxidative stress.
Amara et al. (7) reported reduced coupling in the first dorsal interosseous muscle of elderly vs. younger adults due to similar O2 uptake but lower cellular ATP flux. Some studies have suggested that cellular energy demand is reduced with aging (8), possibly due to decreased protein synthesis (27); however, we did not observe lower ATP demand in our elderly group. Interestingly, elderly subjects who had higher ATP demand had better glucose tolerance, although the significance of this should not be overstated due to the small number of observations (n = 13). We additionally measured fiber type distribution in our subjects, because there is evidence that type II fibers are preferentially lost with aging, resulting in a greater percentage of the more uncoupled type I fibers. This could seemingly lead to overall reduced muscular efficiency but really only reflect a change in the fiber type distribution (7). We did not see a difference in the proportion of fiber types between young and elderly, and furthermore, a greater percentage of type I fibers was associated with a lower mitochondrial efficiency only in the young volunteers. This suggests that age-associated uncoupling is independent of muscle fiber type distribution and in particular may reflect an uncoupling of the more oxidative stress-prone type II fibers (28).
We next investigated whether elderly subjects showed evidence of greater oxidative stress or reduced antioxidant activity, because cumulative free radical damage is thought to reduce mitochondrial function and cause age-associated insulin resistance. SOD activity was similar between young and elderly; however, oxidative stress measured by urinary isoprostanes excretion rate was significantly greater in elderly subjects. This was accompanied by a higher serum concentration of C-reactive protein, a well-accepted marker of nonspecific inflammation commonly observed with aging. Overall, the elderly adults displayed evidence of whole-body oxidative stress and inflammation without up-regulation of cellular antioxidant enzyme activity. Although the cross-sectional nature of our study does not allow for identification of cause-and-effect relationships, we speculate that mild mitochondrial uncoupling may be a response to age-associated oxidative stress as a mechanism to alleviate ROS generation, which is congruent with previous reports (7, 29). Oxidative damage to mitochondrial lipids has been shown to increase proton leak (30). An increase in proton leak reduces coupling efficiency and also reduces the proton-motive force (31). Decreased proton-motive force may reduce the amount of time that H+ sit on ETS complexes I and III, thus reducing the potential for further ROS formation. Interestingly, and supporting this concept, we found that for the entire group, those with more coupled mitochondria had greater SOD activity. However, because ROS production was not directly measured, we cannot determine whether this was due to increased ROS generation in the more coupled mitochondria.
Lastly, we measured the content of several proteins key to mitochondrial biogenesis, including total and phosphorylated AMPK, an important cellular fuel sensor; PGC1-α, the master regulator of mitochondrial biogenesis; and SIRT-1, a NAD+-dependent histone/protein deacetylase that is also widely regarded as a cellular fuel sensor. When activated, AMPK initiates metabolic pathways that restore ATP levels including mitochondrial biogenesis and function (32, 33), thus further helping to reduce oxidative stress (34). Activated AMPK is thought to stimulate mitochondrial biogenesis by activating PGC-1α. Along these lines, we observed a nonsignificant increase in p-AMPKs485 and trend for increased PGC-1α content in elderly subjects, certainly not definitive of increased biogenesis but also in sharp contrast to others who have reported decreased PGC-1α with aging (35).
Interpretability of this study is limited by the relatively small number of subjects, particularly given the methodological variability of our in vivo mitochondrial measurements. Our findings represent data from a study using novel experimental approaches and are meant to lead to further investigation. Taken together, our results show that the capacity to generate ATP after acute depletion was reduced with aging independent of mitochondrial content. Cellular ATP demand and O2 uptake under resting conditions were not different with age; however, elderly subjects showed some evidence for reduced mitochondrial coupling. We theorize that this uncoupling may help to alleviate ROS activity (31), and furthermore, mild uncoupling may help to stimulate mitochondrial biogenesis, because uncoupling reduces the ATP to ADP ratio that can be maintained by the cell (36). These alterations in mitochondrial activity were present along with lower glucose tolerance and increased adiposity in all depots except intramyocellular. Our results are independent of obesity but may have been influenced by physical activity, because the young subjects despite being sedentary were more active on a daily basis.
Our results are based on a small number of healthy, Caucasian, nonobese young and elderly volunteers and may not translate to a larger more diverse population. Additional studies should verify our findings, and future experiments should investigate whether impairments in mitochondrial bioenergetics are truly inherent to the aging process. Such impairment may cause a cascade of adverse metabolic events; alternatively, they may be driven by reduced daily physical activity, thus lower energy demand, leading to lipid oversupply and metabolic consequences.
Acknowledgments
We acknowledge Crystal Traylor, Mary Susan Thomas, and Christina Rowley (clinical staff); Kori Murray and Randy Deen (imaging staff); and Stacy Carling and Shantele Thomas (laboratory staff) for their contribution to this study. We thank Dr. Michal Jazwinski and the Louisiana Healthy Aging Study for helping to recruit some of our elderly volunteers. Finally, we thank Dr. David J. Marcinek for the measurement of myoglobin.
This work was supported by National Institutes of Health Grants P01AG22064, K01DK89005, R01AR41928, and RCAG36606 and Nutrition Obesity Research Center Grant P30DK72476. MRI scan analysis was supported in part by Grant P30DK26687.
Disclosure Summary: The authors declare no conflicts of interest and have nothing to disclose.
Footnotes
- AMPKα
- AMP-activated protein kinase-α
- AT
- adipose tissue
- BMI
- body mass index
- CV
- coefficient of variation
- DXA
- dual-energy x-ray absorptiometry
- ETS
- electron transport system
- FFA
- free fatty acids
- FFM
- fat-free mass
- FM
- fat mass
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- Hb
- hemoglobin
- IHL
- intrahepatic lipid
- IMAT
- intermuscular AT
- IMCL
- intramyocellular lipid
- Mb
- myoglobin
- MRI
- magnetic resonance imaging
- MRS
- magnetic resonance spectroscopy
- OXPHOS
- oxidative phosphoryation
- p-
- phospho-
- PCG1-α
- peroxisome proliferator activated receptor gamma coactivator 1 α
- PCr
- phosphocreatine
- P/O
- number of ATPs produced per pair of electrons traveling through the ETS
- ROS
- reactive oxygen species
- SAT
- sc AT
- SIRT1
- NAD-dependent deacetylase sirtuin-1
- SM
- skeletal muscle
- SOD
- superoxide dismutase
- VAT
- visceral AT
- VL
- vastus lateralis.
References
- 1. Facchini FS, Hua N, Abbasi F, Reaven GM. 2001. Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab 86:3574–3578 [DOI] [PubMed] [Google Scholar]
- 2. Karakelides H, Irving BA, Short KR, O'Brien P, Nair KS. 2010. Age, obesity, and sex effects on insulin sensitivity and skeletal muscle mitochondrial function. Diabetes 59:89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kuk JL, Saunders TJ, Davidson LE, Ross R. 2009. Age-related changes in total and regional fat distribution. Ageing Res Rev 8:339–348 [DOI] [PubMed] [Google Scholar]
- 4. Thompson LV. 2009. Age-related muscle dysfunction. Exp Gerontol 44:106–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harman D. 2003. The free radical theory of aging. Antioxid Redox Signal 5:557–561 [DOI] [PubMed] [Google Scholar]
- 6. Conley KE, Jubrias SA, Esselman PC. 2000. Oxidative capacity and ageing in human muscle. J Physiol 526(Pt 1):203–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Amara CE, Shankland EG, Jubrias SA, Marcinek DJ, Kushmerick MJ, Conley KE. 2007. Mild mitochondrial uncoupling impacts cellular aging in human muscles in vivo. Proc Natl Acad Sci USA 104:1057–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. 2003. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300:1140–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johannsen DL, Calabro MA, Stewart J, Franke W, Rood JC, Welk GJ. 2010. Accuracy of Armband Monitors for Measuring Daily Energy Expenditure in Healthy Adults. Med Sci Sports Exerc 42:2134–2140 [DOI] [PubMed] [Google Scholar]
- 10. Ross R. 1996. Magnetic resonance imaging provides new insights into the characterization of adipose and lean tissue distribution. Can J Physiol Pharmacol 74:778–785 [PubMed] [Google Scholar]
- 11. Gallagher D, Kuznia P, Heshka S, Albu J, Heymsfield SB, Goodpaster B, Visser M, Harris TB. 2005. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr 81:903–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. International Commission on Radiological Protection 1975. Report of the Task Group on Reference Man. ICRP Publication 23. Snyder WS, Cook MJ, Nasset ES, Karhausen LR, Howells GP, Tipton IH, eds. Oxford, UK: Pergamon [Google Scholar]
- 13. Larson-Meyer DE, Newcomer BR, Hunter GR. 2002. Influence of endurance running and recovery diet on intramyocellular lipid content in women: a 1H NMR study. Am J Physiol Endocrinol Metab 282:E95–E106 [DOI] [PubMed] [Google Scholar]
- 14. Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, de Beer R, Graveron-Demilly D. 2001. Java-based graphical user interface for the MRUI quantitation package. Magma 12:141–152 [DOI] [PubMed] [Google Scholar]
- 15. Rico-Sanz J, Thomas EL, Jenkinson G, Mierisová S, Iles R, Bell JD. 1999. Diversity in levels of intracellular total creatine and triglycerides in human skeletal muscles observed by 1H-MRS. J Appl Physiol 87:2068–2072 [DOI] [PubMed] [Google Scholar]
- 16. Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI. 1999. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia 42:113–116 [DOI] [PubMed] [Google Scholar]
- 17. Matsuda M, DeFronzo RA. 1999. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 18. Jubrias SA, Esselman PC, Price LB, Cress ME, Conley KE. 2001. Large energetic adaptations of elderly muscle to resistance and endurance training. J Appl Physiol 90:1663–1670 [DOI] [PubMed] [Google Scholar]
- 19. Amara CE, Marcinek DJ, Shankland EG, Schenkman KA, Arakaki LS, Conley KE. 2008. Mitochondrial function in vivo: spectroscopy provides window on cellular energetics. Methods 46:312–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Conley KE, Blei ML, Richards TL, Kushmerick MJ, Jubrias SA. 1997. Activation of glycolysis in human muscle in vivo. Am J Physiol 273:C306–C315 [DOI] [PubMed] [Google Scholar]
- 21. Blei ML, Conley KE, Odderson IB, Esselman PC, Kushmerick MJ. 1993. Individual variation in contractile cost and recovery in a human skeletal muscle. Proc Natl Acad Sci USA 90:7396–7400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blei ML, Conley KE, Kushmerick MJ. 1993. Separate measures of ATP utilization and recovery in human skeletal muscle. J Physiol 465:203–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bergstrom J. 1975. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 35:609–616 [PubMed] [Google Scholar]
- 24. Davies SS, Zackert W, Luo Y, Cunningham CC, Frisard M, Roberts LJ., 2nd 2006. Quantification of dinor, dihydro metabolites of F2-isoprostanes in urine by liquid chromatography/tandem mass spectrometry. Anal Biochem 348:185–191 [DOI] [PubMed] [Google Scholar]
- 25. Bajpeyi S, Pasarica M, Moro C, Conley K, Jubrias S, Sereda O, Burk DH, Zhang Z, Gupta A, Kjems L, Smith SR. 2011. Skeletal muscle mitochondrial capacity and insulin resistance in type 2 diabetes. J Clin Endocrinol Metab 96:1160–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, Befroy D, Pypaert M, Hardie DG, Young LH, Shulman GI. 2007. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab 5:151–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rooyackers OE, Adey DB, Ades PA, Nair KS. 1996. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci USA 93:15364–15369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anderson EJ, Neufer PD. 2006. Type II skeletal myofibers possess unique properties that potentiate mitochondrial H2O2 generation. Am J Physiol Cell Physiol 290:C844–C851 [DOI] [PubMed] [Google Scholar]
- 29. Marcinek DJ, Schenkman KA, Ciesielski WA, Lee D, Conley KE. 2005. Reduced mitochondrial coupling in vivo alters cellular energetics in aged mouse skeletal muscle. J Physiol 569:467–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brookes PS, Land JM, Clark JB, Heales SJ. 1998. Peroxynitrite and brain mitochondria: evidence for increased proton leak. J Neurochem 70:2195–2202 [DOI] [PubMed] [Google Scholar]
- 31. Brand MD. 2000. Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp Gerontol 35:811–820 [DOI] [PubMed] [Google Scholar]
- 32. Jäger S, Handschin C, St-Pierre J, Spiegelman BM. 2007. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA 104:12017–12022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kahn BB, Alquier T, Carling D, Hardie DG. 2005. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1:15–25 [DOI] [PubMed] [Google Scholar]
- 34. Ruderman N, Prentki M. 2004. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov 3:340–351 [DOI] [PubMed] [Google Scholar]
- 35. Anderson R, Prolla T. 2009. PGC-1alpha in aging and anti-aging interventions. Biochim Biophys Acta 1790:1059–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nicholls DG. 2004. Mitochondrial membrane potential and aging. Aging Cell 3:35–40 [DOI] [PubMed] [Google Scholar]