Abstract
Although the toxicity of metal contaminated soils has been assessed with various bioassays, more information is needed about the biochemical responses, which may help to elucidate the mechanisms involved in metal toxicity. We previously reported that the earthworm, Eisenia fetida, accumulates cadmium in its seminal vesicles. The bio-accumulative ability of earthworms is well known, and thus the earthworm could be a useful living organism for the bio-monitoring of soil pollution. In this short review, we describe recent studies concerning the relationship between earthworms and soil pollutants, and discuss the possibility of using the earthworm as a bio-monitoring organism for soil pollution.
Keywords: earthworm, metal, oxidative DNA damage
1. Introduction
It is well known that earthworms play an important role in the soil macrofauna biomass. They are extremely important in soil formation, principally by consuming organic matter, fragmenting it, and mixing it intimately with soil mineral particles to form water-stable aggregates [1]. In particular, the bioaccumulation ability of earthworms is essential for a bio-monitoring organism [2]. Therefore, the earthworm is likely to be an excellent organism for this purpose.
A bio-monitoring method would be appropriate to evaluate metal toxicity, because of its sensitivity and availability for unknown metabolites. Organisms such as fish, snails, and plants have been employed as bio-monitors [3–5]. Although this approach is useful and promising, it is also somewhat limited, because it could be available only for a specific combination of a living organism with certain substances. Hence, it is important to find appropriate living organisms as bio-monitors for each type of assessment.
Recent research has indicated that the earthworm is a candidate bio-monitor organism for soil pollutants, because it plays an important role in the soil macrofauna biomass. The species Eisenia fetida (Savigny, 1826, E. fetida) is most commonly used in ecotoxicology, and is recognized as a useful bio-monitor for testing the chemical toxicity of soil [6]. In particular, this species’ proximity to the soil contaminants is a merit for the analysis [7,8].
In this short review, we describe the possible use of the earthworm as a bio-monitoring organism for soil pollutants, by summarizing our previous work [9] and that of other researchers.
2. Effects of Metals on Earthworms
Earthworms have been indicated as a candidate bio-monitoring organism for soil pollutants. To establish a bio-monitoring system using earthworms, the effects of various chemical pollutants on earthworms have been studied. The accumulation of both natural and depleted uranium in earthworms was analyzed to evaluate the corresponding biological effects [10]. The study showed that no effects were observed in terms of mortality or weight reduction, but cytotoxic and genetic effects were identified at quite low natural uranium concentrations. Among metals, methyl mercury might be more easily absorbed by and accumulated in earthworms [2], suggesting that the earthworm is an ideal candidate for monitoring methyl mercury. Lee et al. also mentioned that metal bioaccumulation by earthworms could be used as an ecological indicator of metal availability [11]. On the other hand, metal pollution reportedly had no effect on earthworm communities [12].
Chemicals and ecological risk assessments should be based on the effects of the mixture, rather than those of a single compound. In this context, Natal-de-Luz et al. investigated the effects of sludge contaminated with chromium, copper, nickel, and zinc, and soils freshly spiked with the same mixture of metals, on Eisenia andrei [13]. They detected a decrease in the metal bioavailability for earthworms, promoted by the high organic matter content of the sludge. The binary mixture effects of cadmium and zinc on the mortality of Aporrectodea caliginosa were also investigated by Qiu et al. [14]. They reported that the effects of the cadmium and zinc mixtures on the mortality of Aporrectodea caliginosa were mainly antagonistic, and the magnitude of the antagonism was dependent upon both the relative concentrations of cadmium and zinc and their concentration magnitudes. The effects of combinations of metals and other chemical agents, such as pesticides, on earthworms were also investigated. Lister et al. studied the effects of a binary mixture of nickel and chlorpyrifos, an organophosphate insecticide, on Lumbricid earthworms, and found that both chemicals were rapidly accumulated to equilibrium [15]. Although the nickel uptake followed the same pattern as the single chemicals, the rates of chlorpyrifos uptake and elimination were faster, suggesting that a mixture of chemicals in soil might enhance the toxicity to organisms.
Taken together, an analysis using a single chemical compound does not necessarily reflect its toxicity for organisms in the environment. Therefore, when using earthworms as a bio-monitoring organism, careful attention should be paid to the target chemicals’ characteristics. A summary of the recently reported data about the effects of chemical agents in soil on earthworms is provided in Table 1.
Table 1.
Recent reports on the effects of chemical agents in earthworms.
| Species | Chemical agents | Effects | References |
|---|---|---|---|
| Lumbricus rubellus | Cd | Bioaccumulation | Stürzenbaum et al. (2004) [16] |
| Lumbricus rubellus | Cd and Cu | A slight decrease in weight. | Burgos et al. (2005) [17] |
| Eisenia fetida | Cd and Ni | Cd, but not Ni, accumulated and generated 8-oxoguanine in earthworm. | Nakashima et al. (2008) [9] |
| Eisenia fetida | Cu and ciprofloxacin (CIP) | Cu may be partly assimilated as Cu-CIP complexes I by earthworms, changing the bioavailability, subcellular distribution, and toxicity of Cu to earthworms. | Huang et al. (2009) [18] |
| Lumbricus rubellus | Pb and Zn | Accumulated in the posterior alimentary canal. | Andre et al. (2009) [19] |
| Drawida sp., Allolobophora sp., Limnodrilus sp. | Mercury | Mercury was mostly present in inorganic forms in earthworms. The bioaccumulation factors of methyl mercury from soil in earthworms were much higher than those of total mercury. | Zhang et al. (2009) [2] |
| Lumbricus terrestris | TiO2 | An enhanced apoptotic frequency, which was higher in the cuticle, intestinal epithelium and chloragogenous tissue than in the longitudinal and circular musculature. | Lapied et al. (2011) [20] |
| Eisenia fetida | azodrin | Concentration-dependent changes in the morphology and the AChE activity. | Rao and Kavitha (2004) [21] |
| No description | polybrominated diphenyl ethers | Bioaccumulation | Sellström et al. (2005) [22] |
| Eisenia fetida | metalaxyl | Metalaxyl was rapidly assimilated by earthworms, and the bioaccumulation of metalaxyl was enantioselective, with preferential accumulation of the S-enantiomer. | Xu et al. (2011) [23] |
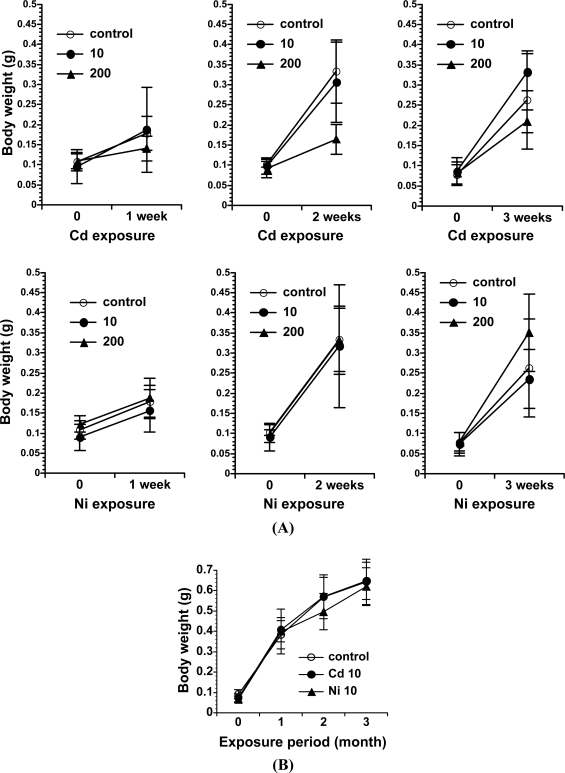
In terms of the relationship between metals and earthworms, cadmium has been extensively studied and is known to affect the earthworm’s physiological state, in terms of bioaccumulation [16], a slight decrease in weight [17], and oxidative DNA damage generation [9]. However, the earthworm is not useful for all chemical substances in soil. For example, nickel is not accumulated in the earthworm, and thus had no effects on growth, as shown in Figure 1 [9].
Figure 1.
All E. fetida were weighed under wet conditions in the short-term (A) and the long-term (B) experiments. Each data point represents the mean of six E. fetida. The treatment of E. fetida with 200 μg Cd/g soil resulted in body weight loss, suggesting Cd-induced growth inhibition. In the long-term experiment, each data point represents the mean of 16 E. fetida, and no significant differences between any groups were observed. (Nakashima et al. [9], Copyright Elsevier.)
Among the factors that define the toxicological effects of metals on organisms are metal binding proteins, such as metallothioneins (MTs). MTs, cysteine-rich cationic proteins, play a critical role in preventing metal toxicity. Two MT isoforms (MT1 and MT2) were identified in the earthworm [24], and MT2 was increased in cadmium-exposed earthworms [25]. Stürzenbaum et al. demonstrated that the earthworm had the intrinsic capacity to efficiency sequester and compartmentalize cadmium via a metallothionein-mediated trafficking pathway, by using X-ray microanalysis, quantitative polymerase chain reaction, and immunohistochemical techniques [16]. Therefore, MT1/MT2 should be considered in the establishment of a bio-monitoring system using earthworms. These findings prompted us to assess the usefulness of earthworms as a bio-monitor. However, there are no established methods for using earthworms for this function.
3. Methods for the Detection of Metal Toxicity by Using Earthworms
In soil pollution, metal contamination is a critical event. To prevent metal toxicity, we must first understand the metal’s features and assess its toxicity. However, the methods used for the assessment of metal toxicity are often not sensitive enough, because metals can be toxic below the technical detection limits. To overcome this limitation, many research efforts have been made to develop detection techniques or assessment methods for metal contamination of soil. Furthermore, the toxic actions of metals sometimes depend on their metabolites generated in living organisms. Thus, adequate methods to assess metal toxicity are difficult to develop.
As we stated above, the earthworm is a candidate organism for the assessment of pollutants, but is not useful for all chemical substances in soil. Therefore, when using the earthworm as a bio-monitoring organism for soil pollutants, it is important to select the appropriate target substances. We have presented several methods using earthworms.
The analysis of earthworm reproduction is a useful method for the evaluation of chemical substances in the environment. The effects of cadmium and zinc on the reproduction of Enchytraeus albidus were investigated. Both metals reportedly affected the enchytraeids’ reproduction [26].
Nahmani et al. suggested that life cycle parameters, such as cocoon production and hatching rate, of Eisenia fetida were more sensitive to metal pollution than survival or weight change [27]. These studies revealed that the earthworm should be useful for the detection of the cytotoxic effects of certain types of chemical agents in soil, by selecting adequate parameters.
4. 8-Oxoguanine Generation in Metal-Treated Earthworm DNA
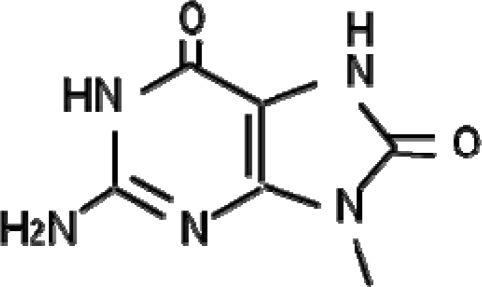
Quantitative and qualitative analyses of the oxidative DNA damage generated in living organisms are useful for the evaluation of the genotoxic effects of soil pollutants, because all aerobic organisms are constantly exposed to oxygen molecules. In particular, 7,8-dihydro-8-oxoguanine (8-oxoguanine, 8-oxo-Gua, Figure 2), a major form of oxidative DNA damage, may have an important role in carcinogenesis, because it causes the GC-to-TA transversion type point mutation [28–30]. 8-oxo-Gua is constantly generated in DNA and the nucleotide pool by reactive oxygen species (ROS), due to exposure to endogenous or exogenous factors.
Figure 2.
Structure of 7,8-dihydro-8-oxoguanine (8-oxo-Gua). 8-oxo-Gua is formed by hydroxylation of guanine at the C-8 position.
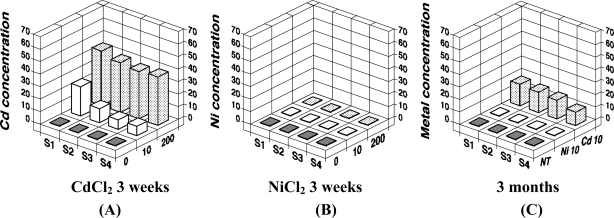
We recently analyzed the accumulation of 8-oxo-Gua in the DNA of E. fetida exposed to heavy metals, to determine if a method using earthworms as a bio-monitor would be useful for the assessment of soil mutagenicity [9]. We employed cadmium and nickel as test metals, because the carcinogenic potentials of cadmium and nickel have been established for humans and animals [31,32], and these metals are known to generate 8-oxo-Gua in DNA [33–36]. In the study, E. fetida were kept in a 20 liter stainless steel tank at an ambient temperature of 24 °C, using mold with skim milk as a food source until metal exposure [Figures 3(A,B)]. Three to six individuals were kept in a 600 mL glass container [Figure 3(C)] containing 50 g of soil with/without metals (CdCl2 or NiCl2). They were exposed to 10 or 200 μg metal/g soil for 1, 2, and 3 weeks or 10 μg metal/g soil for 3 months. As a result, we detected a high level of cadmium accumulation in E. fetida [Figures 4(A,C)]. On the other hand, no Ni accumulation was observed [Figures 4(B,C)].
Figure 3.
(A) Eisenia fetida; (B) E. fetida were kept in a 20 liter stainless steel tank; (C) E. fetida were maintained in a 600 mL glass container when they were exposed to heavy metals.
Figure 4.
Heavy metal accumulation in E. fetida. Each data point represents the mean of three individuals. Heavy metal concentrations were measured by atomic absorption spectrometry, and are expressed as μg per body weight (data are modified from Nakashima et al. [9], Copyright Elsevier).
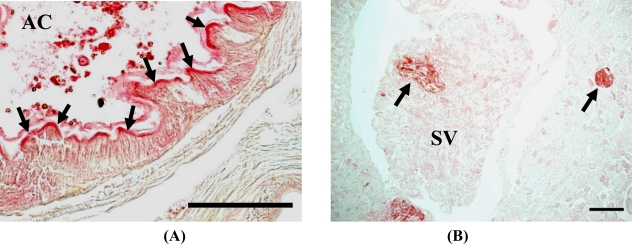
In addition, we observed positive 8-oxo-Gua staining in the gut epithelial layers in almost all samples [Figure 5(A)]. The metal absorption routes include the digestive system and the surface wall [37,38], but the main route is the digestive system. Since gut epithelial layers are frequently exposed to ROS, 8-oxo-Gua accumulation was constantly detected. Although the 200 μg cadmium-exposed E. fetida showed relatively stronger signals at 2 weeks in comparison to the others, almost all of the specimens showed positive signals in the gut epithelial layers, and the differences in the signal strength were too small to conclude that cadmium exposure increased 8-oxo-Gua accumulation in the organs. On the other hand, the positive signals in the seminal vesicles were clearly detected only in E. fetida treated with 10 μg of cadmium at 3 months [Figure 5(B)]. The seminal vesicles are considered as metallothionein (MT)-poor organs. Therefore, it seems reasonable to speculate that a lower level of MT expression is involved in the accumulation of cadmium-induced DNA damage.
Figure 5.
Immunohistochemical analyses of 8-oxo-dG accumulation in alimentary canal (AC) (A) and seminal vesicles (SV) (B) of E. fetida (S1) [data of (B) are modified from Nakashima et al. [9], Copyright Elsevier].
Therefore, we would like to propose the merit of analyzing DNA damage generated in earthworms for the assessment of soil pollution. Besides analysis of 8-oxo-Gua generation, single-cell gel electrophoresis (comet) assay may be a useful tool for evaluation of genotoxic effects of pollutants on earthworms. Bonnard et al. indicated genotoxic effects of an industrially contaminated soil on E. fetida by using comet assay [39]. Although these studies suggested the utility of measuring DNA damage for evaluation, there are presently only a few reports about DNA damage in the earthworm [9,40].
5. Conclusions
Our previous data demonstrated the possible utility of using earthworms as a bio-monitoring organism, by measuring the oxidative DNA damage generated in the earthworms as a bio-monitoring method for assessing soil mutagenicity. However, many points remain unresolved. For example, this method would only be reliable for bio-accumulated metals, such as cadmium, but not for non-bio-accumulated metals, such as nickel, even if they generate 8-oxo-Gua. Furthermore, it is not clear how the pollution level of soil should be evaluated, based on the data from earthworms. Although several studies have been performed, no standardized method has been established, according to our knowledge. Further studies will be required to establish a broader bio-monitoring method using earthworms to assess soil pollution.
Acknowledgments
The authors thank Ki-Hyun Kim, Guest Editor of “Sensing of Toxic and Hazardous Metals in Various Environmental Media”, a special issue of Sensors, for inviting us to write this review article, and Elsevier LTD for permission to reproduce the material in reference [9] as Figures 1, 4 and 5 in this article. This work was supported by a grant from The Foundation for Earth Environment.
References
- 1.Edwards C.A. The Importance of Earthworms as Key Representatives of the Soil Fauna. In: Edwards C.A., editor. Earthworm Ecology. CRC Press LLC; Boca Raton, FL, USA: 2004. pp. 3–11. [Google Scholar]
- 2.Zhang Z.S., Zheng D.M. Bioaccumulation of total and methyl mercury in three earthworm species (Drawida sp., Allolobophora sp., and Limnodrilus sp.) Bull. Environ. Contam. Toxicol. 2009;83:937–942. doi: 10.1007/s00128-009-9872-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harnly M., Seidel S., Rojas P., Fornes R., Flessel P., Smith D., Kreutzer R., Goldman L. Biological monitoring for mercury within a community with soil and fish contamination. Environ. Health Perspect. 1997;105:424–429. doi: 10.1289/ehp.97105424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Regoli F., Gorbi S., Fattorini D., Tedesco S., Notti A., Machella N., Bocchetti R., Benedetti M., Piva F. Use of the land snail Helix aspersa as sentinel organism for monitoring ecotoxicologic effects of urban pollution: An integrated approach. Environ. Health Perspect. 2006;114:63–69. doi: 10.1289/ehp.8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Citterio S., Aina R., Labra M., Chiani A., Fumagalli P., Sgorbati S., Santagostino A. Soil genotoxicity assessment: A new strategy based on biomolecular tools and plant bioindicators. Environ. Sci. Technol. 2002;36:2748–2753. doi: 10.1021/es0157550. [DOI] [PubMed] [Google Scholar]
- 6.Brulle F., Mitta G., Coquerelle C., Vieau D., Lemière S., Leprêtre A., Vandenbulcke F. Cloning and real-time PCR testing of 14 potential biomarkers in Eisenia fetida following cadmium exposure. Environ. Sci. Technol. 2006;40:2844–2850. doi: 10.1021/es052299x. [DOI] [PubMed] [Google Scholar]
- 7.Reinecke A.J., Reinecke S.A. Earthworm as Test Organisms in Ecotoxicological Assessment of Toxicant Impacts on Ecosystems. In: Edwards C.A., editor. Earthworm Ecology. CRC Press LLC; Boca Raton, FL, USA: 2004. pp. 299–320. [Google Scholar]
- 8.Steenbergen N.T.T.M., Iaccino F., De Winkel M., Reijnders L., Peijnenburg W.J.G.M. Development of a biotic ligand model and a regression model predicting acute copper toxicity to the earthworm Aporrectodea caliginosa. Environ. Sci. Technol. 2005;39:5694–5702. doi: 10.1021/es0501971. [DOI] [PubMed] [Google Scholar]
- 9.Nakashima T., Okada T., Asahi J., Yamashita A., Kawai K., Kasai H., Matsuno K., Gamou S., Hirano T. 8-Hydroxydeoxyguanosine generated in the earthworm Eisenia fetida grown in metal-containing soil. Mutat. Res. 2008;654:138–144. doi: 10.1016/j.mrgentox.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Giovanetti A., Fesenko S., Cozzella M.L., Asencio L.D., Sansone U. Bioaccumulation and biological effects in the earthworm Eisenia fetida exposed to natural and depleted uranium. J. Environ. Radioact. 2010;101:509–516. doi: 10.1016/j.jenvrad.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Lee S.H., Kim E.Y., Hyun S., Kim J.G. Metal availability in heavy metal-contaminated open burning and open detonation soil: Assessment using soil enzymes, earthworms, and chemical extractions. J. Hazard. Mater. 2009;170:382–388. doi: 10.1016/j.jhazmat.2009.04.088. [DOI] [PubMed] [Google Scholar]
- 12.van Gestel C.A., Koolhaas J.E., Hamers T., van Hoppe M., van Roovert M., Korsman C., Reinecke S.A. Effects of metal pollution on earthworm communities in a contaminated floodplain area: Linking biomarker, community and functional responses. Environ. Pollut. 2009;157:895–903. doi: 10.1016/j.envpol.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Natal-da-Luz T., Ojeda G., Pratas J., Van Gestel C.A., Sousa J.P. Toxicity to Eisenia andrei and Folsomia candida of a metal mixture applied to soil directly or via an organic matrix. Ecotoxicol. Environ. Saf. 2011;74:1715–1720. doi: 10.1016/j.ecoenv.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Qiu H., Vijver M.G., Peijnenburg W.J. Interactions of cadmium and zinc impact their toxicity to the earthworm Aporrectodea caliginosa. Environ. Toxicol. Chem. 2011;30:2084–2093. doi: 10.1002/etc.595. [DOI] [PubMed] [Google Scholar]
- 15.Lister L.J., Svendsen C., Wright J., Hooper H.L., Spurgeon D.J. Modelling the joint effects of a metal and a pesticide on reproduction and toxicokinetics in Lumbricid earthworms. Environ. Int. 2011;37:663–670. doi: 10.1016/j.envint.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Stürzenbaum S.R., Georgiev O., Morgan A.J., Kille P. Cadmium detoxification in earthworms: From genes to cells. Environ. Sci. Technol. 2004;38:6283–6289. doi: 10.1021/es049822c. [DOI] [PubMed] [Google Scholar]
- 17.Burgos M.G., Winters C., Stürzenbaum S.R., Randerson P.F., Kille P., Morgan J. Cu and Cd effects on the earthworm Lumbricus rubellus in the laboratory: Multivariate statistical analysis of relationships between exposure, biomarkers, and ecologically relevant parameters. Environ. Sci. Technol. 2005;39:1757–1763. doi: 10.1021/es049174x. [DOI] [PubMed] [Google Scholar]
- 18.Huang R., Wen B., Pei Z., Shan X.-Q., Zhang S., Williams P.N. Accumulation, subcellular distribution and toxicity of copper in earthworm (Eisenia fetida) in the presence of Ciprofloxacin. Environ. Sci. Technol. 2009;43:3688–3693. doi: 10.1021/es900061t. [DOI] [PubMed] [Google Scholar]
- 19.Andre J., Charnock J., Stürzenbaum S.R., Kille P., Morgan A.J., Hodoson M.E. Accumulated metal speciation in earthworm populations with multigenerational exposure to metalliferous soils: Cell fractionation and high-energy synchrotron analyses. Environ. Sci. Technol. 2009;43:6822–6829. doi: 10.1021/es900275e. [DOI] [PubMed] [Google Scholar]
- 20.Lapied E., Nahmani J.Y., Moudilou E., Chaurand P., Labille J., Rose J., Exbrayat J.M., Oughton D.H., Joner E.J. Ecotoxicological effects of an aged TiO2 nanocomposite measured as apoptosis in the anecic earthworm Lumbricus terretris after exposure through water, food, and soil. Environ. Int. 2011;37:1105–1110. doi: 10.1016/j.envint.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Rao J.V., Kavitha P. Toxicity of azodrin on the morphology and acetylcholinesterase activity of the earthworm Eisenia foetida. Environ. Res. 2004;96:323–327. doi: 10.1016/j.envres.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Sellström U., de Wit C.A., Lundgren N., Tysklind M. Effect of sewage-sludge application on concentration of higher-brominated diphenyl ethers in soils and earthworms. Environ. Sci. Technol. 2005;39:9064–9070. doi: 10.1021/es051190m. [DOI] [PubMed] [Google Scholar]
- 23.Xu P., Diao J., Liu D., Zhou Z. Enantioselective bioaccumulation and toxic effects of metalaxyl in earthworm Eisenia foetida. Chemosphere. 2011;83:1074–1079. doi: 10.1016/j.chemosphere.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 24.Stürzenbaum S.R., Kille P., Morgan A.J. The identification, cloning and characterization of earthworm metallothionein. FEBS Lett. 1998;431:437–442. doi: 10.1016/s0014-5793(98)00809-6. [DOI] [PubMed] [Google Scholar]
- 25.Stürzenbaum S.R., Winters C., Galay M., Morgan A.J., Kille P. Metal ion trafficking in earthworms. J. Biol. Chem. 2001;36:34013–34018. doi: 10.1074/jbc.M103605200. [DOI] [PubMed] [Google Scholar]
- 26.Novais S.C., Gomes S.I., Gravato C., Guilhermino L., De Coen W., Soares A.M., Amorim M.J. Reproduction and biochemical responses in Enchytraeus albidus (Oligochaeta) to zinc or cadmium exposures. Environ. Pollut. 2011;159:1836–1843. doi: 10.1016/j.envpol.2011.03.031. [DOI] [PubMed] [Google Scholar]
- 27.Nahmani J., Hodson M.E., Black S. Effects of metals on life cycle parameters of the earthworm Eisenia fetida exposed to field-contaminated, metal-polluted soils. Environ. Pollut. 2007;49:44–58. doi: 10.1016/j.envpol.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 28.Wood M.L., Dizdarogle M., Gajewski E., Essigmann J.M. Mechanistic studies of ionizing radiation and oxidative mutagenesis: Genetic effects of a single 8-hydroxyguanine (7-hydro-8-oxoguanine) residue inserted at a unique site in a viral genome. Biochemistry. 1990;29:7024–7032. doi: 10.1021/bi00482a011. [DOI] [PubMed] [Google Scholar]
- 29.Shibutani S., Takeshita M., Grollman A.P. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-OH-dG. Nature. 1991;349:431–434. doi: 10.1038/349431a0. [DOI] [PubMed] [Google Scholar]
- 30.Cheng K.C., Cahill D.S., Kasai H., Nishimura S., Loeb A.P. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G→T and A→C substitutions. J. Biol. Chem. 1992;267:166–172. [PubMed] [Google Scholar]
- 31.Chromium, Nickel, Welding. IARC Monographs; Lyon, France: 1990. Volume 49. [PMC free article] [PubMed] [Google Scholar]
- 32.Beryllium, Cadmium, Mercury and Exposures in the Glass Manufacturing Industry. IARC Monographs; Lyon, France: 1993. Volume 58. [PMC free article] [PubMed] [Google Scholar]
- 33.Hirano T., Yamaguchi Y., Kasai H. Inhibition of 8-hydroxyguanine repair in testes after administration of cadmium chloride to GSH-depleted rats. Toxicol. Appl. Pharmacol. 1997;147:9–14. doi: 10.1006/taap.1997.8260. [DOI] [PubMed] [Google Scholar]
- 34.Dalley H., Hartwig A. Induction and repair inhibition of oxidative DNA damage by nickel (II) and cadmium (II) in mammalian cells. Carcinogenesis. 1997;18:1021–1026. doi: 10.1093/carcin/18.5.1021. [DOI] [PubMed] [Google Scholar]
- 35.Merzenich H., Hartwig A., Ahrens W., Beyersmann D., Schlepegrell R., Scholze M., Timm J., Jöckel K.H. Biomonitoring on carcinogenic metals and oxidative DNA damage in a cross-sectional study. Cancer Epidemiol. Biomark. Prevent. 2001;10:515–522. [PubMed] [Google Scholar]
- 36.Hengstler J.G., Bolm-Audorff U., Faldum A., Janssen K., Reifenrath M., Götte W., Jung D., Mayer-Popken O., Fuchs J., Gebhard S., Bienfait H.G., Schlink K., Dietrich C., Faust D., Epe B., Oesch F. Occupational exposure to heavy metals: DNA damage induction and DNA repair inhibition prove co-exposures to cadmium, cobalt and lead as more dangerous than hitherto expected. Carcinogenesis. 2003;24:63–73. doi: 10.1093/carcin/24.1.63. [DOI] [PubMed] [Google Scholar]
- 37.Saxe J.K., Impellitteri C.A., Peijnenburg W.J., Allen H.E. Novel model describing trace metal concentrations in the earthworm. Eisenia andrei. Environ. Sci. Technol. 2001;35:4522–4529. doi: 10.1021/es0109038. [DOI] [PubMed] [Google Scholar]
- 38.Vijver M.G., Wolterbeek H.T., Vink J.P., van Gestel C.A. Surface adsorption of metals onto the earthworm Lumbricus rubellus and the isopod Porcellio scaber is negligible compared to absorption in the body. Sci. Total Environ. 2005;340:271–280. doi: 10.1016/j.scitotenv.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 39.Bonnard M., Eom I.C., Morel J.L., Vasseur P. Genotoxic and reproductive effects of an industrially contaminated soil on the earthworm Eisenia fetida. Environ. Mol. Mutagen. 2009;50:60–67. doi: 10.1002/em.20436. [DOI] [PubMed] [Google Scholar]
- 40.Li M., Liu Z., Xu Y., Cui Y., Li D., Kong Z. Comparative effects of Cd and Pb on biochemical response and DNA damage in the earthworm Eisenia fetida (Amelida, Oligocheta) Chemosphere. 2009;74:621–625. doi: 10.1016/j.chemosphere.2008.10.048. [DOI] [PubMed] [Google Scholar]