Abstract
Background
Umbilical endometriosis is rare and can be a challenging diagnosis in the absence of classic signs and symptoms.
Case
A case of severe primary spontaneous umbilical endometriosis with foci of plasma cell endometritis and diffuse stromal lymphvascular presence is reported.
Conclusion
Despite a lengthy differential, endometriosis must be considered in the evaluation of an umbilical mass. The presence of plasma cell endometritis and stromal lymphvascular elements in the absence of pelvic endometriosis lend evidence to the theory of lymphvascular transport as an etiology of extra-pelvic endometriosis.
Introduction
Endometriosis, originally described by von Rokitansky in 1860, is the existence and growth of endometrial tissue remote from the uterine cavity1, 2. Extrapelvic endometriosis is noted in up to 12% of patients with known pelvic disease2, 3. Cutaneous endometriosis is most often noted in surgical scars and trocar sites3. The umbilicus is a common site of cutaneous endometriosis, but still comprises less than 1% of all ectopic endometriosis2. Umbilical endometriosis is often associated with previous surgery but does occur primarily. Simultaneous pelvic endometriosis diagnosed by laparoscopic visualization has been documented only 28%– 33% of the time with a known umbilical endometrioma2, 4.
Umbilical endometriosis can present with classic signs and symptoms. Patients describe a firm, dark or bluish mass. Classic symptoms include pain and tenderness, more severe with menses, associated with bleeding or discharge from the umbilicus. However, these pathognomonic symptoms are not as common as one might expect. Only 15% of lesions present with bloody discharge and only half produce cyclical symptoms4. Slow enlargement over years is not uncommon5.
Typical symptoms are not diagnostic and a broad differential diagnosis of an umbilical mass exists. Granuloma, hernia, keloid, nevus, metastatic disease and pyogenic granuloma are causes of umbilical masses >5% of the time. Rarely, malignant transformation of umbilical endometriosis has been reported4. Medical management is rarely definitive unless merely temporizing until menopause or preoperatively. Simple surgical resection is the recommended therapy7.
Case
A 46 year-old black female G2 P2002 presented with a history of uterine prolapse seeking treatment. During examination a superficial umbilical mass was noted. She reported 8 years of slow growth. It caused pain most days, and she had not noted increased pain with menstrual periods. She denied discharge or bleeding from the umbilicus. The patient denied any past medical or surgical history. Gynecological history was significant for 2 uncomplicated vaginal deliveries and regular menstrual flow every 29 days. She denied dyspareunia and dysmenorrhea.
Examination was significant for a 5.0 by 4.0 by 2.0 cm dark, firm, non-mobile mass, which was tender to palpation. The mass replaced the umbilicus entirely. The patient had a 16-week fibroid uterus in addition to stage IV uterine prolapse. The uterus was not confluent with the umbilical mass. Vaginal ultrasound did not elucidate any further information. A CT scan was obtained that revealed a 3.3 × 3.0 cm hyperdense focal cutaneous mass that did not extend deep to the fascia. Laboratory evaluation was significant for a hematocrit of 23, normal white blood cell count of 5.5, normal CEA and negative hemocult. A CA-125 was slightly elevated at 42.7. Core biopsy in the office was consistent with endometriosis.
The patient underwent wide local excision of the umbilicus and mass in conjunction with exploratory laparotomy, total hysterectomy, bilateral salpingoophorectomy, abdominal colposuspension, culdoplasty and midurethral sling. Again the umbilical mass was noted to be entirely suprafascial. There was no evidence of intrabdominal endometriosis. A neoumbilicus was surgically developed upon closure of the midline incision. She tolerated the procedure well. At two month follow up, the patient was asymptomatic and the neoumbilicus was well formed.
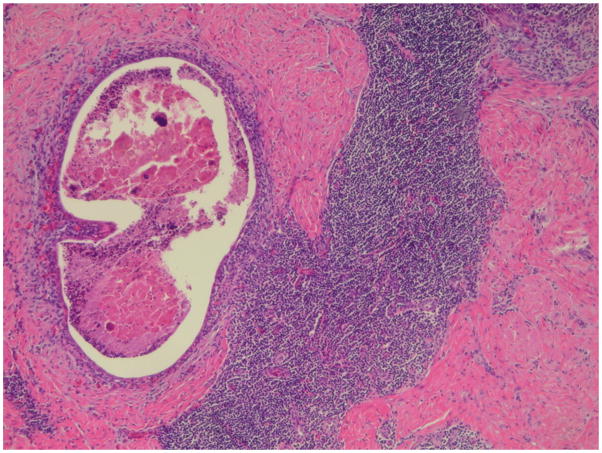
Surgical pathology revealed a 5.0 × 4.0 × 3.0 cm area of endometriosis with negative margins at the umbilicus. Characteristic of cutaneous endometriosis, endometrial glands in a fibrous eosinophilic stroma were noted within the dermis. Additionally, small diffuse foci of stromal lymphvascular tissue (Figure 1) consistent with mild inflammation and plasma cells, suggesting endometritis (Figure 2), were present throughout the umbilical specimen. The uterus, tubes and ovaries were free of endometriosis and endometritis.
Fig. 1.
Foci of umbilical endometriosis on the left with adjacent lymphoid follicleon the right (original magnification 40x, hematoxylin-eosin stain).
Fig. 2.
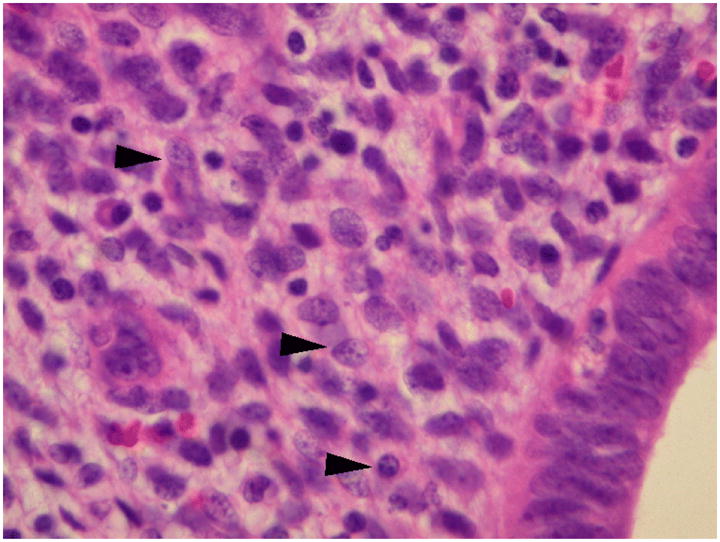
Lymphoplasmacytic infiltrate. Arrowheads denote plasma cells (original magnification 400x, hematoxylin-eosin stain).
Comment
A case of primary symptomatic umbilical endometriosis is discussed. The interesting findings of endometritis within an umbilical tumor as evidenced by the presence of infiltrating plasma cells and lymphvascular elements within the umbilical mass are not commonly reported. These findings add weight to the theory of lymphatic or vascular transportation and reimplantation at the umbilicus5. The presence of inflammatory cells may speak to the non-cyclic pain symptoms. It would be interesting to note whether or not conservative management in the form of antibiotic therapy is useful in diminishing tumor size or pain symptoms.
Although recurrence is thought to be rare7, some report an 11% recurrence rate following surgical treatment4. As a result, margins of at least 0.5 cm are advisable7. In this case, complete resection resulted in the removal of the entire umbilicus. Reconstruction was accomplished easily and with good cosmetic results.
Diagnosis can be troublesome if a suspecting practitioner fails to consider endometriosis. Pathognomonic symptoms are not always present or elucidated in an abbreviated history. The size of this reported lesion was slightly misleading as many of the cases previously reported were significantly smaller. Additionally, the presence of endometritis may have caused more notable constant pain rather than the prototypical cyclic pain symptoms. The differential diagnosis of an umbilical mass is not brief, and more ominous entities such as metastatic carcinoma or melanoma must be ruled out6. Imaging modalities may be useful in characterizing the lesion, however, histology is the gold standard for diagnosis. In this case, computed tomography characterization elucidated the lack of subfascial involvement. Additionally, a CEA and CA-125 were ordered secondary to the presence of a pelvic mass, umbilical mass, and very low hematocrit in the absence of abnormal pelvic or rectal bleeding. Although not definitive, these tests add confidence to a decision to perform primary excision to obtain a definitive diagnosis as well as treatment without the inconvenience of a core biopsy. Fine needle aspiration and percutaneous biopsy may be helpful if endometriosis is suspected, but excisional biopsy is more efficient. Some researchers report undiagnostic aspiration rates as high as 75%7.
Endometriosis must be considered during evaluation of an umbilical mass despite the absence of previous surgery, lack of catamenial symptoms, or bloody discharge. The presence of endometritis in the setting of umbilical endometriosis may help explain constant pain. If endometriosis is strongly suspected, wide excisional biopsy is the most efficient manner of diagnosis and therapy.
Synopsis.
Foci of endometritis may cause noncatemenial symptoms, thus endometriosis must be considered during evaluation of an umbilical mass despite the lack of pathognomonic symptoms.
Acknowledgments
Financial support: Supported in part by the National Institute of Diabetes, Digestive, and Kidney Disease (DK068389 to Holly E. Richter)
References
- 1.Schachrater LR, Tash J, Olgac S, Bochner BH. Umbilical endometriosis. J Urol. 2003;170:2388–2389. doi: 10.1097/01.ju.0000095784.41023.b7. [DOI] [PubMed] [Google Scholar]
- 2.Frischknecht F, Raio L, Fleischmann A, Dreher E, Luscher P, Mueller MD. Umbilical endometriosis. Surg Endosc. 2004;18:347. doi: 10.1007/s00464-003-4245-6. [DOI] [PubMed] [Google Scholar]
- 3.Barbaros U, Iyibozkurt AC, Gulluoglu M, Barbaros M, Erbil Y, Tunali V, et al. Endometriotic umbilical port site metastasis after laparoscopy. 2005;193:1761–1763. doi: 10.1016/j.ajog.2005.05.088. [DOI] [PubMed] [Google Scholar]
- 4.Skidmore RA, Woosley JT, Katz VL. Decidualized umbilical endometriosis. Int J Gynecol Obstet. 1996;52:269–273. doi: 10.1016/0020-7292(95)02612-6. [DOI] [PubMed] [Google Scholar]
- 5.Zollner U, Girschick G, Steck T, Dietl J. Arch Gynecol Obstet. 2002;267:258–260. doi: 10.1007/s00404-002-0438-9. [DOI] [PubMed] [Google Scholar]
- 6.Von Stemm AMR, Meigel WN, Scheidel P, Gocht A. Umbilical enxometriosis. J Eur Acad Dermatol Venereol. 1999;12:30–32. [PubMed] [Google Scholar]
- 7.Zhao X, Lang J, Leng J, Liu Z, Sun D, Zhu L. Abdominal wall endometriomas. Int J Gynecol Obstet. 2005;90:218–222. doi: 10.1016/j.ijgo.2005.05.007. [DOI] [PubMed] [Google Scholar]