Abstract
Hepatitis C virus (HCV), an emerging bloodborne pathogen, causes chronic liver disease frequently except in about 10-20% of infections which undergo spontaneous resolution. Investigating factors that influence viral clearance is essential to understand the natural history of this infection and establishing novel strategies for prevention and treatment. HCV clearance was estimated in a unique cohort of 1260 HIV and HBV negative current drug users enrolled for a hepatitis B vaccination study. It was defined as the inability to detect viral RNA using a PCR method in presence of serum anti-HCV antibody EIA. Associated demographic and socio-behavioral factors including drug use patterns were identified from the enrolled subjects using multivariate regression analysis. 33.3 % (420/1260) of drug users were found positive for anti-HCV antibodies and 14.8% (62/420) of these individuals achieved viral clearance (negative PCR test). Race or ethnicity of the participants was the only significant factor associated with HCV clearance. Hispanics (OR = 3.4, 95% CI: 1.3-8.5, p = 0.01) and Caucasians (OR = 3.1, 95% CI: 1.5-6.6, p = 0.003) had significantly higher odds of clearing the virus compared to African Americans when adjusted for age and gender. None of the socio-behavioral factors including alcohol intake and drug use patterns were significant determinants of HCV clearance. Racial or ethnic differences in HCV clearance were observed in this study suggesting an important role of host genetic susceptibility factors in determining the clinical course of this disease. Further research is needed to examine these genetic associations of host-virus relationships.
Keywords: Drug Users, Hepatitis C, Race, Viral Persistence, African American
Hepatitis C virus (HCV) is best known for its chronic disease phase among all hepatitis causing viruses. With around three percent of the world population infected with HCV, there are an estimated 170 million potentially contagious chronic carriers [Alter and Mast. 1994]. Similarly, 4.1 million HCV infected Americans include 3.2 million chronic cases; ranking HCV as the most common bloodborne infection of the nation [Armstrong et al. 2006]. Chronic HCV infection is primarily responsible for causing hepatocellular carcinoma (HCC) and end stage liver disease, two conditions requiring the majority of liver transplants in the USA [Alter and Mast. 1994]. The annual economic burden of these infections in terms of medical expenses and work loss compensation is estimated to be well above a billion dollars in the USA alone [Kim. 2002]. In the realm of its chronicity, the most intriguing feature of these infections is the consistently observed spontaneous resolution or viral clearance in approximately 15% of HCV-infected individuals [Alter et al. 1992, Micallef et al. 2006, Thomas et al. 2000]. Viral clearance is defined as the failure to detect viral RNA from blood samples in the presence of a positive antibody response. Thus, HCV infection has a highly variable course, ranging from spontaneous resolution to end stage liver disease. Host factors including age, gender, race, level of viraemia, alcohol intake and the nature of infecting HCV genotype have been shown to impact HCV clearance. However, the results from these few studies are contradicting and inconclusive [Alter et al. 1992, Chen et al. 2009, Hofer et al. 2003, Micallef et al. 2006, Page et al. 2009, Santantonio et al. 2003, Seeff. 2002, Thomas et al. 2000]. One of the common limitations of these studies was that the participants had existing co-infections with either human immunodeficiency virus (HIV) or hepatitis B virus (HBV). The potentially significant effect of these interactions on the resulting clinical outcomes of HCV infections was not controlled for in these studies.
Although there have been advances in HCV treatment modalities, the therapeutic response is highly variable and the current treatment regime is extremely expensive with embedded adverse effects. Currently, there is no HCV vaccine available, making it critical that factors associated with the spontaneous resolution or viral clearance in infected individuals be identified and characterized. Since the introduction of a blood screening program for HCV, nearly all newly acquired HCV infections in the USA are the result of sharing needles and/or drug preparation equipments by illicit drug users. Injecting drug users have a higher prevalence of hepatitis C infections compared to the general population with approximately 70% of them testing seropositive for anti-HCV antibodies [Alter and Mast. 1994]. Since this subset of the population has a unique profile and encompass the majority of incident HCV infections in the general population, drug users make an ideal population for studying determinants associated with viral clearance in a natural setting.
The current study was aimed at estimating the frequency of hepatitis C viral clearance and associated factors in a drug using population without concurrent HIV and/or HBV infections.
Materials and Methods
Data were obtained from a cohort of 1,260 drug users enrolled in a hepatitis B vaccination study (DASH project) from February 2004 to October 2007 [Hwang et al. 2010]. The study participants were identified from drug distribution areas, street corners and crack houses in two highly endemic drug-using urban neighborhoods of Houston, TX. Tracking, recruiting and retaining drug users in the study was accomplished with the help of outreach workers employing chain-referral methods. Inclusion criteria for participants in this trial were current illicit drug use confirmed by OnTrack Teststik urine test (Varian Inc, Palo Alto, CA), and negative tests for HIV and HBV infections at the time of screening [Hwang et al. 2010]. Drug users were required to be ≥ 18 years, have a local residence with valid contact information for follow up, report illicit drug use during the 48 hour period prior to screening and demonstrate competency in the informed consent process. Following verbal screening and informed consent, a medical assistant or nurse collected 10 ml of blood from each drug user to assess their respective HIV, HBV and HCV infection status [Hwang et al. 2010] . All data collection procedures and laboratory methods were approved by the Committee for the Protection of Human Subjects at the University of Texas Health Science Center.
Data Collection and Laboratory Methods
The participants provided data using verbally administered questionnaires which were also recorded electronically via computer-assisted personal interviews (CAPI, QDS, Bethesda, MD). They were interviewed to collect information on their demographic, drug-use, social and behavioral characteristics. Determinants of prime interest were age, gender, race or ethnicity, education level, marital status, living arrangements, employment, history of drug use patterns and sexual habits. A series of questions were asked to determine the drug use patterns including if they were currently on a drug, ever used that drug in the past, age when started using that drug and the number of years of drug use, frequency of drug use in past 30 days, frequency and pattern at the time of heaviest use for each of the listed drugs: crack cocaine, powder cocaine, methamphetamine, marijuana, “fry”, heroin, speedball, codeine syrup, alcohol and/or other street drugs. A detailed history of binging for each of these drugs was also recorded for respective participants. Information was collected on binged drug use, amount of drugs used for binging, number of days spent in a drug binge, number of male and female sex partners while on the drug binge, place and time of last binging episode and the reason for stopping it. Drug history also included questions on injecting drugs, age of first injection, frequency and duration of injections, type of drug injected, use of clean needles and sharing of drug use equipment with others. A history of sexually transmitted diseases including Gonorrhea, Herpes, Chlamydia and Trichomonas infections and their treatment was also gathered from these participants. Sexual habits were recorded including the gender and number of partners in past 30 days, if traded sex for drugs or money, use of condoms and use of drugs during sex [Hwang et al. 2010].
HCV infection for this study was defined if the subject tested positive for anti-HCV antibodies by enzyme immunoassay. Blood specimens were tested at enrollment and at follow-up for anti-HCV antibodies using Abbott's AxSYM system (Abbott Laboratories, Chicago, IL). Hepatitis C viral clearance was defined as the failure to detect HCV RNA from blood samples using polymerase chain reaction (PCR) in the presence of anti-HCV antibodies. PCR to detect hepatitis C viral RNA was conducted using the COBAS AMPLICOR MONITOR (Roche Diagnostics, Indianapolis, Indiana) with the ability to detect viral load as low as 60 IU/ml. Controls were run with each batch, and results were determined by the recommended cut off values from the manufacturer for positive, gray zone (intermediate) and negative readings. All gray zone readings were repeated to confirm the results.
Statistical Analyses
Given a fixed sample size of 420 observations and a two-sided test with 0.05 significance level, there was 80% power for the detection of relative risk ratios between 1.82 - 3.28 for an outcome prevalence between 0.1 - 0.2 and exposure prevalence between 0.1 - 0.6 [Schlesselman. 1974].
STATA 11.0 (STATA Corporation, College Station, TX, USA) was used to complete all data analyses. After preliminary data examination for consistency and accuracy, the prevalence of viral clearance was determined by calculating the number of participants with negative PCR tests divided by the total number of participants with positive anti-HCV tests. The prevalence of viral clearance was also stratified by age, gender, race, education, marital status, housing status, intravenous drug use and illicit drugs use; traded sex for money or drugs, men having sex with men and total number of sexual partners in past 30 days.
Unadjusted odds ratios and 95% confidence intervals (CI) with their p-values for each independent variable and viral clearance were calculated. Then, a final model was constructed using a forward selection process by entering variables significant at p<0.25 in the model, one at a time [Mickey and Greenland. 1989]. Variables were retained in the model for their main effect if they were significant at the 0.05 level or their confounding effect if they induced a greater than or equal to 15% change in the odds ratio of another significant variable of primary interest [Rothman KJ and Greenland S. 1998]. Previously eliminated variables (p>0.25) were entered one at a time to ensure that all variables were given the opportunity to appear in the final model. Hosmer – Lemeshow goodness of fit test was used to assess the fit of the model.
Results
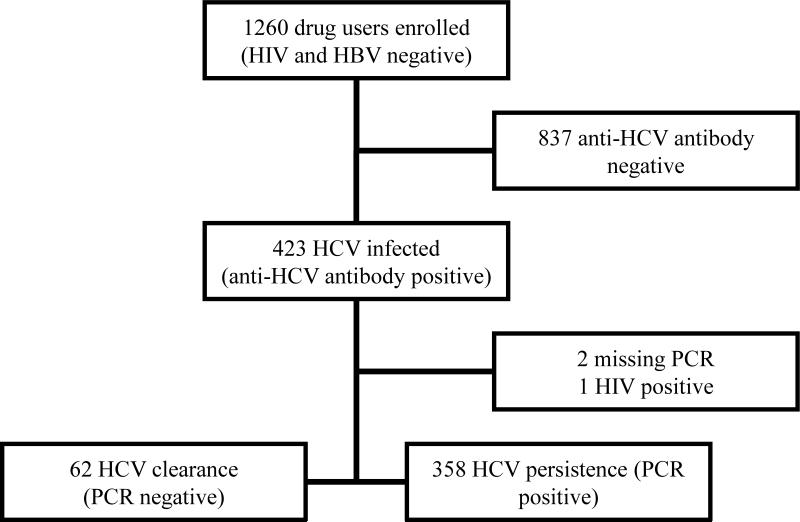
Out of 423 (33.6%) anti-HCV antibody positive drug users from the hepatitis B vaccination study, 420 (99.3%) were included in the final analyses of the current study. Based on their enrollment criteria for the vaccination study, all these study participants were free from HIV and HBV co-infections. The current cohort consisted of 272/378 (72.0%) injecting drug users and 148/882 (16.8%) non-injecting drug users from that study. The majority of the current study population was comprised of 40-49 years old African American males having received some high school education. More than 75% of this population lived without a partner in a temporary residence including the streets of Houston. Their demographic and 30 day drug use profiles stratified by viral clearance status are presented in Table I. Crack cocaine was the drug of choice for these participants; however, the majority of them used more than one drug at a time. The consort diagram (Fig. 1) summarizes distribution of participants, according to viral clearance patterns determined by PCR. 14.8% (62/420) participants cleared their respective hepatitis C infections (negative for HCV RNA by PCR).
Table I.
Demographic, social and behavioral characteristics of subjects with negative and positive PCR post HCV infection
| Total | PCR negative | PCR positive | P-value | |||
|---|---|---|---|---|---|---|
| Study participants | 420 | 62 (14.8) | 358 (85.2) | |||
| Age | 18-29 | 10 (2.4) | 3 (4.8) | 7 (2) | 0.027 | |
| 30-39 | 46 (11) | 6 (9.7) | 40 (11.2) | |||
| 40-49 | 228 (54.3) | 42 (67.7) | 186 (52) | |||
| ≥50 | 136 (32.4) | 11 (17.7) | 125 (34.9) | |||
| Gender | Male | 337 (80.2) | 49 (79) | 288 (80.5) | 0.796 | |
| Female | 83 (19.8) | 13 (21) | 70(19.6) | |||
| Race | African American | 325 (77.4) | 36 (58.1) | 289 (80.7) | <0.001 | |
| Caucasian | 68 (16.2) | 18 (29) | 50 (14) | |||
| Hispanic/Other | 27 (6.4) | 8 (12.9) | 19 (5.3) | |||
| Education | <High School | 28 (6.7) | 4 (6.5) | 24 (6.7) | 0.603 | |
| High School | 310 (73.8) | 43 (69.4) | 267 (74.6) | |||
| Some college | 82 (19.5) | 15 (24.2) | 67 (18.7) | |||
| Housing status | Permanent | 95 (22.6) | 15 (24.2) | 80 (22.4) | 0.748 | |
| Temporary/streets | 325 (77.4) | 47 (75.8) | 278 (77.7) | |||
| Marital status | Without a partner | 344 (81.9) | 54 (87.1) | 290 (81) | 0.253 | |
| With a partner | 76 (18.1) | 8 (12.9) | 68 (19) | |||
| Injecting drug use ever | Yes | 272 (64.8) | 43 (69.4) | 229 (64) | 0.413 | |
| No | 148 (35.2) | 19 (30.6) | 129 (36) | |||
| Injecting drug use* | Yes | 63 (15) | 10 (16.1) | 53 (14.8) | 0.787 | |
| Crack cocaine* | Yes | 376 (89.5) | 55 (88.7) | 321 (89.7) | 0.821 | |
| Powder cocaine* | Yes | 63 (15) | 11 (17.7) | 52 (14.5) | 0.513 | |
| Methamphetamines* | Yes | 14 (3.3) | 2 (3.2) | 12 (3.4) | 0.959 | |
| Fry* | Yes | 3 (0.7) | 1 (1.6) | 2 (0.6) | 0.385 | |
| Marijuana* | Yes | 201 (47.9) | 30 (48.4) | 171 (47.8) | 0.928 | |
| Heroin* | Yes | 31 (7.4) | 4 (6.5) | 27 (7.5) | 0.762 | |
| Speedball* | Yes | 16 (3.8) | 0 (0) | 16 (4.5) | ||
| Codeine* | Yes | 15 (3.6) | 3 (4.8) | 12 (3.4) | 0.563 | |
| Other street drugs* | Yes | 60 (14.3) | 13 (21) | 47 (13.1) | 0.103 | |
| Combination drug* | Used 1 drug | 66 (15.7) | 10(16.1) | 56 (15.6) | 0.731 | |
| Used 2 drugs | 140 (33.3) | 18 (29) | 122 (34.1) | |||
| Used ≥ 3 drugs | 214 (51) | 34 (54.8) | 180 (50.3) | |||
| Traded sex for money/drugs* | Yes | 80 (19.1) | 13 (21) | 67 (18.7) | 0.677 | |
| Men having sex with men | Yes | 50 (11.9) | 8 (12.9) | 42 (11.7) | 0.812 | |
| New sexual partners* | ≥ 1 | 133 (31.7) | 17 (27.4) | 116 (32.4) | 0.126 |
in past 30 days
Fig. 1.
Consort diagram: Distribution of the study population based on HCV status
Of the demographic variables, race was observed to have a significant association with viral clearance. The association did not change significantly after adjustment for age and gender of these participants. Hispanics (OR = 3.4; 95% CI: 1.3 – 8.5, p = 0.01) and Caucasians (OR = 3.1; 95% CI: 1.5 – 6.6, p = 0.003) had increased viral clearance odds compared to African Americans in this study (Table II). A significant trend of decreased capacity of viral clearance was also observed for drug users of increasing age. This effect disappeared after adjusting for race in the final model. For the univariate analyses, drug use variables associated with increased odds for viral clearance were current use of street drugs other than those mentioned earlier, history of marijuana use and history of methamphetamine use. Drug use variables with decreased odds for viral clearance were initiating crack cocaine use after the age of 15 years, initiating methamphetamine use after the age of 15 years and having more than one new sexual partner in the last 30 days. However, none of the drug use indicators including age for initiating drug use and duration of drug use were significant at p<0.05 level to be retained in the final model. There was no significant difference in the rate of HCV clearance between injecting and non-injecting drug users. History, duration and frequency of alcohol use were not associated with hepatitis C viral clearance in these participants. None of the variables related to the sexual habits of these participants were significantly associated with the observed hepatitis C viral clearance.
Table II.
Multivariate analysis of host factors associated with hepatitis C viral clearance
| Unadjusted (N=420) | Adjusted (N =420) | ||||
|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | ||
| Age | |||||
| 18-29 | Reference | ||||
| 30-39 | 0.4 (0.1, 1.7) | 0.199 | n. s. | ||
| 40-49 | 0.5 (0.1, 2.1) | 0.367 | |||
| ≥50 | 0.2 (0.1, 0.9) | 0.037 | |||
| Gender | |||||
| Male | Reference | ||||
| Female | 1.1 (0.6, 2.1) | 0.796 | n. s. | ||
| Race | |||||
| African American | Reference | ||||
| Caucasian | 2.9 (1.5, 5.5) | 0.001 | 3.1* (1.5, 6.6) | 0.003 | |
| Hispanic/Other | 3.4 (1.4, 8.3) | 0.008 | 3.4* (1.3, 8.5) | 0.01 |
adjusted for age and gender
There was no significant interaction identified between any of the examined variables. No additional variables resulted in more than or equal to a 15% change in the main effects to indicate an effect of confounding. The final multiple logistic regression model for predicting hepatitis C viral clearance included age, gender and race of the drug users. Age and gender were included in the final model due to their biological significance reported by previous studies on HCV clearance.
Discussion
This is the first report describing HCV clearance from a community based study targeting injecting and non-injecting drug users. The unique strength of the current study was its ability to determine the host factors associated with HCV clearance in a natural setting of drug users with no viral coinfections such as HIV and/or HBV. This was a major advantage compared to most of the previous studies which could not exclude the confounding effects associated with concomitant HIV and/or HBV infections in their participants. Hepatitis C viral clearance was achieved by 14.8% of the subjects from the cohort as determined by negative HCV RNA PCR tests. This rate was similar to previously reported values by other two previous studies in the USA that examined viral clearance in prior injection drug users and community-acquired hepatitis C infection cases [Alter et al. 1992, Thomas et al. 2000].
A primary focus of the current study was to identify host related factors such as demographic, social and behavioral characteristics, including patterns of drug use as a means of predicting the hepatitis C viral clearance in a select group of individuals. From the current cohort, Non-African race was the only significant factor associated with improved viral clearance. Race has been demonstrated to be an important risk factor in the progression of acute hepatitis C infections to persistent disease in previous studies. A community-based, prospective cohort study in Baltimore [Thomas et al. 2000] reported an adjusted odds ratio of 5.15 (2.60-10.17) for HCV clearance in non-blacks compared to blacks. However, they examined viral clearance in younger individuals (mean age of 34 years) with prior history of injection drug use who were likely to have been co-infected with HIV as compared to the current cohort (mean age of 46 years) who were current drug users free of HIV and HBV co-infections. The African American race has been observed to have unfavorable outcomes in the context of other clinical phases of hepatitis C infections as well. Patients with African ancestry have demonstrated a reduced response rate to antiviral treatments for HCV [Layden-Almer et al. 2003], and an increased risk for HCV-related hepatocellular carcinoma compared to Caucasians [El-Serag et al. 2007]. These racial trends imply host genetic susceptibility as an important factor in determining the variable course of this disease.
Based on the current literature, there are two main mechanisms to explain the genetic basis of the observed host-virus interactions: host immune response and viral entry or replication pathways. The IL-28B locus was found to be associated with increased natural HCV clearance [Thomas et al. 2009] and sustained virologic response to antiviral drug treatment [Ge et al. 2009]. Similarly, class I human leukocyte antigens (HLA) genes, including killer-like immunoglobulin (KIR) ligand and CD4 T cell response mediator, have been implicated in determining HCV infection outcomes (HLA-A*1101, HLA-B*57, HLA-Cw*0102, HLA DRB1*1101 with viral clearance and HLA-A*2301, HLA-Cw*04, HLA DRB1*0701, HLA DRB4*0101, HLA DRB1*11 with viral persistence) [Thio et al. 2002, Thursz et al. 1999]. Racial differences were also observed in these associations between HLA genes and HCV outcomes. Achieving higher rates of viral clearance was seen in African American subjects with HLA-DQB1*0301 and in Caucasian subjects with DRB1*0101 DQB1*0501 genes. Viral persistence was observed in Caucasians carrying DRB1*0301 and DQB1*0201 genes [Thio et al. 2001]. These results highlight the role of immune response related gene polymorphisms in HCV clearance and emphasize the need for studying these genetic factors in the light of ethnic diversity. Chemokine receptors, especially CCR5, have been shown to be involved in cell-mediated immune response to HCV. Although, results from the various studies are still controversial, role of CCR5-delta 32 homozygosity, a genotype which is virtually absent in African and Asian populations, seems to be very relevant in determining the susceptibility and clinical outcomes of hepatitis C infections [Coenen and Nattermann. 2010]. In regards to the second mechanism, LDL receptor has been demonstrated to facilitate viral entry into mammalian cells [Agnello et al. 1999]. Serum or plasma HCV particles are physically associated with low density lipoprotein (LDL) and very low density lipoproteins (VLDL). Hepatocytes, studded with these LDL receptors for lipid regulation, are the primary sites for hepatitis C viral replication. Defects in these receptors or impairment in the lipid metabolism pathways may affect the HCV life cycle. Racial or ethnic differences including atherogenic risk profiles and hepatic lipase foci polymorphisms between African Americans and Caucasians have been characterized, providing a biological explanation for the differences observed in HCV clearance between races [Vega et al. 1998, Wilson. 2000]; however, future studies are warranted to explore further these complex genetic associations.
As observed in the current study, other demographic factors including age and gender were not predictive of HCV clearance. Increasing age of the drug user was independently associated with decreased HCV clearance in the current cohort; however, this association was lost after adjusting for race or ethnicity. This finding is also supported by the study utilizing the Baltimore cohort; as it did not show a statistically significant association between HCV clearance and age, alcohol use or frequency of injecting drugs either [Thomas et al. 2000]. A systematic review on spontaneous viral clearance following acute hepatitis C infections reported female gender and presentation of acute clinical hepatitis with favorable outcomes; however, this systematic review included all the longitudinal studies with heterogeneous populations from diverse geographic locations and ethnicities [Micallef et al. 2006]. Female injecting drug users showed an estimated 3-fold higher rate of viral clearance compared to male injecting drug users in a prospective cohort study of 95 HCV infected injecting drug users [Page et al. 2009]. This study also examined a few different host factors including age, race, drug habits and sexual history; however, no significant associations were identified probably due to a small sample size. In addition, there have been inconsistent reports of other host related factors which might be associated with HCV clearance; however, most of these studies lacked adequate statistical power. For example, a community based study of 40 HCV infected people reported that old age and jaundice offered protection from chronicity; and concluded that viral genotype or viral load were not significant in HCV clearance [Santantonio et al. 2003]. Development of jaundice as a predictor of spontaneous viral clearance was also supported by another small study of 12 patients with acute hepatitis C [Hofer et al. 2003]. A prospective study in Taiwan following 42 children with chronic hepatitis observed a higher spontaneous resolution rate in children with lower levels of HCV RNA at the time of enrollment [Chen et al. 2009]. One of the major strengths of the current study was the large sample size which rendered adequate statistical power for the identification of any significant host factors that may be associated with HCV clearance. None of the other factors tested including alcohol intake, patterns of drug use or sexual behaviors were found to be associated with HCV viral clearance significantly.
One of the limitations of the current study is that it did not examine the effect of virus-related factors which might also play a role in the development of persistent HCV infections. Although, viral genotype and viral load have been identified as very significant factors in HCV treatment response, most investigators reject the idea that these factors predict progression of the disease [Seeff. 2002]. Among the 6 genotypes and 15 HCV subtypes defined to date [Armstrong et al. 2006], only genotype 1b has been reported to be associated with higher rate of disease progression compared to others [Seeff. 2002]. The majority of the current cohort is expected to be infected with the genotype 1 of HCV, a result similar to that observed previously in this population [Mast et al. 2005]. Also, the cross-sectional design of this study cannot establish causation; however, prospective HCV studies are rare due to the practical disadvantage of the long asymptomatic stage. Relevance of race or ethnicity in HCV clearance is not affected by the limitation of this study design. One possible limitation of the study is that it relied on a single negative PCR result to determine HCV clearance. A single negative test may represent fluctuating low levels of viraemia rather than true clearance.
In conclusion, hepatitis C remains a highly prevalent viral infection of illicit drug users and a preventive vaccine is needed to control the spread of disease among high-risk populations successfully. The confirmation that spontaneous HCV viral clearance provides protection from chronic disease provides hope that an effective vaccine can be designed. One of the most important drawbacks in HCV vaccine development has been a lack of understanding of factors associated with protection against chronic HCV infections. African Americans were associated with decreased hepatitis C viral clearance among the drug using population studied and this finding can be applied in the clinical management of individual cases. Although currently not well understood, the impact of race on disease progression and presentation seems to be independent of other virus-related or environmental factors. Future studies are needed to explore host genetic susceptibility and elucidate mechanisms leading to viral clearance. Insight into these features of host-virus relationships will be crucial in the successful development of vaccines, antiviral therapies and progress in the overall knowledge of host immunity to viral infections.
Acknowledgments
Funding/Support:
This study was funded by the National Institute on Drug Abuse, National Institutes of Health.
Abbreviations
- HCV
hepatitis C virus
- anti-HCV
antibody to hepatitis C virus
- HBV
hepatitis B virus
- HIV
human immunodeficiency virus
- CI
confidence interval
- CCR5-delta32
CCR5 gene with 32 base pairs deletion
- Speedball
mixture of heroin and cocaine
- Fry
marijuana dipped in PCP (phencyclidine)
- PCR
polymerase chain reaction
- IL-28B
interleukin 28B
- LDLr
low density lipoprotein receptor
- HLA
human leukocyte antigen, major histocompatibility complex
- OR
odds ratio
Footnotes
The authors do not have a commercial or other association that might pose a conflict of interest.
References
- Agnello V, Ábel G, Elfahal M, Knight GB, Zhang Q. Hepatitis C virus and other Flaviviridae viruses enter cells via low density lipoprotein receptor. Proc Natl Acad Sci U S A. 1999;96:12766–12771. doi: 10.1073/pnas.96.22.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter MJ, Mast EE. The epidemiology of viral hepatitis in the United States. Gastroenterol Clin North Am. 1994;23:437–455. [PubMed] [Google Scholar]
- Alter MJ, Margolis HS, Krawczynski K, Judson FN, Mares A, Alexander WJ, Hu PY, Miller JK, Gerber MA, Sampliner RE, Meeks EL, Beach MJ. The Natural History of Community-Acquired Hepatitis C in the United States. N Engl J Med. 1992;327:1899–1905. doi: 10.1056/NEJM199212313272702. [DOI] [PubMed] [Google Scholar]
- Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- Chen ST, Ni YH, Chen PJ, Chen HL, Jeng YM, Lu MY, Wu JF, Hsu HY, Chang MH. Low viraemia at enrollment in children with chronic hepatitis C favours spontaneous viral clearance. J Viral Hepat. 2009;16:796–801. doi: 10.1111/j.1365-2893.2009.01135.x. [DOI] [PubMed] [Google Scholar]
- Coenen M, Nattermann J. The role of CCR5 in HCV infection. Eur J Med Res. 2010;15:97–101. doi: 10.1186/2047-783X-15-3-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Serag HB, Lau M, Eschbach K, Davila J, Goodwin J. Epidemiology of hepatocellular carcinoma in Hispanics in the United States. Arch Intern Med. 2007;167:1983–1989. doi: 10.1001/archinte.167.18.1983. [DOI] [PubMed] [Google Scholar]
- Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ, Sulkowski M, McHutchison JG, Goldstein DB. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- Hofer H, Watkins-Riedel T, Janata O, Penner E, Holzmann H, Steindl-Munda P, Gangl A, Ferenci P. Spontaneous viral clearance in patients with acute hepatitis C can be predicted by repeated measurements of serum viral load. Hepatology. 2003;37:60–64. doi: 10.1053/jhep.2003.50019. [DOI] [PubMed] [Google Scholar]
- Hwang LY, Grimes CZ, Tran TQ, Clark A, Xia R, Lai D, Troisi C, Williams M. Accelerated hepatitis B vaccination schedule among drug users: a randomized controlled trial. J Infect Dis. 2010;202:1500–1509. doi: 10.1086/656776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WR. The burden of hepatitis C in the United States. Hepatology. 2002;36:s30–s34. doi: 10.1053/jhep.2002.36791. [DOI] [PubMed] [Google Scholar]
- Layden-Almer JE, Ribeiro RM, Wiley T, Perelson AS, Layden TJ. Viral dynamics and response differences in HCV-infected African American and white patients treated with IFN and ribavirin. Hepatology. 2003;37:1343–1350. doi: 10.1053/jhep.2003.50217. [DOI] [PubMed] [Google Scholar]
- Mast EE, Lu-Yu Hwang, Seto DSY, Nolte FS, Nainan OV, Wurtzel H, Alter MJ. Risk Factors for Perinatal Transmission of Hepatitis C Virus (HCV) and the Natural History of HCV Infection Acquired in Infancy. J Infect Dis. 2005;192:1880–1889. doi: 10.1086/497701. [DOI] [PubMed] [Google Scholar]
- Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34–41. doi: 10.1111/j.1365-2893.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. American Journal of Epidemiology. 1989;129:125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- Page K, Hahn JA, Evans J, Shiboski S, Lum P, Delwart E, Tobler L, Andrews W, Avanesyan L, Cooper S, Busch MP. Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incident infection, resolution, and reinfection. J Infect Dis. 2009;200:1216–1226. doi: 10.1086/605947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S, editors. Modern Epidemiology. 2nd ed. Lippincott-Raven; Philadelphia, PA: 1998. [Google Scholar]
- Santantonio T, Sinisi E, Guastadisegni A, Casalino C, Mazzola M, Gentile A, Leandro G, Pastore G. Natural course of acute hepatitis C: a long-term prospective study. Dig Liver Dis. 2003;35:104–113. doi: 10.1016/s1590-8658(03)00007-0. [DOI] [PubMed] [Google Scholar]
- Schlesselman JJ. Sample size requirements in cohort and case-control studies of disease. American Journal of Epidemiology. 1974;99:381–384. doi: 10.1093/oxfordjournals.aje.a121625. [DOI] [PubMed] [Google Scholar]
- Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35–46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- Thio CL, Gao X, Goedert JJ, Vlahov D, Nelson KE, Hilgartner MW, O'Brien SJ, Karacki P, Astemborski J, Carrington M, Thomas DL. HLA-Cw*04 and Hepatitis C Virus Persistence. J Virol. 2002;76:4792–4797. doi: 10.1128/JVI.76.10.4792-4797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thio CL, Karacki P, Astemborski J, Thomas DL, Goedert JJ, Vlahov D, Nelson KE, Hilgartner MW, O'Brien SJ, Marti D, Carrington M. Racial Differences in HLA Class II Associations with Hepatitis C Virus Outcomes. J Infect Dis. 2001;184:16. doi: 10.1086/321005. [DOI] [PubMed] [Google Scholar]
- Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O'Huigin C, Kidd J, Kidd K, Khakoo SI, Alexander G, Goedert JJ, Kirk GD, Donfield SM, Rosen HR, Tobler LH, Busch MP, McHutchison JG, Goldstein DB, Carrington M. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DL, Astemborski J, Rai RM, Anania FA, Schaeffer M, Galai N, Nolt K, Nelson KE, Strathdee SA, Johnson L, Laeyendecker O, Boitnott J, Wilson LE, Vlahov D. The Natural History of Hepatitis C Virus Infection: Host, Viral, and Environmental Factors. JAMA. 2000;284:450–456. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- Thursz M, Yallop R, Goldin R, Trepo C, Thomas HC. Influence of MHC class II genotype on outcome of infection with hepatitis C virus. The Lancet. 1999;354:2119–2124. doi: 10.1016/s0140-6736(99)91443-5. [DOI] [PubMed] [Google Scholar]
- Vega GL, Clark LT, Tang A, Marcovina S, Grundy SM, Cohen JC. Hepatic lipase activity is lower in African American men than in white American men: effects of 5' flanking polymorphism in the hepatic lipase gene (LIPC). J Lipid Res. 1998;39:228–232. [PubMed] [Google Scholar]
- Wilson PWF. Lipids, Lipases, and Obesity : Does Race Matter? Arterioscler Thromb Vasc Biol. 2000;20:1854–1856. doi: 10.1161/01.atv.20.8.1854. [DOI] [PubMed] [Google Scholar]