Abstract
Membranes containing reactive nanoparticles (Fe and Fe/Pd) immobilized in a polymer film (polyacrylic acid, PAA-coated polyvinylidene fluoride, PVDF membrane) are prepared by a new method. In the present work a biodegradable, non-toxic -“green” reducing agent, green tea extract was used for nanoparticle (NP) synthesis, instead of the well-known sodium borohydride. Green tea extract contains a number of polyphenols that can act as both chelating/reducing and capping agents for the nanoparticles. Therefore, the particles are protected from oxidation and aggregation, which increases their stability and longevity. The membrane supported NPs were successfully used for the degradation of a common and highly important pollutant, trichloroethylene (TCE). The rate of TCE degradation was found to increase linearly with the amount of Fe immobilized on the membrane, the surface normalized rate constant (kSA) being 0.005 L/m2h. The addition of a second catalytic metal, Pd, to form bimetallic Fe/Pd increased the kSA value to 0.008 L/m2h. For comparison purposes, Fe and Fe/Pd nanoparticles were synthesized in membranes using sodium borohydride as a reducing agent. Although the initial kSA values for this case (for Fe) are one order of magnitude higher than the tea extract synthesized NPs, the rapid oxidation reduced their reactivity to less than 20 % within 4 cycles. For the green tea extract NPs, the initial reactivity in the membrane domain was preserved even after 3 months of repeated use. The reactivity of TCE was verified with “real” water system.
1. Introduction
Chlorinated organic compounds constitute a large group of pollutants of international concern due to their high toxicity, persistence and various sources of distribution in the environment. In recent years, zero-valent nanoscale metal (especially iron) particles have attracted a growing attention in groundwater remediation of chlorinated solvents [1–3]. For a more rapid and complete dechlorination, a second element is often added, resulting in creation of bimetallic iron-based nanoparticles. Iron has the role to generate hydrogen gas (by corrosion reaction in water) and the second element acts as a hydrogenation catalyst, thus increasing the rate of dechlorination. A variety of systems including Fe/Pt [4], Fe/Ag [5], Fe/Cu [6], Fe/Ni [7, 8] or Fe/Pd [9–12] have been reported. Among these, the latter bimetallic system is the most efficient and most commonly used. Due to their magnetic properties, Fe (or Fe/Pd bimetallic) nanoparticles tend to agglomerate in water rapidly [13] to form micron size or larger aggregates, thus losing their reactivity. Various techniques have been employed to control the particle size, using dispersing agents such as polymers [13–17], surfactants [18] or by coating with a protective layer [13,17,19]. Another approach is to synthesize supported nanoparticles on activated carbon [20], beads [21], particles [22], polyelectrolyte multilayers [23], or membranes, as substrates.
Membranes’ open structure and high internal surface area ensure a high nanoparticle loading and easy active site accessibility. Consequently, they have received a special attention and have been successfully employed as platforms for nanoparticle synthesis [24–31] including several from our own group [24,26,27,30,31]. In recent years, our laboratory has focused on the use of poly(vinylidene fluoride) (PVDF) membrane modified with polyacrylic acid (PAA) by dip coating or solvent phase in situ polymerization of acrylic acid, with subsequent Fe/Pd nanoparticle incorporation.
Our latest efforts were concentrated toward the use of green chemistry to synthesize the Fe/Pd bimetallic supported nanoparticles. Thus, we investigated a method of PAA modification of PVDF membranes using aqueous phase polymerization (green chemistry, no organic solvent used). Further, metal ions were reduced to zero-valent metallic particles using green (without a hazardous impact on the environment) chemicals (reducing agents) as an alternative to the commonly used hydride reducing agent, sodium borohydride. A variety of materials originating from biorenewable natural sources, such as plant surfactants [32], waste biomass [33], vitamins [34,35], aminoacids [36], pomace (wine waste) [37], oleic acid [38], coffee [39] and green tea [39–41] or camphora leaf [42,43] extracts have emerged as replacements for well established chemicals. These materials are nontoxic, biodegradable and act as both dispersive and capping agents, thus minimizing the nanoparticle oxidation and agglomeration. Various metallic particles such as Au, Pd, Pt, Ag and Fe were synthesized using these novel techniques. In addition, a novel method for nanostructured metal oxides (Fe, Co, Mn etc.) fabrication without using of any reducing agent was reported, and utilized only microwave irradiation [44].
Our main aim is to directly synthesize zero valent iron NPs in membrane pores using green tea extract as a reducing agent thus exploiting the abundance of polyphenols. The overall objectives of the current paper are: i) synthesize the membrane supported Fe and Fe/Pd using a green reducing/capping agents, tea extract, ii) determine the dechlorination performances of these “green” supported nanoparticles, and iii) evaluate their long term stability and activity. Our previous publications in this area did not address green chemistry, use of Fe NP only, and particle longevity.
2. Materials and Methods
2.1 Materials
All chemicals used were of reagent grade. Trichloroethylene (TCE), ferrous chloride, and deionized ultrafiltered (DIUF) water were all purchased from Fisher Scientific. Potassium persulfate was purchased from EM Science, acrylic acid, potassium tetrachloropalladate (II), sodium borohydride and 1, 2-dibromoethane (DBE) were purchased from Sigma-Aldrich. Chloride reference solution (100 ppm) was purchased from Thermo Electron Corporation and ethylene glycol (EG) from Mallinckrodt. Hydrophilized PVDF microfiltration membranes, with a thickness of 125 µm and nominal pore size of 650 nm were obtained from Millipore Corporation.
2.2 Methods
2.1.1 PAA Functionalization of Membrane
PVDF membranes were functionalized with poly(acrylic acid) by in situ polymerization of acrylic acid. The polymerization reaction was carried out in aqueous phase and the polymerization solution contained 40 mL deoxygenated DIUF, the initiator potassium persulfate (0.6 g, ~1% wt.), 20 mL acrylic acid, and 1mL cross-linking agent ethylene glycol. This corresponds to 6.15 mol% EG to acrylic acid molar ratio; the ether bond formed between carboxylic group and EG generate the cross-linking PAA network structure [29, 45]. EG is bidentate ligand and binds to carboxylic acid groups in a 1:2 molar ratio; it is important to only do a partial (about 12.3% under our experimental conditions) cross-linking to leave free carboxylic groups (87.7%) for metal entrapment. The PVDF membrane was dipped in the polymerization solution for 2 minutes, sandwiched between two teflon plates and placed in an oven at 90 °C for 4 hours. Nitrogen gas was continuously supplied to remove the oxygen, which acts as an inhibitor for the polymerization reaction [46]. To ensure the proper wetting, hydrophilic PVDF membranes were used for the aqueous phase polymerization.
2.2.2 Fe and bimetallic Fe/Pd nanoparticle synthesis in PAA-functionalized PVDF membranes
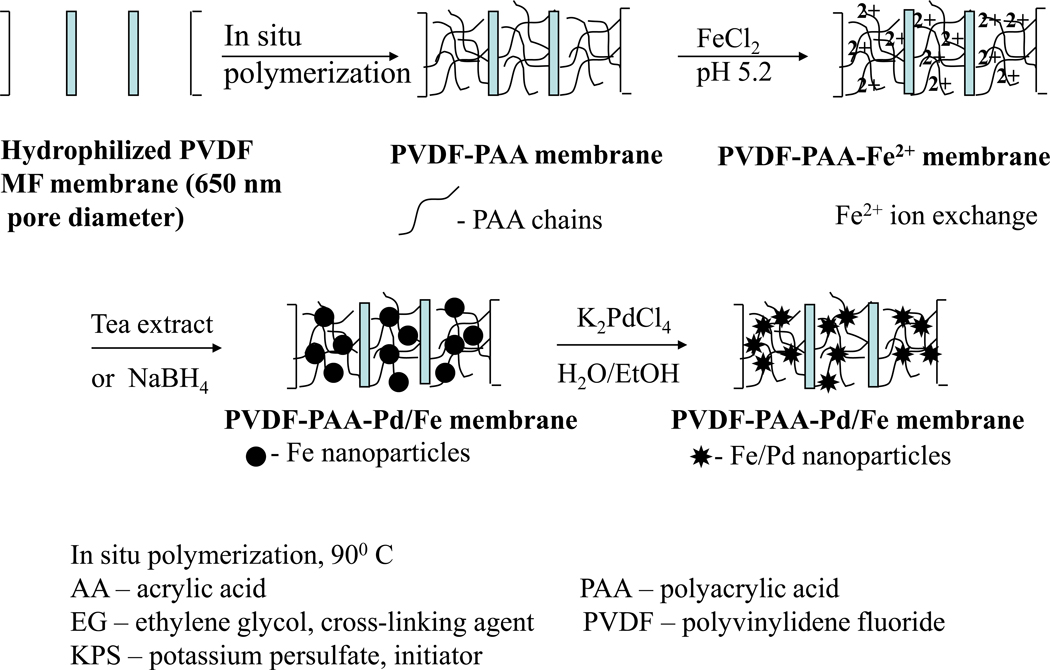
The flowchart for membrane functionalization and nanoparticle synthesis is shown in Figure 1. Prior to Fe2+ ion exchange, PAA-functionalized PVDF membranes were immersed in NaCl (5 to 10 % wt) solution at pH 10 (adjusted with 0.1M NaOH) for at least 3 h to convert the –COOH to COONa form. In the next step, the membrane was washed with DIUF until the pH of the washing solution became neutral. Then, the membrane was immersed in FeCl2 solution at a pH of 5.5 (adjusted with 0.1M NaOH) for 4 h. Typically the feed solution volume and concentration were 200 mL and 180 mg/L Fe2+, respectively. Nitrogen gas was bubbled to minimize Fe2+ oxidation. The Fe2+-modified membrane was immersed in green tea extract (50 ml, 20 g/L) at pH 5, this ensured the formation of Fe nanoparticles. The green tea extract was prepared by immersion of 4g dry green tea leafs in boiling water (200 mL) for 30 minutes; the liquid was filtered using 1) a filter paper and 2) a 200 nm microfiltration (regenerated cellulose) membrane. Fe nanoparticles were prepared using sodium borohydride (typically 50 mL, 10 g/L) as a reducing agent, instead of the tea extract.
Figure 1.
Schematic for PAA coating and Pd/Fe nanoparticle synthesis in PVDF membranes (all green chemistry)
For the Fe/Pd bimetallic nanoparticles, the secondary metal, Pd, was deposited on the Fe nanoparticles by immersing the membrane (typically for 2 h) in a K2PdCl4 solution (20 mL, 25mg/L Pd in a mixture of ethanol/water 90:10 vol%). This was performed in closed vials with vigorous shaking.
2.2.3 Metal Analysis
The amount of Fe captured during ion exchange and Pd deposited on the Fe-modified membrane was determined from material balance. In addition, membranes (after the dechlorination studies were completed) were digested by immersion in nitric acid (0.02L, 35%) thus desorbing the metals in the solution phase. The concentrations for Fe and Pd in the feed, permeate and digested solutions were quantified using a Varian SpectrAA 220 Fast Sequential atomic absorption spectrometer equipped with a Fisher Scientific hollow cathode lamp. For Fe, the lamp was operated at a wavelength of 386.0 nm. The calibration plot was created using 4 different concentrations of Fe ranging from 25 to 200 mg/L with R2 = 0.9995 and average analytical error of 2%. In the case of Pd, the lamp was operated at a wavelength of 246.6 nm and the linear calibration range is between 0.2 and 28 mg/L Pd. The error of analysis was <2%.
2.2.4 TCE degradation analysis
Dechlorination of TCE was conducted by cutting PAA/PVDF membranes containing Fe or Fe/Pd nanoparticles (original membranes were of 13.2 cm2 external area and 125 µm thickness) into small pieces and immersing them in 20 mL sealed vials containing TCE (30 mg/L) solution. Probes were collected with a syringe at different times. TCE samples were extracted in pentane, and analyzed with a Hewlett Packard Series II 5890 GC-MS. In this analysis, the decrease of TCE concentration in time was monitored and DBE was used as an internal standard. The calibration was performed in between 5 and 50 mg/L TCE, linear R2 =0.9899, and the average analytical error was <8.5%.
TCE was also dechlorinated under convective conditions; for this case, the PAA/PVDF membrane containing Fe/Pd nanoparticles was mounted in a filtration cell and a feed solution (0.2 L, 30 mg/L TCE) was permeated through the membrane. Instead of measuring the TCE (volatile) concentration in the permeate solution directly, the concentration of Cl− in the permeate solution was measured using an Accumet combination chloride electrode. Then, based on the assumption of a total dechlorination (1 degraded mol of TCE releases 3 moles of Cl−) this was used to back calculate the concentration of TCE in the permeate solution.
3. Results and Discussion
3.1 Fe and Fe/Pd nanoparticle synthesis and characterization
The procedure for membrane modification and nanoparticle incorporation was described in previous section. Tea extract contains a number of polyphenols, the most common one being epicatechin or its ester forms with gallic acid. Polyphenols can directly complex and then reduce iron to zero valent particles. For example, the reduction potential for epicatechin is 0.57 V, sufficient for the reduction of Fe2+ to Fe0 (−0.44 V). A general reaction can be written as:
where Ar is the phenyl group and n is the number of hydroxyl groups oxidized by Fe2+. Although this is just a proposed mechanism, it was suggested in a variety of studies dealing with the synthesis of Fe [40,41], Pd [39,43], Ag [39,42] and Au [42] metal nanoparticles reduced with polyphenols and other polyol compounds. It was shown (FTIR studies) that most hydroxyl groups were converted to carbonyl during this process [42,43].
After the immersion of Fe2+-modified membrane in the green tea extract, the membrane changes color from white to black (due to the formation of Fe0 nanoparticles on the membrane). The NP size can be controlled by adjusting PAA cross-linking in pores and by modification of reductant to exchanged metal ratio. For comparison purposes, Fe and Fe/Pd nanoparticles were synthesized using the conventional hydride reducing reagent, sodium borohydride. For the Fe/Pd bimetallic nanoparticles, the secondary metal, Pd, was deposited by post-coating reaction of Fe with K2PdCl4 solution.
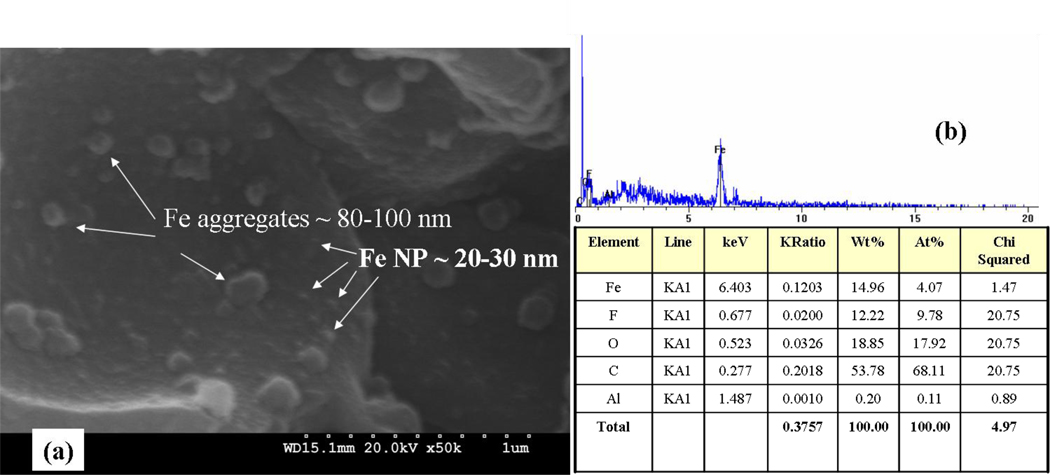
An SEM image for the cross-section of the PVDF/PAA membrane containing Fe nanoparticles is shown in Figure 2a. It can be observed that the base nanoparticles are in the range from 20 to 30 nm and there are some aggregates, between 80 and 100 nm. The Energy Dispersive X-ray (EDX) spectrum (Figure 2b) confirms the presence of Fe and quantitative elemental analysis shows an atomic ratio Fe:O of approximately 1:4. One would expect most of the “O” is from the COO groups of polyacrylic acid. Nanoparticle XRD patterns for both Fe and Fe/Pd (Figure 3) indeed show iron to be in the metallic α-Fe form ((110), (200)) and Pd ((111) and (200)) peaks.
Figure 2.
(a) SEM imaging for the Fe NP (reduced using tea polyphenols), immobilized on a PAA/PVDF membrane (cross-section) and (B) EDX spectrum
Figure 3.
XRD spectra for Fe and Fe/Pd nanoparticles reduced with tea extract
3.2 TCE dechlorination studies
The Fe and bimetallic Fe/Pd nanoparticles have been used to dechlorinate toxic organics, trichloroethylene (a common pollutant in groundwater) being used as a model compound. It is well known that dechlorination by Fe nanoparticles, occurs via electron transfer mechanism whereas in the Fe/Pd bimetallic system Fe generates H2 and Pd acts as a catalyst (the dechlorination occurs on Pd surface).
3.2.1 Fe Nanoparticles
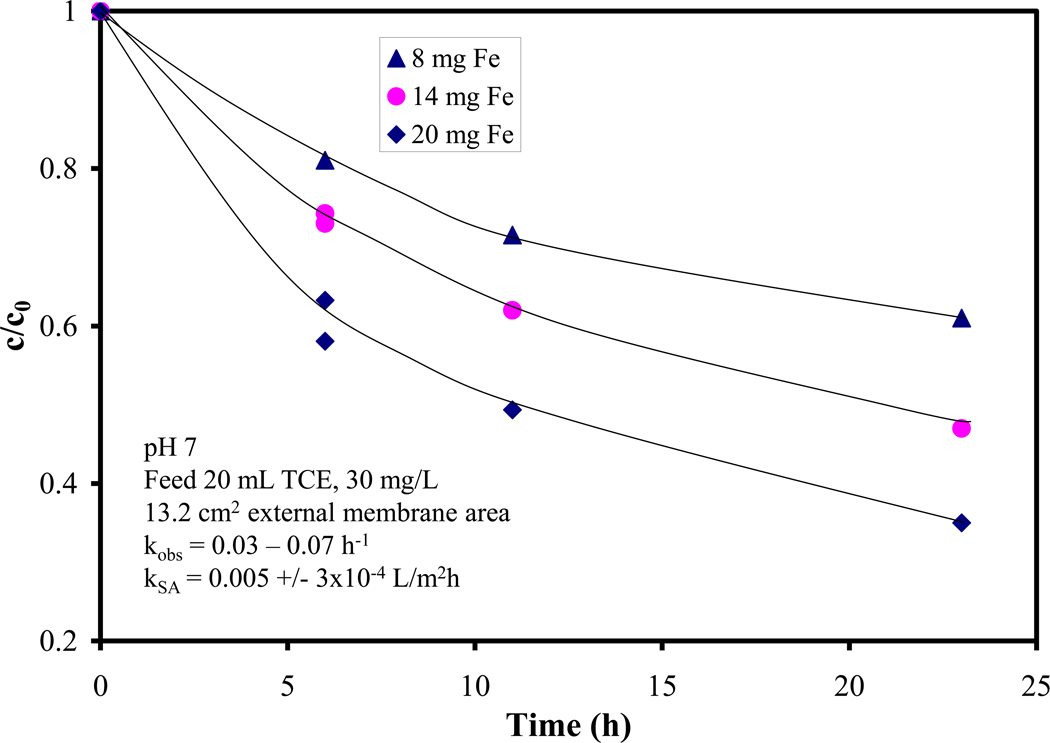
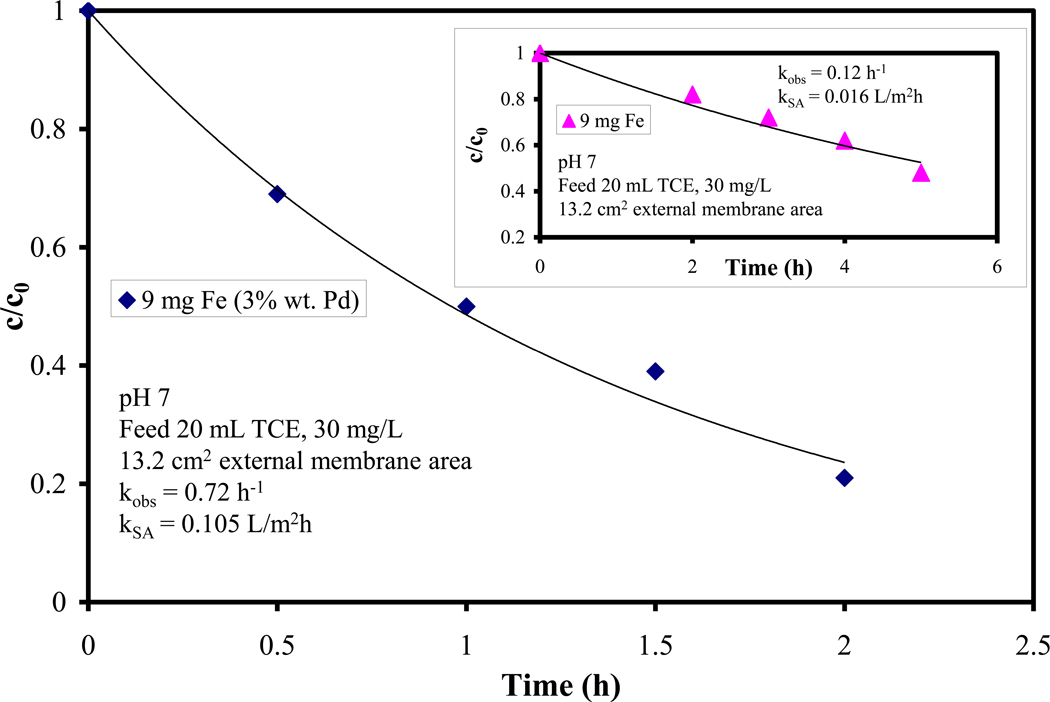
The dechlorination performance for three membranes containing immobilized Fe nanoparticles is shown in Figure 4. Some points were taken in duplicate, these were separate experiments (another cycle) showing a good reproducibility and stability (no nanoparticle deactivation observed). As expected, the rate of degradation increases linearly with increasing Fe loading. The surface normalized rate constant kSA can be written as: dC/dt = kobs C = kSA aS ρm C, where “aS” is the specific surface area of the nanoparticles (m2/g), ρm is the nanoparticle loading (g/L). The specific surface area was determined to be 25 m2/g, assuming discreet, spherical nanoparticles (30 nm diameter, as observed by SEM imaging). For three different loadings kSA was approximately constant, the average value was 0.005 L/m2h and the standard deviation ~ 5%.
Figure 4.
TCE dechlorination using Fe NP immobilized on PAA/PVDF membrane, using tea polyphenols as a reducing agent
To ensure that TCE concentration decrease is due to reaction and not other processes such as evaporation (TCE is volatile) or physical adsorption on the membrane, control experiments were conducted. The same feed of TCE (30 mg/L 0.02 L) in the presence of tea extract (20 g/L) was shaken in a gas tight vial without the membrane; TCE samples were taken after 5, 10, and 24 h, respectively. The concentration of TCE was within 5% of the original value, showing the evaporation does not play a significant role in TCE concentration decrease observed in dechlorination experiments. Secondly, the whole membrane was extracted in pentane and analyzed by GC-MS; no TCE was detected. Therefore, it can be assumed that TCE concentration decrease observed during dechlorination studies is entirely due to reaction with Fe nanoparticles. In addition, dechlorination experiments were conducted with “real” water taken from a contaminated site in Paducah KY; this water contains among others (82 mg/L alkalinity, 293 mg/L total dissolved solids and 1.2 mg/L turbidity), a more detailed description of the water quality can be found in literature [47]. For the same feed concentration of TCE (30 mg/L), the discrepancy between the dechlorination results in “real” and DIUF water was within 5%. This shows a minimal impact of the matrix present in the contaminated site on dechlorination, and that our systems can be applied in the “real world”.
3.2.2 Fe/Pd Nanoparticles and Reaction rate evaluations
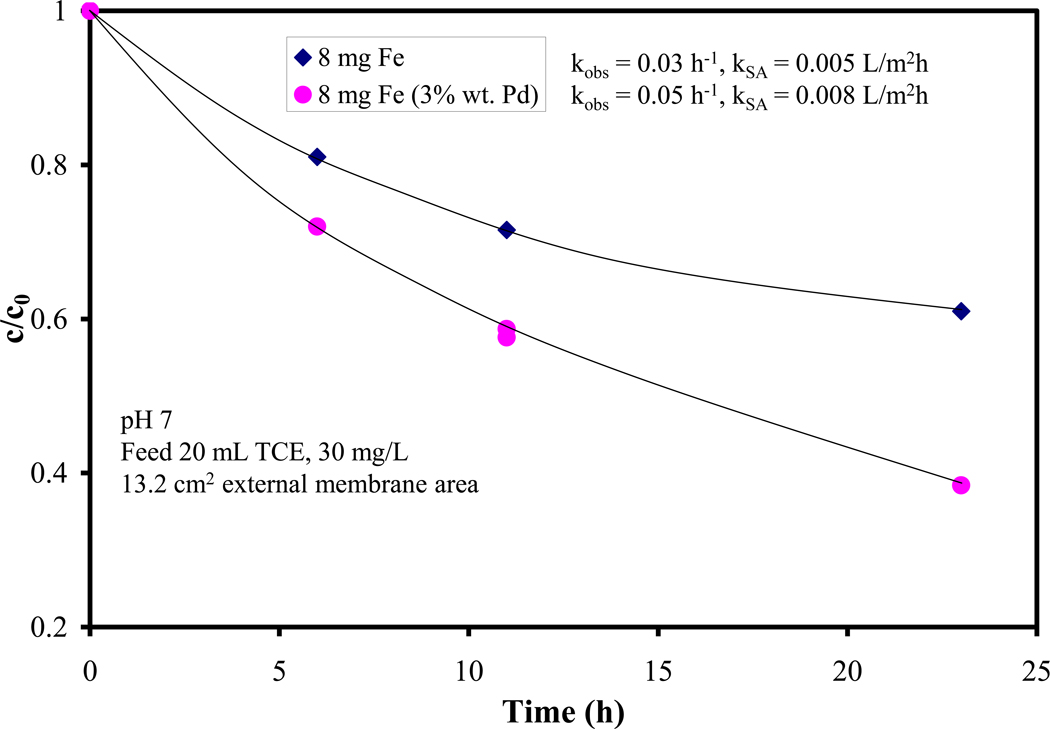
It is well known that the addition of a secondary metal increases the dechlorination performance as shown in Figure 5. A PVDF/PAA membrane containing 8 mg Fe nanoparticles was used in dechlorination studies then it was post-coated with Pd (3 wt%. as of Fe) and the dechlorination experiment was repeated. The addition of a secondary metal led to almost a two-fold increase in kSA value, from 0.005 to 0.008 L/m2h. It is known from literature [14,15] that dechlorination performances by Fe and Fe/Pd nanoparticles (reduced by borohydride) in homogeneous phase, there is a more significant increase for the kSA of the two systems, at least 1 order of magnitude. However, since there is no data available for supported Fe and Fe/Pd nanoparticles on PVDF/PAA membranes, these were synthesized (for comparison purposes) using sodium borohydride as a reducing agent. The dechlorination studies indeed showed a much higher discrepancy (one order of magnitude) in kSA value for the Fe vs Fe/Pd systems (Figure 6). Due to the fact that the tea extract acts as both a reducing and a capping agent, not all of the Fe NP surface becomes available for Pd deposition using post-coating. Consequently, Pd has a much smaller effect on dechlorination by Fe/Pd vs Fe nanoparticles, when reduced by the tea extract as opposed to sodium borohydride.
Figure 5.
TCE dechlorination using Fe and Fe/Pd NP immobilized on PAA/PVDF membrane, using tea polyphenols as a reducing agent
Figure 6.
TCE dechlorination using Fe and Fe/Pd NP immobilized on PAA/PVDF membrane, using NaBH4 as a reducing agent
However, a direct comparison between kSA values for borohydride- and polyphenol-reduced NPs may not be appropriate. In the batch mode operation, kSA is an apparent surface normalized rate constant, not the intrinsic one, and involves both mass transfer and reaction on the catalyst surface. In the case of polyphenol-reduced NPs based on Figure 4 and 5, the reaction is the rate limiting step and not the mass transfer. NP immobilized membranes (which can be cut in small pieces and injected) are more suitable for direct underground applications where lower rates (0.005 to 0.01 L/m2h are acceptable because of the availability of longer contact times. However, an improvement in rate still could be achieved by increasing the loading of Pd, as well as minimizing the amount (and layer thickness) of capping agent on NPs surface, by washing multiple times with the appropriate solvent. These could be a tradeoff between achieving faster rates and still maintaining the NPs longevity. For the highly reactive borohydride-reduced NPs, mass transfer limitations may occur and thus a convective flow experiment was conducted to establish intrinsic reaction rates.
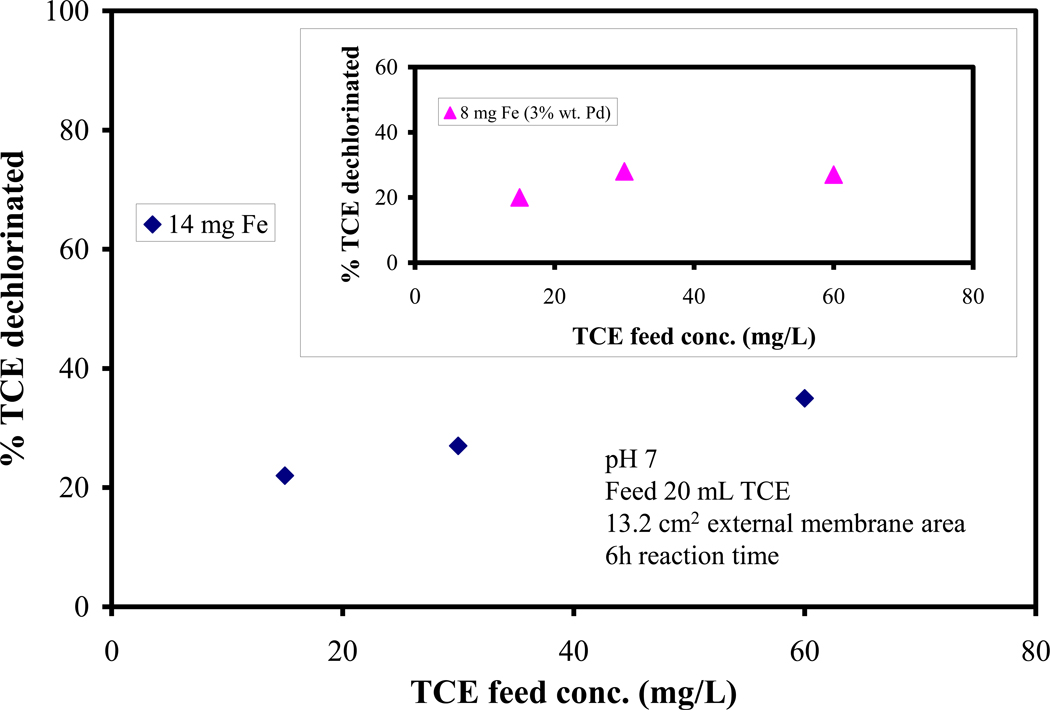
The kinetic parameters were calculated assuming a pseudo first order kinetics (i.e. the rate of dechlorination depends only on the metal loading). To prove the validity of this assumption, it was important to determine the effect of the feed TCE concentration on dechlorination rates. Figure 7 shows a plot of the TCE conversion on Fe and Fe/Pd nanoparticles (for 6 h reaction time) at different feed TCE concentrations. The conversion does not change much (< 20% change) over the feed concentration examined, showing that pseudo first order assumption can be used for kinetic data processing.
Figure 7.
The effect of feed concentration on TCE dechlorination using Fe NP (reduced by tea extract) immobilized on PAA/PVDF membrane
3.2.3 Use of Convective flow (residence time variations)
Another important aspect of the membrane-supported nanoparticles is its ability to be deployed under convective flow conditions. This was tested for one of the membranes, Fe/Pd (11.2 mg Fe, 3 wt% Pd as Fe) reduced with borohydride. For this experiment, the membrane was mounted in a filtration cell and a solution of TCE (30 mg/L) was passed through. Under convective conditions, 27% TCE dechlorination was attained for 1.1 min residence time in the membrane (flux of 1.6 × 10−4 cm3/cm2s at 11.7 bar). The residence time was calculated as τ = V/(AJv), where V is the membrane volume, A is the external area (13.2 cm2), and Jv is the permeation flux (cm3/cm2/s). In addition, V = εAL, where ε is the porosity (70% on average, from manufacturer’s data) and L is the membrane thickness (125 µm). Using this residence time, the rate constant, kobs and kSA was determined. Assuming CSTR the values were 19.8 h−1 (kobs) and 1.32 L/m2h (kSA), respectively. Thus, the kSA value for the Fe/Pd nanoparticles changed by one order of magnitude (from 0.105 to 1.32 L/m2h), when operated in batch vs convective mode. This shows that there are indeed diffusive limitations in batch mode for uncoated brohydride reduced NPs.
3.2.4 Nanoparticle longevity and Fe recapture
As mentioned previously, major benefit of using tea extract (epicatechin) type capping agents is to minimize Fe oxidation and to maintain particles in non-agglomerated form without loss of activity. It is well studied the aging of the Fe nanoparticles (reduced with borohydride) occurs not only due to dechlorination, but due to the presence of common groundwater dissolved constituents, including oxygen [48]. Figure 8 shows photos of the four membranes (Fe and Fe/Pd reduced by sodium borohydride and tea extract) after TCE dechlorination. Initially, all four membranes were black (not shown here). It can be observed that the Fe membrane reduced by borohydride showed the most pronounced oxidation (orange color). This is followed by Fe/Pd membrane (also reduced by borohydride), showing dark brown spots. On the other hand, both Fe and Fe/Pd membranes retained the black color even after much longer reaction times (23h vs 2 or 5) and multiple cycles. Moreover, to study the long term stability, one membrane (8 mg Fe reduced with tea extract) was stored in water and tested periodically over a period of 3 months and it showed dechlorination rates similar (within 15%) with the initial value. This trend is shown in Figure 9. Moreover, for the case of borohydride reduced Fe nanoparticles, the activity decreases to less than 20 % of the initial value after only 4 cycles.
Figure 8.
NP-immobilized on PAA/PVDF membranes after TCE dechlorination a) Fe and b) Fe/Pd (reduced by NaBH4), c) Fe and d) Fe/Pd (reduced by tea extract)
Figure 9.
Normalized TCE dechlorination (vs 1st cycle) for Fe NP-immobilized on PAA/PVDF membranes, reduced by NaBH4 and tea extract
It is important to mention that Fe0 (the reactant) oxidizes to Fe2+/ Fe3+ during dechlorination, and it is recaptured by the membrane-bound carboxylic acid groups on the membrane. Experimental verification by Fe analysis indeed showed no loss. This can be potentially regenerated back to the metal form by reducing agents. In contrast to homogeneous phase nanoparticles applications, this approach provides no loss of NPs and soluble iron in the solution phase.
4. Conclusions
The current study reports for the first time the synthesis of Fe and bimetallic Fe/Pd nanoparticles on a membrane support, using a “green” reducing agent - tea extract, which acts as both reducing and capping agent. The latter ensures a high nanoparticle longevity and resistance to oxidation. We have successfully demonstrated a membrane-based approach for the reductive destruction of a model toxic chlorinated organic, a common pollutant, TCE. The dechlorination can be performed in convective mode, using pump and treat approach or in batch mode, when membrane-immobilized NP’s can be injected underground. For all cases, the surface area normalized reaction rates were quantified.
Research Highlights.
Provides a new approach in terms of green (use of green tea extract) nanoparticle synthesis and enhancement of stability/longevity
In-situ, green synthesis of about 30 nm metal nanoparticles in PAA functionalized microfiltration membranes for degradation of pollutants
Successfully demonstrated membrane-based approach for the reductive degradation of a model toxic chlorinated organic, a common pollutant, trichloroethylene (TCE) from water
Acknowledgement
This work was supported by NIEHS-SRP, NIEHS-SRP Supplement grant and by DOE-KRCEE.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liu YQ, Majetich SA, Tilton RD, Sholl DS, Lowry GV. TCE dechlorination rates, pathways, and efficiency of nanoscale iron particles with different properties. Environ. Sci. Technol. 2005;39:1338–1345. doi: 10.1021/es049195r. [DOI] [PubMed] [Google Scholar]
- 2.Liu YQ, Lowry GV. Effect of particle age (Fe-o content) and solution pH on NZVI reactivity: H-2 evolution and TCE dechlorination. Environ. Sci. Technol. 2006;40:6085–6090. doi: 10.1021/es060685o. [DOI] [PubMed] [Google Scholar]
- 3.Thompson JM, Chisholm BJ, Bezbaruah AN. Reductive dechlorination of chloroacetanilide herbicide (Alachlor) using zero-valent iron nanoparticles. Environ. Eng. Sci. 2010;27:227–232. [Google Scholar]
- 4.Zhang WX, Wang CB, Lien HL. Treatment of chlorinated organic contaminants with nanoscale bimetallic particles. Catal. Today. 1998;40:387–395. [Google Scholar]
- 5.Xu Y, Zhang WX. Subcolloidal Fe/Ag particles for reductive dehalogenation of chlorinated benzenes. Ind. Eng. Chem. Res. 2000;39:2238–2244. [Google Scholar]
- 6.Zhu N, Luan H, Yuan S, Chen J, Wu X, Wang L. Effective dechlorination of HCB by nanoscale Cu/Fe particles. J. Hazard. Mater. 2010;176:1101–1105. doi: 10.1016/j.jhazmat.2009.11.092. [DOI] [PubMed] [Google Scholar]
- 7.Tee YH, Grulke E, Bhattacharyya D. Role of Ni/Fe nanoparticle composition on the degradation of trichloroethylene from water. Ind. Eng. Chem. Res. 2005;44:7062–7070. [Google Scholar]
- 8.Tee YH, Bachas LG, Bhattacharyya D. Degradation of trichloroethylene and dichlorobiphenyls by iron-based bimetallic nanoparticles. J. Phys. Chem. C. 2009;113:9454–9464. doi: 10.1021/jp809098z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lien HL, Zhang WN. Nanoscale Pd/Fe bimetallic particles: Catalytic effects of palladium on hydrodechlorination. Appl. Cat. B-Environmental. 2007;77:110–116. [Google Scholar]
- 10.Zhu BW, Lim TT. Catalytic reduction of chlorobenzenes with Pd/Fe nanoparticles: reactive sites, catalyst stability, particle aging, and regeneration. Environ. Sci. Technol. 2007;41:7523–7529. doi: 10.1021/es0712625. [DOI] [PubMed] [Google Scholar]
- 11.Yan W, Herzing AA, Li XQ, Kiely CJ, Zhang WX. Structural evolution of Pd-doped nanoscale zero-valent iron (nZVI) in aqueous media and implications for particle aging and reactivity. Environ. Sci. Technol. 2010;44:4288–4294. doi: 10.1021/es100051q. [DOI] [PubMed] [Google Scholar]
- 12.Nagpal V, Bokare AD, Rajeev CC, Rhode CV, Paknikar KM. Reductive dechlorination of γ-hexachlorocyclohexane using Fe-Pd bimetallic nanoparticles. J. Hazard. Mater. 2010;175:680–687. doi: 10.1016/j.jhazmat.2009.10.063. [DOI] [PubMed] [Google Scholar]
- 13.Lu AH, Salabas EL, Schuth F. Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007;46:1222–1244. doi: 10.1002/anie.200602866. [DOI] [PubMed] [Google Scholar]
- 14.He F, Zhao D, Liu J, Roberts CB. Stabilization of Fe-Pd nanoparticles with sodium carboxymethyl cellulose for enhanced transport and dechlorination of trichloroethylene in soil and groundwater. Ind. Eng. Chem. Res. 2007;46:29–34. [Google Scholar]
- 15.He F, Zhao D. Hydrodechlorination of trichloroethene using stabilized Fe-Pd nanoparticles: Reaction mechanism and effects of stabilizers, catalysts and reaction conditions. Appl. Cat. B-Environmental. 2008;84:533–540. [Google Scholar]
- 16.Kanel SR, Goswami RR, Clement TP, Barnett MO, Zhao D. Two dimensional transport characteristics of surface stabilized zero-valent iron nanoparticles in porous media. Environ. Sci. Technol. 2008;42:896–900. doi: 10.1021/es071774j. [DOI] [PubMed] [Google Scholar]
- 17.Leng Y, Sato K, Li JG, Ishigaki T, Iijima M, Kamiya H, Yoshida T. Iron nanoparticles dispersible in both ethanol and water for direct silica coating. Powder Technol. 2009;196:80–84. [Google Scholar]
- 18.Zhu BW, Lim TT, Feng J. Influences of amphiphiles on dechlorination of a trichlorobenzene by nanoscale Pd/Fe: adsorption, reaction kinetics, and interfacial interactions. Environ. Sci. Technol. 2008;42:4513–4519. doi: 10.1021/es800227r. [DOI] [PubMed] [Google Scholar]
- 19.Wang W, Zhou M, Jin Z, Li T. Reactivity characteristics of poly(methyl methacrylate) coated nanoscale iron particles for trichloroethylene remediation. J. Hazard. Mater. 2010;173:724–730. doi: 10.1016/j.jhazmat.2009.08.145. [DOI] [PubMed] [Google Scholar]
- 20.Choi H, Agarwal S, Al-Abed SR. Adsorption and simultaneous dechlorination of PCBs on GAC/Fe/Pd: mechanistics aspects and reactive capping barrier concept. Environ. Sci. Technol. 2009;43:488–493. doi: 10.1021/es8015815. [DOI] [PubMed] [Google Scholar]
- 21.Kim H, Hong HJ, Jung J, Kim SH, Yang JW. Degradation of trichloroethylene (TCE) by nanoscale zero-valent iron (nZVI) immobilized on alginate bed. J. Hazard. Mater. 2010;176:1038–1043. doi: 10.1016/j.jhazmat.2009.11.145. [DOI] [PubMed] [Google Scholar]
- 22.Zheng T, Zhan J, He J, Day C, Lu Y, McPherson GL, Piringer G, John VT. Reactivity characteristics of nanoscale zerovalent iron-silica composites for trichloroethylene remediation. Environ. Sci. Technol. 2008;42:4494–4499. doi: 10.1021/es702214x. [DOI] [PubMed] [Google Scholar]
- 23.Huang Q, Shi X, Pinto RA, Petersen EJ, Weber J. Tunable synthesis and immobilization of zero-valent iron nanoparticles for environmental applications. Environ. Sci. Technol. 2008;42:8884–8889. doi: 10.1021/es8015588. [DOI] [PubMed] [Google Scholar]
- 24.Meyer DE, Wood K, Bachas LG, Bhattacharyya D. Degradation of chlorinated organics by membrane-immobilized nanosized metals. Environ. Progress. 2004;23:232–242. [Google Scholar]
- 25.Wu L, Ritchie SMC. Enhanced dechlorination of trichloroethylene by membrane-supported Pd-coated iron nanoparticles. Environ. Progress. 2008;27:218–224. [Google Scholar]
- 26.Xu J, Bhattacharyya D. Fe/Pd Nanoparticle immobilization in microfiltration membrane pores: Synthesis, characterization, and application in the dechlorination of polychlorinated biphenyls. Ind. Eng. Chem. Res. 2007;46:2348–2359. [Google Scholar]
- 27.Xu J, Bhattacharyya D. Modeling of Fe/Pd nanoparticle-based functionalized membrane reactor for PCB dechlorination at room temperature. J. Phys. Chem. C. 2008;112:9133–9144. doi: 10.1021/jp7097262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim H, Hong HJ, Lee YJ, Shin HJ, Yang JW. Degradation of trichloroethylene by zero-valent iron immobilized in cationic exchange membrane. Desalination. 2008;223:212–220. [Google Scholar]
- 29.Wang X, Chen C, Liu H, Ma J. Preparation and characterization of PAA/PVDF membrane-immobilized Pd/Fe nanoparticles for dechlorination of trichloroacetic acid. Water Res. 2008;42:4656–4664. doi: 10.1016/j.watres.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Lewis S, Smuleac V, Montague A, Bachas LG, Bhattacharyya D. Iron-functionalized membranes for nanoparticle synthesis and reactions. Sep. Sci. Technol. 2009;44:3289–3311. doi: 10.1080/01496390903212805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smuleac V, Bachas LG, Bhattacharyya D. Aqueous-phase synthesis of PAA in PVDF membrane pores for nanoparticle synthesis and dichlorobiphenyl degradation. J. Membr. Sci. 2010;346:310–317. doi: 10.1016/j.memsci.2009.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nadagouda MN, Hoag G, Collins J, Varma RS. Green synthesis of Au nanostructures at room temperature using biodegradable plant surfactants. Cryst. Growth Des. 2009;9:4979–4983. [Google Scholar]
- 33.Liu Z, Zhang F-S. Nano-zerovalent iron contained porous carbon developed from waste biomass for the adsorption and dechlorination of PCBs. Biores. Technol. 2010;101:2562–2564. doi: 10.1016/j.biortech.2009.11.074. [DOI] [PubMed] [Google Scholar]
- 34.Nadagouda MN, Varma RS. A greener synthesis of core (Fe, Cu)-shell (Au, Pt, Pd, and Ag) nanocrystals using aqueous vitamin C. Cryst. Growth Des. 2007;7:2582–2587. [Google Scholar]
- 35.He F, Liu J, Roberts CB, Zhao D. One-step “green” synthesis of Pd nanoparticles of controlled size and their catalytic activity for trichloroethene hydrodechlorination. Ind. Eng. Chem. Res. 2009;48:6550–6557. [Google Scholar]
- 36.Baruwati B, Polshettiwar V, Varma RS. Glutathion promoted expeditious green synthesis of silver nanoparticles in water using microwaves. Green Chem. 2009;11:926–930. [Google Scholar]
- 37.Baruwati B, Varma RS. High value products from waste: grape pomace extract - a three-in-one package for the synthesis of metal nanoparticles. ChemSusChem. 2009;2:1041–1044. doi: 10.1002/cssc.200900220. [DOI] [PubMed] [Google Scholar]
- 38.Li L, Ding J, Xue J. A facile green approach for synthesizing monodisperse magnetite nanoparticles. J. Mater. Res. 2010;25:810–813. [Google Scholar]
- 39.Nadagouda MN, Varma RS. Green synthesis of silver and palladium nanoparticles at room temperature using coffee and tea extract. Green Chem. 2008;10:859–862. [Google Scholar]
- 40.Nadagouda MN, Castle AB, Murdock RC, Hussain SM, Varma RS. In vitro biocompatibility of nanoscale zerovalent iron particles (NZVI) synthesized using tea polyphenols. Green Chem. 2010;12:114–122. [Google Scholar]
- 41.Hoag GE, Collins JB, Holcomb JL, Hoag JR, Nadagouda MN, Varma RS. Degradation of bromothymol blue by ‘greener’ nano-scale zero-valent iron synthesized using tea polyphenols. J. Mater. Chem. 2009;19:8671–8677. [Google Scholar]
- 42.Huang JL, Li QB, Sun DH, Lu YH, Su YB, Yang X, Wang HX, Wang YP, Shao WY, He N, Hong JQ, Chen CX. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotech. 2007;18:1–11. [Google Scholar]
- 43.Yang X, Li Q, Wang H, Huang J, Lin L, Wang W, Sun D, Su Y, Opiyo JB, Hong L, Wang Y, He N, Jia L. Green synthesis of palladium nanoparticles using broth of Cinnamomum camphora leaf. J. Nanopart. Res. 2010;12:1589–1598. [Google Scholar]
- 44.Polshettiwar V, Baruwati B, Varma RS. Self-assembly of metal oxides into three-dimensional nanostructures: synthesis and application in catalysis. ACSNano. 2009;3:728–736. doi: 10.1021/nn800903p. [DOI] [PubMed] [Google Scholar]
- 45.Xu J, Dozier A, Bhattacharyya D. Synthesis of nanoscale bimetallic particles in polyelectrolyte membrane matrix for reductive transformation of halogenated organic compounds. J. Nanopart. Res. 2005;7:449–467. [Google Scholar]
- 46.Gabriel EM, Gillberg GE. In situ modification of microporous membranes. J. Appl. Pol. Sci. 2003;48:2081–2090. [Google Scholar]
- 47.Meyer DE, Hampson S, Ormsbee L, Bhattacharyya D. A study of groundwater matrix effects for the destruction of trichloroethylene using Fe/Pd nanoaggregates. Environ. Progress. 2009;28:507–518. doi: 10.1002/ep.10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reinsch BC, Forsberg B, Penn RL, Kim CS, Lowry GV. Chemical transformations during aging of zerovalent iron nanoparticles in the presence of common groundwater dissolved constituents. Environ. Sci. Technol. 2010;44:3455–3461. doi: 10.1021/es902924h. [DOI] [PubMed] [Google Scholar]