Abstract
Modulation of single-cell responses by compound stimuli (target plus flankers) extending outside the cell’s receptive field (RF) may represent an early neural mechanism for encoding objects in visual space, enhancing their perceptual saliency. The spatial extent of contextual modulation is wide. The size of the RF is known to be dynamically variable. It has been suggested that RF expansion when target contrast decreases is the real cause of effects attributed to modulation by flankers. This is not the case. We directly compared, in the same cells, the extent of RF size changes when stimulus contrast decreased with that revealed by systematically changing the target-and-collinear-flankers separation. We found that RF expansion at low contrast was not universal, and that the spatial extent of RF expansion, when it existed, was smaller than that of collinear flanker modulation. We conclude that the two processes in striate cortex work independently from each other.
Electronic supplementary material
The online version of this article (doi:10.1007/s00221-009-2057-1) contains supplementary material, which is available to authorized users.
Keywords: Spatial integration, Collinear facilitation, Contrast dependency, Receptive field size dynamics, Elliptic Gabor, Cat striate cortex
Introduction
Neuronal responses elicited by a pattern stimulus presented in the RF (target) are often modified by stimuli concurrently presented outside the RF (flankers). Response modulation by flankers can be either facilitative or suppressive, depending on various factors including the orientation difference between the target and flankers (Blakemore and Tobin 1972; Maffei and Fiorentini 1976; Nelson and Frost 1985; Li and Li 1994; Kapadia et al. 1995; Sillito et al. 1995; Levitt and Lund 1997; Polat et al. 1998) and differences in luminance contrast inside and outside the RF (Levitt and Lund 1997; Polat et al. 1998; Sengpiel et al. 1998; Mizobe et al. 2001; Chen et al. 2001; Sadakane et al. 2006). Collinear facilitation is important for contextural integratation of objects in space; this has been shown in physiology (Kapadia et al. 1995; Polat et al. 1998; Chisum et al. 2003) and in human psychophysics (Polat and Sagi 1993; Kapadia et al. 1995).
Recent findings suggest that the RF center is not static, but dynamically expands in size when contrast of target stimulus decreases: the RF size at low target contrast is as much as thrice that at high contrast (Kapadia et al. 1999; Sceniak et al. 1999; Cavanaugh et al. 2002a, b). One might ask whether the expansion could account for collinear facilitation by flankers. When target contrast decreases, might the flankers fall inside the excitatory center of the expanded RF, and thus directly generate additional response? The answer is no, since the collinear, high-contrast flankers we used, by themselves, never elicit a response de novo in the absence of a target (Polat et al. 1998; Mizobe et al. 2001; Chen et al. 2001). The flanker effects, if present, can only be modulatory. A similar sentiment is argued by other authors in a recent study with different experimental design (Ichida et al. 2007).
The expansion of RF size at low stimulus contrast may be explained by models of RF dynamics based on network-driven, primarily excitatory mechanisms (Sceniak et al. 1999; Cavanaugh et al. 2002a) or on a recurrent network model (Angelucci and Bressloff 2006). In the majority of striate cortical cells we studied, collinear facilitation was seen at low target contrast when flankers were in or beyond the nominal suppressive region (Chen et al. 2001; the present study). In almost half of these cells, the strength of collinear facilitation was seen to continually increase with increasing target contrast. According to the aforementioned models of RF dynamics, for these cells, the RF center size should be shrinking, putting high-contrast flankers, if anything, farther away from the excitatory center. This observation further supports the position that collinear facilitation is not a consequence of the RF expansion at low contrast. Indeed, low-contrast expansion of the RF is not an universal phenomenon: an important result of the present study is the observation of instances of low-contrast contraction of the RF.
In this study we examined, in the same cells, the sign and extent of RF size changes at low contrast and the effects of RF response modulation by collinear flankers in the periphery. We found: (1) collinear facilitation outside the RF at low target contrast, (2) both expansion and contraction along the collinearity axis of the RF at low contrast, and (3) persistence over distance of response modulation by collinear flankers.
Materials and methods
Animal preparation and recording
Adult cats were used for single-cell recording from primary visual cortex. Cats were anesthetized with isoflurane (<0.8%) mixed with an N2O:O2 mixture (70:30), supplemented by continuous i.v.-infusion of Na-pentothal [0.5–4 mg/(kg h)], and paralyzed with pancuronium bromide [0.2–0.4 mg/(kg h), iv] under artificial respiration. The infusion solution was isotonic, sterile 5% lactated Ringer’s solution delivered through the radial vein at a rate that depended on the animal’s body weight (3.3–4.7 ml/h). Prophylactic doses of antibiotic (Ancef ~30 mg/kg iv) were given 30 min before the start of preparatory surgery and thereafter twice daily (im) in 3-day recording sessions. The following vital signs were monitored continuously: heart rate, respiration rate, electrocardiogram, rectal temperature (37.5°C), electroencephalograms, end-tidal CO2 level in the exhalant gas (PCO2, 3–6%), blood oxygen saturation level, and the shape of capnograms. The last three were obtained using the Cosmo capnograph/pulse oximeter (Novametrix Med Syst Inc).
The animal’s nictitating membranes were retracted with 10% neo-synephrine and the pupils were dilated with 1% atropine-sulfate eye drops. An artificial pupil (diameter 5 mm) was placed in front of the eye, which was covered with a contact lens having no power. The animal was monocularly refracted with trial lenses to a viewing distance of 40 cm. We used tungsten-in-glass microelectrodes (Levick 1972) with an exposed tip of 13–16 μm (impedance, ~4 MΩ at 1KHz) placed near the projection site of central vision (P 2–4 mm; L ~1.5 mm). Single-cell activity was fed to an AC preamplifier (Grass P15). Recorded cells had RFs within 10 deg eccentricity. The experimental protocol was approved by the Institutional Animal Care and Use Committee at the host institute, conforming to the principles regarding the care and use of animals laid down in the NIH Guidelines, and adopted by the Society for Neuroscience.
RF search
After initially estimating the orientation range and optimal orientation of the cell under study, based on the minimal response field (Barlow et al. 1967) plotted by a moving hand-held light slit, we more precisely determined orientation tuning by stimulating the cell with high-contrast, square-wave gratings that were drifting over the RF at ~2 Hz. The drifting gratings covered the entire field of the monitor screen (30 × 40 cm). Response measure (spikes per second) was derived from the total number of spikes accumulated in post-stimulus time histograms over the recording period of 10 s/trial. Orientations covering a range surrounding the manually determined optimal orientation were displayed, and the one yielding maximum response was taken as an improved estimate. Next, using thus-determined orientation, a range of spatial frequencies was displayed, and again, the one producing the maximum response was taken as the operative estimate. Stimulus presentations and data collection were done using a custom software package on a Macintosh G4 computer.
It was imperative to be sure of the stability of RF’s location and size. Therefore, we ran RF mapping automatically, using a homing scheme involving patch subdivision and M-sequence stimulation (see Suppl Fig. 1). An M-sequence is a pseudorandom, binary sequence and its strength as applied to vision research has been well established clinically in multifocal electroretinogram recording from the human eye (e.g., Sutter 1992) and some application to cortical physiology in an animal model (Kitano et al. 1994; Kasamatsu et al. 1998, 2005). The initial patches used were squares showing drifting square-wave gratings. These were adequate to locate the RF center. Final measures of RF sizes were obtained using Gabor patches (2D sinusoidal luminance gratings embedded in circular Gaussian-weighted envelopes).
Size-tuning test: standard and along the collinearity axis
A square patch of drifting, high-contrast square-wave grating was superimposed on the RF center determined by the M-sequence stimulation scheme described above (see the legend of Suppl Fig. 1). The patch size was then changed over a wide range, in a size-increasing manner or randomly (standard size tuning). In several cells, tests run in the two manners yielded comparable results. Typically six discrete sizes were tested, the smallest being the one estimated from the hand-plotted RF. The number of measurement repetitions was 3–10, the choice primarily depending on the cell’s firing rate.
A unique feature we included here is size tuning tests carried out along the collinearity axis. The size of an elliptical Gabor patch centered on the RF was systematically increased in six steps with different aspect ratios (from 1:1 to 1:5) along the collinearity axis. Contrast of these elongated targets (ETs) was set at three levels: low (10 or 20%), intermediate (30 or 50%) and high (80 or 100%). All combinations of sizes and contrasts were tested again in blocks. Within each block contrast is fixed while the size of stimulus was randomized. High contrast was always tested first.
Experimental design
The current study comprised two parts: (1) Preliminary—standard size tuning with a size-varying, square patch of drifting, high-contrast square-wave grating and (2) Main—contrast- and configuration-dependent dynamics of RFs using appropriately fitted Gabor patches or elliptical analogs (see below). In both parts, orientations and spatial frequencies of gratings were chosen to match the preferred values of the cell’s RF, obtained previously as explained above. The mean grating luminance was 45 cd/m2 and background luminance was kept at a mesopic level (~1 cd/m2). Contrast response curves were obtained by successively sweeping target Gabor contrast from low to high in 15 even steps. Contrast threshold of RF responses was determined by linear extraolation of the contrast response curve to the back ground noise level attained with stimulus of zero contrast. Laminar loci of recorded cells were reconstructed on histological sections following an established method in our laboratory (e.g., Mizobe et al. 2001). We collected data in the following order: (1) contrast sweep with the Gabor patch (target) alone; (2) contrast sweep with the target plus a pair of high-contrast flankers at three different distances; (3) contrast sweep with ETs having various aspect ratios; (4) ET size tuning at three levels of contrasts (low, intermediate and high); and (5) contrast sweep with ET and flankers at the tip of its extension. Not all recorded cells were necessarily studied with a complete battery of these tests. The stability of recording determined the total duration of recording time for each cell, which was usually shorter than 1 h. The cell shown in Fig. 1 lasted for nearly 3 h.
Fig. 1.
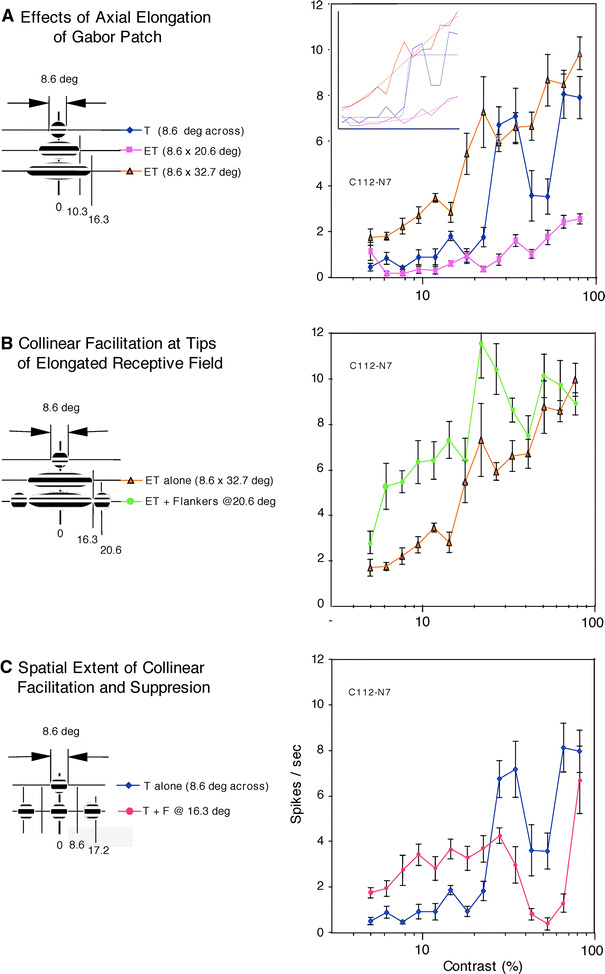
The spatial extent of collinear facilitation and suppression tested with the elongated target (ET) made of Gabor patches elongated along the collinearity axis and compound stimuli made of round Gabor patches (8.6 deg across). The data shown are from a cell (C112-N7) that was recorded from layer 5 in the medial bank. a Contrast response curves obtained by stimulation with ETs having different aspect ratios (1:1, 1:2.4, and 1:3.8). When the ratio was 1:2.4 (purple squares), the maximal extent of the elongated Gabor was 10.3 deg from the RF center, the contrast response curve showed a very shallow slope indicating the presence of strong suppression at moderate-to-high contrast. With greater elongation (aspect ratio 3.8, brown triangles), the Gabor patch reached 16.3 deg from the RF center, a distance corresponding to the site of the flankers’ center (c, red circles), we obtained strong facilitation at low-to-moderate contrast. The response magnitude in spikes per second was plotted against percent contrast values on a logarithmic scale. Error bars are ±1 SEM (standard error of means). These conventions apply here and in others. Inset sigmoid curve fit, shown in dotted lines, for each of the three contrast response curves in solid lines. b Facilitation evoked by concurrent presentation of high-contrast flankers with the maximally elongated Gabor patch (aspect ratio 3.8, green circles). Contrast response function is shifted to the left, indicating the presence of pure contrast gain control. c Contrast response curves obtained under different stimulus configurations, as schematically shown in the inset left of the curves. T is the target Gabor patch placed on the RF (blue diamonds). F is a pair of flankers placed at 16.3 deg from the center of the RF center. The properties of flanker Gabor patches were identical to those of the target, except that the flanker contrast was always fixed high while the target contrast ranged widely as shown on the abscissa of the diagram. When the cell was stimulated with the target plus collinear, high-contrast flankers, responses were enhanced at low-to-moderate target contrast and suppressed at high target contrast (red circles). The cell was thus classified as a type-I cell of Chen et al. (2001)
Results
Receptive field size, contrast threshold and surround suppression
We recorded from 106 single neurons in V1 of 7 cats. The average size of the minimal response field was 2.6 ± 1.4 deg × 2.2 ± 2.5 deg (mean ± SD) and these values were as expected (Walker et al. 2000; Cavanaugh et al. 2002a), smaller than the RF size determined by peaks or asymptotes in standard size-tuning curves (see definition above) for the same 106 neurons (4.4 ± 2.8 deg). The common distinction of cell types based on the spatial summation property inside the RF (simple vs. complex cells) was not formally carried out in the present study, since such a distinction has borne no suggestive relation to the sign or extent of lateral integrations (Mizobe et al. 2001).
In 75 of the 106 cells, physiological attributes such as contrast threshold, surround suppression, contrast-dependent dynamics of the RF size and distance effects on flanker modulation were quantitatively studied using raw data averaged over comparable measurements. Average contrast threshold was 20.3 ± 11.4% (see “Discussion”). In the majority of cells that showed a clear, single response peak, followed by a low plateau or leveling (N = 47), the minimal response registered in standard size-tuning curves after the peak was down to on average 42% of the peak response. Meanwhile in 20 cells that showed multiple minor peaks after the main peak, the cellular response went down to only 73% of the main peak. For the remaining eight cells, standard size tuning was not applicable because there was no ultimate peak within the display limitation of 13 deg top to bottom. Thus, in standard size-tuning tests, the majority of our cells showed strong or moderate surround suppression with increasing stimulus size around the RF, which corroborates the literature.
Facilitation outside the expanded RF
The common premise (see Introduction) is that the RF, surrounded by a suppressive region, expands at low contrast. To understand the nature of dynamic changes in excitatory RF center size, we made detailed examinations of physiological properties in the region immediately outside the presumably expanded RF. We used ETs having elliptical form with major axis lying along the cell’s preferred orientation rather than the round, size-varying targets that are used commonly. Sensitivity of detecting elongated Gabor patches was studied in humans, psychophysically (Polat and Tyler 1999; see also Foley et al. 2007; Meese and Hess 2007) and by visual evoked potential recording (Polat and Norcia 1998). The direct motivation for the choice of ETs is that the restriction of stimuli to the axial region (flanker collinearity) is important in eliciting facilitative response modulation (see Fig. 10, Mizobe et al. 2001). Stimulating with large round patches (that include off-axis portions) tends to eliminate facilitation, as does on-axis placement of small, round, orthogonally oriented flankers (Polat et al. 1998; Mizobe et al. 2001). In some tests, discrete flankers were placed immediately adjacent to the ET along the major axis. The flankers’ diameter was equal to the target’s minor diameter, and their contrast was set high. The flankers’ orientation was either collinear or orthogonal to that of the target.
Data for a layer-5 cell are shown in Fig. 1. We first generated a baseline contrast response curve (Fig. 1a, blue diamonds). Next, we stimulated with an ET having an aspect ratio of 2.4 (8.6 × 20.6 deg). This stimulus resulted in strong suppression of the cell’s responses at moderate-to-high contrast (Fig. 1a, purple squares). However, facilitation took over, especially at low-to-moderate contrast, when the ET’s length further increased to 32.7 deg with aspect ratio of 3.8 (Fig. 1a, brown triangles). It is likely that stimulation with a more elongated ET activated a facilitative zone outside a suppressive one in the RF surround.
Next, we wanted to directly elucidate the nature of the region immediately outside the ETs tested above. We placed a pair of high-contrast Gabor patches (8.6 × 8.6 deg) adjacent to the longitudinal edges of the ET, centering on sites 20.6 deg from the RF center (Fig. 1b inset). The contrast response curve obtained under this stimulus configuration (ET plus flankers) is further shifted toward the left, away from the control curve obtained without the flankers (Fig. 1b, green circles vs. brown triangles), indicating facilitation—especially at low-to-moderate contrast—by flankers placed at 20.6 deg from the RF center. To interpret this finding as a result of activation of an expanded part of the RF center at low contrast, we would have to accept that the RF center of this cell is now as large as 49.7 deg across along the collinear axis, six times diameter obtained at high contrast. No such gigantic RFs of single cells have been reported in cat striate cortex, even in the infragranular laminae, in which one tends to encounter cells with large RFs.
For the same cell, Fig. 1c shows two contrast response curves that correspond to a set of configurations of stimulus arrangements organized along the collinearity axis as shown in Fig. 1c inset: (1) target Gabor (8.6 deg across) alone (blue diamonds); and (2) target plus a pair of high-contrast flankers (each 8.6 deg across), centered 16.3 deg from the RF center, providing a substantial gap between target and flanker (red circles). When collinear flankers were concurrently presented with the target (red circles), we obtained relatively strong facilitation at low-to-moderate contrast (20% or lower). For the same set of stimulus configurations, at high contrast, the cell tended to show weaker response than that of the target alone. This cellular behavior suggests that of type-I cells of Chen et al. (2001)—facilitation at low target contrast and suppression at high target contrast.
Taken together, the above findings indicate that the response modulation affected by collinear flankers is a neural mechanism underlying gain control (Chen et al. 2001). Such gain control may be most likely independent of mechanisms supporting contrast-dependent changes in RF size, since the spatial extent of collinear flanker modulation seems to be much larger than that of contrast-dependent dynamics of RF size.
RF expansion and contraction
Next, we directly investigated the apparent changes in the RF size at low stimulus contrast. Using ETs in place of standard Gabor patches, we carried out a new series of size-tuning tests. The length of a collinearly elongated Gabor patch centered on the RF was systematically increased. Contrast of the ETs was set at three levels: low (10, 20%), moderate (30, 50%) and high (80, 100%).
Confirming the earlier reports (Cavanaugh et al. 2002a, b), we found some cells in which RFs defined by the response peak using ETs showed clear expansion at low contrast. In Fig. 2a, at 100% contrast (green diamonds) the cell’s responses to ETs peaked or asymptoted around ~10 deg in length along the collinear axis. But, when stimulated at 20% contrast (red circles) the response strength of this cell continuously increased upon increase in the major axis of an ET (maximal stretch of 25 deg). Thus, with ET stimulation, this cell shows RF expansion at low contrast, as it did with standard size-tuning stimulation. The cell had a moderate RF size (5.7 deg across) with relatively low contrast threshold (9.4%). In Fig. 2b, when flankers are added to the target (purple squares), there is a mostly upward shift of the contrast response curve in a wide range of contrasts (a few points at moderate values are excepted). At the target contrast of 20%, for example, the response magnitude increased several-fold by the concurrent presentation of high-contrast flankers 11 deg from the RF center (see Fig. 2b inset). This finding may, at first glance, support the notion that stronger responses obtained with collinear flankers were in effect caused by the high-contrast flankers falling inside the RF expanded at low contrast. But, this interpretation has basic problems, as we explain here. First, there is increased response when a high-contrast target is supplemented by high-contrast flankers that lie well beyond the peak in the ET-derived size-tuning curve, which is a clear expression of collinear facilitation (see Fig. 1b), and which is at odds with the expected high-contrast RF shrinking that would put the flankers in the suppressive surround. Second, for the largest ET we examined (37.1 deg), there is a large jump in the contrast response curve at low-to-moderate contrast. This is hard to reconcile with the general expectation that the contrast-dependent dynamic would vary smoothly in all its parameters. The large jump to near saturation level appears at ~10% contrast (Fig. 2b, yellow triangles) and is maintained for all higher contrasts. Yet, the size-tuning curves at low contrast (20%, Fig. 2a, red circles) show a smooth, shallow increase in spiking, more like what one would expect.
Fig. 2.
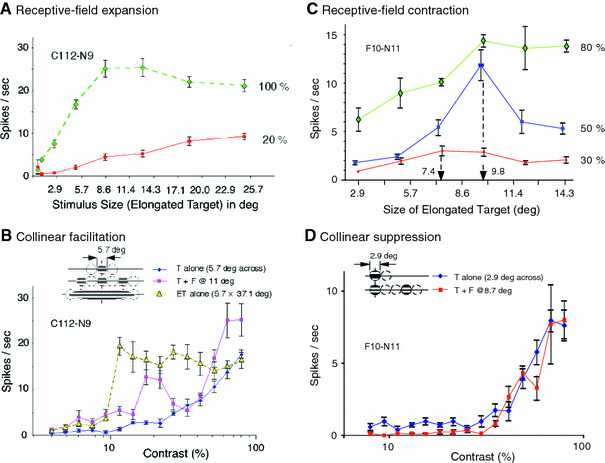
RF size changes with change in stimulus contrast. RF expansion and contraction are exemplified by two cells, C112-N9 and F10-N11. For each cell, in a preliminary test with high-contrast, square-wave, drifting gratings, the cell’s RF was characterized. We then fit a Gabor patch whose size was the same as, or slightly larger than that determined above. Other stimulus parameters were those determined as preferred. The Gabor patch was expanded elliptically, usually in six steps, with increasing aspect ratios, along the collinearity axis of the cell’s RF. Stimulus contrast was set at three levels: low (10 or 20%), moderate (30 or 50%) and high (80 or 100%). a RF expansion upon decreasing stimulus contrast (cell C112-N9). The size of the RF, as defined by the peak response in size-tuning curves with the elongated Gabor target (ET), was ~10 deg in diameter at 100% contrast (green diamonds) and expanded to about 25 deg upon stimulation at 20% contrast (red circles). b In the same cell, a pair of high-contrast flankers placed 11 deg from the center of the RF strongly enhanced RF responses, especially at ~20% contrast, a sign of collinear facilitation in that the contrast response curve shifts to the left (purple squares). When stimulated by a single Gabor whose elongated edge along the major axis reached to the region probed with high-contrast flankers (see inset), the collinear facilitation noted above was augmented still further (yellow triangles). c RF contraction upon decreasing contrast. This cell (F10-N11) had a relatively small RF of 2.9 deg (across) when stimulated by a target Gabor (aspect ratio 1:1) alone. When stimulated with ETs, the peak response was attained at 80% contrast when stimulus spanned 9.8 deg (green diamonds). The location of the peak remained the same with stimulation at 50% contrast (blue squares). It went down to 7.4 deg at 30% contrast (red circles). d The same cell showed subtle suppression of contrast response at low-to-moderate contrast when tested with the target plus flankers at 8.7 deg (red squares), countering the possibility that high-contrast flankers falling in the region of an expanded RF might generate more spike than the target alone (blue diamonds) does
The major result of this study is that expansion of the RF is not universal (see also Fig. 6a of Ichida et al. 2007). Indeed, to our great surprise, for many cells, the RFs measured with ETs contracted when stimulus contrast was lowered. The cell shown in Fig. 2c exemplifies this phenomenon. First, it had a relatively small RF (2.4 deg across) as defined by a clear peak in the standard size-tuning curve obtained with round Gabors, followed by, maximally, 39% reduction in response strength with increasing stimulus size (data not shown). The cell was then tested with ETs having various aspect ratios, as shown in Fig. 2c. At both 80 and 50% contrast (green diamonds and blue squares), the RF size measured with ETs was 9.8 deg along the major axis of the ET. Significantly, at 30% contrast (red circles), the RF size is reduced to 7.4 deg. We also note that for this cell the addition of high-contrast flankers caused no increase in response at low target contrast. Rather, when the cell was stimulated with the target plus flankers at 8.7 deg (Fig. 2d, red squares), contrast response function was suppressed, albeit in a subtle manner, at low-to-moderate target contrast, showing the involvement of gain control mechanism.
To quantify the extent of RF expansion and contraction, we made the following population analysis using a total of 48 cells: first, for each of 32 cells for which we have RF size data for both the low- and high-contrast range, we formed the ratio of the RF size at low contrast to that at high contrast (Ichida et al. 2007). Ratios of greater than one correspond to expansion with decreasing contrast, and those less than one correspond to contraction. The shaded columns in Fig. 3a show the distribution of the ratios in form of a frequency histogram. One sees immediately that cells contracting (16, left side of Fig. 3a) outnumber those expanding (12, right side). This is hardly consistent with a hypothesis of universal expansion. Four cells remained unchanged (low/high ratio = 1).
Fig. 3.
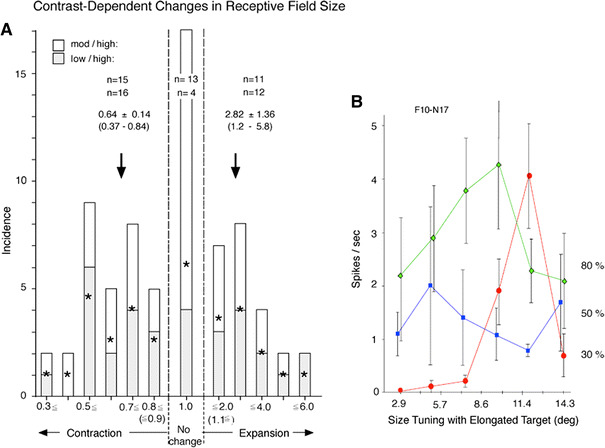
a The distribution of RF size-change indices based on a total of 71 data points derived from 48 cells in which the RF size was measured at high contrast and at one or both moderate and low contrast. The index was calculated, for each cell, by dividing the RF size obtained at the available moderate or low contrast by that at high contrast. There was no systematic difference in the incidence between the indices derived from moderate/high (39 data points, open columns) and those for low/high (32 data points, shaded columns), irrespective of the direction and extent of changes in the RF size. Therefore, the two sub-classes are collapsed here to show the average incidence (stars) at different direction and extent of RF changes. The grand average RF index for expanded cells (index > 1) was 2.82 ± 1.36 (mean ± one standard deviation, N = 23) and that for contraction (index < 1), 0.64 ± 0.14 (N = 31). The former value is comparable to the published data for the RF expansion in monkeys (Sceniak et al. 1999; Cavanaugh et al. 2002a; Ichida et al. 2007). The remaining 17 data points from 16 cells showed no change (index = 1) upon decreasing stimulus contrast. b An example of cell that showed inconsistent changes in RF size when stimulus contrast was reduced from high (80%) to two intermediate levels (30 and 50%)
Likewise, for 39 cells, we have RF size data for both the moderate- and high-contrast ranges. The open columns in Fig. 3a show the distribution of the size ratios derived of these data. Again, the number of contracting cells (15) is a bit larger than the number of expanding cells (11). Thirteen cells remained unchanged. Thus, the results of our examination of contrast-dependent changes in RF size summarized in Fig. 3a indicate more complexity in the contrast effect on RF size than is allowed by the currently prevailing view that RF expansion at low contrast is universal. In particular, we must conclude that, contrary to the previous suggestions, consistent RF expansion as stimulus contrast is reduced is not an universal phenomenon; indeed it is uncommon. Even including inconsistent encounters where the size expansion attained at moderate contrast was not maintained when contrast was further reduced to low, we detected expansion only in ~30% of the cases (Fig. 3a). Cells in our sample sometimes show contraction as contrast levels decrease from high to moderate followed by expansion upon further contrast decrease to lower (for example, Fig. 3b). The disparate cellular behavior presumably reflects variation of local connectivity patterns underlying contrast-dependent changes in RF size.
Phyisological correlates
We next looked for any cellular properties that may relate to these contrast-dependent changes in RF size. We found in cells that contracted with decreasing contrast, RF size determined by the peak or asymptote of standard size tuning curves was significantly smaller than that of cells that expanded (p < 0.02 t-test, Fig. 4a). Among the contracting cells, but not others, the extent of contraction was found to be related to the strength of response suppression measured in standard size tuning tests (Fig. 4b, linear regression coefficient r = −0.50 p < 0.01 t-test). That is, the more suppression in the standard size tuning curves, the more RF contraction at low contrast. However, the average strength of response suppression did not differ between the three groups (contracting 0.54 of the peak response; no change 0.47; expanding 0.52). Contrast threshold also failed to distinguish the three groups.
Fig. 4.
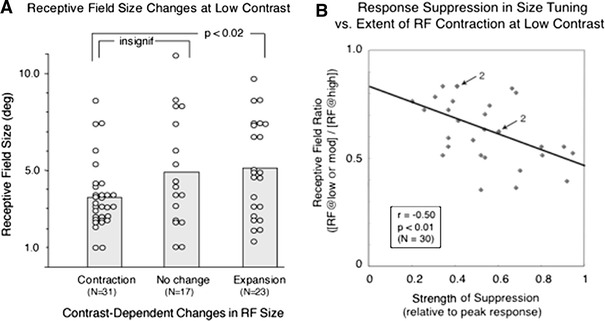
Physiological correlates. a Relation between the RF size and its changes at low contrast, based on 71 data points derived from 48 cells (same cells that appear in Fig. 3). The average RF size determined by the peak or asymptote in the standard size tuning curves turned out to be significantly smaller in the 31 contracting cells (3.6 ± 1.7 deg) than that of the 23 expanding cells (5.1 ± 2.5 deg) at p < 0.02. But, the difference between the contracting cells and 17 no change cells (4.9 ± 2.9 deg) did not reach a significant level. b The extent of RF contraction at low or moderate contrast is related to the strength of response suppression in RF size tuning. The scatter diagram shows the distribution of 30 RF size ratios in the contracted cells (contraction index as calculated above). See Fig. 3 legend on the ordinate plotted against respective values of response suppression relative to the peak response in standard size tuning curves. The RF size ratios are significantly related to the strength of response suppression measured in standard size tuning tests with regression coefficient r = −0.50 at p < 0.01 (Student t test). The number 2 with the arrow indicates two points overlapping
Distance effects on response modulation by flankers
Consistent with the notion that the collinear facilitation and suppression are indeed central to lateral interactions, we were able to trace such effects over substantial distance in visual space along the collinearity axis.
In our previous study (Chen et al. 2001), we found the presence of four types of lateral effects on the RF response effected by concurrently presented collinear flankers: facilitation at low target contrasts and suppression at high contrasts (type I 37.4%), facilitation that increases with increasing contrast (type II 29.3%), suppression that increases with increasing contrast (type III 10.1%) and suppression at low contrasts with facilitation at high contrasts (type IV 8.1%). In that study, 15.2% did not show effects (no effect NE).
In the present population, two main measurements (RF size tuning tested along the collinearity axis with ETs under different target contrast values, and flanker modulation effects at different distances between the target and flankers) were successfully carried out for 40 cells. In all except one of these cells, three distances between the target and flankers were tested (see below). The 119 data points, which represented distances up to, on average, 16.6 deg from the center of the RF, were collected. The present assessment of modulation types was based on, as before (Chen et al. 2001), significant differences between the average response magnitude for the target alone and target-plus-flankers conditions at more than two contrast values along the entire span of the contrast response function.
First, we confirmed the presence of the four types (five groups including NE cases) of modulation effects, though sampling incidence of each of the four types was different in the present population, increasingly facilitative type-II cells being the most common subtype (34.2%), followed by increasingly suppressive type-III cells (20.0%). Cross-over type cells (14.2%) remained the third most common type (see Fig. 5 left).
Fig. 5.
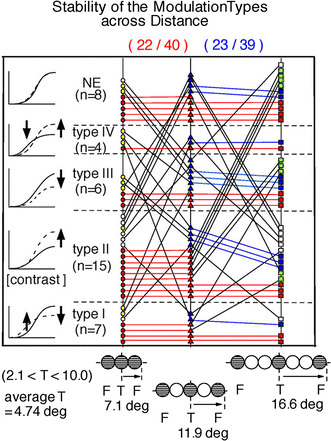
Stability assessment of the RF response modulation with concurrently presented collinear flankers at three distances. The enumerated cell types shown in the left column indicate the qualitative change in contrast response with the addition of flankers. The point of the figure is that the type of a cell is not constant, but rather, may depend on the flanker spacing. It is of interest that some cells that show one flanker effect at one spacing show another effect or no effect (NE) at another spacing. Following the oblique and horizontal lines allow one to see how individual cells changed type with spacing. However, the interest here is not so much how a single cell behaves as in the general pattern (and statistics) of changes, and one may get an impression of these things from the figure as a whole. There were 40 cells for which typing information was available and which were among the 48 cells of Fig. 3a. In these 40 cells, the distribution of different modulation types (Chen et al. 2001) were as follows, when the separation between the target and flankers was minimal with no overlap: type I, 7 cells; type II, 15 cells; type III, 6 cells; type IV, 4 cells and no effect, 8 cells (left column). Across these five groups, the majority (22 of 40 cells) showed the same typing when the center-to-center separation between the target on the RF and collinear flankers in the periphery increased from the minimum (0 gap, no-overlap) to distance corresponding to the patch size used as the target (red circles–red triangles). Likewise, the modulation type for 23 of 39 cells (blue triangles–blue squares) remained the same between separation equal to one target patch (1 gap) and that of two patches (2 gaps). Seven of the 40 cells maintained the same modulation types when the comparison was made between the 2-gap and 0-gap conditions. In about one-third (13 cells) of the 40 cells, the modulation type was invariant over all separations (red circles–red triangles–red squares). In the present analysis, the average size of a target was 4.7 deg (range 2.1–10.0 deg). For the same cells, in general, the spatial extent of the modulatory effects found here is much wider than the expansion and contraction range seen in Fig. 2, indicating that the underlying mechanisms must be different
Does target-flanker separation affect cell typing? To test this question, we investigated the modulatory effects with a pair of collinear flankers placed at three loci outside the RF: immediately adjacent (0 gap), separated by distance equal to the diameter of one target patch (1 gap), and separated by distance equal to the diameter of two target patches (2 gaps). The average extent of the surveyed separation was 7.1, 11.9, and 16.6 deg, respectively (see Fig. 5 bottom inset).
Across these five groups, the majority (22 of the 40 cells) showed the same phenotypes when the separation between the target (in the RF) and collinear flankers (outside it) increased from the minimum (0 gap, red circles) to distance corresponding to one patch size (Fig. 5, one gap middle column). Again, modulation types of the majority of cells (23 of 39 cells) remained the same between separation equal to one target (1 gap) and two targets (2 gaps) (Fig. 5, middle and right columns). In 20 cells, the modulation type remained the same between the no-gap and two-gap conditions (Fig. 5, white squares and circles). Also, in one-third (13 cells) of the 40 cells studied (Fig. 5, red circles–triangles–squares), the modulation types were invariant for all three separations tested. These findings show that the effects of flanker separation on modulation types are somewhat stable under the present experimental condition.
More importantly, Fig. 5 shows that same modulatory effects, in general, continue out to relatively large distance from the RF center. And, as one would expect, they tend to diminish with distance, as reflected in the number of NE cells, which slightly increases with flanker separation. On the other hand, at the larger distance of 16.6 deg from the RF center (two gaps, Fig. 5 right column), except for the 11 cells in NE group, the sampling incidence of all others is found either staying the same as, or reducing from, that at the one-gap separation.
Laminar localization of cells modulated by collinear flankers
Recording sites of 128 cells were successfully recovered on Nissl-stained histological sections obtained from 16 tracks in 7 cats. Forty-seven cells (37%) were found in the supragranular laminae, 30 cells (23%) in the granular lamina, and 51 cells (40%) in the infragranular laminae.
In 71 of the 128 cells, we were able to obtain information about RF response modulation types, here including the NE group. For these cells, the sampling incidence of cells in the three main sub-laminae did not significantly differ from that of the 128 cells in the full population: 28 cells in supragranular, 19 cells in granular, and 24 cells in infragranular laminae. This finding is consistent with the trend noted earlier (Mizobe et al. 2001) that the proportions of modulated cells were relatively equal among the three sublaminae.
However, when we distinguish among the modulation types, including the NE group, we do see inequalities in the laminar distribution. In particular, (1) while type-II cells tended to be found in infragranular laminae, type-III cells clearly favored supragranular laminae in the present population (χ2 test df = 2, p < 0.02). (2) Type-I and -IV cells, and cells in the NE group seemed more or less equally found in the three sublaminae.
Discussion
RF expansion at low contrast is not universal
Various cortical models (Sceniak et al. 1999, 2001; Cavanaugh et al. 2002a, b) attempt to explain RF expansion at low stimulus contrast (linear subtractive Difference of Gaussian (DoG), Gaussian or nonlinear divisive normalization). Aside from quantitative differences in their dynamic properties, as discussed in detail by Cavanaugh et al. (2002a), these models perform in much same way, based on network-driven primarily excitatory mechanisms. They all share the geometric premise that excitation and inhibition are spatially superimposed in such a way that excitation dominates at the RF center and inhibition dominates in a much wider surrounding region. This relatively simple, center-surround spatial organization is also a basic feature of our ellipsoidal model (see Suppl Fig. 2).
Recently, Angelucci et al. (2002), and Angelucci and Bressloff (2006) pointed out the inadequacy of the DoG model favored by previous authors. They proposed a recurrent network model, based on the a priori assumptions that (1) the RF contracts at high contrast and expands at low contrast, and (2) the excitatory and inhibitory mechanisms share the same spatial extent. In that model, referencing Bair et al. (2003), Angelucci and Bressloff (2006) emphasize the primary role of feedback afferent from higher visual areas (see below). We, however, did not find RF expansion at low contrast to be universal, which puts our findings at odds with the assumptions of the previous authors. Instead, we found expansion in some cells, contraction in others, and no change in a final portion.
It may be argued that this discrepancy comes from a peculiarity of the present cell population, which showed low firing rate (order of 10 spikes/s) instead of the more commonly reported rate of ~100 spikes/s (e.g., Cavanaugh et al. 2002a; see also Kasamatsu et al. (2001)). We believe that the low firing rate we saw was a consequence of a strict anesthesia regimen during recording that was required in the approved protocol. Interestingly, the cells’ network properties, other than firing rate, remained close to the normal, including the presence of the same four subtypes of lateral modulation (Chen et al. 2001).1 It is possible that, while the RF size expands at low contrast in highly excitable state as reported earlier, the reduced firing rates reflected the cells’ behavior in a less-excitable state, which may have led to our novel finding of RF contraction at low contrast.
What other features in the present study may have let us see contraction here while the phenomenon escaped detection in earlier studies? First, the species difference—monkeys were used in the above-cited studies, while cats were used in ours. Second, because of the low firing rate, the excitation and inhibition balance of the network properties detected in our studies may be shifted in such a way that the RF geometry is altered. Third, and importantly, in the previous studies by others, the test stimulus was a disk or annulus concentric with the RF, while we used elliptical or other non-round stimulus configurations along the collinearity axis.
With round stimuli, the effect of surround suppression might increase rapidly with increasing stimulus size because of the increase in stimulus area that lies in the surround (strong summation of surround suppression; e.g., Kitano et al. 1994). This area effect does not directly explain RF expansion at low contrast. Yet, in conjunction with non-linearities associated with an underlying recurrent network, an explanation is possible; it is observed that when a cell’s RF and its surround are stimulated with round, high-contrast targets of increasing size, the response magnitude rapidly peaks followed by strong suppression (in the majority of cases) or asymptotes (minority), most likely due to strong recurrent inhibition derived from local networks (see a model in Fig. 7 of Kasamatsu et al. 2005). However, with round low-contrast stimuli, the recurrent inhibition underlying response suppression may not develop proportionally strongly, in effect dynamically altering the shape or salience of the suppressive region. The hypothesized, nonlinear, weak inhibition, with increase in stimulus diameter, still leads to relatively increased spiking, showing shallow size-tuning curves. This may be interpreted as RF expansion. Note that this does not imply that the maximum amount of spiking is great with low contrast stimuli; only that the spiking continues to slowly increase as the stimulus gets larger. In fact, it is experimentally observed that the average response magnitude attained by low-contrast stimuli is substantially lower than that by high-contrast stimuli, reducing physiological significance of the expanded RF at low contrast.
In contrast, the ETs used in the present study drive surround suppression only weakly. This is so because the elliptical stimuli, being confined to the neighborhood of the collinear axis, have relatively less of their area in the suppressive surround. Our thesis is that functional networking is fundamentally different along the collinear axis compared to off-axis. In particular, facilitative effects are concentrated on-axis (model shown in Fig. 10 of Mizobe et al. 2001). The ETs used in present study preferentially drive the collinear part of the total network, generating more excitation than inhibition. At the same time, relatively weak surround suppression generated by the ETs would be expected to imply a relatively small contribution of recurrent inhibition derived from self and near-by cells. This in turn might allow the contributions to spike generation of long-range lateral interactions, both excitatory and inhibitory in nature, to be more effective than otherwise, opening wider possibilities for network behavior. In particular, contraction of the RF with decreased contrast might be explained. It is instructive that, using iso-oriented (our term, collinear) annuli of varying radii, which less involve the non-collinear region outside the RF, Ichida et al. (2007) were able to show the presence of response facilitation from the RF surround, which is nominally supposed to be suppressive.
Network implication of collinear modulation
The extensive lateral spread of modulation effects may not be explainable solely by cell-pair networks within striate cortex (one cell sensing the target and the other the flanker)—a more elaborate set of connectivities may be required. A possibility is that collinear facilitation may arise from interactions among inputs that directly drive visuocortical cells; in particular, large suppressive surround of lateral geniculate nucleus (LGN) cells provides inhibitory input, and strong facilitation results from disinhibition (Vidyasagar 1987). It has been observed that surround suppression is successively strengthened through the stages of thalamocortical transformation (Sadakane et al. 2006). However, contribution of surround suppression in the LGN to the collinear facilitation we obtain using discrete flanker patches is likely to be small for the following reasons: first, the spatial extent of collinear modulation reported is wide (Mizobe et al. 2001; see also Fig. 5 of this study), substantially wider than that for LGN cells (Jones et al. 2000). Second, though the presence of contrast-dependent suppression of surround stimulation was also shown for LGN cells, its effectiveness in terms of stimulus contrast was significantly lower than that obtained for visuocortical cells (Sadakane et al. 2006). Third, and most importantly, in the LGN there was no significant difference in the strength of suppression between iso-oriented (i.e., collinear) and orthogonally oriented grating stimulus placed in the surround (Solomon et al. 2002).
Another candidate scheme for explaining modulation is based on feedback projections from higher cortical areas such as the mid-temporal area (Bair et al. 2003; Angelucci and Bressloff 2006; Ichida et al. 2007). It is critical to the argument that these areas may send feedback afferent to V1 much faster than generally thought (Bair et al. 2003). Bair et al. (2003) found relatively short conduction delay of surround suppression effects. We find these authors’ deductions unconvincing as follows: Figure 6a of Bair et al. compares time courses of suppression of the activity of a single cell in the presence of various configurations of surround stimulation. In particular, both a “far surround” (one starting far from the RF and extending still further away), and a “near surround” (starting nearer to the RF but containing the region covered by the far surround), generated the same steep time course to maximal suppression.
Bair et al. interpreted their data (typified by Fig. 6a) to imply that the observed relatively short conduction delay of surround suppression is distance independent in general, and thus possibly mediated by feedback from higher areas. This conclusion is not supported by the data shown in their Fig. 6b, in which strong activation with near surround stimulation resulted in early and steep suppression while far surround stimulation resulted in later and less steep suppression (distance dependence). For the cell shown in Fig. 6a, the suppression lasted longer with near surround stimulation than with far. This might be a result of the fact that the near stimulation affected more cells (mass effect). If suppression effects were local as suggested by Crook et al. (2002), not involving feedback afferents, one would expect the situation of Fig. 6b to be general, since not only are suppression-generating elements closer with near surround stimulation, but there are also more of them. Both conditions would seemingly imply quicker suppression onset. However, that conclusion is not necessary. The fact that the onset was the same for both types of surrounds in Fig. 6a could be explained if the suppression pathway was already saturated, even by the weaker far-surround activation. We think this is likely because of the large size of stimulated area relative to the RF center size. In addition, the combined effect of the use of opioid anesthesia and strong RF stimulation may have contributed to saturation.
In light of this discussion, we cannot rely on the scheme promoted by Bair et al. (2003) to account for the modulatory effect of surround stimulation. Incidentally, the average conduction speed of ~1 m/s, which Bair et al. concluded to be considerably faster than expected for horizontal cortical connections previously implicated in surround suppression, is in fact comparable to that of the slow distributed component of local field potential generated within cat striate cortex (Kitano et al. 1994; Kasamatsu et al. 2005). This type of local field potential is closely related to response suppression and also related to the membrane potentials that eventually control the excitability of the local circuits (see below).
Angelucci and Bressloff’s model (2006 presents two difficulties: one, discussed above, is the assumption of distance independence inherited from Bair et al. (2003) and the other, more serious, the invalid assumption of RF expansion at low contrast, which has been inherited by Ichida et al. (2007). In fact, we have observed instances of the reverse behavior. In the majority of striate cortical cells that we studied, collinear facilitation was seen at low target contrast when flankers were in or beyond the nominal suppressive region (Chen et al. 2001 and the present study). In almost half of these cells, the strength of collinear facilitation was seen to continually increase with increasing target contrast. According to the aforementioned models of RF dynamics, for these cells the RF center size should be shrinking, putting high-contrast flankers, if anything, farther away from the excitatory center. Thus, collinear facilitation is certainly not a consequence of the expansion of the RF at low contrast, as suggested previously (Cavanaugh et al. 2002a, b).
In short, there is good reason to believe that neither expansion of RF at low contrast nor collinear modulation causes the other; rather that the two are separate functional entities. We expect (and to that extent, agree with Cavanaugh et al.) that both derive from a common set of fixed anatomical connectivities producing different effects due to different dynamic balance of excitation and inhibition generated in the same local circuits (Yoshimura et al. 2000). In this context, it is intriguing that the modulatory process is more controlled by GABAB receptors that have weaker conductance with much longer time constant than GABAA receptors: postsynaptic activation of visuocortical cells due to direct thalamic afferent is controlled more by GABAA receptors and the activation due to lateral input within cortex more by GABAB receptors (Kasamatsu et al. 2005).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure 1 (GIF 155 KB)
The main purpose of the present study was to examine in detail changes in receptive-field (RF) size under different stimulus contrast conditions. Imperative is to realistically determine the RF center as well as its size when a cell under study is stimulated with a single, optimally selected stimulus. To facilitate this, we carried out objective RF mapping using M-sequence-driven pattern stimuli (see the main text for refs). Top, We started our survey with an area of 18 cm × 18 cm (a) at 40 cm from the animal’s eye in a matrix of 3 × 3, with each stimulus patch size being 6 cm × 6 cm (8.6 deg × 8.6 deg). The matrix size successively reduced to 9 cm × 9 cm (b), 6 cm × 6 cm (c) and finally 3 cm × 3 cm (d) as indicated to the right. To the left, successively shrinking image of the central patch of each of the four matrices is shown in different colors. Which one of the 9 patches in the matrix is filled with the stimulus pattern at a given moment is determined following an M sequence of an appropriate length. The base interval of the onset of randomly presented stimuli was a multiple (usually around 10) of 14 msec and each presentation lasted for 150 msec. In practice, since we routinely superimposed the center of the matrix onto the center of the manually plotted RF, if our initial estimate is appropriate, we expect to have a measurable response only in the central patch of the 9-patch matrix. When it turns out that we are able to secure the central response only, this means that beyond any doubt, the RF is confined to the central patch of 6 cm × 6 cm. Bottom a-d, Each round of the M-sequence–driven stimulus presentation was repeated 5 times and the average response magnitude (expressed in spikes/sec) at each of the 9 patches was calculated immediately after the completion of stimulation and the resulting kernels plotted at the corresponding patches. A set of examples of thus-obtained kernels are shown in a-d. Here, we also used a color scheme based on an area-sum of power of each trace, red being for strong responses and blue for weak ones. Assisted by this color scheme, it was clear during recording that RF mapping rapidly proceeded, having successively captured the RF of this cell at the central patch of a given matrix (see Methods for detail).
This file is unfortunately not in the Publisher's archive anymore:
Fig. 2. An ellipsoidal model of the receptive-field organization of single visuocortical cells. A, Three distinct types of visual space may be distinguished outside the RF center (classical receptive field, CRF) that is excitatory: 1) A modulatory region (shown in red) outside the CRF, yet within the boundary determined by annular minimal response field (annular minimal response field, AMRF, as defined by Cavanaugh et al. 2002a, 2002b). 2) An inhibitory surround (shown in blue), lying outside of AMRF. The spatial extent of inhibitory surround is not well defined. This is the basic premise of most models (Sceniak et al. 1999). The part of the AMRF outside the CRF supports both excitatory and inhibitory modulation effects. 3) Based on the present findings, we propose the existence of another modulatory zone beyond inhibitory surround yet within the maximal extent of collinear modulation (MECM) whose effects on RF response can be either excitatory or inhibitory depending on other experimental constraints. Note that the modulatory region shown in red and demarcated by the outer boundaries of region 1 and the inner boundary of region 2 (which equals the outer boundary of region 1) is defined by delicate balances between exitatory and inhibitory influences that depend on the geometry (disk shaped or annular) of the stimulus presented. Both boundaries lie within both the excitatory and inhibitory regions of the classical “Mexican hat” picture. (TIFF 868 kb)
Acknowledgments
Supported by NIH grants, EY-012413 (TK) and EY-06883 (Core). We thank Dr. M. Carandini for helpful comments on an early draft of the paper and Ms. M. Mejia for histology.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
The validity of cell typing without prior modeling of the contrast- and collinearity-dependent behavior of single striate cells was addressed elsewhere (Miller and Kasamatsu 2005)
References
- Angelucci A, Bressloff PC. Contribution of feedforward, lateral and feedback connections to the classical receptive field center and extra-classical receptive field surround of primate V1 neurons. Prog Brain Res. 2006;154:93–120. doi: 10.1016/S0079-6123(06)54005-1. [DOI] [PubMed] [Google Scholar]
- Angelucci A, Levitt JB, Walton EJS, Hupé JM, Bullier J, Lund JS. Circuits for local and global signal integration in primary visual cortex. J Neurosci. 2002;22:8633–8646. doi: 10.1523/JNEUROSCI.22-19-08633.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bair W, Cavanaugh JR, Movshon JA. Time course and time-distance relationships for surround suppression in macaque V1 neurons. J Neurosci. 2003;23:7690–7701. doi: 10.1523/JNEUROSCI.23-20-07690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB, Blakemore C, Pettigrew JD. The neural basis of binocular depth discrimination. J Physiol (Lond) 1967;193:327–342. doi: 10.1113/jphysiol.1967.sp008360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C, Tobin EA. Lateral inhibition between orientation detectors in the cat’s visual cortex. Exp Brain Res. 1972;15:439–440. doi: 10.1007/BF00234129. [DOI] [PubMed] [Google Scholar]
- Cavanaugh JR, Bair W, Movshon JA. Nature and integration of signals from the receptive field center and surround in macaque V1 neurons. J Neurophysiol. 2002;88:2530–2546. doi: 10.1152/jn.00692.2001. [DOI] [PubMed] [Google Scholar]
- Cavanaugh JR, Bair W, Movshon JA. Selectivity of spatial distribution of signals from the receptive field surround in macaque V1 neurons. J Neurophysiol. 2002;88:2547–2556. doi: 10.1152/jn.00693.2001. [DOI] [PubMed] [Google Scholar]
- Chen CC, Kasamatsu T, Polat U, Norcia AM. Contrast response characteristics of long-range lateral interactions in cat striate cortex. NeuroReport. 2001;12:655–661. doi: 10.1097/00001756-200103260-00008. [DOI] [PubMed] [Google Scholar]
- Chisum HJ, Moose F, Fitzpatrick D. Emergent properties of layer 2/3 neurons reflect the collinear arrangement of horizontal connections in tree shrew visual cortex. J Neurosci. 2003;23:2947–2960. doi: 10.1523/JNEUROSCI.23-07-02947.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook JM, Engelmann R, Löwell S. GABA-inactivation attenuates collinear facilitation in cat primary visual cortex. Exp Brain Res. 2002;143:295–302. doi: 10.1007/s00221-002-1007-y. [DOI] [PubMed] [Google Scholar]
- Foley JM, Varadharajan S, Koh CC, Farias MCQ. Detection of Gabor patterns of different sizes, shapes, phases and eccentricities. Vis Res. 2007;47:85–107. doi: 10.1016/j.visres.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichida JM, Schwabe L, Bressloff PC, Angelucci A. Response facilitation from the “suppressive” receptive field surround of macaque V1 neurons. J Neurophysiol. 2007;98:2168–2181. doi: 10.1152/jn.00298.2007. [DOI] [PubMed] [Google Scholar]
- Jones HE, Andolina IM, Oakely NM, Murphy PC, Sillito AM. Spatial summation in geniculate nucleus and visual cortex. Exp Brain Res. 2000;135:279–284. doi: 10.1007/s002210000574. [DOI] [PubMed] [Google Scholar]
- Kapadia MK, Ito M, Gilbert CD, Westheimer G. Improvement in visual sensitivity by changes in local context: parallel studies in human observers and in V1 of alert monkeys. Neuron. 1995;15:843–856. doi: 10.1016/0896-6273(95)90175-2. [DOI] [PubMed] [Google Scholar]
- Kapadia MK, Westheimer G, Gilbert CD. Dynamics of spatial summation in primary visual cortex of alert monkeys. Proc Natl Acad Sci USA. 1999;96:12073–12078. doi: 10.1073/pnas.96.21.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamatsu T, Kitano M, Sutter EE, Norcia AM. Lack of lateral inhibitory interactions in visual cortex of monocularly deprived cats. Vis Res. 1998;38:1–12. doi: 10.1016/S0042-6989(97)00158-2. [DOI] [PubMed] [Google Scholar]
- Kasamatsu T, Polat U, Pettet MW, Norcia AM. Colinear facilitation promotes reliability of single-cell responses in cat striate cortex. Exp Brain Res. 2001;138:163–172. doi: 10.1007/s002210100675. [DOI] [PubMed] [Google Scholar]
- Kasamatsu T, Mizobe K, Sutter EE. Muscimol and baclofen differentially suppress retinotopic and nonretinotopic responses in visual cortex. Vis Neurosci. 2005;22:839–858. doi: 10.1017/S0952523805226135. [DOI] [PubMed] [Google Scholar]
- Kitano M, Niiyama K, Kasamatsu T, Sutter EE, Norcia AM. Retinotopic and nonretinotopic field potentials in cat visual cortex. Vis Neurosci. 1994;11:953–977. doi: 10.1017/S0952523800003904. [DOI] [PubMed] [Google Scholar]
- Levick WR. Another tungsten microelectrode. Med Biol Eng. 1972;10:510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Levitt JB, Lund JS. Contrast dependence of contextual effects in primate visual cortex. Nature. 1997;387:73–76. doi: 10.1038/387073a0. [DOI] [PubMed] [Google Scholar]
- Li C-Y, Li W. Extensive integration field beyond the classical receptive field of cat’s striate cortical neurons—classification and tuning properties. Vis Res. 1994;34:2337–2355. doi: 10.1016/0042-6989(94)90280-1. [DOI] [PubMed] [Google Scholar]
- Maffei L, Fiorentini A. The unresponsive regions of visual cortical receptive fields. Vis Res. 1976;16:1131–1139. doi: 10.1016/0042-6989(76)90253-4. [DOI] [PubMed] [Google Scholar]
- Meese TS, Hess RF. Anisotropy for spatial summation of elongated patches of grating: a tale of two tails. Vis Res. 2007;47:1880–1892. doi: 10.1016/j.visres.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Miller R, Kasamatsu T (2005) Principal patterns of response modulation by collateral flankers in cat visual cortex. Society for Neuroscience 35th annual meeting, abstract 389.7
- Mizobe K, Polat U, Pettet MW, Kasamatsu T. Facilitation and suppression of single striate-cell activity by spatially discrete pattern stimuli presented beyond the receptive field. Vis Neurosci. 2001;18:377–391. doi: 10.1017/S0952523801183045. [DOI] [PubMed] [Google Scholar]
- Nelson JI, Frost BJ. Intracortical facilitation among co-oriented, co-axially aligned simple cells in cat striate cortex. Exp Brain Res. 1985;61:54–61. doi: 10.1007/BF00235620. [DOI] [PubMed] [Google Scholar]
- Polat U, Norcia AM. Elongated physiological summation pools in the human visual cortex. Vis Res. 1998;38:3735–3741. doi: 10.1016/S0042-6989(97)00426-4. [DOI] [PubMed] [Google Scholar]
- Polat U, Sagi D. Lateral interactions between spatial channels: suppression and facilitation revealed by lateral masking experiments. Vis Res. 1993;33:993–999. doi: 10.1016/0042-6989(93)90081-7. [DOI] [PubMed] [Google Scholar]
- Polat U, Tyler CW. What pattern the eye sees best. Vis Res. 1999;39:887–895. doi: 10.1016/S0042-6989(98)00245-4. [DOI] [PubMed] [Google Scholar]
- Polat U, Mizobe K, Pettet MW, Kasamatsu T, Norcia AM. Collinear stimuli regulate visual responses depending on cell’s contrast threshold. Nature. 1998;391:580–584. doi: 10.1038/35372. [DOI] [PubMed] [Google Scholar]
- Sadakane O, Ozeki H, Naito T, Akasaki T, Kasamatsu T, Sato H. Contrast-dependent, contextual response modulation in primary visual cortex and lateral geniculate nucleus of the cat. Eur J Neurosci. 2006;23:1633–1642. doi: 10.1111/j.1460-9568.2006.04681.x. [DOI] [PubMed] [Google Scholar]
- Sceniak MP, Ringach DL, Hawken MJ, Shapley R. Contrast’s effect on spatial summation by macaque V1 neurons. Nat Neurosci. 1999;2:733–739. doi: 10.1038/11197. [DOI] [PubMed] [Google Scholar]
- Sengpiel F, Baddley RJ, Freeman TCB, Harrad R, Blakemore C. Different mechanisms underlie three inhibitory phenomena in cat area 17. Vis Res. 1998;38:2067–2080. doi: 10.1016/S0042-6989(97)00413-6. [DOI] [PubMed] [Google Scholar]
- Sillito AM, Grieve KL, Jones HE, Cudeiro J, Davis J. Visual cortical mechanisms detecting focal orientation discontinuities. Nature. 1995;378:492–496. doi: 10.1038/378492a0. [DOI] [PubMed] [Google Scholar]
- Solomon SG, White AJ, Martin PR. Extraclassical receptive field properties of parvocellular, magnocellular, and kinocellular cells in the primate lateral geniculate nucleus. J Neurosci. 2002;22:338–349. doi: 10.1523/JNEUROSCI.22-01-00338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter EE. A deterministic approach to nonlinear systems analysis. In: Pinter RB, Nabet B, editors. Nonlinear vision: determination of neural receptive fields. Function and networks. Boca Raton: CRC Press; 1992. pp. 171–220. [Google Scholar]
- Vidyasagar TR. A model of striate response properties based on geniculate anisotropies. Biol Cybern. 1987;57:11–23. doi: 10.1007/BF00318712. [DOI] [PubMed] [Google Scholar]
- Walker GA, Ohzawa I, Freeman RD. Suppression outside the classical cortical receptive field. Vis Neurosci. 2000;17:379–3769. doi: 10.1017/S0952523800173055. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Sato H, Imamura K, Watanabe Y. Properties of horizontal and vertical inputs to pyramidal cells in the superficial layers of the cat visual cortex. J Neurosci. 2000;20:1931–1940. doi: 10.1523/JNEUROSCI.20-05-01931.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1 (GIF 155 KB)
The main purpose of the present study was to examine in detail changes in receptive-field (RF) size under different stimulus contrast conditions. Imperative is to realistically determine the RF center as well as its size when a cell under study is stimulated with a single, optimally selected stimulus. To facilitate this, we carried out objective RF mapping using M-sequence-driven pattern stimuli (see the main text for refs). Top, We started our survey with an area of 18 cm × 18 cm (a) at 40 cm from the animal’s eye in a matrix of 3 × 3, with each stimulus patch size being 6 cm × 6 cm (8.6 deg × 8.6 deg). The matrix size successively reduced to 9 cm × 9 cm (b), 6 cm × 6 cm (c) and finally 3 cm × 3 cm (d) as indicated to the right. To the left, successively shrinking image of the central patch of each of the four matrices is shown in different colors. Which one of the 9 patches in the matrix is filled with the stimulus pattern at a given moment is determined following an M sequence of an appropriate length. The base interval of the onset of randomly presented stimuli was a multiple (usually around 10) of 14 msec and each presentation lasted for 150 msec. In practice, since we routinely superimposed the center of the matrix onto the center of the manually plotted RF, if our initial estimate is appropriate, we expect to have a measurable response only in the central patch of the 9-patch matrix. When it turns out that we are able to secure the central response only, this means that beyond any doubt, the RF is confined to the central patch of 6 cm × 6 cm. Bottom a-d, Each round of the M-sequence–driven stimulus presentation was repeated 5 times and the average response magnitude (expressed in spikes/sec) at each of the 9 patches was calculated immediately after the completion of stimulation and the resulting kernels plotted at the corresponding patches. A set of examples of thus-obtained kernels are shown in a-d. Here, we also used a color scheme based on an area-sum of power of each trace, red being for strong responses and blue for weak ones. Assisted by this color scheme, it was clear during recording that RF mapping rapidly proceeded, having successively captured the RF of this cell at the central patch of a given matrix (see Methods for detail).


