Abstract
Abstract: Spontaneous iron accumulation in hepatocytes was observed in a 7-week-old female Han Wistar GALAS rat. Very fine yellowish brown pigments, which showed a positive reaction with Berlin Blue stain, were apparent in the cytoplasm close to the bile canaliculi, with a diminishing periportal-to-centrilobular gradient. There were also differences in distribution between and within lobes. Transmission electron microscopy revealed cytosolic ferritin and pericanalicular siderosomes in hepatocytes. No degeneration or necrotic changes were observed, and non-hepatocyte cells did not demonstrate any obvious accumulation of iron. There were no abnormalities in the animal other than this finding in the liver.
Keywords: iron overload, iron accumulation, hepatocyte, spontaneous, rats
Iron can accumulate in the liver under a variety of conditions, including systemic iron-loading, some types of hepatitis and alcoholic liver disease1. Hemochromatosis is a well-known congenital metabolic disorder disease in which iron accumulates predominantly in liver parenchymal cells in the early phase and subsequently in liver Kupffer cells, the pancreas, the heart, endocrine glands and so on2. It is clear that the fundamental abnormality existing in this condition is excessive absorption of iron in the duodenum.
In a toxicological study, we recently encountered a female rat at 7 weeks of age with iron overloading in the liver. Because this animal was a control fed a normal diet, the abnormality was presumed to be spontaneous. There has only been one previous article documenting spontaneous iron loading in rats3. According to that report, iron deposition began to be observed in animals around 9 weeks of age at very low incidences (0.07% in males and 0.56% in females). Here, we describe the characteristics of the changes seen in a young animal.
The 4-week-old female BrlHan: WIST@Jcl(GALAS) (SPF) rat was purchased from Charles River Japan Inc. as one batch of breeding to be utilized for a 2-week feeding toxicological study. The animal was housed with one cage mate in a suspended aluminum cage with a stainless-steel wire-mesh front and floor. The room temperature and relative humidity were controlled at 22–26°C and 40–70%, respectively. The room air was changed 10 or more times per hour. Fluorescent tube lighting was employed to provide a 12-hr light/12-hr dark cycle (lighting period, 0800–2000). After a 7-day period of quarantine and acclimation, this animal was assigned to the control group and fed the control diet, CRF-1 (Oriental Yeast, Co. Ltd., Japan). After the 2-week treatment period, the animal was killed under ether anesthesia along with the other animals and subjected to a complete necropsy. The organs removed were fixed in 10% neutral buffered formalin solution. Routinely prepared paraffin embedded sections were stained with haematoxylin and eosin for light microscopic examination. A liver section was also stained with Berlin Blue for hemosiderin. Pieces of liver, originally fixed in 10% neutral buffered formalin solution, were refixed in 2.5% glutaraldehyde solution, post-fixed in 2% osmium tetroxide, dehydrated and embedded in epoxy resin for transmission electron microscopic examination. Ultrathin sections were stained with uranyl acetate and lead citrate. This experiment was performed in accordance with The Guide for Animal Care and Use of Sumitomo Chemical Co., Ltd.
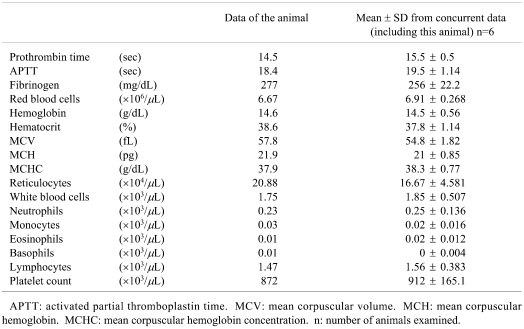
There was no evidence of any abnormal condition in this animal in regard to clinical observations, body weight, food consumption, hematology (Table 1), blood biochemistry (Table 2) and necropsy. The serum ferritin, transferrin and iron saturation levels were not measured. Although no abnormality was noted at necropsy, the liver preserved in 10% neutral buffered formalin solution did show a change in color. The caudal process of the caudal lobe and parts of the area of the left lateral lobe and right medial lobe were reddish brown (Fig. 1). There was a tendency for areas showing abnormal color to be close to the porta hepatis.
Table 1. Hematology.
Table 2. Blood biochemistry.
Fig. 1.
A liver preserved in formalin solution. Note the reddish brown coloration.
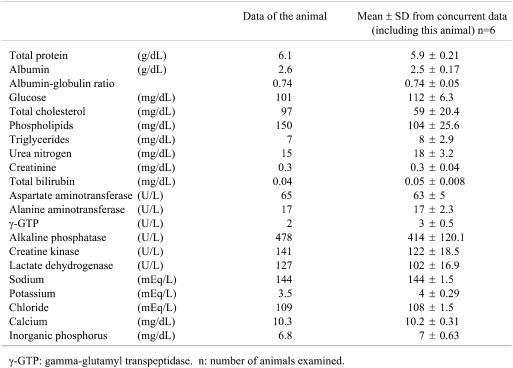
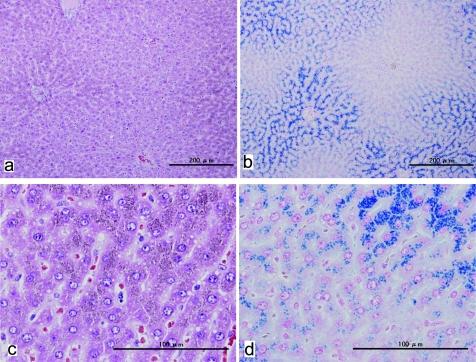
Microscopically, no abnormalities were recognized in any organs except for the liver in this animal. The spleen also demonstrated no variation from normal. Very fine yellowish-brown pigments were found in the hepatocytes with an uneven distribution among the lobes, corresponding to the gross lesion (Figs. 2, 3). The pigmentation was strongest around periportal areas. The staining intensity was weaker in midzonal areas, and very weak to absent in centrilobular hepatocytes. Thus, there was a clear diminishing periportal-to-centrilobular gradient. Regarding the distribution of the pigments in the cell, those were aggregating close to bile canaliculi. Berlin Blue staining revealed conclusive evidence that the pigments were hemosiderin. No degeneration or necrotic changes were observed. Kupffer cells did not exhibit appreciable accumulation of iron.
Fig. 2.
The liver tissue shows uneven positive staining with Berlin Blue. a. H.E staining, b. Berlin Blue staining.
Fig. 3.
Very fine yellowish brown pigments showing a positive reaction with Berlin Blue stain are apparent in hepatocytes. The pigments in the cytoplasm are close to bile canaliculi, with a diminishing periportal-to-centrilobular gradient. a, c. H.E stain. b, d. Berlin Blue stain.
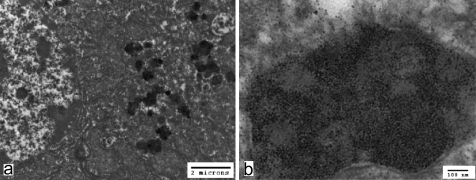
Transmission electron microscopy revealed cytosolic ferritin and pericanalicular lysosomes, or siderosomes, in periportal hepatocytes (Fig. 4). Many single membrane limited bodies filled with electron opaque aggregates were observed. They varied in density and had irregular-shaped deposits, sometimes including fine particles, and corresponded to pigmented granules on light microscopy. Furthermore, very fine particles were also observed diffusely, not enveloped by any membrane in the cytosol. These particles were considered to be ferittin based on morphological criteria including a size of around 5–7 nm2. Other organelles and the bile canaliculi were normal.
Fig. 4.
a. Irregular bodies filled with electron-opaque aggregates are apparent in hepatocytes. b. Very fine particles (ferritin) are distributed diffusely, without any enveloping membrane in the cytosol, as well as in single membrane-limited bodies.
According to a previous report on spontaneous iron overload in SD rats3, onset involves only hepatocyte pigmentation, the degree of iron overload increases with age and there is no relation to spleen hemosiderin deposition. The authors described iron accumulation as beginning in the pericanalicular siderosomes of periportal hepatocytes and extending the progressively to the entire lobule and non-hepatocytic cells, like Kupffer cells in the sinusoids and other macrophages around the bile ducts in portal tracts. In addition, no significant inflammatory cell infiltration, degeneration, necrosis or fibrosis were noted.
In humans, primary or genetic hemochromatosis is an inherited disease characterized by excessive absorption of iron from a diet with normal iron content and progressive iron loading of parenchymal tissues such as the liver. The changes in humans are irreversible, and liver cell iron overload leads to cirrhosis and eventual hepatocellular carcinomas in some cases. However, in the early stages, only iron accumulation in hepatocytes is apparent, with a pericanalicular polarization, along with a diminishing periportal-to-centrilobular gradient2, almost the same as our female rat.
In experimental animals, there have been other reports of iron overload in the liver4–7. However, almost all examples of experimentally induced hemochromatosis differed from our case because early iron deposition occurs primarily in non-hepatocytic cells and may involve other organs4. Only with dietary iron overload, in the very early stages, iron accumulation begin in hepatocytes predominantly5.
The origin of the spontaneous iron overload observed in our animal is not clear but might be related to an increase of duodenal iron absorption or an imbalance between iron absorption and excretion capacity at the level of the cell. Although the liver iron concentration, blood iron concentration, transferrin and ferritin were not examined, there is a similarity with human hereditary hemochromatosis.
The pathogenesis of different iron overloading in hepatocytes between and within lobes, is also unclear, but strongly suggests that the iron deposition is attributable to altered metabolism rather than an underlying genetic defect. IL-1beta, IL-6 and TNF-alfa stimulate transferrin-bound iron uptake by parenchymal cells of the liver8, and heterogenic iron deposition cases have been reported in chronic hepatitis or cirrhosis patients. An absence of iron accumulation in preneoplastic and neoplastic lesions has been described in the rat liver with chemical induced hemosiderosis6. In some human biopsy specimens from patients with chronic viral hepatitis, unusual foci of irregular hepatocellular iron storage have been described9. Furthermore, interpretation of hepatic iron content in small biopsy samples is particularly difficult, such as in case of thalassemia patients with an uneven hepatic iron distribution10.
In conclusion, the histological features of the present case of uncertain pathogenesis, point to similarities in the typical hemochromatosis in humans.
Acknowledgments
We wish to thank the members of the Kansai Conference of Toxicologic Pathology (KCTP) for their advice.
References
- 1.Batts KP. Iron overload syndromes and the liver. Modern Pathology. 20: S31–S39 2007 [DOI] [PubMed] [Google Scholar]
- 2.Iancu TC, Deugnier Y, Halliday JW, Powell LW, Brissot P. Ultrastructural sequences during liver iron overload in genetic hemochromatosis. J Hepatol. 27: 628–638 1997 [DOI] [PubMed] [Google Scholar]
- 3.Masson R, Roome NO. Spontaneous iron overload in Sprague-Dawley rats. Toxicol Pathol. 25: 308–316 1997 [DOI] [PubMed] [Google Scholar]
- 4.Ward RJ, Florence AL, Baldwin D, Abiaka C, Roland F, Ramsey MH, Dickson DP, Peters TJ, Crichton RR. Biochemical and biophysical investigations of the ferrocene-iron-loaded rat. An animal model of primary haemochromatosis. Eur J Biochem. 202: 405–410 1991 [DOI] [PubMed] [Google Scholar]
- 5.Whittaker P, Hines FA, Robl MG, Dunkel VC. Histopathological evaluation of liver, pancreas, spleen, and heart from iron-overloaded Sprague-Dawley rats. Toxicol Pathol. 24: 558–563 1996 [DOI] [PubMed] [Google Scholar]
- 6.Williams GM, Yamamoto RS. Absence of stainable iron from preneoplastic and neoplastic lesions in rat liver with 8-hydroxyquinoline-induced siderosis. J Natl Cancer Inst. 49: 685–692 1972 [PubMed] [Google Scholar]
- 7.Powell LW, McKeering LV, Halliday JW. Alterations in tissue ferritins in iron storage disorders. Gut. 16: 909–912 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirayama M, Kohgo Y, Kondo H, Shintani N, Fujikawa K, Sasaki K, Kato J, Niitsu Y. Regulation of iron metabolism in HepG2 cells: a possible role for cytokines in the hepatic deposition of iron. Hepatology. 18: 874–880 1993 [DOI] [PubMed] [Google Scholar]
- 9.Lefkowitch JH, Yee HT, Sweeting J, Green PH, Magun AM. Iron-rich foci in chronic viral hepatitis. Hum Pathol. 29: 116–118 1998 [DOI] [PubMed] [Google Scholar]
- 10.Ambu R, Crisponi G, Sciot R, Van Eyken P, Parodo G, Iannelli S, Marongiu F, Silvagni R, Nurchi V, Costa V, Faa G, Desmet VJ. Uneven hepatic iron and phosphorus distribution in beta-thalassemia. J Hepatol. 23: 544–549 1995 [DOI] [PubMed] [Google Scholar]