Abstract
Background
Childhood overweight has become a serious health problem among children and adolescents in the United States. No previous study, to our knowledge, has analyzed the effect of body mass index (BMI) on range of motion and carrying angle of the elbow joint in a healthy pediatric population. The primary objective of this study was to determine the effect of BMI on orthopedic parameters of the elbow joint, including range of motion, flexion, extension, and carrying angle.
Study Participants and Methods
Healthy children age 2 to 18 years (mean 12.0 ± 3.9 years) were recruited at an urban pediatric orthopedic clinic as pediatric orthopedic patients or as the siblings or friends of patients. Measures of range of motion (flexion and extension) and carrying angle of 226 elbows and of BMI from 113 study participants were analyzed.
Results
BMI was negatively correlated with right and left elbow range of motion (r = −0.54, P <0.01; r = −0.43, P <0.01) and right and left elbow flexion (r = −0.59, P <0.01; r = −0.50, P <0.01). BMI had a positive correlation with right elbow extension (r = 0.20, P = 0.04). BMI did not correlate with left elbow extension or right or left carrying angle. After adjustment for age, sex, and ethnic group, BMI was associated with right (P <0.01) and left (P <0.01) elbow range of motion.
Conclusions
These data demonstrate that increased BMI in children is negatively correlated with range of motion of the elbow joint. Further studies are needed to evaluate the consequences of impaired range of motion associated with overweight on activity levels and energy expenditure in growing children and adolescents.
Keywords: Pediatric, Body mass index, Orthopedic surgery, Anthropometric, Elbow joint, Range of motion, Childhood overweight, Obesity
Childhood overweight has become a serious public health concern over the past several decades. Overweight in a child is defined as a body mass index (BMI) that is at or above the 95th percentile for age and sex, and risk for overweight is a BMI from the 85th to the 95th percentile for age and sex (1). Data from the National Health and Nutrition Examination Surveys demonstrate that the prevalence of overweight in children 2 to 5 years old and 6 to 11 years old has more than doubled (from 5.0% to 10.3% and 6.5% to 15.8%, respectively) and in those 12 to 19 years old has tripled (5.0%–16.1%) over the past 35 years (2). Also alarming is that 22.6% of 2- to 5-year-olds, 31.2% of 6- to 11-year-olds, and 30.9% of 12- to 19-year-olds are overweight or at risk for overweight (2). Overweight in childhood has many negative health consequences, which include eating disorders, hypertension, sleep disorders, atherosclerosis, a potential link to the recent increase in early-onset type 2 diabetes mellitus, and obesity in adulthood (and its associated increase in morbidity and mortality) (3). Overweight in childhood has been correlated with a greater risk for adverse psychosocial consequences, including lowered self-esteem and depression (4). Last, overweight children are more likely to be victims or perpetrators of bullying behaviors than are their normal-weight peers (5). All of these factors may contribute to decreased participation in sports and exercise by overweight children and an overall decrease in health-related quality of life.
A major goal of therapy with children who are overweight or at risk for overweight is maintenance of baseline weight. Diet modification, increased daily energy expenditure through increased physical activity such as sports and exercise, and other behavioral modifications are recommended methods to lower a child’s BMI (6). We hypothesize that overweight children have significantly more soft tissue around their elbow joints that may lead to decreased range of motion in the elbow joint caused by soft-tissue impediment. The potential loss of elbow range of motion associated with increased BMI in turn could affect the ability of overweight children to function at the same physical level as non-obese children. For example, elbow range of motion is required for pitching a baseball or shooting a free-throw in basketball (7,8). Decreased range of motion could make it more difficult for overweight children to increase their daily activity as a method for BMI reduction. Additionally, decreased ROM could lead to a small but cumulative decline in energy expenditure caused by relatively decreased muscle movement and body activity.
Other studies have examined the relationship between range of motion and BMI (9,10). BMI was associated with reduced flexion in the elbow joint in an older adult population (9). Soucie et al (10) reported that in patients with hemophilia, increased BMI was a significant risk factor independent of joint hemorrhage for decreased range of motion. Our study is the first, to our knowledge, to analyze the effect of BMI on range of motion of the elbow joint in a healthy pediatric population.
The purpose of this study was to conduct an initial investigation to determine whether the BMI of healthy children, without previous trauma or disease process affecting the elbow, had an impact on range of motion and carrying angle of the elbow joint. The elbow joint was selected for this initial investigation because it is required for participation in many common sports, is simple to measure, has 1 major axis of rotation, and has a finite active range of motion.
STUDY PARTICIPANTS AND METHODS
A cross-sectional study of 113 study participants (226 elbows) was conducted in a pediatric orthopedic clinic in 2004. Individuals were recruited when they entered the clinic and were excluded if they had a history of previous trauma or disease affecting the elbow joint. Study participants included clinic patients and the siblings of clinic patients. Demographic information was obtained from parents and included parental classification of the child’s ethnicity. Institutional review board approval was obtained for the project, and consent was obtained for all participants before they participated in the study.
BMI (kg/m2) was calculated as weight (kg) divided by height (m2) (2). Height and weight were either self-reported or measured in the clinic, depending on when the most recent measurement was obtained for that individual. Height was converted from feet-inches to centimeters, and weight was converted from pounds to kilograms. BMI was then converted to the correlating z score by use of information from the website of the Centers for Disease Control and Prevention (11). Age and ethnicity were also self-reported or reported by the parent/guardian.
Standardized range of motion and carrying angle of the elbow were measured with a Lafayette Gollehon Extendable Goniometer (Lafayette Instrument Co, Lafayette, IN) (Fig. 1). The person stood or sat with the shoulder adducted and flexed in the neutral position and with the elbow, wrist, and interphalangeal joints extended.
FIG. 1.
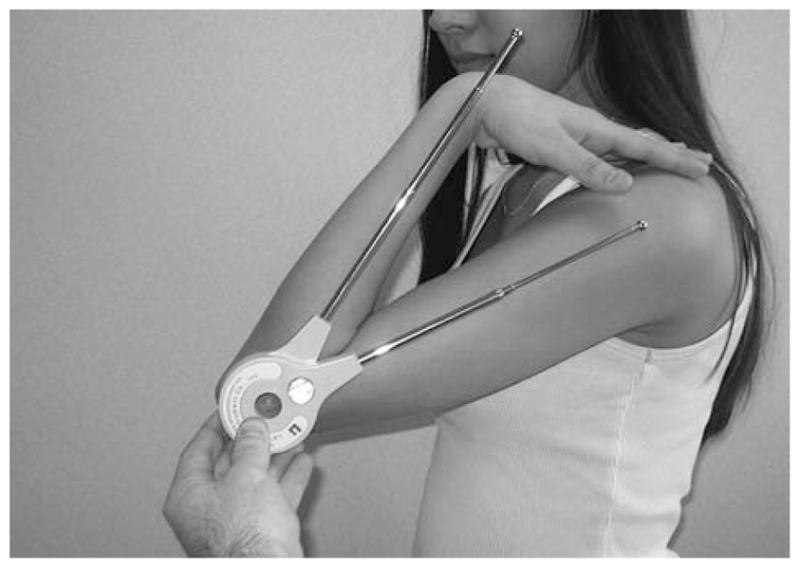
The Lafayette Gollehon Extendable Goniometer (Lafayette Instrument Co, Lafayette, IN), measuring active elbow flexion.
Elbow range of motion was assessed by aligning the goniometer on the lateral aspects of both arms to measure flexion and extension. The fulcrum of the goniometer was aligned anteroinferior to the lateral condyle, approximately even with the center of the arcs formed by the trochlear sulcus and capitellum. The center of the arcs formed by the trochlear sulcus and capitellum was identified as the center of the axis of rotation for the elbow joint (12). The distal arm of the goniometer was aligned parallel to the long axis of the forearm. The proximal arm of the goniometer was aligned parallel to the shaft of the humerus. The person then actively flexed and extended the arm through the full range of motion.
Flexion was measured with 0° defined as the starting point (no flexion). Extension was measured similarly (0° as the starting point), but greater extension was defined as a more negative measurement. Total range of motion was then calculated by subtracting extension from flexion.
Carrying angle was also measured on both upper limbs. The person stood or sat with a fully extended arm, a supinated forearm, and a flexed and adducted shoulder. The goniometer was placed on the anterior surface of the upper limb and was aligned with the ulna distally, the goniometer’s hinge with the cubital fossa, and the humerus proximally.
One investigator (D.W.G.) obtained all of the measurements for the study to prevent intertester variability. For the first 35 study participants, flexion and extension measurements were obtained in triplicate and averaged for each individual elbow to ensure reproducibility. A Pearson correlation coefficient of 0.94 for flexion and 0.96 for extension was found when the first measurement obtained was compared with the mean for all 3 measurements. The mean difference between the first and second flexion measurements was 0.01° ± 3.03° and between the second and third flexion measurements was 0.11° ± 2.16°. The mean difference between the first and second extension measurements was 0.37° ± 2.84° and between the second and third extension measurements was 0.51° ± 2.59°. Because of the excellent correlation between initial and average measurements, single measurements were obtained by D.W.G. for the remaining 78 participants.
Laterality (handedness) was self-reported by participants or their parents. Children under age 3 or those who had not begun using 1 hand predominantly were excluded from laterality analysis.
Data were analyzed by use of an unpaired t test to assess differences between sexes, Pearson correlation coefficient for correlation, and analysis of variance for differences between ethnic groups using Stata 7.0. Independent predictors of BMI z score were assessed by use of multivariate linear regression. In the multivariate regression models, range of motion and carrying angle measurements were adjusted for age, sex, and ethnic group. The Asian/Pacific Islander group was removed from the multivariate analysis because of the small sample size of this group.
RESULTS
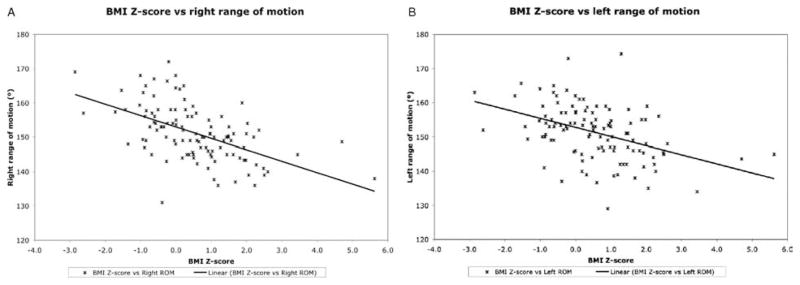
In all, 113 individuals were enrolled in the study. Of those 113, 73 (65%) were male and 40 (35%) were female. The mean age was 11.96 ± 3.89 years (range 2–18). Their ethnicity was as follows: white 47.8% (54), African American 31.9% (36), Hispanic/Latino 18.6% (21), and Asian/Pacific Islander 1.8% (2). Their weights were characterized as follows: 20 were overweight (BMI >95th percentile), 15 were at risk for overweight (BMI 85th–95th percentile), 72 were normal weight (BMI 15th–85th percentile), and 6 were underweight (BMI <15th percentile). Their mean BMI z score was 0.55 ± 1.29 (Table 1). Total range of motion had a negative correlation with increasing BMI z score (right r = −0.54, P <0.01; left r = −0.43, P <0.01) (Table 2, zFig. 2). Both right and left flexions also showed moderate negative correlations with increasing BMI score (right flexion n = 113, r = −0.59, P <0.01; left flexion n = 113, r = −0.50, P <0.01) (Table 2). Right extension had a positive correlation with increasing BMI (r = 0.20, P = 0.04), but left extension did not show a significant correlation (r = 0.12, P = 0.21). Thus, right extension decreased (became more positive) with increasing BMI, resulting in decreased range of motion. Carrying angle did not correlate with BMI for the right or left arm (right r = 0.02, P = 0.87; left r = 0.003, P = 0.97) (Table 2).
TABLE 1.
Demographic and orthopedic variables
| Mean | Median | SD | |
|---|---|---|---|
| Age, y | 11.96 | 12.60 | 3.91 |
| BMI z score | 0.55 | 0.50 | 1.29 |
| Range of motion, ° | 151.16 | 151.00 | 7.89 |
| Right flexion, ° | 145.75 | 147.00 | 14.98 |
| Left flexion, ° | 145.91 | 147.50 | 14.89 |
| Right extension, ° | −3.86 | −3.00 | 5.43 |
| Left extension, ° | −3.86 | −4.00 | 5.96 |
| Carrying angle, ° | 10.35 | 10.00 | 4.61 |
TABLE 2.
Correlation between BMI z score, age, and orthopedic parameters according to Pearson correlation coefficient (r)
| BMI z score
|
Age
|
|||
|---|---|---|---|---|
| r | P | r | P | |
| Right range of motion | −0.54 | <0.01 | 0.18 | 0.05 |
| Left range of motion | −0.43 | <0.01 | 0.25 | <0.01 |
| Right flexion | −0.59 | <0.01 | 0.35 | <0.01 |
| Left flexion | −0.50 | <0.01 | 0.45 | <0.01 |
| Right extension | 0.20 | 0.04 | 0.09 | 0.34 |
| Left extension | 0.12 | 0.21 | 0.06 | 0.50 |
| Right carrying angle | 0.02 | 0.87 | 0.25 | <0.01 |
| Left carrying angle | 0.003 | 0.97 | 0.31 | <0.01 |
FIG. 2.
A, BMI z score versus right total range of motion. Linear regression demonstrates negative correlation between BMI z score and range of motion in the right elbow joint (r = −0.54, P <0.01). B, BMI z score versus left total range of motion. Linear regression demonstrates negative correlation between BMI z score and range of motion in the left elbow joint (r = −0.43, P <0.01).
Other demographic variables (age, sex, and ethnicity) were analyzed in relation to range of motion, flexion, and carrying angle (Tables 2 and 3). The Asian/Pacific Islander individuals were not included in analysis of ethnicity because of the small number of people in this group. Age correlated significantly with range of motion (right r = 0.18, P = 0.05; left r = 0.25, P <0.01), flexion (right r = 0.35, P <0.01; left r = 0.45, P <0.01) and carrying angle (right r = 0.25, P <0.01; left, r = 0.31, P <0.01) (Table 2). Girls showed a significantly increased range of motion (right P = 0.04; left P = 0.01) and carrying angle (right P = 0.11; left P <0.01) in comparison with the mean measurements in boys. Ethnicity affected right and left range of motion (P ≤ 0.01) (Table 3). We found no statistically significant relationships between laterality (handedness) and range of motion, extension, flexion, and carrying angle (results not shown).
TABLE 3.
Demographics in relation to orthopedic parameters: range of motion (ROM) and carrying angle
| Characteristic | Right ROM Mean ± SD, P value |
Left ROM Mean ± SD, P value |
Right carrying angle Mean ± SD, P value |
Left carrying angle Mean ± SD, P value |
|---|---|---|---|---|
| Sex | ||||
| M | 149.83 ± 7.35 | 149.79 ± 7.56 | 9.44 ± 4.80 | 10.93 ± 4.03 |
| F | 153.00 ± 8.14 | 153.62 ± 7.84 | 11.03 ± 5.00 | 13.28 ± 5.13 |
| P = 0.04 | P = 0.01 | P = 0.11 | P <0.01 | |
| Ethnicity | ||||
| White | 153.07 ± 8.40 | 153.52 ± 8.13 | 9.37 ± 4.38 | 11.56 ± 4.15 |
| P <0.01 | P <0.01 | P = 0.20 | P = 0.32 | |
| African American | 147.58 ± 5.83 | 147.03 ± 6.17 | 11.19 ± 5.39 | 12.61 ± 4.55 |
| Hispanic | 151.24 ± 7.15 | 152.05 ± 7.05 | 9.57 ± 5.18 | 10.81 ± 5.51 |
To determine which factors affect range of motion and carrying angle after adjusting for other variables, we included age, ethnicity, sex, and BMI in a multivariate analysis (Table 4). Multivariate analysis demonstrated that BMI independently predicted range of motion for both arms (right P <0.01; left P <0.01). Thus, a BMI z score increase of 1 (ie, 50th to 84th percentile) was associated with elbow range of motion decrease by 2.92° for the right arm and 1.95° for the left arm.
TABLE 4.
Multivariate linear regression analysis of parameters affecting elbow range of motion and carrying angle
| Measurement, n | Range of motion (R)* |
Range of motion (L)† |
Carrying angle (R)‡ |
Carrying angle (L)§ |
||||
|---|---|---|---|---|---|---|---|---|
| β (SE) | P | β (SE) | P | β (SE) | P | β (SE) | P | |
| BMI z score | −2.92 (0.51) | P <0.01 | −1.95 (0.53) | P <0.01 | 0.09 (0.38) | P = 0.81 | 0.19 (0.34) | P = 0.59 |
| Age | 0.18 (0.16) | P = 0.33 | 0.39 (0.17) | P = 0.03 | 0.33 (0.12) | P <0.01 | 0.37 (0.11) | P <0.01 |
| Sex | ||||||||
| [M] | P = 0.02 | P <0.01 | P = 0.08 | P <0.01 | ||||
| F | 2.95 (1.27) | P = 0.02 | 3.48 (1.31) | P <0.01 | 1.68 (0.94) | P = 0.08 | 2.38 (0.85) | P <0.01 |
| Ethnicity | ||||||||
| [White] | P = 0.08 | P <0.01 | P = 0.20 | P = 0.46 | ||||
| African American | −2.50 (1.45) | P = 0.09 | −4.31 (1.50) | P <0.01 | 1.95 (1.08) | P = 0.07 | 1.16 (0.97) | P = 0.23 |
| Hispanic | 1.26 (1.72) | P = 0.47 | 1.36 (1.78) | P = 0.45 | 1.02 (1.27) | P = 0.43 | 0.12 (1.15) | P = 0.92 |
Adjusted r2 = 0.33.
Adjusted r2 = 0.31.
Adjusted r2 = 0.08.
Adjusted r2 = 0.13. Variable [ ] = baseline; β = beta coefficient; SE = standard error.
Sex affected right range of motion (P = 0.02), left range of motion (P <0.01), and left carrying angle (P <0.01) and neared significance for right carrying angle (P = 0.08). Girls had a greater range of motion (by 2.95° for the right elbow; 3.48° for the left elbow) and carrying angle (by 1.68° for the right elbow; 2.38° for the left elbow) than did boys (Table 4). Sex differences in range of motion were not due to BMI differences (mean BMI z scores were 0.54 for boys and 0.52 for girls; P = 0.94).
Ethnicity also seemed to influence range of motion in linear multivariate analysis (P <0.01 for the left elbow and P = 0.08 for the right elbow when analysis of variance was used to test for significance of ethnicity in the multivariate regression). Our multivariate results indicated that African-Americans have decreased left range of motion (P <0.01) in comparison with whites (Table 4). Ethnicity did not correlate with right (P = 0.20) or left (P = 0.46) carrying angle (Table 4).
DISCUSSION
These results demonstrate a clear correlation between decreased range of motion and increased BMI in healthy children. This could be due to increased soft tissue around the elbow joint that results in a mechanical block to complete flexion. Our results show that increased BMI is associated with decreased flexion; both arms demonstrated a negative association between BMI and flexion. Thus, soft tissue mass may interfere with complete flexion of the elbow joint in children with larger BMIs.
Carrying angle demonstrated no correlation with BMI. These results suggest that carrying angle is not affected by increased tissue mass around the elbow joint, but rather by changes in the bony anatomy of the elbow joint during maturation.
Significantly decreased range of motion could lead to the difficulty overweight children have with weight maintenance and BMI reduction. Lazzer et al (13) found that obese adolescents spend more time during the day in light activities than do nonobese adolescents. However, obese individuals spend significantly less time in moderate activities and sports than do nonobese individuals. Increased BMI may limit a child’s ability to participate fully in athletics and moderate activities because of decreased range of motion. This study demonstrated a decrease in elbow joint range of motion, and we speculate that further studies will find a decrease in range of motion of other joints in relation to BMI. This hindrance on range of motion will decrease the ability of overweight children to raise daily energy expenditure, compounding the weight problem. This may contribute to other factors preventing an increase in daily energy expenditure, such as low self-esteem, depression, and low fitness levels. Our data on the elbow joint support the conclusion of Soucie et al (10) that increased BMI correlates with a decrease in range of motion of multiple joints (hips, knees, shoulders, elbows, and ankles). The elbow joint is the most commonly affected joint in the upper limb of patients with hemophilia (14). Inasmuch as Soucie et al (10) included only patients with hemophilia, it is difficult to determine whether BMI caused the sequelae of hemophilia to be more severe, thereby decreasing joint range of motion, or whether BMI alone decreased range of motion. Our data suggest that BMI is correlated with decreased range of motion in healthy children. However, owing to the cross-sectional nature of this study, we cannot conclude that BMI is a direct cause of decreased elbow range of motion.
One potential weakness of this study is that the height and weight data were self-reported by some of the participants, whereas others were measured and weighed in the clinic. This caused decreased precision in our measurements. Previous studies have demonstrated that self-reported height and weight correlate with actual height and weight, although children will tend to under-report weight and overreport height (15–19). These studies suggest that our study participants who self-reported height and weight most likely reported a lower weight and taller height than their actual measurements. This would cause us to miss children that are at risk for or are overweight.
These results demonstrated a decrease in elbow range of motion of 2° to 3° for each z score increase of 1. This most likely does not affect a child with a z score of 1.6 (almost overweight) because the elbow range of motion would be limited only by approximately 3° to 5°. However, as a child becomes more overweight a z score of 5.6 (the highest BMI z score in this study population) would have elbow range of motion limited by approximately 11° to 17° compared with a child with a z score of 0. This is a substantial amount of elbow range of motion limitation that could conceivably limit participation in activities that require full elbow range of motion.
These results can be generalized to the normal pediatric population. Although the study participants were recruited at a pediatric orthopedic clinic, the majority were healthy siblings of patients who had come along for the clinic visit. The patients who were included were carefully selected to exclude anyone with a previous pathological condition of the elbow joint or a disease process thought to affect the elbow joint.
The impact of decreased range of motion on physical activity must be explored further. Overweight children expend more energy than do lean children at the same level of physical activity (20). However, the overweight child must want to participate in activities to begin expending energy. If increased BMI leads to decreased range of motion, this could in turn hinder participation in sports and exercise, thereby decreasing physical activity and decreasing energy expenditure. Inasmuch as the decreased energy expenditure can lead to an increased BMI, a vicious cycle is established. The decreased range of motion may additionally lead to decreased energy expenditure for the same type of activity because the amount of muscle activity will be less and the overall and cumulative energy expended over time will be diminished. When combined with the factors listed earlier—depression, lower self-esteem, increased bullying or victimization by bullies, and negative health consequences—loss of elbow range motion makes it even more difficult for overweight children to lower their BMI.
One note of interest is that laterality (handedness) did not correlate with range of motion on either the dominant or the nondominant side. These results indicate that although the right or left arm may be used more frequently for daily activities such as writing or throwing a ball, range of motion is more dependent on factors such as genetic predisposition to flexibility or soft tissue mass around the joint.
Overall, this study provides preliminary data demonstrating that increased BMI is correlated with a decrease in pediatric elbow joint range of motion. More systematic investigations are needed to confirm our results. Additionally, the influence of pediatric BMI on range of motion of other joints in growing children and adolescents requires further study.
Studies evaluating the functional range of motion of joints required for children to engage fully in sports and exercise are needed. These would help determine whether the results reported here have a clinically significant impact on overweight children’s ability to participate in sports and exercise. These findings will have serious implications in planning exercise and other strategies aimed at preventing and treating overweight in the pediatric population.
Acknowledgments
Supported in part by NIH Grant K24 DK060617 (M.B.H.). No source of funding had any involvement in the development, execution, or analysis of the study.
Footnotes
None of the authors has any potential conflict of interest, real or perceived.
References
- 1.Krebs NF, Jacobson MS. Prevention of pediatric overweight and obesity. Pediatrics. 2003;112:424–30. doi: 10.1542/peds.112.2.424. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Flegal KM, Carroll MD, et al. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288:1728–32. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 3.Fowler-Brown A, Kahwati LC. Prevention and treatment of overweight in children and adolescents. Am Fam Physician. 2004;69:2591–8. [PubMed] [Google Scholar]
- 4.Sjoberg RL, Nilsson KW, Leppert J. Obesity, shame, and depression in school-aged children: a population-based study. Pediatrics. 2005;116:e389–92. doi: 10.1542/peds.2005-0170. [DOI] [PubMed] [Google Scholar]
- 5.Janssen I, Craig WM, Boyce WF, et al. Associations between overweight and obesity with bullying behaviors in school-aged children. Pediatrics. 2004;113:1187–94. doi: 10.1542/peds.113.5.1187. [DOI] [PubMed] [Google Scholar]
- 6.Barlow SE, Dietz WH. Obesity evaluation and treatment: Expert Committee recommendations. The Maternal and Child Health Bureau, Health Resources and Services Administration and the Department of Health and Human Services. Pediatrics. 1998;102:E29. doi: 10.1542/peds.102.3.e29. [DOI] [PubMed] [Google Scholar]
- 7.Escamilla RF, Fleisig GS, Zheng N, et al. Kinematic comparisons of 1996 Olympic baseball pitchers. J Sports Sci. 2001;19:665–76. doi: 10.1080/02640410152475793. [DOI] [PubMed] [Google Scholar]
- 8.Button C, MacLeod M, Sanders R, et al. Examining movement variability in the basketball free-throw action at different skill levels. Res Q Exerc Sport. 2003;74:257–69. doi: 10.1080/02701367.2003.10609090. [DOI] [PubMed] [Google Scholar]
- 9.Escalante A, Lichtenstein MJ, Hazuda HP. Determinants of shoulder and elbow flexion range: results from the San Antonio Longitudinal Study of Aging. Arthritis Care Res. 1999;12:277–86. [PubMed] [Google Scholar]
- 10.Soucie JM, Cianfrini C, Janco RL, et al. Joint range-of-motion limitations among young males with hemophilia: prevalence and risk factors. Blood. 2004;103:2467–73. doi: 10.1182/blood-2003-05-1457. [DOI] [PubMed] [Google Scholar]
- 11.Clinical Growth Charts: United States. Centers for Disease Control and Prevention, National Center for Health Statistics. [Accessed August 31, 2004];2000 http://www.cdc.gov/growthcharts.
- 12.London JT. Kinematics of the elbow. J Bone Joint Surg Am. 1981;63:529–35. [PubMed] [Google Scholar]
- 13.Lazzer S, Boirie Y, Bitar A, et al. Assessment of energy expenditure associated with physical activities in free-living obese and nonobese adolescents. Am J Clin Nutr. 2003;78:471–9. doi: 10.1093/ajcn/78.3.471. [DOI] [PubMed] [Google Scholar]
- 14.Hogh J, Ludlam CA, Macnicol MF. Hemophilic arthropathy of the upper limb. Clin Orthop. 1987;218:225–31. [PubMed] [Google Scholar]
- 15.Abalkhail BA, Shawky S, Soliman NK. Validity of self-reported weight and height among Saudi school children and adolescents. Saudi Med J. 2002;23:831–7. [PubMed] [Google Scholar]
- 16.Brener ND, McManus T, Galuska DA, et al. Reliability and validity of self-reported height and weight among high school students. J Adolesc Health. 2003;32:281–7. doi: 10.1016/s1054-139x(02)00708-5. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Patterson CM, Hills AP. A comparison of self-reported and measured height, weight and BMI in Australian adolescents. Aust N Z J Public Health. 2002;26:473–8. doi: 10.1111/j.1467-842x.2002.tb00350.x. [DOI] [PubMed] [Google Scholar]
- 18.Goodman E, Hinden BR, Khandelwal S. Accuracy of teen and parental reports of obesity and body mass index. Pediatrics. 2000;106 (1 Pt 1):52–8. doi: 10.1542/peds.106.1.52. [DOI] [PubMed] [Google Scholar]
- 19.Elgar FJ, Roberts C, Tudor-Smith C, et al. Validity of self-reported height and weight and predictors of bias in adolescents. J Adolesc Health. 2005;37:371–5. doi: 10.1016/j.jadohealth.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Norman AC, Drinkard B, McDuffie JR, et al. Influence of excess adiposity on exercise fitness and performance in overweight children and adolescents. Pediatrics. 2005;115:e690–6. doi: 10.1542/peds.2004-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]