Abstract
Background
Treatment of ulcerative proctitis has not been well studied in pediatric populations. We conducted an open-label trial to evaluate the clinical efficacy of a mesalamine suppository (500 mg) to treat pediatric patients with mild to moderate ulcerative proctitis.
Methods
Pediatric patients (5–17 years of age) with ulcerative proctitis were enrolled for baseline evaluations, including a flexible sigmoidoscopic (or colonoscopic) assessment with biopsies performed at study entry. Eligible patients were started on mesalamine suppositories (500 mg) at bedtime. Two follow-up visits were scheduled after 3 and 6 weeks of treatment. The dose could be increased to 500 mg twice daily at the week 3 follow-up visit if deemed appropriate by the investigator based on the Disease Activity Index (DAI) assessment. The primary outcome measure was a DAI derived from a composite score of stool frequency, urgency of defecation, rectal bleeding, and general well-being.
Results
Forty-nine patients were included in the intent-to-treat analysis. The mean DAI value decreased from 5.5 at baseline to 1.6 and 1.5 at weeks 3 and 6, respectively (P < 0.0001). Only 4 patients had their dose increased to 500 mg twice daily at week 3. Forty-one patients experienced at least one adverse event, most of which were deemed mild and unrelated to study therapy. The most common treatment-emergent adverse events were gastrointestinal (n = 30, 61.2%).
Conclusions
This study showed that a daily bedtime dose of a 500 mg mesalamine suppository is safe and efficacious in children with ulcerative proctitis.
Keywords: mesalamine, ulcerative proctitis, pediatric patients, colitis, disease activity index, inflammatory bowel disease, colitis
Ulcerative proctitis constitutes a large portion of newly diagnosed ulcerative colitis cases.1–4 It is defined as disease limited to the distal colon (the rectum), not extending beyond 15–20 cm proximal to the anal verge. The first line treatment for ulcerative proctitis is 5-aminosalicylic acid (5-ASA/mesalamine), administered as an oral medication or as rectal suppositories (1 g/day), enemas, foams, or gels (1–4 g/day).5–7 The therapeutic effect of mesalamine is attributed to its local concentration on the intestinal mucosa and not to its serum levels, which are very low due to a rapid metabolism of mesalamine into its main acetylated metabolites excreted in urine.8 Studies have shown that higher concentrations of the drug in inflamed colonic mucosa enhance response to treatment.9,10
Current treatment options aim at inducing and maintaining remission of disease-related symptoms and decrease the risk of drug-related adverse events.11 Mesalamine 500 mg suppositories have been used worldwide for more than 20 years with proven safety and efficacy in the adult population.5,12–16 Most adult patients respond to a twice daily dosing, in the morning and at bedtime. However, about 20% of ulcerative colitis patients have disease onset before 20 years of age.17–19 Pediatric patients will thus require long-term maintenance therapy with 5-aminosalicylate (5-ASA).20 Even though the presenting symptoms and therapeutic management options are similar in adults and children, data on the efficacy and safety of short- and long-term mesalamine treatment in pediatric patient populations are lacking.21 Clearly, clinical trials are needed for pediatric patients with inflammatory bowel disease (IBD) in general and ulcerative proctitis in particular. This is especially true considering the recently documented increase in the incidence rate of IBD among pediatric patients (from 9.5 per 100,000 in 1994 to 11.4 per 100,000 in 2005).19
In the present study we evaluated the clinical efficacy and safety of mesalamine 500 mg suppositories in a population of pediatric patients with ulcerative proctitis.
PATIENTS AND METHODS
Patients
Pediatric patients, 5–17 years of age, were enrolled if they had ulcerative proctitis confirmed by flexible sigmoidoscopy or colonoscopy and biopsy performed within 7 days of the baseline visit.
Patients were excluded if they were diagnosed with any other digestive disease (e.g., Crohn’s disease, irritable bowel syndrome, or proctitis secondary to infection, radiation, etc.). Additionally, patients were excluded if inflammatory changes were observed grossly or found microscopically beyond the rectum (more than 15 cm from the anal margin; a biopsy at 20 cm had to be normal). Patients were excluded if they were not on a stable oral dose of 5-ASA or sulfasalazine (oral dose of no more than 60 mg/kg/day) or if any new form of mesalamine (oral, enema, or suppositories) had been used in the 30 days preceding baseline. Patients were also excluded if they were using any other medications including corticosteroids, immuno-modulators, probiotics, or biologics within the month preceding baseline evaluation. Additional exclusion criteria included presence of any contraindication to the use of mesalamine or other related products (e.g., history of hypersensitivity to aminosalicylates), presence of clinically significant impairment of renal or hepatic function, a clinically significant urinary tract obstruction, history of idiopathic pancreatitis, presence of coagulation disorders or use of anticoagulant drugs, including aminosalicylic acid, pregnancy or lactation or if of childbearing potential but not using reliable contraception, or use of any other experimental drug in the 30 days prior to enrollment. Only one patient was a smoker and was not excluded.
Institutional Review Board (IRB) approvals for the study were obtained at each site. Written informed consent and assent were obtained from parents and patients prior to enrollment, as required by each IRB.
Study Design
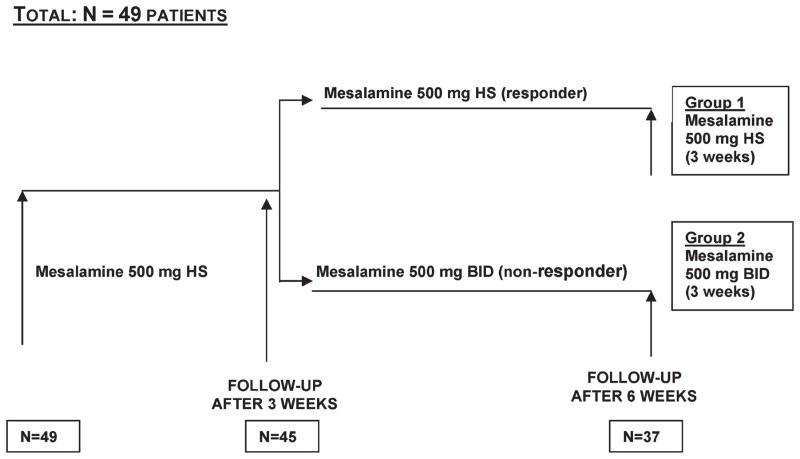
The study was a multicenter, open-label, non-randomized, single group design (Fig. 1) in pediatric patients enrolled at 15 sites across Canada, the United States, and Poland between September 2002 and April 2006. Following informed consent, all patients meeting inclusion/exclusion criteria were assigned to the same treatment group: mesalamine (Canasa or Salofalk, Axcan Pharma, Mont St-Hilaire, QC, Canada) 500 mg suppositories, taken every day at bedtime for a 3-week treatment period. Subsequently, patients either remained on that regimen for another 3 weeks if their response was deemed adequate as evaluated by the investigator according to the modified Disease Activity Index (DAI) score at the week 3 visit, or their dose regimen was increased to 500 mg suppositories twice daily (morning and at bedtime) if their response was deemed inadequate.
FIGURE 1.
Schematic diagram of the study design. HS, once a day at bedtime; BID, twice daily.
The study entailed three visits over 6 weeks: 1) a baseline evaluation, 2) one follow-up visit after 3 weeks of treatment, and 3) a final follow-up visit after an additional 3 weeks of treatment. At baseline, medical history and demographic data were collected. Screening assessments involved a complete investigation to confirm the patient’s diagnosis of ulcerative proctitis by a flexible sigmoidoscopy or colonoscopy and three mucosal biopsies at 5, 10, and 20 cm from the anal margin.
The proctitis was graded by a 15-point DAI modified from Walmsley et al22 (Table 1). Similar to Walmsley’s Simple Clinical Colitis Activity Index, the DAI used in this study consisted of scores for six clinical disease components (stool frequency (day), stool frequency (night), urgency of defecation, blood in stool, extracolonic features, and on the general well-being of the patient) that are represented in a semiquantitative rating scale. A DAI score of ≥4 and <11 on a 15-point index (excluding the extracolonic features score) corresponded to a mild to moderate severity in ulcerative proctitis symptoms (inclusion criterion).
TABLE 1.
Disease Activity Index (DAI) Scoring System*
| Clinical Criterion | Score/Subscales | |
|---|---|---|
| Stool Frequency (Day) | 0 | 0–2 Stools/day |
| 1 | 3–4 Stools/day | |
| 2 | 5–7 Stools/day | |
| 3 | ≥8 Stools/day | |
| Stool Frequency (Night) | 0 | Absence of stool |
| 1 | 1–2 Stools/night | |
| 2 | ≥3 Stools/night | |
| Urgency of defecation | 0 | No urgency |
| 1 | Hurry | |
| 2 | Immediately | |
| 3 | Incontinence | |
| Blood in stool | 0 | No blood found |
| 1 | Trace | |
| 2 | Occasionally frank | |
| 3 | Usually frank | |
| General well being | 0 | Very well |
| 1 | Slightly below par | |
| 2 | Poor | |
| 3 | Very poor | |
| 4 | Terrible | |
| Extracolonic features | 1 | Per manifestation of Arthritis/arthralgias, Fever, Erythema nosodum, Episcleritis/uveitis |
Modified from Walmsley et al.20 Total score is calculated by adding the evaluated scores for each of the first 5 clinical features. The extracolonic features were not included in the score calculations.
A pathologist blinded to dosage classified the severity of histological inflammation of the rectal biopsies. Tissue appearance score ranged from 0 to 3 (0 = normal; 1 = minimal increase in acute and chronic inflammatory cells with no morphological distortion or crypt abscess; 2 = moderately increased inflammatory infiltrates with occasional crypt abscess formation and some crypt distortion; 3 = markedly increased inflammatory infiltrates with diffuse crypt abscesses and/or destruction), based on an established scoring system.23
Patients also underwent routine hematology, serum chemistry, urinalysis, stool culture, a pregnancy test if indicated, and pANCA (perinuclear antineutrophil cytoplasmic antibody) and ASCA (anti-Saccharomyces cerevisiae antibody) testing. All patients were asked to complete a daily diary in which they were to record bowel frequency, blood in stools, urgency, retention time of suppository, general well-being, other symptoms, as well as any concomitant medication.
Within 7 days of completing the first 3-week treatment period (i.e., study days 22–28), the patients returned to the hospital/clinic to undergo the first follow-up visit. Adverse events and concomitant medications were then reported. General assessment of adverse events was performed by reviewing the patient daily diaries and by open-ended questioning of the parent(s) or legal guardian(s) and the patient. At this follow-up visit the patients also underwent a physical examination and the investigator determined the Physician Global Assessment (PGA).24 This scale is based on the physician’s overall clinical assessment of the patient’s symptoms, physical exam, laboratory data, and the sigmoidoscopic findings (when applicable) and ranges from 1 to 5 (1 = much improved; 2 = minimally improved, 3 = no change, 4 = worse, 5 = much worse). An evaluation of the patient’s DAI scores, as performed at visit 1, was also repeated. DAI scoring was performed based on the overall clinical assessment of the physician of the patient’s symptoms and symptoms collected in the patient diary over the 3 days immediately preceding the study visit.
Patients who had a satisfactory response to therapy (DAI scores decreased by 25% or more, and the investigator deemed that a greater reduction was not necessary) continued on the same dosage (500 mg daily) until the sixth week of study. Patients who had an inadequate response to treatment (i.e., their DAI had not improved by at least 25%, or the investigator deemed that a greater reduction was safe and beneficial) had their dosage increased to 500 mg twice daily. If response to treatment was deemed inadequate from a safety or efficacy (after the dose increase) viewpoint, the patient was withdrawn from the trial and completed the end of study evaluations.
Within 7 days of completing the second three weeks of treatment (i.e., study days 43–49), the physical examination and clinical laboratory (hematology, biochemistry, and urinalysis), tests were repeated including mucosal biopsies at 5, 10, and 20 cm from the anal margin. Adverse events and concomitant medication were recorded.
Statistical Analysis
Our goal was to recruit 45 patients to demonstrate a 50% reduction in DAI total score between week 6 and baseline, at a significance level of 5% and a power of 80%. The intent-to-treat (ITT) population represented the primary analysis population and included all enrolled patients who received at least one dose of treatment. The primary efficacy parameter was the change from baseline in the total DAI score to week 6 using the Last Observation Carried Forward (LOCF) method for missing data. Secondary efficacy parameters included the change from baseline for each individual component of the DAI (to weeks 3 and 6); the change in the total DAI score from baseline to week 3 and from week 3 to week 6; remission rate at weeks 3 and 6; responder rate at weeks 3 and 6; correlation between pANCA and ASCA results and response to treatment at week 6; and PGA. A patient was considered in remission if the postbaseline DAI score was ≤3 and the postbaseline “Blood in Stool” DAI subscale score was <3. A patient was considered a responder if the postbaseline DAI score declined by 25% from the baseline DAI score.
Statistical inference (Wilcoxon signed rank test) was performed on the DAI scores (individual and total). A 0.05 significance level was used in all tests of pre- and post-treatment differences. Spearman correlations were computed between baseline pANCA and ASCA results and response to treatment week 6.
Descriptive statistics performed on all efficacy parameters are presented for both therapeutic regimens combined. Quantitative parameters are summarized by the population size (N for sample size and n for available data), mean, standard deviation, median, minimum, and maximum values. The population size (N for sample size and n for available data) and percentage (based on available data) for each class of qualitative parameters are presented. Drop-outs were not replaced and were included in the data analysis to the extent that these data were available.
Since only four patients received the 500 mg twice daily dosing, no descriptive or inferential statistics were performed by dosing regimen.
Safety was assessed on the basis of adverse events (AEs), clinical laboratory evaluations, vital signs, and physical examination. Only descriptive statistics were conducted on safety parameters. SAS software (Cary, NC; v. 8.2 or higher, Windows XP) was used to generate all statistical outputs. Since the total number of patients/site was small, all sites were pooled together and statistical analyses were not adjusted for site.
RESULTS
Following informed consent, 54 patients were screened and 49 (25 males and 24 females) were enrolled. Patients were excluded for a normal biopsy at baseline that ruled out proctitis (n = 1), disease extending beyond the rectum, to the 20-cm biopsy (n = 2), and biopsy confirming Crohn’s disease (n = 2). The study population was male (51%) and primarily Caucasian (87.8%) with a mean age of 12.5 ± 3.51 (SD) years (Table 2). Forty-one (83.7%) subjects had a history of ulcerative proctitis, with a mean time since onset of 1.7 ± 1.82 years. Only 36.7% of recruited patients were already taking a stable dose of 5-ASA or sulfasalazine (average dose 2.0 g/day) at dosages compliant with the protocol.
TABLE 2.
Demographic and Baseline Characteristics (N = 49)
| Parameter | Statistic | Mesalamine 500 mg (HS or BID) |
|---|---|---|
| Gender | n | 49 |
| Male | n (%) | 25 (51.0%) |
| Female | n (%) | 24 (49.0%) |
| Age (years) | n | 49 |
| Mean (SD) | 12.5 (3.51) | |
| Median (Min, Max) | 13.0 (5, 17) | |
| Race | n | 49 |
| Caucasian | n (%) | 43 (87.8%) |
| Black | n (%) | 3 (6.1%) |
| Other | n (%) | 3 (6.1%) |
| Smoking | n | 49 |
| Smoker | n (%) | 1 (2.0%) |
| Non-Smoker | n (%) | 48 (98.0%) |
| History of UP | n | 49 |
| No | n (%) | 8 (16.3%) |
| Yes | n (%) | 41 (83.7%) |
| If Yes, Time Since Onset (years) | n | 41 |
| Mean (SD) | 1.7 (1.82) | |
| Median (Min, Max) | 1.0 (0, 8) | |
| pANCA at Baseline | n | 48 |
| Positive | n (%) | 13 (27.1%) |
| Negative | n (%) | 35 (72.9%) |
| ASCA at Baseline | n | 48 |
| Positive | n (%) | 2 (4.2%) |
| Negative | n (%) | 46 (95.8%) |
| Disease Activity Index (DAI) at Baseline | n | 49 |
| 4 | n (%) | 12 (24.5%) |
| 5 | n (%) | 19 (38.8%) |
| 6 | n (%) | 9 (18.4%) |
| 7 | n (%) | 5 (10.2%) |
| 8 | n (%) | 2 (4.1%) |
| 9 | n (%) | 1 (2.0%) |
| 10 | n (%) | 0 |
| 11 | n (%) | 1 (2.0%) |
UP = Ulcerative Proctitis; HS = one a day at bedtime; BID = twice daily; pANCA = perinuclear antineutrophil cytoplasmic antibody; ASCA = anti-Saccharomyces cerevisiae antibody.
Of the 49 enrolled patients, 45 patients (91.8%) remained on the 500 mg dosage for the entire duration of their involvement in the trial. Only four patients (8.2%) had their dose increased from 500 mg to 1000 mg (500 mg twice daily) at the first follow-up visit. The dose increase was due to worsening or no change in the patient’s condition, as evaluated by the PGA despite a DAI close to 4 in three of these four patients.
Thirty-seven patients (75.5%) completed the trial. Nearly 25% (n = 12) of patients terminated early due to treatment-emergent adverse event (n = 1), biopsy reading not confirming ulcerative proctitis diagnosis (n = 7), treatment failure (n = 2), C. difficile in stools (n = 1), or poor protocol compliance (n = 1). Of note is that patients could be enrolled in the study pending biopsy and stool culture results. If proctitis was not confirmed in these patients they were withdrawn from the study but included in the safety analysis. Among the 37 patients who completed the 6-week trial, 29 (78.4%) underwent all study procedures, while eight patients (21.6%) did not (most missed follow-up stool culture).
Treatment compliance, assessed by suppository returns, was 97.9% ± 5.8% from baseline to week 3 and 96.7% ± 9.1% from week 3 to week 6.
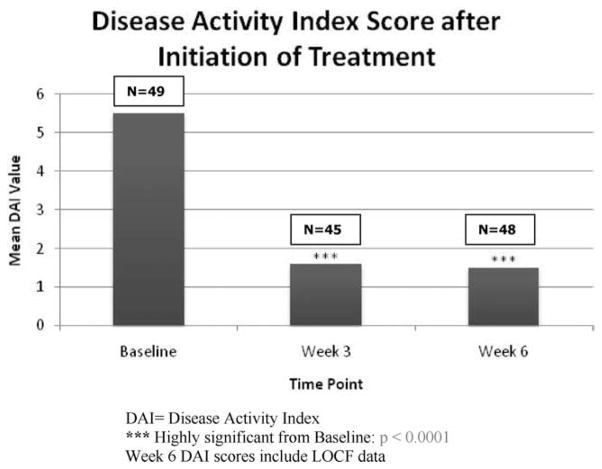
The ITT population (n = 49) had DAI mean scores at baseline of 5.5 ± 1.4 (range 4–11) (Tables 3, 4). Significant reductions from baseline were observed in mean DAI scores at week 3 (1.6 ± 2.0; P < 0.0001) and at week 6 (1.5 ± 1.9; P < 0.0001) (Fig. 2). The primary efficacy endpoint was the change in DAI score from baseline to week 6. At week 6 the mean DAI score had decreased by −4.0 ± 2.1 (P < 0.0001 versus baseline) (Table 3). No differences were observed in the change in DAI score between weeks 3 and 6.
TABLE 3.
Disease Activity Index (DAI) Score (Intent-To-Treat-Population; N=49)
| Time Point | Statistic | Mesalamine 500 mg (HS or BID*) |
|---|---|---|
| Baseline | n | 49 |
| Mean (SD) | 5.5 (1.4) | |
| Median (Min, Max) | 5.0 (4, 11) | |
| Week 3 | n | 45 |
| Mean (SD) | 1.6 (2.0) | |
| Median (Min, Max) | 1.0 (0, 8) | |
| Change from Baseline to Week 3 | n | 45 |
| Mean (SD) | −3.7 (1.8) | |
| Median (Min, Max) | −4.0 (−8, 2) | |
| P-valuea | <0.0001 | |
| Week 6 LOCF | n | 48 |
| Mean (SD) | 1.5 (1.9) | |
| Median (Min, Max) | 1.0 (0, 6) | |
| Change from Baseline to Week 6 | n | 48 |
| Mean (SD) | −4.0 (2.1) | |
| Median (Min, Max) | −4.0 (−11, 2) | |
| P-valuea | <0.0001 | |
| Change from Week 3 to Week 6 | n | 45 |
| Mean (SD) | 0.0 (1.7) | |
| Median (Min, Max) | 0.0 (−6, 6) | |
| P-valueb | 0.9657 |
P-value from a Wilcoxon Signed Rank Test.
HS= one a day at bedtime; BID= twice daily.
TABLE 4.
Individual DAI Components and PGA After Mesalamine Treatment (500 mg HS or BID) in the ITT Population, N = 49
| Time Point | Stool Frequency Day | Stool Frequency Night | Urgency of Defecation | Blood in Stools | General Well Being | PGAb |
|---|---|---|---|---|---|---|
| Baseline | ||||||
| N | 49 | 49 | 49 | 49 | 49 | |
| Mean (SD) | 0.9 (0.8) | 0.4 (0.6) | 1.2 (0.8) | 2.0 (1.0) | 0.9 (0.7) | – |
| Median (Min, Max) | 1.0 (0, 3) | 0.0 (0, 2) | 1.0 (0, 3) | 2.0 (0, 3) | 1.0 (0, 3) | |
| Week 3 | ||||||
| N | 45 | 45 | 45 | 45 | 45 | 44 |
| Mean (SD) | 0.3 (0.5) | 0.1 (0.3) | 0.3 (0.6) | 0.4 (0.8) | 0.5 (0.7) | 1.5 (0.8) |
| Median (Min, Max) | 0.0 (0, 2) | 0.0 (0, 1) | 0.0 (0, 3) | 0.0 (0, 3) | 0.0 (0, 2) | 1.0 (1, 4) |
| Change from Baseline to Week 3 | ||||||
| N | 45 | 45 | 45 | 45 | 45 | |
| Mean (SD) | −0.6 (0.8) | −0.3 (0.5) | −0.8 (0.9) | −1.5 (1.1) | −0.5 (0.7) | – |
| Median (Min, Max) | −1.0 (−3, 1) | 0.0 (−2, 1) | −1.0 (−3, 2) | −2.0 (−3, 1) | −1.0 (−2, 1) | |
| P-valuea | <0.0001 | 0.0017 | <0.0001 | <0.0001 | <0.0001 | |
| Week 6 LOCF | ||||||
| N | 48 | 48 | 48 | 48 | 48 | 47 |
| Mean (SD) | 0.4 (0.6) | 0.0 (0.6) | 0.3 (0.6) | 0.4 (0.8) | 0.4 (0.6) | 1.6 (1.1) |
| Median (Min, Max) | 0.0 (0, 2) | 0.0 (−2, 1) | 0.0 (0, 2) | 0.0 (0, 3) | −0.5 (−2, 0) | 1.0 (1, 4) |
| Change from Baseline to Week 6 LOCF | ||||||
| N | 48 | 48 | 48 | 48 | 48 | |
| Mean (SD) | −0.5 (1.0) | −0.4 (0.6) | −0.9 (0.9) | −1.6 (1.1) | −0.6 (0.6) | – |
| Median (Min, Max) | 0.0 (−3, 2) | 0.0 (−2, 1) | −1.0 (−3, 1) | −2.0 (−3, 0) | −0.5 (−2, 0) | |
| P-valuea | 0.0007 | 0.0002 | <0.0001 | <0.0001 | <0.0001 | |
| Change from Week 3 to Week 6 LOCF | ||||||
| N | 45 | 45 | 45 | 45 | 45 | 44 |
| Mean (SD) | 0.2 (0.6) | −0.1 (0.3) | 0.0 (0.5) | 0.0 (0.8) | −0.1(0.4) | 0.2 (1.1) |
| Median (Min, Max) | 0.0 (−1, 2) | 0.0 (−1, 0) | 0.0 (−1, 2) | 0.0 (−2, 3) | 0.0 (−1, 1) | 0.0 (−3, 3) |
| P-valuea | 0.1133 | 0.2500 | >0.999 | 0.6415 | 0.5078 | 0.3789 |
P-value from a Wilcoxon Signed Rank Test.
Collected only at weeks 3 and 6; HS= one a day at bedtime; BID= twice daily; DAI= disease activity index; PGA= physician global assessment; SD= standard deviation.
FIGURE 2.
DAI scores throughout study visits. DAI, Disease Activity Index; ***highly significant from baseline. For week 6 DAI scores the LOCF method was used for missing data.
Significant differences were observed for all individual DAI components between baseline and week 3 and baseline and week 6 (Table 4), although no statistical differences were observed in individual DAI components between week 3 and week 6.
Data were available from 45 patients who could be analyzed for response and remission status. Response was achieved in the vast majority of patients (n = 42; 93.3%) at week 3 and at week 6 (n = 44; 91.7%). Similarly, the majority of patients met criteria for remission at week 3 (n = 37; 82.2%) and at week 6 (n = 39; 81.3%).
At baseline the majority of subjects were negative for pANCA (n = 35; 72.9%) and ASCA (n = 46; 95.8%). Patient response to treatment did not correlate with either pANCA or ASCA in this study population.
Descriptive statistics for the ITT population are presented for PGA scores obtained at week 3 and week 6 (see Table 4). At week 3 physicians assessed 85.4% of patients (n = 41) as “minimally improved” (n = 6) or “much improved” (n = 35). Similarly, in the PGA scores at week 6 the physicians assessed 81.25% of patients (n = 39) as “minimally improved” (n = 5) or “much improved” (n = 34). By weeks 3 and 6, very few patients were assessed as “no change” (week 3: n = 4; (8.3%); week 6 n = 2; (4.2%), “worse” (week 3: n = 3 (6.3%); week 6 n = 7; (14.6%) or “much worse” (week 3: n = 0; 0%; week 6 n = 0; 0%).
All 49 patients included in the safety population received at least one dose of study drug. The total extent of exposure to study treatment was 41.0 ± 9.3 days. A total of 41 patients out of 49 (83.7%) reported at least one treatment-emergent adverse event (TEAE) and a total of 169 TEAEs. The majority of TEAEs (69.2%) were mild and were considered not related to study therapy. The most common TEAEs judged to possibly be related to study drug were in the category of gastrointestinal disorders and nervous system disorders, the most frequent in each respective category being abdominal pain (16.4%) and headache (8.2%). Three serious TEAEs were reported by two patients: one case of anemia requiring a blood transfusion (considered two events) and one case of emotional disorder. None were considered to be related to study therapy. Gastrointestinal disorders (mostly abdominal pain, diarrhea, or vomiting) represented the TEAEs reported by the highest number of patients (n = 30, 61.2%). Other TEAEs included: 1) burning/heat sensation at suppository administration site (n = 9, 18.4%); 2) headache (n = 13, 26.5%); and 3) retrosternal pain/heartburn (n = 6, 12.2%). Hematology, serum chemistry, and urinalysis laboratory values remained within normal ranges and/or the observed changes were deemed not clinically significant.
DISCUSSION
Our study substantiates the clinical efficacy and safety of mesalamine 500 mg suppositories taken once a day for the treatment of ulcerative proctitis in pediatric patients age 5–17 years. Improvement of the proctitis, as measured by DAI scores, was documented within 3 weeks in the majority of patients. Moreover, each individual component (stool frequency and urgency, bloody stools, extra-intestinal manifestations, and general well-being) of the DAI score also showed significant improvement.
Our results demonstrate that the 500 mg daily suppository was an effective and well-tolerated dose for this pediatric patient population with mild to moderate disease activity at baseline. All clinical parameters and physician assessments were clearly improved by week and maintained through week 6. Several authors reported on the topic of improving adherence to treatment in patients with IBD and the factors that influence it,25–27 notably the dosing regimen. Compliance is always much higher during a study, where monitoring and follow-up is more intense than in the typical clinical setting. The rate of nonadherence to medication regimens in IBD patients is estimated between 40% and 60%,28–30 strongly supporting a once daily dosing plan.25–27,31,32 While compliance was not an endpoint for our study, we suspect that the once-daily dosing has an added advantage in ensuring better compliance in patients, especially in pediatric patients.
While the exact time to response and remission with the 500 mg dose of rectal mesalamine could not be established utilizing this study design, the vast majority of patients met the criteria of treatment responders after 3 weeks of treatment and continued to meet the responder criteria after 6 weeks. The same is true for the ≈80% of patients who met remission criteria at both weeks 3 and 6. Supporting these data is the observation that the mean total DAI score and all six individual component scores showed significant improvements indicative of clinical response after the first 3 weeks of treatment. This may suggest that the time to maximal efficacy may be less than 3 weeks; however, additional clinical trials would need to be conducted. The clinical effects of mesalamine rectal therapy were also maintained for the additional 3 weeks of therapy. In contrast, in a study in adult patients with ulcerative proctitis receiving mesalamine 1000 mg (either 500 mg twice daily or 1000 mg at bedtime), utilizing similar clinical endpoints, patients demonstrated significant clinical improvement at week 3 and reported additional significant improvements in DAI scores from week 3 to week 6.33
In the adult population the presence of pANCA and ASCA serum antibodies has been found to be clinically relevant in the diagnosis of IBD. Changes in these serum antibodies have been shown to correlate with disease activity in adults.34–38 It is believed that these serologic markers do not provide the same value in children.21 In this particular pediatric study, no correlations could be detected between patients’ responses to treatment (responder versus not responder) at week 6 and baseline pANCA or ASCA results (positive versus negative).
The majority of TEAEs were mild to moderate, transient, nonserious, and not related to study therapy. Very few patients were unable to tolerate the therapy, as evidenced by the very low discontinuation and high adherence rates. The most common TEAEs related to study drug included abdominal pain and headache. The safety profile of mesalamine suppositories in this pediatric population is comparable to that reported for adults.33,39 The most prevalent TEAEs noted in both children and adults were related to gastrointestinal complaints. No new safety issues were identified in this study or treatment populations. In adults, rare cases of acute intolerance syndrome, characterized by cramping, acute abdominal pain, and bloody diarrhea, sometimes fever, headache, and a rash, have been reported.40,41 These reactions were not observed in our study; however, physicians should be aware of these events when using mesalamine therapy in children.
One limitation to our open-label and uncontrolled study is that we did not use a comparator to 5-ASA such as sulfasalazine or corticosteroids to determine how remission rates compare with the other treatments. The use of a placebo group was not used due to ethical considerations. Since 5-ASA is recommended as first-line treatment of ulcerative colitis, we did not include a second treatment arm in the trial. Also, since suppository is the ideal formulation to use for the treatment of proctitis, no other first- or second-line comparator was available. Another limitation to our study is the 6 weeks duration of follow-up. In longer-term clinical trials, ≈80% of patients with mild and 90% of those with moderate ulcerative colitis become asymptomatic within 6 months of treatment (sulfasalazine, 5-ASA, and/or corticosteroids), although within 1 year 27% require corticosteroid treatment.42,43 Future studies are needed that follow patients for 1 year or longer and evaluate long-term remission rates and the frequency of relapse in this young patient population. Of note is that rectally administered 5-ASA has been suggested as the best available therapy for maintenance of remission.7
This study confirms the efficacy and safety of 5-ASA suppositories in children and adolescents age 5–17 years suffering from ulcerative proctitis. The majority of subjects met both responder and remission criteria after 3 and 6 weeks of treatment. Mesalamine therapy was also well tolerated and no new safety concerns were identified.
Acknowledgments
The authors thank all centers and investigators who participated in the study, mainly 1) Canada: Drs. G. Aumais, S. Forget, D.M. Israel, A. Otley, L. Smith, H. Huynh; 2) the USA: Drs. M. Dubinsky, D.A. Gremse, E. Hoffenberg, J. Hyams, M. Lamet, P. Mamula, J. Markowitz, P. Rufo, R.L. Sigmon, V. Tolia; 3) Poland: Profs. K. Fyderek, F. Iwanczak, K. Karczewska, M. Krawczynski, M. Korzon, I. Malecka-Planeta, and M. Czerwionka-Szaflarska. We also thank the following for assistance: Carine Abdul-Hamid, Anna Kazmierczak, and Laura Aber, Rozina Jadoon, Dr. Luc Oligny, Rolland Gaudet, and Danielle de Montigny.
Sponsored by Axcan Pharma Inc. (Quebec, Canada). MBH is supported in part by NIH Grant DK060617.
Footnotes
Presented in abstract form at the DDW 2009 meeting in Chicago, IL.
References
- 1.Kildebo S, Nordgaard K, Aronsen O, et al. The incidence of ulcerative colitis in Northern Norway from 1983 to 1986. The Northern Norwegian Gastroenterology Society. Scand J Gastroenterol. 1990;25:890–896. doi: 10.3109/00365529008997609. [DOI] [PubMed] [Google Scholar]
- 2.Langholz E. Ulcerative colitis. An epidemiological study based on a regional inception cohort, with special reference to disease course and prognosis. Dan Med Bull. 1999;46:400–415. [PubMed] [Google Scholar]
- 3.Miner PB., Jr . Clinical features, course, laboratory findings, and complications in ulcerative colitis. In: Kirsner JB, editor. Inflammatory Bowel Disease. Philadelphia: W.B. Saunders; 2000. [Google Scholar]
- 4.Tysk C, Jarnerot G. Ulcerative proctocolitis in Orebro, Sweden. A retrospective epidemiologic study, 1963–1987. Scand J Gastroenterol. 1992;27:945–950. doi: 10.3109/00365529209000168. [DOI] [PubMed] [Google Scholar]
- 5.Marshall JK, Irvine EJ. Putting rectal 5-aminosalicylic acid in its place: the role in distal ulcerative colitis. Am J Gastroenterol. 2000;95:1628–1636. doi: 10.1111/j.1572-0241.2000.02180.x. [DOI] [PubMed] [Google Scholar]
- 6.Regueiro M, Loftus EV, Jr, Steinhart AH, et al. Clinical guidelines for the medical management of left-sided ulcerative colitis and ulcerative proctitis: summary statement. Inflamm Bowel Dis. 2006;12:972–978. doi: 10.1097/01.mib.0000231496.92013.85. [DOI] [PubMed] [Google Scholar]
- 7.Regueiro M, Loftus EV, Jr, Steinhart AH, et al. Medical management of left-sided ulcerative colitis and ulcerative proctitis: critical evaluation of therapeutic trials. Inflamm Bowel Dis. 2006;12:979–994. doi: 10.1097/01.mib.0000231495.92013.5e. [DOI] [PubMed] [Google Scholar]
- 8.Jewel DP. Ulcerative colitis. In: Sleisenger MH, Fordtran JS, editors. Gastrointestinal Disease. Philadelphia: W.B. Saunders; 1993. pp. 1305–1330. [Google Scholar]
- 9.Naganuma M, Iwao Y, Ogata H, et al. Measurement of colonic mucosal concentrations of 5-aminosalicylic acid is useful for estimating its therapeutic efficacy in distal ulcerative colitis: comparison of orally administered mesalamine and sulfasalazine. Inflamm Bowel Dis. 2001;7:221–225. doi: 10.1097/00054725-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Frieri G, Giacomelli R, Pimpo M, et al. Mucosal 5-aminosalicylic acid concentration inversely correlates with severity of colonic inflammation in patients with ulcerative colitis. Gut. 2000;47:410–414. doi: 10.1136/gut.47.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gionchetti P, Venturi A, Rizzello F, et al. Retrograde colonic spread of a new mesalazine rectal enema in patients with distal ulcerative colitis. Aliment Pharmacol Ther. 1997;11:679–684. doi: 10.1046/j.1365-2036.1997.00183.x. [DOI] [PubMed] [Google Scholar]
- 12.Campieri M, Gionchetti P, Belluzzi A, et al. Topical treatment with 5-aminosalicylic in distal ulcerative colitis by using a new suppository preparation. A double-blind placebo controlled trial. Int J Colorectal Dis. 1990;5:79–81. doi: 10.1007/BF00298473. [DOI] [PubMed] [Google Scholar]
- 13.Farup PG, Hovde O, Halvorsen FA, et al. Mesalazine suppositories versus hydrocortisone foam in patients with distal ulcerative colitis. A comparison of the efficacy and practicality of two topical treatment regimens. Scand J Gastroenterol. 1995;30:164–170. doi: 10.3109/00365529509093256. [DOI] [PubMed] [Google Scholar]
- 14.Gionchetti P, Rizzello F, Venturi A, et al. Comparison of oral with rectal mesalazine in the treatment of ulcerative proctitis. Dis Colon Rectum. 1998;41:93–97. doi: 10.1007/BF02236902. [DOI] [PubMed] [Google Scholar]
- 15.van Hogezand RA, van Hees PA, van Gorp JP, et al. Double-blind comparison of 5-aminosalicylic acid and acetyl-5-aminosalicylic acid suppositories in patients with idiopathic proctitis. Aliment Pharmacol Ther. 1988;2:33–40. doi: 10.1111/j.1365-2036.1988.tb00668.x. [DOI] [PubMed] [Google Scholar]
- 16.Mamula P, Markowitz J, Baldassano R. Clinical features and natural history of pediatric inflammatory diseases. In: Sartor R, Sandborn W, editors. Kirsner’s Inflammatory Bowel Diseases. Philadelphia: WB Saunders; 2004. pp. 555–565. [Google Scholar]
- 17.Grimm IS, Friedman LS. Inflammatory bowel disease in the elderly. Gastroenterol Clin North Am. 1990;19:361–389. [PubMed] [Google Scholar]
- 18.Heyman MB, Kirschner BS, Gold BD, et al. Children with early-onset inflammatory bowel disease (IBD): analysis of a pediatric IBD consortium registry. J Pediatr. 2005;146:35–40. doi: 10.1016/j.jpeds.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 19.Benchimol EI, Guttmann A, Griffiths AM, et al. Increasing incidence of paediatric inflammatory bowel disease in Ontario, Canada: evidence from health administrative data. Gut. 2009;58:1490–1497. doi: 10.1136/gut.2009.188383. [DOI] [PubMed] [Google Scholar]
- 20.Griffiths AM, Sherman PM. Colonoscopic surveillance for cancer in ulcerative colitis: a critical review. J Pediatr Gastroenterol Nutr. 1997;24:202–210. doi: 10.1097/00005176-199702000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Kelsen J, Baldassano RN. Inflammatory bowel disease: the difference between children and adults. Inflamm Bowel Dis. 2008;14:S9–S11. doi: 10.1002/ibd.20560. [DOI] [PubMed] [Google Scholar]
- 22.Walmsley RS, Ayres RC, Pounder RE, et al. A simple clinical colitis activity index. Gut. 1998;43:29–32. doi: 10.1136/gut.43.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott BB, Goodall A, Stephenson P, et al. Rectal mucosal plasma cells in inflammatory bowel disease. Gut. 1983;24:519–524. doi: 10.1136/gut.24.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–1629. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 25.Cerveny P, Bortlik M, Kubena A, et al. Nonadherence in inflammatory bowel disease: results of factor analysis. Inflamm Bowel Dis. 2007;13:1244–1249. doi: 10.1002/ibd.20189. [DOI] [PubMed] [Google Scholar]
- 26.Hawthorne AB, Rubin G, Ghosh S. Review article: medication non-adherence in ulcerative colitis—strategies to improve adherence with mesalazine and other maintenance therapies. Aliment Pharmacol Ther. 2008;27:1157–1166. doi: 10.1111/j.1365-2036.2008.03698.x. [DOI] [PubMed] [Google Scholar]
- 27.Moshkovska T, Stone M, Baker R, et al. Qualitative investigation of patient adherence to 5-aminosalicylic acid therapy in patients with ulcerative colitis. Inflamm Bowel Dis. 2008;14:763–768. doi: 10.1002/ibd.20404. [DOI] [PubMed] [Google Scholar]
- 28.Kane SV, Cohen RD, Aikens JE, et al. Prevalence of nonadherence with maintenance mesalamine in quiescent ulcerative colitis. Am J Gastroenterol. 2001;96:2929–2933. doi: 10.1111/j.1572-0241.2001.04683.x. [DOI] [PubMed] [Google Scholar]
- 29.Shale MJ, Riley SA. Studies of compliance with delayed-release mesalazine therapy in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18:191–198. doi: 10.1046/j.1365-2036.2003.01648.x. [DOI] [PubMed] [Google Scholar]
- 30.Bernal I, Domenech E, Garcia-Planella E, et al. Medication-taking behavior in a cohort of patients with inflammatory bowel disease. Dig Dis Sci. 2006;51:2165–2169. doi: 10.1007/s10620-006-9444-2. [DOI] [PubMed] [Google Scholar]
- 31.Lakatos PL, Lakatos L. Once daily 5-aminosalicylic acid for the treatment of ulcerative colitis; are we there yet? Pharmacol Res. 2008;58:190–195. doi: 10.1016/j.phrs.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Robinson A. Review article: improving adherence to medication in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2008;27(suppl 1):9–14. doi: 10.1111/j.1365-2036.2008.03604.x. [DOI] [PubMed] [Google Scholar]
- 33.Lamet M, Ptak T, Dallaire C, et al. Efficacy and safety of mesalamine 1 g HS versus 500 mg BID suppositories in mild to moderate ulcerative proctitis: a multicenter randomized study. Inflamm Bowel Dis. 2005;11:625–630. doi: 10.1097/01.mib.0000171277.70404.40. [DOI] [PubMed] [Google Scholar]
- 34.Jaskowski TD, Litwin CM, Hill HR. Analysis of serum antibodies in patients suspected of having inflammatory bowel disease. Clin Vaccine Immunol. 2006;13:655–660. doi: 10.1128/CVI.00034-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quinton JF, Sendid B, Reumaux D, et al. Anti-Saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic au-toantibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1998;42:788–791. doi: 10.1136/gut.42.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Satsangi J, Landers CJ, Welsh KI, et al. The presence of anti-neutrophil antibodies reflects clinical and genetic heterogeneity within inflammatory bowel disease. Inflamm Bowel Dis. 1998;4:18–26. doi: 10.1097/00054725-199802000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Sendid B, Quinton JF, Charrier G, et al. Anti-Saccharomyces cerevisiae mannan antibodies in familial Crohn’s disease. Am J Gastroenterol. 1998;93:1306–1310. doi: 10.1111/j.1572-0241.1998.00415.x. [DOI] [PubMed] [Google Scholar]
- 38.Zholudev A, Zurakowski D, Young W, et al. Serologic testing with ANCA, ASCA, and anti-OmpC in children and young adults with Crohn’s disease and ulcerative colitis: diagnostic value and correlation with disease phenotype. Am J Gastroenterol. 2004;99:2235–2241. doi: 10.1111/j.1572-0241.2004.40369.x. [DOI] [PubMed] [Google Scholar]
- 39.Marteau P, Florent C. Comparative, open, randomized trial of the efficacy and tolerance of slow-release 5-ASA suppositories once daily versus conventional 5-ASA suppositories twice daily in the treatment of active cryptogenic proctitis: French Pentasa Study Group. Am J Gastroenterol. 2000;95:166–170. doi: 10.1111/j.1572-0241.2000.01679.x. [DOI] [PubMed] [Google Scholar]
- 40.SALOFALK mesalamine suppository monograph. Axcan Pharma Inc; 2004. [Google Scholar]
- 41.CANASA mesalamine suppository monograph. Axcan Pharma Inc; 2008. [Google Scholar]
- 42.Cuffari C, Present DH, Bayless TM, et al. Optimizing therapy in patients with pancolitis. Inflamm Bowel Dis. 2005;11:937–946. doi: 10.1097/01.mib.0000179469.86500.ac. [DOI] [PubMed] [Google Scholar]
- 43.Kirschner B. The medical management of inflammatory bowel disease in children. In: Kirsner JB, editor. Inflammatory Bowel Disease. Philadelphia: WB Saunders; 2000. pp. 578–597. [Google Scholar]