In a large international ecological study, comparing urinary sodium excretion and stomach cancer mortality in 39 countries, Joossens et al (1996) concluded that ‘Salt intake, measured as 24-hour urine sodium excretion, is likely the rate-limiting factor of stomach cancer mortality at the population level’. On the basis of human observational and animal experimental data, as well as mechanistic plausibility, the 2003 report from the joint World Health Organization/Food and Agriculture Organization Expert Consultation (WHO/FAO) concluded that salt-preserved food and salt ‘probably’ increase the risk of gastric cancer (WHO/FAO, 2003). In fact, there is substantial evidence that the risk of gastric cancer is increased by high intakes of some traditionally preserved salted foods, especially meats and pickles, and with salt per se (Palli, 2000; Tsugane, 2005). The World Cancer Research Fund (WCRF) report (2007) concluded that ‘salt is a probable cause of stomach cancer’, and that there is robust evidence for the mechanisms operating in humans.
In the UK, the Committee on Medical Aspects of Food Policy (COMA) panel on Dietary Reference Values (Department of Health, 1991) advised that sodium (Na) intakes should be maintained below 3.2 g (or 8.0 g of salt) per day and set the reference nutrient intake (RNI) for men and women at 1.6 g of sodium (or 4.0 g of salt) per day. Following this, COMA's Cardiovascular Review Group recommended that salt intake should be gradually reduced further to a daily average of 6 g (Department of Health, 1994). This recommendation was also accepted in the food and health action plan ‘Choosing a better diet’ (Department of Health, 2005).
In this section, we consider the population-attributable fraction of stomach cancer associated with an intake of salt >6 g per day.
Methods
The relative risk (RR) of stomach cancer in relation to salt intake has been taken from the meta-analysis of cohort studies (WCRF, 2007), suggesting a RR of 1.08 per g per day, an excess RR of 0.08 per g. The durations of follow-up in the two studies contributing to this pooled value (van den Brandt et al, 2003; Tsugane et al, 2004) were 6.3 and 11 years, respectively. The latent period, or interval between ‘exposure’ to salt and the appropriate increase in risk of cancers of the stomach, is therefore taken to be 10 years, and the 2010 fraction of avoidable cancers is based on an estimate of salt intake in 2000–2001. Table 1 shows the results from the 2000–2001 National Diet and Nutrition Survey in which average daily urinary excretion of salt was 11 g per day in men and 8.1 g per day in women (Food Standards Agency, 2003).
Table 1. Urinary salt excretion in grams per day in Great Britain, 2000–2001.
|
Urinary salt excretion (grams per
day) by age group (years)
|
|||||
|---|---|---|---|---|---|
| Sex | 19–24 | 25–34 | 35–49 | 50–64 | 19–64 |
| Men | 11.0 | 11.4 | 11.1 | 10.5 | 11.0 |
| Women | 9.1 | 8.7 | 8.0 | 7.5 | 8.1 |
From National Diet and Nutrition Survey, Food Standards Agency (2003).
On the basis of an excess risk of 0.08 per gram of salt per day, the risk of stomach cancer associated with an intake of x g salt per day in excess of the recommended 6 g per day is as follows:
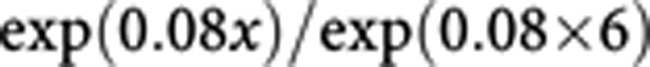 |
so that, in the lowest consumption category (women in the age group of 50–64 years), where average salt intake (x) is 7.5 g per day, the RR is as follows:
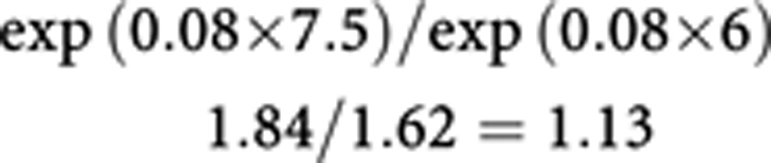 |
Table 2 shows the estimated intake of salt in 2000–2001 (Food Standards Agency, 2003), and the RRs of stomach cancer (by sex and age group) associated with the excess intake, compared with the recommended level of 6 g per day.
Table 2. Salt intake (grams per day, 2000–2001) and associated relative risk of stomach cancer.
|
Age group (years)
|
|||||
|---|---|---|---|---|---|
| Salt intake 2000–2001 | 19–24 | 25–34 | 35–49 | 50–64 | 19–64 |
| Men | |||||
| Mean grams per day | 11.0 | 11.4 | 11.1 | 10.5 | 11.0 |
| Excess grams per day | 5.0 | 5.4 | 5.1 | 4.5 | 5.0 |
| RR for this excess | 1.49 | 1.54 | 1.50 | 1.43 | 1.49 |
| Women | |||||
| Mean grams per day | 9.1 | 8.7 | 8.0 | 7.5 | 8.1 |
| Excess grams per day | 3.1 | 2.7 | 2.0 | 1.5 | 2.1 |
| RR for this excess | 1.28 | 1.24 | 1.17 | 1.13 | 1.18 |
Abbreviations: RR=relative risk (of stomach cancer).
Results
Table 3 shows the estimated number of cases of stomach cancer ‘caused’ in 2010 by the excessive consumption of salt in 2000–2001. These excess cases are calculated as (observed–expected), where the number expected=(observed/RR). Approximately 24% of stomach cancer cases can be attributed to this cause.
Table 3. Stomach cancer cases in the UK in 2010 due to intake of salt >6 g daily.
|
Age (years)
|
Stomach cancer
|
All cancera
|
||||||
|---|---|---|---|---|---|---|---|---|
| At exposure | At outcome | Obs. | Relative risk | Excess attrib. cases | PAF (%) | Obs | Excess attrib. cases | PAF (%) |
| Men | ||||||||
| 19–24 | 34–39 | 25 | 1.49 | 8 | 33.0 | 1792 | 8 | 0.5 |
| 25–34 | 40–49 | 159 | 1.54 | 56 | 35.1 | 6794 | 56 | 0.8 |
| 35–49 | 50–64 | 828 | 1.50 | 277 | 33.5 | 37 617 | 277 | 0.7 |
| 50–64 | ⩾65 | 3443 | 1.43 | 1041 | 30.2 | 108 729 | 1041 | 1.0 |
| All ages | 4467 | 1382 | 30.9 | 158 667 | 1382 | 0.9 | ||
| Women | ||||||||
| 19–24 | 34–39 | 28 | 1.28 | 6 | 22.0 | 3607 | 6 | 0.2 |
| 25–34 | 40–49 | 95 | 1.24 | 18 | 19.4 | 13 667 | 18 | 0.1 |
| 35–49 | 50–64 | 361 | 1.17 | 53 | 14.8 | 41 338 | 53 | 0.1 |
| 50–64 | ⩾65 | 2067 | 1.13 | 234 | 11.3 | 92 439 | 234 | 0.3 |
| All ages | 2577 | 312 | 12.1 | 155 584 | 312 | 0.2 | ||
| All | ||||||||
| 19–24 | 34–39 | 52 | 14 | 27.1 | 5400 | 14 | 0.3 | |
| 25–34 | 40–49 | 254 | 74 | 29.2 | 20 461 | 74 | 0.4 | |
| 35–49 | 50–64 | 1189 | 331 | 27.8 | 78 955 | 331 | 0.4 | |
| 50–64 | ⩾65 | 5510 | 1275 | 23.1 | 201 167 | 1275 | 0.6 | |
| All ages | 7044 | 1694 | 24.0 | 314 251 | 1694 | 0.5 | ||
Abbreviations: attrib.=attributable; Obs.=observed cases; PAF=population-attributable fraction.
Excluding non-melanoma skin cancer.
The excess number of cases is also expressed in terms of cancer as a whole. About 0.5% of cancers in 2010 are due to salt consumption in excess of the recommended daily maximum of an average of 6 g.
Discussion
The difficulties in estimating salt consumption in epidemiological studies probably contribute to the very heterogeneous findings; nevertheless, the consensus view, most recently expressed in the WCRF report (2007), is that salt intake (as well as sodium intake and salty and salted foods) is a probable cause of gastric cancer. The ‘optimum exposure level’, against which the risk of actual exposure was evaluated, was chosen as that recommended in the report of the Committee on Medical Aspects of Food Policy (Department of Health, 1994) and the UK government's food and health action plan ‘Choosing a better diet’ (Department of Health, 2005). This recommendation (less than 6 g of salt per day) was based on general health considerations, and mostly guided by the well-established link between salt and blood pressure. High salt intake is a major contributor to high blood pressure, which increases the risk of heart disease and stroke (MacGregor, 1999), and there is evidence that reductions in dietary salt can reduce blood pressure and the long-term risk of cardiovascular events (Cook et al, 2007). Nevertheless, it seems to be a reasonable (and attainable) target with respect to reduction in the risk of gastric cancer. The calculation of excess risk assumes a simple log-linear increase in the risk of gastric cancer with increasing salt intake. The evidence for this is somewhat equivocal: it is apparent for total salt use in cohort but not case–control studies, whereas for sodium intake it was also apparent in case–control studies; for salted and salty foods, the reverse was observed (dose–response relationship in case–control but not cohort studies; WCRF, 2007).
In general, diets of Western communities contain amounts of sodium that are far in excess of any physiological need and many times the recommended daily sodium requirement. The likely adverse effect on cancer risk in the UK is small, as the incidence of gastric cancer is low (gastric cancer ranks only 13th in terms of incidence in the UK, with incidence rates well below the European average (CRUK, 2011)). Average consumption in the UK is around 10 g per day, and had shown little change between 1986–7 and 2001 (Food Standards Agency, 2004). Although individuals can limit their personal consumption by avoiding salt in cooking, or adding salt at the table, around 75% of salt in the diet is from processed foods. In 2005, the Food Standards Agency developed proposals for voluntary targets for salt levels in a wide range of food categories (85 categories in total) as a guide for the food industry. There has subsequently been some progress on voluntary salt reductions by the industry (Department of Health, 2009). There is no direct evidence from intervention studies of the benefit of reduced salt intake with respect to gastric cancer. In Japan, the national dietary policy has resulted in declines in dietary salt intake, and there has been an equivalent reduction in the incidence of gastric cancer (Tominaga and Kuroishi, 1997); however, there have been other changes in prevalence of gastric cancer risk factors – notably in prevalence of infection with Helicobacter pylori (Kobayashi et al, 2004) – and thus the part played by salt reduction is far from clear.
See acknowledgements on page Si.
Footnotes
The author declares no conflict of interest.
References
- Cancer Research UK (CRUK) (2011) UK Stomach Cancer Incidence Statistics, http://info.cancerresearchuk.org/cancerstats/types/stomach/incidence/
- Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK (2007) Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOTP). Br Med J 334: 885–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health (1991) Report on Health and Social Subjects: 41. Dietary Reference Values for Food Energy and Nutrients for the United Kingdom. HMSO: London [PubMed] [Google Scholar]
- Department of Health (1994) Nutritional Aspects of Cardiovascular Disease. Report on Health and Social Subjects 46. The Stationery Office: London [Google Scholar]
- Department of Health (2005) Choosing a Better Diet: A Food and Health Action Plan. http://www.dh.gov.uk/en/Consultations/Closedconsultations/DH_4084430
- Department of Health (2009) Healthcare: Salt, http://www.dh.gov.uk/en/Healthcare/Bloodpressure/DH_4084299
- Food Standards Agency (2003) National Diet and Nutrition Survey: Adults Aged 19 to 64, Vol. 3. Vitamin and Mineral Intake and Urinary Analytes. http://www.food.gov.uk/multimedia/pdfs/ndnsv3.pdf
- Food Standards Agency (2004) National Diet and Nutrition Survey: Adults aged 19 to 64, Vol. 5. Summary Report. http://www.food.gov.uk/multimedia/pdfs/ndns5full.pdf
- Joossens JV, Hill MJ, Elliott P, Stamler R, Lesaffre E, Dyer A, Nichols R, Kesteloot H (1996) Dietary salt, nitrate and stomach cancer mortality in 24 countries. European Cancer Prevention (ECP) and the INTERSALT Cooperative Research Group. Int J Epidemiol 25: 494–504 [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Kikuchi S, Lin Y, Yagyu K, Obata Y, Ogihara A, Hasegawa A, Miki K, Kaneko E, Mizukoshi H, Sakiyama T, Tenjin H (2004) Trends in the incidence of gastric cancer in Japan and their associations with Helicobacter pylori infection and gastric mucosal atrophy. Gastric Cancer 7: 233–239 [DOI] [PubMed] [Google Scholar]
- MacGregor GA (1999) Nutrition and blood pressure. Nutr Metab Cardiovasc Dis 9: 6–15 [PubMed] [Google Scholar]
- Palli D (2000) Epidemiology of gastric cancer: an evaluation of available evidence. J Gastroenterol 35: S84–S89 [PubMed] [Google Scholar]
- Tominaga S, Kuroishi T (1997) An ecological study on diet/nutrition and cancer in Japan. Int J Cancer 10(Suppl): 2–6 [DOI] [PubMed] [Google Scholar]
- Tsugane S (2005) Salt, salted food intake, and risk of gastric cancer: epidemiologic evidence. Cancer Sci 96: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugane S, Sasazuki S, Kobayashi M, Sasaki S (2004) Salt and salted food intake and subsequent risk of gastric cancer among middle-aged Japanese men and women. Br J Cancer 90: 128–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brandt PA, Botterweck AA, Goldbohm RA (2003) Salt intake, cured meat consumption, refrigerator use and stomach cancer incidence: a prospective cohort study (Netherlands). Cancer Causes Control 14: 427–438 [DOI] [PubMed] [Google Scholar]
- World Health Organization/Food and Agriculture Organization (WHO/FAO) (2003) Diet, Nutrition and The Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation. WHO Technical Report Series 916. WHO: Geneva [PubMed] [Google Scholar]
- World Cancer Research Fund (WCRF) Panel (2007) Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. World Cancer Research Fund: Washington, DC [Google Scholar]


