Several studies suggest that regular physical exercise protects against development of breast cancer, large bowel cancer and endometrial cancer, independently of the effect of physical exercise in reducing body weight. The World Cancer Research Fund (WCRF, 2007) summarizes the evidence as ‘convincing’ for cancers of the colon, and ‘probable’ for cancers of the breast (in post-menopausal women only) and endometrium (WCRF, 2007).
The evidence that individuals with high levels of physical activity throughout their lives are at lower risk for colon cancer was considered ‘sufficient’ in the International Agency for Research on Cancer (IARC) evaluation (IARC, 2002), and convincing by WHO/FAO (2003). The relationship between physical exercise and risk of rectal cancer is much less certain (IARC, 2002; Wei et al, 2004; Friedenreich et al, 2006), although a few studies (e.g., Slattery et al, 2003) have shown an inverse association.
In relation to physical activity and risk of breast cancer, IARC (2002) concluded that, although studies have not been entirely consistent, the overall results support a reduction in risk with higher levels of activity. Evidence for a dose–response effect was found in most of the studies that examined the trend. The majority of studies have focused on postmenopausal breast cancer, although there is also some evidence for a protective effect of physical activity on premenopausal disease. In some of the studies, the nature of the physical activity (recreational, occupational, and household) has appeared to be of importance, too.
A recent review and meta-analysis of 13 studies (Voskuil et al, 2007) concluded, like WCRF (2007), that physical activity seems to be associated with a reduction in the risk of endometrial cancer, which is independent of body weight.
The Department of Health (2004) target for physical exercise is that everyone should aim to take at least 30 min of physical activity on five or more days of the week. This physical activity should be of at least moderate intensity – similar to brisk walking. Activity can be taken in bouts of 10–15 min, allowing for accumulation of activity throughout the day.
In this section, we examine how much cancer of the colon, female breast and endometrium observed in 2010 might be attributed to a deficit in physical activity in the population below this recommended minimum.
Methods
Most studies of the effect of physical activity on cancer risk present results in terms of categories of activity (high/medium/low, or as quantiles of the population studied). In order to quantify the effect of change to exercise intensity on the health of the population, risk must be quantified in relation to energy expenditure in MET. (MET means metabolic equivalent, and is used to describe the intensity of activities. One MET is defined as the energy spent sitting quietly (equivalent to (4.184 kJ) per kg per hour), while, for example, moderate activity corresponds to 3–6 METs, and vigorous activity to >6 METs. A table showing MET values of different activities is available at http://www.cdc.gov/nccdphp/dnp a/physical/pdf/PA_Intensity_table_2_1.pdf).
At least four recent studies provide estimates of risk of colon cancer in relation to physical activity (adjusted for body mass index) expressed as MET hours: Giovannucci et al (1995), Chao et al (2004), Friedenreich et al (2006) and Wolin et al (2007).
At least four recent studies provide estimates of risk of breast cancer in relation to physical activity (adjusted for body mass index) expressed as MET hours: Friedenreich et al (2001), Carpenter et al (2003), McTiernan et al (2003) and Lahmann et al (2007).
For each of these studies, the relative risk (RR) per MET-hour per week was estimated by assuming a log-linear relationship between exposure and risk, so that:
where x is the exposure level (in MET-hours per week).
We used the simple mean of the RR per unit exposure in each study.
The values were as follows:
Post-menopausal breast cancer: RR 0.9955 per MET-hour per week.
Colon cancer: RR 0.9940 per MET-hour per week.
The value for post-menopausal breast cancer in the meta-analysis of WCRF (2007) is very similar – for postmenopausal breast cancer and recreational activity, an RR of 0.97 per 7 MET-hours per week.
As we are concerned with quantifying the effect of a deficit in exercise, we calculate the increase in risk associated with a decreased exercise intensity of 1 MET-hour per week as
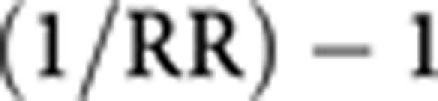 |
The values were the following:
0.00457 for post-menopausal breast cancer.
0.00602 for colon cancer.
With respect to endometrial cancer, none of the case–control and cohort studies so far reported provide results with respect to physical activity in comparable units. It is thus not possible to estimate directly the relationship in terms of RR (or ERR) per MET-hour per week. However, in a large case–control study in Sweden, comparable results are given for post-menopausal breast cancer (Moradi et al, 2000a) and endometrial cancer (Moradi et al, 2000b). The strength of the association between recreational and occupational activity, comparing the lowest with the highest activity categories, is more or less the same for both cancers (RR=1.3). Therefore, the values per MET-hour per week estimated for breast cancer (as above) have also been used for endometrial cancer.
The latent period, or interval between ‘exposure’ to an inadequate level of physical activity, and the appropriate increase in risk of these cancers (or, conversely, the duration of exercise required to eliminate the excess risk of a suboptimal level) are not known. For the four cohort studies contributing to the estimate of RR of colon cancer in this analysis (Giovannucci et al, 1995; Chao et al, 2004; Friedenreich et al, 2006; Wolin et al, 2007) the mean duration of follow-up was 8.9 years. In the two cohort studies contributing to the estimate for breast cancer (McTiernan et al, 2003; Lahmann et al, 2007) it was shorter (5.6 years), while in the seven cohort studies in the meta-analysis of endometrial cancer by Voskuil et al (2007) mean duration of follow-up was 14.8 years. We chose to assume the same latency for all three of 10 years and thus examine the effects on cancers occurring in 2010 from suboptimal levels of physical activity in 2000.
The minimum target would envisage all those doing less than 30 min, 5 days a week, to move to this minimum (but no increase in exercise levels in those individuals already achieving the target level).
The National Diet and Nutrition Survey (FSA, 2004) provides tables showing reported levels of physical exercise, by age group and sex, for adults aged 19–64 in a sample of households in Great Britain in 2000–2001. There are four categories: moderate exercise of 30 min duration for 5 or more days a week, 1–2 days, 3–4 days and <1 day per week. In all, 36% of men and 26% of women aged 19–64 were already at the target level of at least 30 min moderate physical exercise on at least 5 days a week. For those of age >65, equivalent data were obtained from the Health Survey for England (Health and Social Care Information Centre, 2010) by averaging the values in the surveys of 1997 and 2003. The middle category (1–4 days moderate exercise per week) was split into two (1–2 and 3–4), based on the ratios observed at ages 50–64 in the 2000–2001 National Diet & Nutrition Survey.
The results for all adult age groups (ages 19 and above) are shown in Table 1.
Table 1. Estimated percentage of the population performing physical activity at the level given, in 2000.
|
% Performing physical
activitya with
specified frequency per week
|
||||
|---|---|---|---|---|
| Age (years) | <1 day | 1 or 2 days | 3 or 4 days | ⩾5 days |
| Deficit in METs per week | 13.5 | 10.5 | 4.5 | 0 |
| Men | ||||
| 19–24b | 21 | 14 | 17 | 49 |
| 25–34b | 12 | 21 | 21 | 46 |
| 35–49b | 20 | 27 | 19 | 34 |
| 50–64b | 30 | 28 | 18 | 24 |
| 65–74c | 52.6 | 20.1 | 12.9 | 14.5 |
| ⩾75c | 73.0 | 11.9 | 7.6 | 7.5 |
| All (19+)d | 28.2 | 22.9 | 17.5 | 31.5 |
| Women | ||||
| 19–24b | 20 | 36 | 15 | 29 |
| 25–34b | 14 | 29 | 26 | 30 |
| 35–49b | 21 | 29 | 24 | 25 |
| 50–64b | 24 | 34 | 21 | 22 |
| 65–74c | 57.0 | 20.1 | 12.4 | 10.5 |
| ⩾75c | 81.7 | 8.7 | 5.4 | 4.0 |
| All (19+)d | 31.6 | 27.3 | 19.4 | 21.5 |
Abbreviations: METs=metabolic equivalents.
Defined as moderate intensity activity of at least 30 min duration.
From National Diet and Nutrition Survey, FSA (2004).
From Health Survey for England (2009 trend tables), average for 1997 and 2003; the middle physical activity category (1–4 days per week) was split into 1–2 and 3–4 days per week in proportions observed at ages 50–64 in the National Diet and Nutrition Survey, FSA (2004).
Based on UK 2000 population.
Assuming that exercise of moderate intensity is equivalent to 6 METS, so that 30 min of moderate exercise consumes 3 MET-hours, we estimate the deficit in MET hours below the recommended level of 15 per week (3 × 5). Thus, for the proportion of the population exercising moderately 3–4 days a week, the deficit is, on average, 4.5 MET-hours per week (15−[3 × 3.5]), for those exercising 1–2 days 10.5 MET-hours per week and for those exercising less than 1 day per week 13.5 MET-hours (15−[3 × 0.5]) per week. These values are shown in the first row of Table 1.
Population-attributable fractions (PAFs) were calculated for each sex-age group in Table 1 according to the usual formula:
where px is the proportion of population in exercise category x and ERRx the excess RR in exercise category x.
ERRx is calculated as
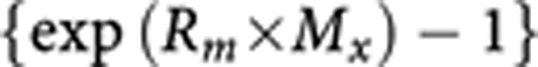 |
where Rm is the increase in risk for a deficit of 1 MET-hour per week and Mx is the deficit in MET-hours per week (less than 15) in exercise category x.
Results
Table 2 shows the estimated PAF and the number of cases of breast, endometrial and colon cancer ‘caused’ in 2010 by the deficit in exercise (in 2000), by age group and sex. The excess number of cases is also expressed in terms of cancer as a whole.
Table 2. Cancer cases diagnosed in 2010, attributable to below-target exercise level in 2000.
|
Age (years)
|
Breast (post-menopausal)
|
Uterus (endometrium)
|
Colon
|
All cancersa
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| At exposure | At outcome (+10 years) | PAF | Observed cases | Excess attributable cases | PAF | Observed cases | Excess attributable cases | PAF | Observed cases | Excess attributable cases | Observed cases | Excess attributable cases |
| Men | ||||||||||||
| 19–24 | 29–34 | — | — | — | — | — | — | 0.031 | 58 | 1.8 | 1333 | 1.8 |
| 25–34 | 35–44 | — | — | — | — | — | — | 0.029 | 216 | 6.2 | 4124 | 6.2 |
| 35–49 | 45–59 | — | — | — | — | — | — | 0.038 | 1451 | 55.5 | 22388 | 55.5 |
| 50–64 | 60–74 | — | — | — | — | — | — | 0.046 | 5331 | 247.3 | 68043 | 247.3 |
| 65–74 | 75–84 | — | — | — | — | — | — | 0.058 | 4339 | 250.6 | 44085 | 250.6 |
| ⩾75 | ⩾85 | — | — | — | — | — | — | 0.067 | 1598 | 106.9 | 16064 | 106.9 |
| Total (%) | — | — | — | — | — | — | 13044 | 668.3 (5.1%) | 158667 | 668.3 (0.4%) | ||
| Women | ||||||||||||
| 19–24 | 29–34 | 0 | 582 | 0 | 0.032 | 28 | 0.9 | 0.043 | 60 | 2.5 | 2248 | 3.4 |
| 25–34 | 35–44 | 0 | 3857 | 0 | 0.028 | 211 | 5.9 | 0.037 | 236 | 8.6 | 8619 | 14.5 |
| 35–49 | 45–59 | 0.032 | 14 628 | 461.7 | 0.032 | 1926 | 60.8 | 0.042 | 1301 | 54.0 | 31 631 | 576.5 |
| 50–64 | 60–74 | 0.035 | 17 194 | 602.8 | 0.035 | 3844 | 134.8 | 0.046 | 3914 | 180.3 | 54 966 | 917.9 |
| 65–74 | 75–84 | 0.046 | 7584 | 352.4 | 0.046 | 1570 | 73.0 | 0.061 | 3873 | 235.9 | 35 386 | 661.2 |
| ⩾75 | ⩾85 | 0.054 | 4367 | 237.1 | 0.054 | 605 | 32.8 | 0.071 | 2299 | 163.3 | 20 050 | 433.3 |
| Total (%) | 48 385 | 1654.1 (3.4%) | 8195 | 308.1 (3.8%) | 11 732 | 644.7 (5.5%) | 155 584 | 2606.9 (1.7%) | ||||
| Persons | ||||||||||||
| 19–24 | 29–34 | 582 | 0 | 28 | 0.9 | 117 | 4.3 | 3582 | 5.2 | |||
| 25–34 | 35–44 | 3857 | 0 | 211 | 5.9 | 452 | 14.8 | 12 743 | 20.7 | |||
| 35–49 | 45–59 | 14 628 | 461.7 | 1926 | 60.8 | 2752 | 109.6 | 54 019 | 632.1 | |||
| 50–64 | 60–74 | 17 194 | 602.8 | 3844 | 134.8 | 9245 | 427.6 | 123 009 | 1165.2 | |||
| 65–74 | 75–84 | 7584 | 352.4 | 1570 | 73.0 | 8212 | 486.4 | 79 472 | 911.8 | |||
| ⩾75 | ⩾85 | 4367 | 237.1 | 605 | 32.8 | 3897 | 270.2 | 36 114 | 540.1 | |||
| Total (%) | 48 385 | 1654.1 (3.4%) | 8195 | 308.1 (3.8%) | 24 776 | 1312.9 (5.3%) | 314 251 | 3275.2 (1.0%) | ||||
Abbreviations: PAF=population-attributable fraction.
Excluding non-melanoma skin cancer.
An estimated 3.4% of breast cancer cases, 3.8% of endometrial cancer cases and 5.3% of colon cancer cases are attributable to exercising less than the minimum recommended. The 5.3% of colon cancers correspond to 3.3% of large bowel cancers (colon and rectum). This corresponds to 1.0% of all cancer cases, 0.4% in men and 1.7% in women.
Discussion
Although a beneficial effect of physical activity levels on the risk of various cancers has been observed in various individual studies – notably for cancers of the lung, pancreas and prostate – the evidence is far from conclusive, and in this section we include only those (colon, post-menopausal breast cancer and endometrial cancer) for which the evidence is considered convincing or probable in the reviews of WRCF (2007) or ‘sufficient’ by IARC (2002).
There are several probable mechanisms underlying the protective effects of physical activity (McTiernan et al, 1998; Quadrilatero and Hoffman-Goetz, 2003). They include modification of levels of metabolic hormones and growth factors, improvement of the anti-tumour immune system, promotion of antioxidant defence and DNA repair. Physical activity may reduce exposure to endogenous oestrogens implicated in breast and endometrial cancer. With respect to colon cancer, physical activity can speed up the transit of food, with reduced exposure to intraluminal carcinogens. Changes to levels of insulin, prostaglandin and bile acids reduce proliferation of mucosal cells.
The current estimate – that around 1% of cancers in the UK may be related to physical inactivity (below a modest aspiration of 30 min five times a week) – is similar to the estimate of Doll and Fau (2003), that less than 1% of cancer deaths are due to physical inactivity. However, the estimates of the proportions of cancers related to inadequate ‘physical activity’ in the UK in 2002 by WCRF (2009) are substantially higher: 12% of colorectal cancer, 12% of breast cancer and 30% of endometrial cancer. There are several reasons for these larger estimates. WCRF selected ‘representative’ studies from which to take the RRs; only one (for colon cancer) is referenced to be from their own meta-analyses (WCRF, 2007), where the value (1.33 for more than 150 hours exercise per week vs none) is not actually reported. The RR for endometrial cancer, 0.57 for ⩾60 minutes of non-occupational exercise per day compared with <30 min (Schouten et al, 2004), is particularly significant. The reference category (optimum physical activity) was different for the three cancers, and in all was higher than the equivalent of 5 × 30 min of moderate exercise per week. Finally, the translation of the exposure prevalence (from the National Diet and Nutrition Survey) to the categories used in the calculation of three different estimates was particularly imaginative.
Using a modelling approach, de Vries et al (2010) estimated that, in 2040, 8.7% of colon cancer cases in men and 17.4% in women would be due to suboptimal levels of physical activity during the previous 20 years. The higher percentages than those estimated in the current analysis (4.9% in men and 5.3% in women) are due to several differences in the methods. The main difference is the dichotomizing of the population into ‘optimal’ and inactive, with RRs from a meta-analysis of leisure-time activity and colon cancer (Samad et al, 2005) that suggested substantial risk in inactive individuals vs those ‘physically active’ (1.28 in men, 1.41 in women), which contrasts with the risk of 1.09 in the least active group of Tables 1 and 2 relative to the optimum of 5 × 30 min of moderate exercise per week (substantially less active than the baseline category of Samad et al, 2005).
Based on short-term trend data from the Health Survey for England (Health and Social Care Information Centre, 2010), it does seem that, in the period 1997–2009, there has been an increase in the proportion of persons exercising five or more times per week. There is evidence from reviews that interventions to increase individual exercise levels in community, health-care and occupational settings can be successful (Hillsdon et al, 2005), although at present there is little review-level evidence of the effectiveness of modifications to the built environment in increasing physical activity in the general population.
See acknowledgements on page Si.
Footnotes
The author declares no conflict of interest.
References
- Carpenter CL, Ross RK, Paganini-Hill A, Bernstein L (2003) Effect of family history, obesity and exercise on breast cancer risk among postmenopausal women. Int J Cancer 106: 96–102 [DOI] [PubMed] [Google Scholar]
- Chao A, Connell CJ, Jacobs EJ, McCullough ML, Patel AV, Calle EE, Cokkinides VE, Thun MJ (2004) Amount, type, and timing of recreational physical activity in relation to colon and rectal cancer in older adults: the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev 13: 2187–2195 [PubMed] [Google Scholar]
- Department of Health (2004) At Least Five a Week: Evidence on the Impact of Physical Activity and Its Relationship to Health. A Report from the Chief Medical Officer. http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4080994
- de Vries E, Soerjomataram I, Lemmens VE, Coebergh JW, Barendregt JJ, Oenema A, Møller H, Brenner H, Renehan AG (2010) Lifestyle changes and reduction of colon cancer incidence in Europe: a scenario study of physical activity promotion and weight reduction. Eur J Cancer 46: 2605–2616 [DOI] [PubMed] [Google Scholar]
- Doll R, Fau PJ (2003) Epidemiology of cancer. In Oxford Textbook of Medicine, Warrell DA, Cox TM, Firth JD, Benz Jr EJ (eds). Oxford University Press: Oxford [Google Scholar]
- Food Standards Agency (FSA) (2004) National Diet and Nutrition Survey: Adults Aged 19 to 64, Vol. 4. Table 5.4: Nutritional Status, Physical Measurements and Physical Activity Levels. http://www.food.gov.uk/multimedia/pdfs/ndnsfour.pdf
- Friedenreich C, Norat T, Steindorf K, Boutron-Ruault MC, Pischon T, Mazuir M, Clavel-Chapelon F, Linseisen J, Boeing H, Bergman M, Johnsen NF, Tjonneland A, Overvad K, Mendez M, Quiros JR, Martinez C, Dorronsoro M, Navarro C, Gurrea AB, Bingham S, Khaw KT, Allen N, Key T, Trichopoulou A, Trichopoulos D, Orfanou N, Krogh V, Palli D, Tumino R, Panico S, Vineis P, Bueno-de-Mesquita HB, Peeters PH, Monninkhof E, Berglund G, Manjer J, Ferrari P, Slimani N, Kaaks R, Riboli E (2006) Physical activity and risk of colon and rectal cancers: the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev 15: 2398–2407 [DOI] [PubMed] [Google Scholar]
- Friedenreich CM, Courneya KS, Bryant HE (2001) Influence of physical activity in different age and life periods on the risk of breast cancer. Epidemiology 12: 604–612 [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC (1995) Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med 122: 327–334 [DOI] [PubMed] [Google Scholar]
- Health and Social Care Information Centre (2010) Health Survey for England – 2009: Trend Tables. http://www.ic.nhs.uk/statistics-and-data-collections/health-and-lifestyles-related-surveys/health-survey-for-england/health-survey-for-england--2009-trend-tables
- Hillsdon M, Foster C, Cavill N, Crombie, Naidoo B (2005) The effectiveness of public health interventions for increasing physical activity among adults: a review of reviews. Evidence Briefing. Health Development Agency. http://www.nice.org.uk/aboutnice/whoweare/aboutthehda/hdapublications/p103.jsp [Google Scholar]
- International Agency for Research on Cancer (IARC) WHO (2002) IARC Handbooks of Cancer Prevention: Weight Control and Physical Activity, Vol. 6. International Agency for Research on Cancer: Lyon [Google Scholar]
- Lahmann PH, Friedenreich C, Schuit AJ, Salvini S, Allen NE, Key TJ, Khaw K-T, Bingham S, Peeters PHM, Monninkhof E, Bas Bueno-de-Mesquita H, Wirfält E, Manjer J, Gonzales CA, Ardanaz E, Amiano P, Quirós JR, Navarro C, Martinez C, Berrino F, Palli D, Tumino R, Panico S, Vineis P, Trichopoulou A, Bamia C, Trichopoulos D, Boeing H, Schulz M, Linseisen J, Chang-Claude J, Clavel Chapelon F, Fournier A, Boutron-Ruault M-C, Tjønneland A, Føns Johnson N, Overvad K, Kaaks R, Riboli E (2007) Physical activity and breast cancer risk: the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev 16: 36–42 [DOI] [PubMed] [Google Scholar]
- McTiernan A, Kooperberg C, Wise E, Wilcox S, Coates R, Adams-Campbell LL, Woods N, Okene J (2003) Recreational physical activity and the risk of breast cancer in post menopausal women. The Womens Health Initiative Cohort Study. JAMA 290: 1331–1336 [DOI] [PubMed] [Google Scholar]
- McTiernan A, Ulrich C, Slate S, Potter J (1998) Physical activity and cancer etiology: associations and mechanisms. Cancer Causes Control 9: 487–489 [DOI] [PubMed] [Google Scholar]
- Moradi T, Nyrén O, Zack M, Magnusson C, Persson I, Adami HO (2000a) Breast cancer risk and lifetime leisure-time and occupational physical activity (Sweden). Cancer Causes Control 11: 523–531 [DOI] [PubMed] [Google Scholar]
- Moradi T, Weiderpass E, Signorello LB, Persson I, Nyrén O, Adami HO (2000b) Physical activity and postmenopausal endometrial cancer risk (Sweden). Cancer Causes Control 11: 829–837 [DOI] [PubMed] [Google Scholar]
- Quadrilatero J, Hoffman-Goetz L (2003) Physical activity and colon cancer: a systematic review of potential mechanisms. J Sport Med Phys Fit 43: 121–138 [PubMed] [Google Scholar]
- Samad AK, Taylor RS, Marshall T, Chapman MA (2005) A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal Dis 7: 204–213 [DOI] [PubMed] [Google Scholar]
- Schouten LJ, Goldbohm RA, van den Brandt PA (2004) Anthropometry, physical activity, and endometrial cancer risk: results from The Netherlands Cohort Study. J Natl Cancer Inst 96: 1635–1638 [DOI] [PubMed] [Google Scholar]
- Slattery ML, Edwards S, Curtin K, Ma K, Edwards R, Holubkov R, Schaffer D (2003) Physical activity and colorectal cancer. Am J Epidemiol 158: 214–224 [DOI] [PubMed] [Google Scholar]
- Voskuil DW, Monninkhof EM, Elias SG, Vlems FA, van Leeuwen FE (2007) Task force physical activity and cancer. Physical activity and endometrial cancer risk, a systematic review of current evidence. Cancer Epidemiol Biomarkers Prev 16: 639–648 [DOI] [PubMed] [Google Scholar]
- Wei EK, Giovannucci E, Wu K, Rosner B, Fuchs CS, Willett WC, Colditz GA (2004) Comparison of risk factors for colon and rectal cancer. Int J Cancer 108: 433–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin KY, Lee IM, Colditz GA, Glynn RJ, Fuchs C, Giovannucci E (2007) Leisure-time physical activity patterns and risk of colon cancer in women. Int J Cancer 121: 2776–2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Cancer Research Fund (WCRF)/American Institute for Cancer Research (AICR) (2009) Policy and Action for Cancer Prevention. Food, Nutrition and Physical Activity: A Global Perspective. AICR: Washington, DC [Google Scholar]
- World Cancer Research Fund (WCRF) Panel (2007) Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. World Cancer Research Fund: Washington, DC [Google Scholar]
- World Health Organization (WHO)/Food and Agriculture Organization (FAO) (2003) Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation WHO Technical Report Series 916. WHO: Geneva [PubMed] [Google Scholar]


