The infectious agents that have been identified as definitely or probably carcinogenic to humans (Groups 1 and 2A) in the International Agency for Research on Cancer (IARC) monograph series are shown in Table 1. They include hepatitis B (HBV) and C (HCV) viruses, human papillomaviruses (HPV), human immunodeficiency virus (HIV) and T-lymphotropic virus type-1 (HTLV-1), Epstein–Barr virus (EBV) and human herpesvirus 8 (HHV8), and the bacterium Helicobacter pylori.
Table 1. Major human infection-associated malignancies.
| Malignancy | Agent (group) |
|---|---|
| Carcinoma | |
| Bladder | Schistosoma haematobium (blood fluke) |
| Cervix | HPV (papillomavirus) |
| Liver | HBV (hepadnavirus) |
| HCV (flavivirus) | |
| Bile duct | Opisthorchis viverrini (liver fluke) |
| Nasopharynx | EBV (herpesvirus) |
| Stomach | Helicobacter pylori (bacterium) |
| Lymphoma | |
| Adult T-cell | HTLV-I (retrovirus) |
| Burkitt | EBV (herpesvirus) |
| Hodgkin | EBV (herpesvirus) |
| Sarcoma | |
| Kaposi | HHV8 (herpesvirus) |
Abbreviations: EBV=Epstein–Barr virus; HBV=hepatitis B virus; HCV=hepatitis C virus; HHV8=human herpesvirus 8; HPV=human papillomavirus; HTLV-I=human T-cell lymphotropic virus type I.
From Mueller et al (2005).
In addition to these associations, there is substantial evidence for a causative relationship between chronic infection with hepatitis C virus (HCV) and non-Hodgkin lymphoma (NHL), an association that has been the subject of several recent systematic reviews (Gisbert et al, 2003; Matsuo et al, 2004; dal Maso and Franceschi, 2006).
Methods
Attributable fractions
For most infections, calculation of the population-attributable fraction (PAF) relies on the classic formula for population-attributable risk (Cole and Macmahon, 1971):
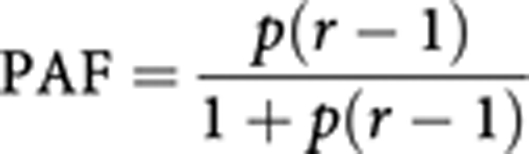 |
where r represents the relative risk of exposure, and p its prevalence in the population. The formula results in a proportion that is applied to the total number of incident cases in the UK population, to obtain the number of cases that can theoretically be attributed to the factor in that population (PAF). Its application requires identification of data on the prevalence of the exposure to the ‘causative’ agents in the UK population, as well as the corresponding relative risks. This method is used to estimate the number of cancers due to HBV, HCV, H. pylori and HIV (NHL).
For EBV, the prevalence of relevant infection is hard to define, as the virus infects almost everyone in childhood or adolescence and persists in latent form in B-lymphocytes throughout life. Clearly, agents other than EBV are essential co-factors in carcinogenesis, and EBV-attributable cancers are defined as those in which EBV-DNA can be demonstrated in tumour cells.
For the oncogenic HPVs, it is generally accepted that almost all cancers of the cervix uteri are the result of infection (Walboomers et al, 1999), so the AF is 100%. At other sites, the prevalence of infection in normal subjects is hard to define, so use of the classic Cole–MacMahon formula is inappropriate; as for EBV, the HPV-attributable cancers are defined as those in which HPV-DNA can be demonstrated in tumour cells.
Results
Human papillomavirus
IARC (2005) considers that there is convincing evidence that infection with HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 or 66 can lead to cervical cancer. For HPV 16, the evidence further supports a causal role in cancer of the vulva, vagina, penis, anus, oral cavity and oropharynx and a limited association with cancer of the larynx and periungual skin. HPV 18 also shows a limited association with cancer at most of these sites. Evidence for associations of HPV types of genus beta with squamous-cell carcinoma of the skin is limited for the general population. There is some evidence that HPVs are involved in squamous-cell carcinoma of the conjunctiva, but inadequate evidence for a role of HPVs in cancer of the esophagus, lung, colon, ovary, breast, prostate, urinary bladder, and nasal and sinonasal cavities.
With respect to cancer of the cervix, oncogenic HPV may be detected by PCR in virtually all cases of cervix cancer, and it is generally accepted that the virus is necessary for development of cancer, and that all cases of this cancer can be ‘attributed’ to infection (Walboomers et al, 1999).
With respect to squamous-cell cancers of the vulva and vagina, carcinoma of the penis, and anal cancer, published studies do not allow quantification of relative risk and infection prevalence, because they are generally small in size, and usually do not include comparable measurement of prevalence of infection at these sites in normal subjects. In order to estimate attributable fractions, therefore, approximate estimates of the proportion of cancer cases infected with HPV in various series are used.
The prevalence of HPV in vaginal cancer is about 60–65% in studies using PCR methodology (Daling et al, 2002; IARC, 2005); an overall HPV prevalence of 63% is assumed. About 20–50% of vulvar cancers contain oncogenic HPV DNA (Madeleine et al, 1997; Herrero and Munoz, 1999), but only the basaloid and warty type that tends to be associated with vulvar intraepithelial neoplasia is caused by HPV infection (prevalence 75–100%), and only 2–23% of the keratinizing carcinomas harbour HPV (Trimble et al, 1996); an overall HPV prevalence of 40% is assumed. For anal cancer, in a large series of cases from Denmark and Sweden, 95% and 83% of cancers involving the anal canal in women and men, respectively, were positive for oncogenic HPV (Frisch et al, 1999), and an AF of 90% is assumed. For penile cancer, HPV DNA was found in 30% of 71 cases of penile cancer from Brazil (Bezerra et al, 2001) and in 42% of 148 cases from the USA and Paraguay (Rubin et al, 2001); the AF is assumed to be 40%.
HPV has a role in the aetiology of a fraction of cancers of the oral cavity and pharynx (Shah, 1998), although the major risk factors are tobacco and alcohol. A review of more than 5000 tumours of the upper aerodigestive tract (Kreimer et al, 2005a) found that prevalence of HPV DNA in specimens from Europe was 16.0% (95% CI, 13.4–18.8) for oral cancers, 28.2% (95% CI, 24.4–32.2) for tumours of the oro-pharynx and 21.3% in squamous cell carcinomas (SCCs) of the larynx. However, HPV may not be of aetiological relevance in all such cases. Van Houten et al (2001) found that only 45% of DNA-positive cases showed E6 mRNA expression, indicative of viral activity. Kreimer et al (2005b) observed that about 50% of head and neck cancers had a high viral load of HPV, and serologic antibodies to HPV16 virus-like particles and HPV16 E6 and E7 proteins were detected in most of these cases. We assume therefore that 8% oral cancers, 14% oropharyngeal cancers and 10.6% laryngeal cancers are HPV-related.
HPV (any type) is estimated to be responsible for 5088 cancers occurring in the UK in 2010 (1.6% of all cancers), comprising 2691 cervix cancers, 1685 cases of ano-genital cancer and 712 cases of upper aerodigestive tract cancer (Table 2).
Table 2. Estimated numbers of HPV-related cancers, UK (2010).
|
Observed cases
|
HPV-related
|
||||
|---|---|---|---|---|---|
| Male | Female | Male | Female | Excess attributable cases (PAF) | |
| Upper aerodigestive cancers | |||||
| Cervix uteri | 2691 | 2691 | 2691 (100) | ||
| Oral cavity | 2284 | 1421 | 183 | 114 | 296 (8.0) |
| Oropharynx | 981 | 323 | 138 | 45 | 184 (14.1) |
| Larynx | 1803 | 386 | 191 | 40 | 232 (10.6) |
| Anogenital cancers | |||||
| Anus | 364 | 621 | 328 | 559 | 887 (90.0) |
| Vulva | 1128 | 451 | 451 (40.0) | ||
| Vagina | 251 | 157 | 157 (62.5) | ||
| Penis | 475 | 190 | 190 (40.0) | ||
| Total (8 sites) | 5907 | 6821 | 1030 | 4058 | 5088 (40.0) |
Abbreviations: HPV=human papillomavirus; PAF=population-attributable fraction (%).
Helicobacter pylori
Helicobacter pylori was classified as being carcinogenic for humans in 1994 (IARC, 1994a). It is considered to be causally associated with both carcinoma of the stomach and gastric lymphoma.
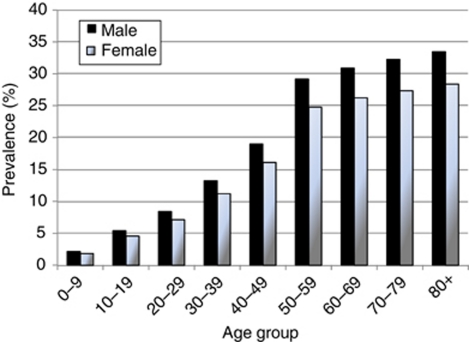
Surveys of H. pylori show that prevalence gradually increases with age. Several studies have suggested that this represents a birth-cohort effect, with infection becoming progressively less common in recent generations (Banatvala et al, 1993; Kosunen et al, 1997; Roosendaal et al, 1997). In the UK the most comprehensive data on prevalence derive from the serological surveys of 10 000 serum samples collected in England and Wales in 1986 and 1996 (Vyse et al, 2002). Prevalence was related to decade of birth and increased from 4% in those born during the 1980s to 30% in those born before 1940; analysis by decade of birth showed no significant difference between samples collected in 1986 and 1996. Estimated prevalence of active infection varied by region and was highest in London.
We estimated prevalence in the UK population in 2000 from the data provided by Vyse et al (2002), assuming that prevalence in Scotland and Northern Ireland was the same as that observed in the North of England (Figure 1).
Figure 1.
Estimated prevalence of Helicobacter pylori in the UK in 2000.
Gastric carcinoma
The most satisfactory evidence on the magnitude of the risk is from prospective studies. Retrospective case–control studies are limited in observing H. pylori infection after the development of cancer. H. pylori tends to disappear as intestinal metaplasia and atrophy develop, so that prevalence of infection may be seriously under-estimated in cases, even if anti-H. pylori antibody is used as an indicator of infection. Several case–control studies nested within cohorts have now been published, in which infection is evaluated in cases and controls before the onset of disease. In a meta-analysis by the Helicobacter and Cancer Collaborative Group (2001), including 12 prospective studies yielding 1228 gastric cancer cases, the OR for the association between H. pylori infection and the subsequent development of gastric cancer was 2.36 (95% CI 1.98–2.81). Analysing cancers of the gastric cardia, the most proximal portion of the stomach and non-cardia separately, they found no increase in risk for cardia cancers (OR 0.99), while the overall risk for non-cardia cancers was 2.97 (95% CI 2.34–3.77). The risk varied with the interval between sample collection and cancer diagnosis (as might be expected, if infection is progressively lost as gastric atrophy develops). The increase in risk was 5.9-fold (95% CI 3.4–10.3) for H. pylori positivity 10 years or more prior to diagnosis. The associations were not related to histological type of gastric cancer (intestinal vs diffuse) or sex.
The proportion of gastric cancer cases occurring at the cardia, compared with elsewhere in the stomach, can be estimated from cancer registry data from England (2007), and from the results of 11 registries throughout the UK in 1998–2005 (Curado et al, 2007). In both sets, a variable proportion of gastric cancer cases (40–65%) are registered without specification of subsite. However, fitting a linear regression model of the proportion of cardia cancers vs the proportion of unspecified registrations suggests that mis-specification of site is more or less random. The predicted proportion of cardia cancers (with zero non-specification) is 51.9% in men and 38.9% in women. Age-specific proportions in the UK were estimated based on the distribution by age in England (2007).
With a relative risk of 5.9 and prevalence of infection in 2000 (10 years earlier) as shown in Figure 1, the attributable fraction of non-cardia gastric cancer cases in 2010 is 61% in men and 59% in women. This represents 2231 cases, 29.2% of all stomach cancers in men and 36.0% in women, or 0.7% of all cancers.
Gastric lymphoma
One of the two large American cohort studies of H. pylori also examined the incidence of gastric NHL and found that these cases showed elevated titres of antibody to H. pylori (Parsonnet et al, 1994). The relative risk was 6.3 (95% CI 2.0–19.9). Gastric NHL is rather a rare tumour, comprising about 5% of all NHL (Newton et al, 1997).
Assuming that 5% of NHL cases in UK are localised to the stomach, there were about 580 new cases in 2010. With a relative risk of 6, and the estimated prevalence of infection in 2000 (Figure 1), 2.8% of NHL cases (327) would be attributable to H. pylori.
Epstein–Barr virus
EBV is considered to be a group I carcinogen by IARC (1997), with conclusive evidence with respect to carcinogenicity in Burkitt lymphoma, NHL in immunosuppressed subjects, sino-nasal angiocentric T-cell lymphoma, Hodgkin lymphoma and nasopharyngeal carcinoma. The evidence concerning other cancers for which an association with EBV has been demonstrated (lymphoepithelial carcinomas, gastric adenocarcinoma and smooth muscle tumours in immunosuppressed subjects) was considered to be inconclusive.
EBV and NHL
Burkitt lymphoma: Burkitt lymphoma is a rare cancer in UK. There were an estimated 158 new cases in 2010. In North America and Europe, about one-fifth to one-third have demonstrable virus in tumour tissue, or elevated antibody titres to EBV (Lenoir et al, 1984; Gutierrez et al, 1992). The numbers of EBV-attributable Burkitt lymphoma cases in UK is therefore about 39.
Other NHL: EBV can cause lymphoproliferative diseases in individuals with immune dysfunction (Lenoir and Delecluse, 1989). Lymphomas arising in immunocompromised individuals are relatively rare, except in the case of AIDS, some of which are associated with EBV. The proportion of AIDS-related lymphomas is estimated in the context of HIV-related cancers (below). The proportion of NHLs that occur in immunocompromised individuals, excluding AIDS (hereditary, syndromes, iatrogenic), or are cases of the rare EBV-associated sino-nasal angiocentric T-cell lymphoma, is impossible to estimate. It must be a very small (<1%) fraction of NHLs, so there is no numerical allowance for these cases in the estimates.
EBV and Hodgkin lymphoma
Case–control studies generally demonstrate higher titres of anti-EBV antibodies in cases of Hodgkin lymphoma than in controls (Evans and Gutensohn, 1984). In a large prospective study, Mueller et al (1989) found that elevated antibody titres precede diagnosis by several years – the actual relative risks (2.6 and 3.7 for IgG and IgA capsid antigens, 4.0 for EBNA and 2.6 for early antigen (diffuse)) and prevalence of raised titres correspond to attributable risks of 30–45%.
Sensitive techniques are able to detect EBV nucleic acids in 25–50% of Hodgkin lymphomas, where it is located in the Reed–Sternberg cells (Weiss et al, 1989; Armstrong et al, 1992). The association with EBV appears to depend upon age. In the childhood age range about 80% of cases are EBV positive (Weinreb et al, 1996), whereas in young adults the proportion is about 15% (Jarrett et al, 1991). In older age groups, EBV positivity appears to be relatively high (70–75%) (Jarrett et al, 1991; Gledhill et al, 1991). In part, this pattern is determined by the frequency of different histological subtypes of Hodgkin lymphoma. The mixed cellularity subtype predominates in childhood, while the nodular sclerosing subtype accounts for the marked peak in young adults. The frequency of EBV positivity is much greater (5–15-fold) in mixed cellularity than in nodular sclerosing Hodgkin lymphoma. Nevertheless, it seems that, even allowing for cell type, age (more childhood cases are EBV positive) and level of socio-economic development are independent predictors of the association (Glaser et al, 1997). For the purposes of estimation, the attributable fraction at ages 0–14 is taken to be 80%, 20% at ages 15–44, and 70% at ages >45. Of the UK total of 1709 new cases in 2010, 773, or 45.2% of the total, are estimated to be EBV related.
EBV and nasopharyngeal carcinoma
The involvement of EBV in nasopharyngeal cancer (NPC) appears to be with undifferentiated carcinomas of the nasopharynx. In low-risk areas, about 10–25% of NPC is of type 1 (keratinising), which is less often infected. It is assumed that 90% of cases of the estimated 446 NPC cases occurring in the UK in 2010 are infected, a total of 401 cases, or 5.8% of all cancers or of the oral cavity and pharynx.
Summary: EBV
EBV is the third most important cancer-causing infection in the UK, responsible for an estimated 1213 cases in 2010 (0.4% of cancers), comprising 773 cases of Hodgkin disease, 401 nasopharyngeal cancers and 39 Burkitt lymphoma cases.
Hepatitis viruses
The role of chronic infection with the viruses of hepatitis B and C in the aetiology of liver cancer is well established (IARC, 1994b). More recently, on the basis of a substantial number of case–control and cohort studies, an association between HCV and NHL has been demonstrated (Gisbert et al, 2003; Matsuo et al, 2004; dal Maso and Franceschi, 2006).
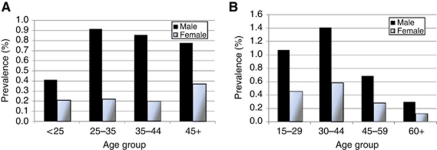
Prevalence of hepatitis B surface antigen (HBsAg) positivity and HCV antibodies in the serum of the general population of UK is unknown, as no true random survey results exist. Age-specific prevalence is published for first-time blood donors (subjects found to be positive are obviously excluded from becoming repeat donors), although these are healthy individuals, with a prevalence much lower than the average in the population. An estimate of the prevalence of hepatitis C in adults (aged 15–59), based on a model incorporating all relevant samples, was made for England in 2003 (HPA, 2006); this estimate had not been updated by the end of 2010. These data were assumed for UK, with an estimate of prevalence at ages >60 based on sero-surveys of laboratory samples in England and Wales in the 1990s (Balogun et al, 2002).
For hepatitis B, the estimated population prevalence, based on sero-surveys of laboratory samples in England and Wales in 1996 (Gay et al, 1999), was used to adjust the observed age–sex-specific prevalence data from blood donors in England in 1995–2008 (HPA, 2009), and further adjusted upwards, to allow for the rather higher prevalence in blood donors in UK, compared with those in England (HPA, 2009).
The estimated prevalences are shown in Figure 2.
Figure 2.
Estimated age- and sex-specific prevalence of carriers of (A) HBsAG and (B) anti-HCV antibodies in the UK.
HBV and HCV and liver cancer
The IARC Monograph (1994b) summarises the results of some 15 cohort studies and 65 case–control studies worldwide, examining the association between seropositivity for HBsAg, indicating chronic infection with HBV, and the risk of hepatocellular carcinoma. The cohort studies yield relative risk estimates of 5.3–148, while the majority of case–control studies yield relative risk estimates between 3 and 30. Some of these studies were able to address potential confounding by aflatoxin, hepatitis C infection, alcohol drinking and tobacco consumption, and the IARC Monograph (1994b) overall evaluation assessed HBV as carcinogenic to humans. A recent meta-analysis by Cho et al (2011) found an odds ratio for mono-infection with a HBV of 13.5 for all 47 studies included, and 20.3 for the four studies in low-prevalence areas (such as UK). A relative risk of 20 is assumed in the current analysis.
The magnitude of the risk of liver cancer associated with chronic ‘infection’ with HCV became evident as the results of studies using second- and third-generation anti-HCV ELISA tests or detection of HCV RNA (by reverse transcription polymerase chain reaction) became available. In a meta-analysis of studies, Donato et al (1998) estimated the relative risk in HCV antibody-positive subjects who were HBsAg negative as 17.3. The more recent meta-analysis by Cho et al (2011) found an odds ratio of 23.8 in seven studies from low-prevalence countries. We assume a relative risk of 20 for HCV infection.
Assuming relative risks of chronic infection by these viruses of 20, and that joint infection by both HBV and HCV is very rare, we estimate the fraction of liver cancers attributable to the two viruses to be just 15.9% (567 of 3568 cases). This represents 0.18% of cancers in the UK in 2010.
HCV and NHL
At least three meta-analyses (Gisbert et al, 2003; Matsuo et al, 2004; dal Maso and Franceschi, 2006) have been conducted to evaluate the strength of the relationship between HCV and NHL. The most recent of these (dal Maso and Franceschi, 2006) gives a rather lower estimate for the relative risk (2.5) than the earlier studies. However, as it considered all NHL (not just B-cell neoplasms) and took into account differences in age between cases and controls, it is probably the most valid estimate. A recent report pooling the results of seven case–control studies (de Sanjose et al, 2008) gave a similar result (odds ratio of 1.8). Using the value of 2.5, and the estimated prevalence of HCV infection in the UK in 2010 (Figure 2), one can estimate the fraction of NHL attributable to HCV as just 0.5% (53 of 11 602 cases).
HIV and HHV8
In 1996, an IARC working group concluded that HIV was carcinogenic to humans, an assessment based on the strong link between infection with the virus and two cancers: Kaposi sarcoma (KS) and NHL (IARC, 1996). These two diseases, along with cancer of the cervix, are considered to be ‘AIDS-defining conditions’ – that is, a HIV-positive subject with these cancers is considered to have AIDS (CDC, 1992). Subsequently, increased risks for several other cancers have been reported. The most convincing data come from a follow-up of cohorts of HIV-positive subjects, comparing the occurrence of cancers with the number expected in the general population. Such studies suggest increased risks of several cancers, especially Hodgkin disease, anal cancer, seminoma, myeloma, and, less certainly, cancers of the lip, brain and lung (Goedert et al, 1998; Frisch et al, 2001; Grulich et al, 2002).
The evaluation by IARC (1997) considered that the evidence for a role of KSHV/HHV8 in the causation of KS was ‘compelling, but as yet limited’. However, it is now generally accepted to be the principal cause of the disease (Boshoff and Weiss, 2001). The effect of HIV is probably through immunosuppression – allowing HHV-8 to escape control and thereby increasing viral load, for example.
HIV/HHV8 and KS
Prior to the epidemic of HIV/AIDS, KS was a very rare cancer in the UK. Grulich et al (1992) calculated the incidence in England and Wales in 1971–80 as 0.14 per million (same in males and females).
Because of the enormous increase in risk in subjects infected with HIV (1000–5000 times the risk in the general population (Serraino et al, 1997)), the increasing incidence of KS was the first obvious manifestation of the AIDS epidemic. Before 1990, up to a third of AIDS cases developed KS at some point (Hoover et al, 1993; Lundgren et al, 1995). The introduction of antiretroviral therapy (HAART) for treating HIV in adults has caused a decline in the incidence of KS in Western countries (International Collaboration on HIV and Cancer, 2000); in the USA, for example, the incidence of KS in men aged 20–54 in the cancer registries of the SEER program fell from 17.2 per 105 in 1990–1 to 2.4 in 2000–2001 (Ries et al, 2004).
The estimated number of KS cases in UK in 2010 is 172. All might be ascribed to infection with HHV-8. Based on the rates in the pre-AIDS era (Grulich et al, 1992), the number of cases expected was 10. The difference (162 cases) is considered to be attributable to HIV infection, while all cases of KS are attributable to infection with HHV-8.
HIV and NHL
The increased frequency of NHL in AIDS was noted in 1982 (Ziegler et al, 1982). Since then, the elevated risk has been confirmed in studies in the United States and Europe (Casabona et al, 1991; Beral et al, 1991). About 3% of AIDS cases present with a lymphoma, but lymphomas may occur in up to 10% of AIDS cases at some point. Almost all lymphomas in AIDS cases are of B-cell type. The cohort study of Coté et al. (1997) estimated the excess risk in AIDS to be about 160 times that in HIV-negative subjects. Risk is highest for high-grade lymphomas; especially diffuse immunoblastic (relative risk=630) and undifferentiated Burkitt lymphomas (relative risk=220). Extra nodal lymphomas are more common in AIDS than usual (Beral et al, 1991), although it is probably because of the great excess of CNS lymphomas (15-fold increase); other extra nodal lymphomas are not in excess (Coté et al. 1997). Males are more commonly affected – but it could be that this is simply because of risk-group differences.
EBV is present in two-thirds of AIDS-related lymphomas (Hamilton–Dutoit et al, 1993) and may have an important role in lymphomagenesis (IARC, 1996, 1997). The frequency varies by lymphoma type – it is found in almost all CNS lymphomas, 70–80% of immunoblastic lymphomas and 30–40% of small-cell/Burkitt-type lymphomas.
The availability of HAART in recent years has resulted in a decline in the risk of NHL in HIV-infected individuals; it fell from 0.62% per year in the pre-HAART era (1992–1996) to 0.36% when HAART regimens were widely available (1996–1999) (International Collaboration on HIV and Cancer, 2000). In the USA, cohort studies suggest that the relative risk of NHL in HIV-positive subjects since the introduction of HAART is about 6.5 (Hessol et al, 2004; Engels et al, 2008), and this figure is used to estimate the attributable fraction.
The overall prevalence of HIV infection in the UK in 2009 was estimated to be 2.89 per 1000 in men aged 15–59 and 1.46 per 1000 in women (HPA, 2010). Prevalence in childhood and those over 60 is much lower: 0.09 and 0.46 per 1000, respectively (HPA, 2010; UK CGHSS, 2006).
Based on these estimated prevalences, and a relative risk of 6.5, only 52 cases of NHL in men and 16 in women (69 total) would have been attributable to HIV in 2010. The numbers seem small, but they are broadly in line with the numbers of cases expected based on observed incidence rates of NHL in HIV-positive subjects in recent years – for example, 1.8 per 1000 in the Swiss cohort in 2002–6 (Polesel et al, 2008) and 0.97 in three US states in 1996–2002 (Engels et al, 2008). With these rates, 120 cases of NHL would have occurred in HIV-positive subjects in the UK, compared with 11 expected, an excess of 109.
HIV and other cancers
Hodgkin lymphoma: Several prospective studies suggest that the risk of Hodgkin lymphoma is increased some 10-fold in HIV-infected subjects (Goedert et al, 1998; dal Maso et al, 2001; Grulich et al, 2002). Case series document unusually aggressive disease, including a higher frequency of the unfavourable histological subtypes (mixed cellularity and lymphocyte depleted), advanced stages and poor therapeutic response compared with the behaviour of HD outside of the HIV setting. It is not clear whether most or all of these cases of Hodgkin lymphoma are related to EBV, all of which have already been attributed to infection with this virus. A separate calculation of HIV-attributable cases has not been carried out.
HPV-associated cancers: HPV-associated malignancies – most notably cancer of the cervix uteri and anal cancers – occur frequently in patients with HIV infection and AIDS (Frisch et al, 2000). In part, this may simply reflect the lifestyle factors associated with both infections – HIV-positive individuals are more likely to be infected by HPV. On the other hand, HIV may alter the natural history of HPV-associated oncogenesis through loss of immune control, facilitating infection with HPV or enhancing its persistence in cells and therefore increasing the development of squamous intraepithelial lesions (SIL). These cancers have already been attributed to infection with HPV.
HIV infection has been found to be associated with an increased risk of conjunctival SCC in follow-up of cohorts of HIV-positive subjects in the USA (Goedert and Coté, 1995; Frisch et al, 2001). With a relative risk of 10, about 1% of cases might be attributable to HIV, given the prevalence of infection in the UK. As only 23 cases of conjunctival cancer were registered in England in 2007, the number of attributable cases is ignored.
Summary: HIV-related cancer
In all, 172 cases of KS and 69 cases of NHL were caused by HIV and/or HHV-8 in 2010. Of the KS cases, 162 are attributed to infection with HIV.
Human T lymphotropic virus
The evidence for the causal role of HTLV-1 in acute T-cell leukaemia/lymphoma (ATL) is compelling (IARC, 1996). Prevalence of HTLV infection in UK is available for first-time blood donors (HPA, 2009). Overall, it is 4.7 per 100 000 in men and 10.7 per 100 000 in women, strongly increasing with age. Based on the recorded incidence of ATL (ICD 91.5) in England in 2007, 25 cases would have been expected in the UK population.
Summary
Table 3 summarises the quantification of cancers attributable to infections in the UK in 2010. The estimate is 3925 (2.5% of all cancers) in men and 5820 (3.7% of all cancers) in women. Of the total of 9745, the infectious agents making the largest contribution are HPV (5088 cases, 1.6% of all cancers), H. pylori (2559 cases, 0.8%) and EBV (1213 cases, 0.4%).
Table 3. Estimated numbers of cancers attributable to different infectious agents, UK 2010.
|
Estimated number of cancer cases by
infectious agent
|
|||||||
|---|---|---|---|---|---|---|---|
| Cancer site | HPV | H. pylori | EBV | HBV and HCV | HIV and KSHV | HTLV | Excess attributable cases (PAF) |
| Males | |||||||
| Oral cavity and pharynx | 321 | 241 | 562 (0.35) | ||||
| Larynx | 191 | 191 (0.12) | |||||
| Stomach | 1304 | 1304 (0.82) | |||||
| Anus | 328 | 328 (0.21) | |||||
| Liver | 446 | 446 (0.28) | |||||
| Kaposi | 147 | 147 (0.09) | |||||
| External genitalia | 190 | 190 (0.12) | |||||
| Non-Hodgkin lymphoma | 182 | 31 | 38 | 52 | 12 | 316 (0.20) | |
| Hodgkin lymphoma | 442 | 442 (0.28) | |||||
| Total | 1030 | 1486 | 713 | 484 | 200 | 12 | 3925 |
| % of all cancera | 0.65 | 0.94 | 0.45 | 0.31 | 0.13 | 0.01 | 2.5% |
| Females | |||||||
| Oral cavity and pharynx | 159 | 160 | 319 (0.21) | ||||
| Larynx | 40 | 40 (0.03) | |||||
| Stomach | 927 | 927 (0.60) | |||||
| Anus | 559 | 559 (0.36) | |||||
| Liver | 121 | 121 (0.08) | |||||
| Kaposi | 25 | 25 (0.02) | |||||
| Cervix uteri | 2691 | 2691 (1.73) | |||||
| External genitalia | 608 | 608 (0.39) | |||||
| Non-Hodgkin lymphoma | 145 | 9 | 14 | 16 | 13 | 197 (0.13) | |
| Hodgkin lymphoma | 331 | 331 (0.21) | |||||
| Total | 4058 | 1072 | 501 | 135 | 41 | 13 | 5820 |
| % of all cancersa | 2.61 | 0.69 | 0.32 | 0.09 | 0.03 | 0.01 | 3.7% |
| Persons | |||||||
| Oral cavity and pharynx | 480 | 401 | 881 (0.28) | ||||
| Larynx | 232 | 232 (0.07) | |||||
| Stomach | 2231 | 2231 (0.71) | |||||
| Anus | 887 | 887 (0.28) | |||||
| Liver | 567 | 567 (0.18) | |||||
| Kaposi | 172 | 172 (0.05) | |||||
| Cervix uteri | 2691 | 2691 (0.86) | |||||
| External genitalia | 798 | 798 (0.25) | |||||
| Non-Hodgkin lymphoma | 327 | 39 | 53 | 69 | 24 | 513 (0.16) | |
| Hodgkin disease | 773 | 773 (0.25) | |||||
| Total | 5088 | 2559 | 1213 | 619 | 241 | 24 | 9745 |
| % of all cancersa | 1.62 | 0.81 | 0.39 | 0.20 | 0.08 | 0.01 | 3.1% |
Abbreviations: EBV=Epstein–Barr virus; H. pylori=Helicobacter pylori; HBV and HCV=hepatitis B and C viruses; HIV and KSHV=human immunodeficiency virus and human herpesvirus 8/Kaposi sarcoma; HPV=human papillomaviruses; HTLV=human T lymphotropic virus type 1; PAF=population-attributable fraction.
Excluding non-melanoma skin cancer.
The cancers for which an infectious aetiology is most important are cervix uteri (2691 cases in 2010), stomach (2231) and the upper aerodigestive tract (mouth, pharynx and larynx – 1113 cases).
Discussion
Worldwide, 17.8% of all cancers are attributable to infections (Parkin, 2006), with a higher percentage in developing countries (26.3%), and an average of 7.7% in developed countries reflecting the higher prevalence of infection with the major causative agents (hepatitis viruses, HPV, H. pylori, HIV). The proportion in the UK is around half of the average for developed countries, and very similar to the estimate (3.5%) for the Netherlands (van Lier et al, 2008).
The results are dependent on the assumptions made about relative risk, and accuracy of the estimates of prevalence of infection in the general population. For some of the associations – especially in relation to HPV and anogenital cancers – the estimate of attributable fraction was based on the proportion of tumours in which the virus (as viral DNA) could be detected. The reason is mainly that prevalence of infection in the same tissues of normal individuals is usually unknown. This may overestimate attributable fractions, by including some cancer cases in which the presence of the virus was coincidental, without, for example, expressing viral oncoproteins. The estimate of HPV-attributable cancers of the oral cavity, pharynx and larynx attempts to compensate for this, by estimating the number of aetiologically relevant infections (expressing E6 and E7 proteins, for example).
The estimate of infection-attributable cancer is a conservative one. Some other associations between infections and human cancers, for which there is reasonable evidence for causality, have not been taken into account. EBV has been detected in several types of cancer, other than those attributed to it in this analysis, with the most suggestive evidence implicating it in the aetiology of gastric cancer (Takada, 2002; Herrmann and Niedobitek, 2003). Chlamydia trachomatis infection has been shown to increase the risk of developing SCC of the cervix (Smith et al, 2004). In any case, no case of cancer has been attributed to more than one infectious agent, so that the numbers of infection-attributable cases can be calculated for different populations. Thus, for example, the risk of cancer of the cervix uteri may be increased by HIV infection (Frisch et al, 2001) as well as C. trachomatis, but as all cases are attributed to HPV, none are included as HIV-related cancers. In addition, the estimates of relative risk for those associations accepted as causal that have been used in the calculations are deliberately modest. For example, the relative risk of liver cancer due to infection with hepatitis B is based on measurement of serum HBsAg. However, viral DNA can be found in many liver cancers without evidence of infection based on HBs antigenaemia or antibody to HCV (Paterlini et al, 1990). The relative risk of non-cardia gastric cancer in relation to infection with H. pylori that was used (5.9) may also be too modest; more sensitive techniques for estimating the presence of H. pylori (for example, by detecting bacterial DNA) have much higher relative risks (Mitchell et al, 2008), but as the prevalence estimates are based on the presence of anti-H. pylori antibody, we use the relative risk estimates based on the same techniques. Accepting that all non-cardia gastric cancers are caused by infection (H. pylori and/or EBV) as well as 10% of NHL are caused by HCV (independent of HIV) would not, however, greatly change the overall estimate.
See acknowledgements on page Si.
Footnotes
The author declares no conflict of interest.
References
- Armstrong AA, Weiss LM, Gallagher A, Jones DB, Krajewski AS, Angus B, Brown G, Jack AS, Wilkins BS, Onions DE (1992) Criteria for the definition of Epstein-Barr virus association in Hodgkin's disease. Leukemia 6: 869–874 [PubMed] [Google Scholar]
- Balogun MA, Ramsay ME, Hesketh LM, Andrews N, Osborne KP, Gay NJ, Morgan-Capner P (2002) The prevalence of hepatitis C in England and Wales. J Infect 45: 219–226 [DOI] [PubMed] [Google Scholar]
- Banatvala N, Mayo K, Mégraud F, Jennings R, Deeks JJ, Feldman RA (1993) The cohort effect and Helicobacter pylori. J Infect Dis 168: 219–221 [DOI] [PubMed] [Google Scholar]
- Beral V, Peterman T, Berkelman R, Jaffe H (1991) AIDS-associated non-Hodgkin lymphoma. Lancet 337: 805–809 [DOI] [PubMed] [Google Scholar]
- Bezerra AL, Lopes A, Landman G, Alencar GN, Torloni H, Villa LL (2001) Clinicopathologic features and human papillomavirus DNA prevalence of warty and squamous cell carcinoma of the penis. Am J Surg Pathol 25: 673–678 [DOI] [PubMed] [Google Scholar]
- Boshoff C, Weiss RA (2001) Epidemiology and pathogenesis of Kaposi’s sarcoma-associated herpesvirus. Phil Trans Roy Soc Lond B 356: 517–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casabona J, Melbye M, Biggar RJ, the AIDS Registry Contributors (1991) Kaposi’s sarcoma and non-Hodgkin’s lymphoma in European AIDS cases. Int J Cancer 47: 49–53 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control (1992) 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Morb Mortal Wkly Rep 41: 1–19 [PubMed] [Google Scholar]
- Cho LY, Yang JJ, Ko PL, Park B, Shin A, Lim MK, Oh JK, Park S, Kim YJ, Shin HR, Yoo KY, Park SK (2011) Coinfection of hepatitis B and C viruses and risk of hepatocellular carcinoma: systematic review and meta-analysis. Int J Cancer 128: 176–184 [DOI] [PubMed] [Google Scholar]
- Cole P, MacMahon B (1971) Attributable risk percent in case-control studies. Brit J Prev Soc Med 25: 242–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coté TR, Biggar RJ, Rosenberg PS, Devesa SS, Percy C, Yellin FJ, Lemp G, Hardy C, Geodert JJ, Blattner WA (1997) Non-Hodgkin’s lymphoma among people with AIDS: incidence, presentation and public health burden. AIDS/Cancer Study Group. Int J Cancer 73: 645–650 [DOI] [PubMed] [Google Scholar]
- Curado M, Edwards B, Shin H (2007) Cancer Incidence in Five Continents, Vol. I to XI. IARC Scientific Publications No. 160. IARC: Lyon [Google Scholar]
- Dal Maso L, Franceschi S (2006) Hepatitis C virus and risk of lymphoma and other lymphoid neoplasms: a meta-analysis of epidemiologic studies. Cancer Epidemiol Biomarkers Prev 15: 2078–2085 [DOI] [PubMed] [Google Scholar]
- Dal Maso L, Serraino D, Franceschi S (2001) Epidemiology of AIDS-related tumours in developed and developing countries. Eur J Cancer 37: 1188–1201 [DOI] [PubMed] [Google Scholar]
- Daling JR, Madeleine MM, Schwartz SM, Shera KA, Carter JJ, McKnight B, Porter PL, Galloway DA, McDougall JK, Tamimi H (2002) A population-based study of squamous cell vaginal cancer: HPV and cofactors. Gynecol Oncol 84: 263–270 [DOI] [PubMed] [Google Scholar]
- de Sanjose S, Benavente Y, Vajdic CM, Engels EA, Morton LM, Bracci PM, Spinelli JJ, Zheng T, Zhang Y, Franceschi S, Talamini R, Holly EA, Grulich AE, Cerhan JR, Hartge P, Cozen W, Boffetta P, Brennan P, Maynadié M, Cocco P, Bosch R, Foretova L, Staines A, Becker N, Nieters A (2008) Hepatitis C and non-Hodgkin lymphoma among 4784 cases and 6269 controls from the International Lymphoma Epidemiology Consortium. Clin Gastroenterol Hepatol 6: 451–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato F, Boffetta P, Puoti M (1998) A meta-analysis of epidemiological studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma. Int J Cancer 75: 347–354 [DOI] [PubMed] [Google Scholar]
- Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, Grigg R, Hylton T, Pawlish KS, McNeel TS, Goedert JJ (2008) Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer 123: 187–194 [DOI] [PubMed] [Google Scholar]
- Evans AS, Gutensohn NM (1984) A population-based case control study of EBV and other viral antibodies among persons with Hodgkin’s disease and their siblings. Int J Cancer 34: 147–157 [DOI] [PubMed] [Google Scholar]
- Frisch M, Biggar RJ, Engels EA, Goedert JJ (2001) AIDS-Cancer Match Registry Study Group. Association of cancer with AIDS-related immunosuppression in adults. JAMA 285: 1736–1745 [DOI] [PubMed] [Google Scholar]
- Frisch M, Biggar RJ, Goedert JJ (2000) Human papillomavirus associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst 92: 1500–1510 [DOI] [PubMed] [Google Scholar]
- Frisch M, Fenger C, van den Brule AJC, Sørensen P, Meijer CJLM, Walboomers JMM, Adami H-O, Melbye M, Glimelius B (1999) Variants of squamous cell carcinoma of the anal canal and perianal skin and their relation to human papillomaviruses. Cancer Res 59: 753–757 [PubMed] [Google Scholar]
- Gay NJ, Hesketh LM, Osborne KP, Farrington CP, Morgan-Capner P, Miller E (1999) The prevalence of hepatitis B infection in adults in England and Wales. Epidemiol Infect 122: 133–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisbert JP, Garcia-Buey L, Pajares JM, Moreno-Otero R (2003) Prevalence of hepatitis C virus infection in B-cell non-Hodgkin's lymphoma: systematic review and meta-analysis. Gastroenterology 125: 1723–1732 [DOI] [PubMed] [Google Scholar]
- Glaser SL, Lin RJ, Stewart SL, Ambinder RF, Jarrett RF, Brousset P, Pallesen G, Gulley ML, Khan G, O’Grady J, Hummel M, Preciado MV (1997) Epstein-Barr virus-associated Hodgkin’s disease: epidemiologic characteristics in international data. Int J Cancer 70: 375–382 [DOI] [PubMed] [Google Scholar]
- Gledhill S, Gallagher A, Jones DB, Krajewski AS, Alexander FE, Klee E, Wright DH, O’Brien C, Onions DE, Jarrett RF (1991) Viral involvement in Hodgkin's disease: detection of clonal type A Epstein-Barr virus genomes in tumour samples. Br J Cancer 64: 227–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert JJ, Coté TR (1995) Conjunctival malignant disease with AIDS in USA. Lancet 346: 257–258 [DOI] [PubMed] [Google Scholar]
- Goedert JJ, Coté TR, Virgo P, Scoppa SM, Kingma DW, Gail MH, Jaffe ES, Biggar RJ (1998) Spectrum of AIDS-associated malignant disorders. Lancet 351: 1833–1839 [DOI] [PubMed] [Google Scholar]
- Grulich AE, Beral V, Swerdlow AJ. (1992) Kaposi's sarcoma in England and Wales before the AIDS epidemic. Br J Cancer 66: 1135–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grulich AE, Li Y, McDonald A, Correl PK, Law MG, Kaldor JM (2002) Rates of non-AIDS-defining cancers in people with HIV infection before and after AIDS diagnosis. AIDS 16: 1155–1161 [DOI] [PubMed] [Google Scholar]
- Gutierrez MI, Bhatia K, Barriga F, Diez B, Muriel FS, de Andreas ML, Epelman S, Risueno C, Magrath IT (1992) Molecular epidemiology of Burkitt's lymphoma from South America: differences in breakpoint location and Epstein-Barr virus association from tumors in other world regions. Blood 79: 3261–3266 [PubMed] [Google Scholar]
- Hamilton-Dutoit SJ, Raphael M, Audouin J, Diebold J, Lisse I, Pedersen C, Oksenhendler E, Marelle L, Pallesen G (1993) In situ demonstration of Epstein-Barr virus small RNAs (EBER 1) in acquired immunodeficiency syndrome-related lymphomas: correlation with tumor morphology and primary site. Blood 82: 619–624 [PubMed] [Google Scholar]
- Health Protection Agency (HPA) (2006) Hepatitis C in England: An Update. Health Protection Agency Centre for Infections: London. http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1196942171852 [Google Scholar]
- Health Protection Agency (HPA) (2009) NBS/Health Protection Agency Infection Surveillance. Six Month Summary Report No. 29: data to end of March 2009. Health Protection Agency: London. http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1241418683965 [Google Scholar]
- Health Protection Agency (HPA) (2010) HIV in the United Kingdom: 2010 Report. Health Prot Rep 2010 4: 47. http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1287145367237 [Google Scholar]
- Helicobacter and Cancer Collaborative Group (2001) Gastric cancer and Helicobacter pylori: a combined analysis of 12 case-control studies nested within prospective cohorts. Gut 49: 347–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero R, Munoz N (1999) Human papillomavirus and cancer. In Infections and Human Cancer. Cancer Surveys, Weiss RA, Beral V, Newton R (eds) Vol. 33, pp 75–98. Cold Spring Harbor Laboratory Press: Cold Spring Harbor [Google Scholar]
- Hessol NA, Seaberg EC, Preston-Martin S, Massad LS, Sacks HS, Silver S, Melnick S, Abulafia O, Levine AM; for the WIHS Collaborative Study Group (2004) Cancer risk among participants in the women's interagency HIV study. J Acquir Immune Defic Syndr 36: 978–985 [DOI] [PubMed] [Google Scholar]
- Hoover DR, Black C, Jacobson LP, Martinez-Maza O, Seminara D, Saah A, Von Roenn J, Anderson R, Armenian HK (1993) Epidemiologic analysis of Kaposi's sarcoma as an early and later AIDS outcome in homosexual men. Am J Epidemiol 138: 266–278 [DOI] [PubMed] [Google Scholar]
- IARC (2005) Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 90, Human Papillomaviruses. IARC: Lyon [PMC free article] [PubMed] [Google Scholar]
- IARC (1994a) Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 61, Schistosomes, Liver Flukes and Helicobacter Pylori. IARC: Lyon [PMC free article] [PubMed] [Google Scholar]
- IARC (1994b) Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 59, Hepatitis Viruses. IARC: Lyon [PMC free article] [PubMed] [Google Scholar]
- IARC (1997) Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 70, Infections with Epstein-Barr Virus and Human Herpes Viruses. IARC: Lyon [PMC free article] [PubMed] [Google Scholar]
- IARC (1996) Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 67, Human Immunodeficiency Viruses and Human T-cell Lymphotropic Viruses. IARC: Lyon [PMC free article] [PubMed] [Google Scholar]
- International Collaboration on HIV and Cancer (2000) Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J Natl Cancer Inst 92: 1823–1830 [DOI] [PubMed] [Google Scholar]
- Jarrett RF, Gallagher A, Jones DB, Alexander FE, Krajewski AS, Kelsey A, Adams J, Angus B, Gledhill S, Wright DH (1991) Detection of Epstein-Barr virus genomes in Hodgkin's disease: relation to age. J Clin Pathol 44: 844–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosunen TU, Aromaa A, Knekt P, Salomaa A, Rautelin H, Lohi P, Heinonen OP (1997) Helicobacter antibodies in 1973 and 1994 in the adult population of Vammala, Finland. Epidemiol Infect 119: 29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreimer AR, Clifford GM, Boyle P, Franceschi S (2005a) Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 14: 467–475 [DOI] [PubMed] [Google Scholar]
- Kreimer AR, Clifford GM, Snijders PJ, Castellsagué X, Meijer CJ, Pawlita M, Viscidi R, Herrero R, International Agency for Research on Cancer (IARC) Multicenter Oral Cancer Study Group (2005b) HPV16 semiquantitative viral load and serologic biomarkers in oral and oropharyngeal squamous cell carcinomas. Int J Cancer 115: 329–332 [DOI] [PubMed] [Google Scholar]
- Herrmann K, Niedobitek G. (2003) Epstein-Barr virus-associated carcinomas: facts and fiction. J Pathol 199: 140–145 [DOI] [PubMed] [Google Scholar]
- Lenoir GM, Delecluse HJ (1989) Lymphoma and the immuno-compromised host. In Immune Disorders and Opportunistic Infections, Revillard J-P, Wierzbicki N (eds). Foundation Franco-Allemande: Suresness, pp 173–183 [Google Scholar]
- Lenoir GM, Philip T, Sohier R (1984) Burkitt-type lymphoma: EBV association and cytogenetic markers in cases from various geographic locations. In Pathogenesis of Leukaemias and Lymphomas: Environmental Influences, Progress in Cancer Research and Therapy, Magrath IT, O’Conor GT, Ramot B (eds), Vol. 27. Raven Press: New York [Google Scholar]
- Lundgren JD, Melbye M, Pedersen C, Rosenberg PS, Gerstoff J (1995) Changing patterns of Kaposi’s sarcoma in Danish acquired immunodeficiency syndrome patients with complete follow-up. Am J Epidemiol 141: 652–658 [DOI] [PubMed] [Google Scholar]
- Madeleine MM, Daling JR, Carter JJ, Wipf GC, Schwartz SM, McKnight B, Kurman RJ, Beckmann AM, Hagensee ME, Galloway DA (1997) Cofactors with human papillomavirus in a population-based study of vulvar cancer. J Natl Cancer Inst 89: 1516–1523 [DOI] [PubMed] [Google Scholar]
- Matsuo K, Kusano A, Sugumar A, Nakamura S, Tajima K, Mueller NE (2004) Effect of hepatitis C virus infection on the risk of non-Hodgkin's lymphoma: a meta-analysis of epidemiological studies. Cancer Sci 95: 745–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell H, English DR, Elliott F, Gengos M, Barrett JH, Giles GG, Forman D (2008) Immunoblotting using multiple antigens is essential to demonstrate the true risk of Helicobacter pylori infection for gastric cancer. Aliment Pharmacol Ther 28: 903–910 [DOI] [PubMed] [Google Scholar]
- Mueller N, Evans A, Harris NL, Comstock GW, Jellum E, Magnus K, Orentreich N, Polk BF, Vogelman J (1989) Hodgkin's disease and Epstein-Barr virus. Altered antibody pattern before diagnosis. New Engl J Med 320: 689–695 [DOI] [PubMed] [Google Scholar]
- Mueller NE, Birmann B, Parsonnet J, Schiffman M, Stuver S (2005) Infectious agents. In Cancer Epidemiology and Prevention, Schottenfeld D, Fraumeni JF Jr (eds) 3rd edn. Oxford University Press: Oxford, UK [Google Scholar]
- Newton R, Ferlay J, Beral V, Devesa SS (1997) The epidemiology of non-Hodgkin’s lymphoma: comparison of nodal and extra-nodal sites. Int J Cancer 72: 923–930 [DOI] [PubMed] [Google Scholar]
- Parkin DM (2006) The global health burden of infection-associated cancers in the year 2002. Int J Cancer 118: 3030–3044 [DOI] [PubMed] [Google Scholar]
- Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, Orentreich N, Vogelman JH, Friedman GD (1994) Helicobacter pylori infection and gastric lymphoma. N Engl J Med 330: 1267–1271 [DOI] [PubMed] [Google Scholar]
- Paterlini P, Gerken G, Nakajima E, Terre S, D’Errico A, Grigioni W, Nalpas B, Franco D, Wands J, Kew M (1990) Polymerase chain reaction to detect hepatitis B virus DNA and RNA sequences in primary liver cancers from patients negative for hepatitis B surface antigen. N Engl J Med 323: 80–85 [DOI] [PubMed] [Google Scholar]
- Polesel J, Clifford GM, Rickenbach M, Dal Maso L, Battegay M, Bouchardy C, Furrer H, Hasse B, Levi F, Probst-Hensch NM, Schmid P, Franceschi S, Swiss HIV Cohort Study (2008) Non-Hodgkin lymphoma incidence in the Swiss HIV Cohort Study before and after highly active antiretroviral therapy. AIDS 22: 301–306 [DOI] [PubMed] [Google Scholar]
- Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, Mariotto A, Feuer EJ, Edwards BK (2004) (eds). SEER Cancer Statistics Review, 1975–2001. National Cancer Institute: Bethesda, MD. http://seer.cancer.gov/csr/1975_2001/ [Google Scholar]
- Roosendaal R, Kuipers EJ, Buitenwerf J, van Uffelen C, Meuwissen SG, van Kamp GJ, Vandenbroucke-Grauls CM (1997) Helicobacter pylori and the birth cohort effect: evidence of a continuous decrease of infection rates in childhood. Am J Gastroenterol 92: 1480–1482 [PubMed] [Google Scholar]
- Rubin MA, Kleter B, Zhou M, Ayala G, Cubilla AL, Quint WGV, Pirog EC (2001) Detection and typing of human papillomavirus DNA in penile carcinoma. Am J Pathol 159: 1211–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serraino D, Pezzotti P, Dorrucci M, Alliegro MB, Sinicco A, Rezza G (1997) Cancer incidence in a cohort of human immunodeficiency virus seroconverters: HIV Italian seroconversion study group. Cancer 79: 1004–1008 [DOI] [PubMed] [Google Scholar]
- Shah KV (1998) Do human papillomavirus infections cause oral cancer? J Natl Cancer Inst 90: 1585–1586 [DOI] [PubMed] [Google Scholar]
- Smith JS, Bosetti C, Munoz N, Herrero R, Bosch FX, Eluf-Neto J, Meijer CJ, Van Den Brule AJ, Franceschi S, Peeling RW (2004) IARC multicentric case-control study. Chlamydia trachomatis and invasive cervical cancer: a pooled analysis of the IARC multicentric case-control study. Int J Cancer 111: 431–439 [DOI] [PubMed] [Google Scholar]
- Takada K (2000) Epstein-Barr virus and gastric carcinoma. Mol Pathol 53: 255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble CL, Hildesheim A, Brinton LA, Shah KV, Kurman RJ (1996) Heterogeneous etiology of squamous carcinoma of the vulva. Obstet Gynecol 87: 59–64 [DOI] [PubMed] [Google Scholar]
- The UK Collaborative Group for HIV and STI Surveillance (UKCGHS) (2006) A Complex Picture. HIV and Other Sexually Transmitted Infections in the United Kingdom: 2006. Health Protection Agency Centre for Infections: London. http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1194947365435 [Google Scholar]
- van Houten VM, Snijders PJ, van den Brekel MW, Kummer JA, Meijer CJ, van Leeuwen B, Denkers F, Smeele LE, Snow GB, Brakenhoff RH (2001) Biological evidence that human papillomaviruses are etiologically involved in a subgroup of head and neck squamous cell carcinomas. Int J Cancer 93: 232–235 [DOI] [PubMed] [Google Scholar]
- van Lier EA, van Kranen HJ, van Vliet JA, Rahamat-Langendoen JC (2008) Estimated number of new cancer cases attributable to infection in the Netherlands in 2003. Cancer Lett 272: 226–231 [DOI] [PubMed] [Google Scholar]
- Vyse AJ, Gay NJ, Hesketh LM, Andrews NJ, Marshall B, Thomas HI, Morgan-Capner P, Miller E (2002) The burden of Helicobacter pylori infection in England and Wales. Epidemiol Infect 128: 411–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N (1999) Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 189: 12–19 [DOI] [PubMed] [Google Scholar]
- Weinreb M, Day PJR, Niggli F, Powell JE, Raafat F, Hesseling PB, Schneider JW, Hartley PS, Tzortzatou-Stathopoulou F, Khalek ER, Mangoud A, El-Safy UR (1996) The role of Epstein-Barr virus in Hodgkin's disease from different geographical areas. Arch Dis Child 74: 27–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LM, Movahed LA, Warnke RA, Sklar J (1989) Detection of Epstein-Barr viral genomes in Reed-Sternberg cells of Hodgkin’s disease. New Engl J Med 320: 502–506 [DOI] [PubMed] [Google Scholar]
- Ziegler JL, Drew WL, Miner RC, Mintz L, Rosenbaum E, Gershow J, Lennette ET, Greenspan J, Shillitoe E, Beckstead J, Casavant C, Yamamoto K (1982) Outbreak of Burkitt’s-like lymphoma in homosexual men. Lancet 2: 631–633 [DOI] [PubMed] [Google Scholar]