Abstract
The potential use of carbonyl sulfide (COS) as tracer of CO2 flux into the land biosphere stimulated research on COS interactions with leaves during gas exchange. We carried out leaf gas-exchange measurements of COS and CO2 in 22 plant species representing deciduous and evergreen trees, grasses, and shrubs, under a range of light intensities, using mid-infrared laser spectroscopy. A narrow range in the normalized ratio of the net uptake rates of COS (As) and CO2 (Ac), leaf relative uptake (As/Ac × [CO2]/[COS]), was observed, with a mean value of 1.61 ± 0.26, which is advantageous to the use of COS in photosynthesis research. Notably, increasing COS concentrations between 250 and 2,800 pmol mol−1 (enveloping atmospheric levels) enhanced stomatal conductance (gs) to a variable extent in most plants examined (up to a normalized enhancement factor [ fe = (gs-max − gs-min)/gs-min] of 1). This enhancement was completely abolished in carbonic anhydrase (CA)-deficient antisense lines of both C3 and C4 plants. We suggest that the stomatal response is mediated by CA and may involve hydrogen sulfide formed in the reaction of COS and water with CA. In all species examined, the uptake rates of COS and CO2 were highly correlated, but there was no relationship between the sensitivity of stomata to COS and the rate of COS uptake (or, by inference, hydrogen sulfide production). The basis for the observed stomatal sensitivity and its variations is still to be determined.
Carbonyl sulfide (COS) is a ubiquitous constituent of the atmosphere. Its concentration in the background atmosphere is 500 ± 100 pmol mol−1 (i.e. about a factor of 1 million less abundant than CO2; Montzka et al., 2007), but its concentration near vegetation may vary over a much wider range depending on proximity to sources, such as biomass burning or urban pollution, or sinks, such as leaves and soils (Montzka et al., 2007; Blake et al., 2008; Campbell et al., 2008). We recently published studies that focused on the physiology of COS uptake by leaves, advancing the goal of using measurements of this trace gas to help quantify the contributions of gross primary productivity and ecosystem respiration to net carbon exchange at local and regional scales (Campbell et al., 2008; Stimler et al., 2010a, 2011). This approach relies on the knowledge of the relative ratio of the COS/CO2 uptake rates at the leaf level [leaf relative uptake {LRU} = (As/Ac) × (Cac/Cas), where A is uptake rate, Ca is ambient concentration, and superscripts s and c denote COS or CO2, respectively]. Both COS and CO2 fluxes into leaves are influenced by physical limitations along the diffusion pathway (Kluczewski et al., 1985; Goldan et al., 1988; Stimler et al., 2010a), followed by hydration reactions. COS reaction with the enzyme carbonic anhydrase (CA) in the presence of water results in the production of CO2 and hydrogen sulfide (H2S) in an exergonic, one-way, reaction (Protoschill-Krebs et al., 1996; Yonemura et al., 2005; Liu et al., 2010):
Equation 1 indicates the important role of CA in COS uptake and its potential significance in modifying the concentrations of H2S inside leaves. H2S, in turn, has been implicated in a range of possible biological effects (for a recent review, see Wang, 2010). Limited information is available, however, on the variations in the rate of this process and its relation to the rate of CO2 uptake among plant species (Sandoval-Soto et al., 2005; Yonemura et al., 2005; Stimler et al., 2010a, 2011).
In this study, we focus on the effect of COS concentration on stomatal conductance (gs). This effect was first noted in an early study of COS uptake by plants, which showed that COS within the range of natural variation appeared to influence gs (Goldan et al., 1988). Recently, we confirmed this observation, showing that increasing COS concentration from 0 to 2,500 pmol mol−1 (parts per trillion) resulted in a large increase in gs in leaves of three species of C3 plants under otherwise constant and optimal conditions (Stimler et al., 2010a). Given these observations, we suggest that COS concentration may be a significant and hitherto unrecognized variable in studies of gs. For example, gases used in laboratory studies may or may not contain COS. Artificial air mixed from standard grades of N2, oxygen, and CO2 in our laboratory is free of COS, while urban air might contain 2,000 pmol mol−1 COS. Uncontrolled variation in COS concentration could complicate the interpretation of studies of gs. We also note that this effect may provide insights into the mechanisms regulating gs.
The objective was, first, to examine the stimulation of gs by COS in a range of species including major functional groups (deciduous and evergreen trees, shrubs, and grasses) and both C3 and C4 photosynthetic types, and second, to take advantage of existing antisense constructs to the enzyme CA (Price et al., 1994; Cousins et al., 2006) to examine the importance of this enzyme for both the uptake of COS and the enhancement of gs by COS. Since CA catalyzes the conversion of COS to CO2 and H2S, the involvement of CA in the stomatal response to COS may also indicate the participation of H2S produced in the mesophyll.
RESULTS AND DISCUSSION
gs Response to COS
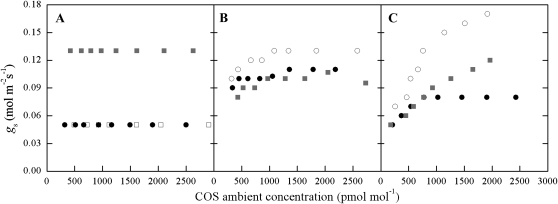
We recently reported (Stimler et al., 2010a) increasing gs in response to increasing ambient COS concentrations within the range observed under natural conditions (Montzka et al., 2007). Here, we extend this study to examine the variations in the gs response to COS among 22 plant species exposed to ambient COS concentrations in the range of 250 to 2,800 pmol mol−1 (enveloping the mean atmospheric concentration of approximately 500 pmol mol−1). The response of gs with increasing COS was quite variable and could not be easily characterized by vegetation or functional type (Fig. 1). We calculated the relative enhancement ( fe) for each species across the COS range used [ fe = (gs-max − gs-min)/gs-min)] and grouped the species in Table I and Figure 1 as follows: (1) no effect ( fe < 0.1); (2) moderate effect (0.1 > fe < 0.3); and (3) high effect ( fe > 0.3). The gs enhancement observed here showed different characteristics from those reported by Goldan et al. (1988) that indicated a sharp reduction in leaf resistance, mainly at low, subambient COS concentrations. Stimler et al. (2010a) reported large enhancements of up to fe of about 2 in Rosa sinensis, Salvia officinalis, and Capsicum annuum, with a linear response across a range of ambient COS concentrations. At present, we do not understand the basis for these differences, but the results presented here indicate that gs response to COS is prevalent, can be highly variable, and cannot be readily predicted at present.
Figure 1.
gs (mol m−2 s−1) during COS response experiments in representative plants from each group reported in Table I, with normalized gs of fe < 0.1 (A), fe < 0.3 (B), and fe > 0.3 (C). For the variations in fe in each group, see Table I. Experiments were conducted under light intensity of 1,189 μmol photons m−2 s−1, atmospheric concentration of CO2 (approximately 400 μmol mol−1), temperature of approximately 23°C, and RH of approximately 75%. A, Citrus maxima (white squares), Citrus madurensis (black circles), and Quercus robur pedunculiflora (gray squares). B, Crocosmia × crocosmiiflora [aurea × pottsii] (white circles), Diospyros virginiana (black circles), and Cestrum nocturnum (gray squares). C, Eucalyptus camaldulensis (white circles), Antigonon leptopus (black circles), and Diospyros digyna (gray squares).
Table I. Minimum and maximum rates of COS flux (As; pmol m−2 s−1), gs (mmol m−2 s−1), and the enhancement factor (fe) during COS response experiments.
fe = (gs-max − gs-min)/gs-min. sd values for measurements on different leaves are indicated (n = 4–6) as well as means and sd of individual fe values. Experiments were conducted under atmospheric concentrations of CO2 (approximately 400 μmol mol−1), temperature of approximately 23°C, RH of approximately 75%, and minimum and maximum concentrations of COS were approximately 250 and 2,000 pmol mol1.
|
As |
gs |
|||||
| Species | Type | Minimum | Maximum | Minimum | Maximum | fe |
| fe < 0.1 | ||||||
| Agapanthus africanus | Grass | 5.0 (0.3) | 24.0 (0.6) | 40 | 44 (5.7) | 0.10 (0.14) |
| Citrus madurensis | Evergreen | 2.2 (0.1) | 18.2 (3.7) | 40 (14) | 43 (9.9) | 0.10 (0.14) |
| Ficus neriifolia | Evergreen | 3.5 | 45.1 | 20 | 20 | 0.00 |
| Macadamia | Evergreen | 2.8 (1.8) | 31.8 (0.5) | 65 (21) | 70 (28.3) | 0.06 (0.09) |
| Quercus roburpedunculiflora | Deciduous | 2.8 (2.7) | 41.0 (1.1) | 120 (14) | 129 (2.12) | 0.08 (0.11) |
| fe < 0.3 | ||||||
| Cestrum nocturnum | Shrub | 2.9 (2.5) | 27.8 (0.3) | 60 (28) | 70 (28.3) | 0.19 (0.09) |
| Citrus maxima | Evergreen | 1.1 (1.3) | 33.4 (11.6) | 50 | 60 (14.1) | 0.20 (0.28) |
| Diospyros virginiana | Deciduous | 2.8 (0.8) | 21.1 (0.3) | 110 (28) | 125 (21.2) | 0.15 (0.10) |
| Jasminum sambac | Shrub | 12.4 (10.3) | 37.6 (5.7) | 95 (21) | 105 (35.4) | 0.09 (0.13) |
| Passiflora edulis | Shrub | 8.4 (3.7) | 32.7 (3.7) | 90 (42) | 110 (42.4) | 0.25 (0.38) |
| Quisqualis indica | Deciduous | 1.0 (0.4) | 33.2 (7.6) | 85 (35) | 95 (35.4) | 0.13 (0.05) |
| Viburnum tinus | Shrub | 2.1 (0.8) | 23.7 (14.2) | 50 (20) | 57 (11.5) | 0.22 (0.38) |
| fe > 0.3 | ||||||
| Antigonon leptopus | Deciduous | 11.3 (7.3) | 36.8 (0.7) | 115 (92) | 155 (106) | 0.44 (0.23) |
| Belamcanda chinensis | Grass | 17.7 | 44.6 | 50 | 100 | 1.00 |
| Crocosmia × crocosmiiflora [aurea × pottsii] | Grass | 9.6 (0.8) | 80.0 (8.2) | 115 (21) | 150 (28.3) | 0.30 (0.01) |
| Eucalyptus camaldulensis | Evergreen | 4.6 (4.9) | 56.0 (3.5) | 70 | 135 (75) | 0.93 (0.71) |
| Flaveria bidentis | Herbaceous | 5.5 (0.03) | 53.8 (0.03) | 191 (31) | 266 (34) | 0.44 (0.32) |
| Limonium perezii | Shrub | 3.0 (0.4) | 27.9 (5.5) | 55 (7) | 75 (7.0) | 0.37 (0.05) |
| Nicotiana tabacum | Herbaceous | 4.5 (3.8) | 51.6 (12.4) | 125 (25) | 175 (7.1) | 0.44 (0.17) |
| Salvia longispicata × Salvia farinacea | Evergreen | 5.3 (4.4) | 95.8 (6.8) | 166 (42) | 220 (72.1) | 0.30 (0.12) |
| Diospyros digyna | Evergreen | 1.8 (1.5) | 34.0 (5.4) | 60 (14) | 105 (21.2) | 0.84 (0.79) |
While a strong correlation between As and both gs and ambient COS concentrations was observed in all leaves (r2 of the linear best fit line = 0.63–0.97 for different species; for a more detailed discussion of this aspect, see Stimler et al., 2010a), there was no correlation between As and fe among the plant species examined. For example, Eucalyptus camaldulensis, which had among the highest fe values (0.93), had a As = 4.5 pmol m−2 s−1, while Ficus carica, which showed no enhancement, had an As = 3.5 pmol m−2 s−1 at 350 pmol mol−1 COS. Maximum observed As values at high COS (approximately 2,500 pmol mol−1) ranged among species between 18.2 and 95.8 pmol m−2 s−1, consistent with previously reported values (Taylor et al., 1983; Kesselmeier and Merk, 1993; Kesselmeier et al., 1999; Geng and Mu, 2004; Stimler et al., 2010a). Therefore, there are no clear relationships between the sensitivity of stomata to COS and the rate of COS uptake.
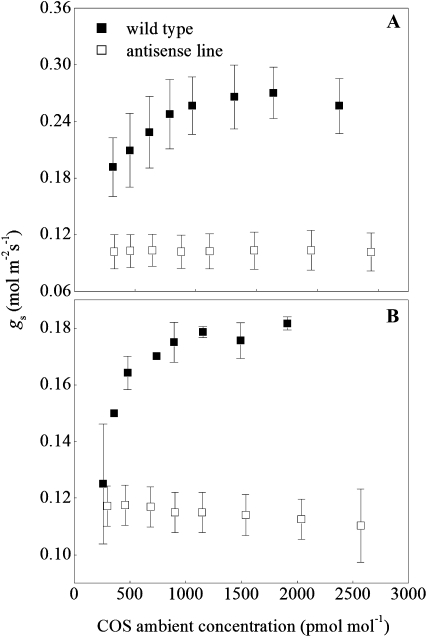
To better understand the basis of the gs enhancement, we examined CA-deficient antisense lines of both C3 and C4 plants (Fig. 2; Stimler et al., 2011). Wild-type plants of both the C3 Nicotiana tabacum and the C4 Flaveria bidentis exhibited strong gs enhancement in response to increasing COS, with fe = 0.44 on average (Table I). This enhancement was completely abolished in the CA-deficient plants. These plants showed constant gs values across the wide range of ambient COS concentrations (Fig. 2), with gs values of 0.10 ± 0.0001 mol m−2 s−1 for F. bidentis and 0.11 ± 0.002 mol m−2 s−1 for N. tabacum. Clearly, increasing ambient COS concentration in itself could not influence gs. As also shown by Stimler et al. (2011), COS uptake was also abolished in the antisense plants, supporting the hypothesis that COS uptake is critically dependent on the catalysis of the hydrolysis of COS to CO2 and H2S. Only when CA was active, converting the COS to H2S, did the gs enhancement occur.
Figure 2.
gs (mol m−2 s−1) to COS during COS response experiments in the wild type (WT) and CA-deficient antisense lines of F. bidentis (A) and N. tabacum (B). Antisense lines were characterized with 2% and 10% of CA activity compared with the wild-type plants in A and B, respectively (Cousins et al., 2006). Conditions are as indicated in Figure 1. Error bars represent sd of four to six leaves.
Variability in LRU among Plant Species
As part of our survey, we also examined the coupling between the rate of COS uptake (As) and that of CO2 uptake (Ac). This is necessary, first, to check to what extent stomatal sensitivity to COS influences variations in the COS/CO2 uptake ratios. This could provide indications of whether the COS effect is only on gs or also on other (e.g. metabolic) processes. Second, estimating the range of variation in the COS/CO2 uptake ratio across species and functional types is critical for assessing the effectiveness of COS as a tracer of CO2 fluxes. Recent studies show the potential of using COS as a tracer of photosynthetic CO2 uptake by land plants (Montzka et al., 2007; Blake et al., 2008; Campbell et al., 2008). This is supported by the close links between the seasonal dynamics of atmospheric COS and CO2 at regional and atmospheric boundary layer scales (Montzka et al., 2004, 2007; Blake et al., 2008; Campbell et al., 2008) as well as at the leaf level (Kesselmeier and Merk, 1993; Sandoval Soto et al., 2005; Yonemura et al., 2005; Stimler et al., 2010a, 2011). The application of COS as a tracer in this context relies on knowledge of the relative COS/CO2 uptake rates at the leaf level: LRU = (As/Ac) × ([CO2]/[COS]), where Ac and As are the uptake rates of COS and CO2, respectively, and the square brackets indicate the respective ambient concentrations.
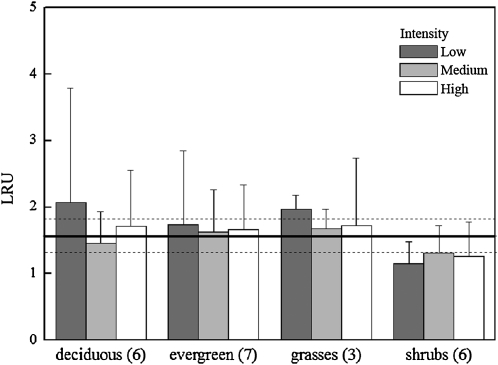
Information on the variations in LRU among plant species is limited, and available studies (Sandoval-Soto et al., 2005; Yonemura et al., 2005; Stimler et al., 2010a, 2011) cover only a limited number of species. Our survey included 22 plant species covering different functional types (Table II; summarized in Fig. 3). We observed a relatively narrow range of LRU values across the 22 plant species examined. Under near-ambient concentrations of COS and CO2 and at room temperature, and across a 10× range in light intensities, an overall average LRU value of 1.61 ± 0.26 (n = 125) was observed. No inherent differences were apparent among the vegetation groups, with deciduous trees, evergreen trees, and grasses showing LRU of 1.75 ± 0.3, 1.68 ± 0.05, and 1.79 ± 0.16, respectively. Individual measurements of LRU ranged from 0.56 ± 0.24 in the evergreen Persea americana to 5.04 ± 2.82 in the deciduous tree species Psidium cattleianum. These LRU values under low light levels were generally more variable, possibly due to the sensitivity to light of photosynthesis, but not of COS uptake, as also noted by Stimler et al. (2010a) in light response measurements. Separating the data between trees and nontrees indicated mean LRU values of 1.7 ± 0.9 and 1.42 ± 0.53, respectively. Shrubs had a mean LRU value of 1.24 ± 0.08. These values are generally consistent with those reported previously (Kesselmeier and Merk, 1993; Sandoval-Soto et al., 2005; Yonemura et al., 2005; Stimler et al., 2010a). The result of a narrow LRU range across plant species is clearly advantageous to the use of COS in photosynthetic CO2-uptake studies. The lack of correlation between the LRU of a species and the sensitivity of its stomata to COS may indicate that the COS effect is largely limited to gs, influencing both COS and CO2 diffusional fluxes into the leaf with little effect on the ratio (LRU; for a discussion of the codiffusion of COS and CO2, see Stimler et al., 2010a).
Table II. Mean LRU values of the plant species used in this study and measured under low, moderate, and high light intensities (179, 352, and 1,889 μmol m−2 s−1, respectively), approximately 500 pmol mol−1 COS and approximately 400 μmol mol−1 CO2, temperature of approximately 23°C, and RH of approximately 75%.
Values represent means (±sd) of three to four measurements on different leaves.
| Light Intensity |
||||||
| Vegetation Type | Low |
Moderate |
High |
|||
| Trees | ||||||
| Deciduous | ||||||
| Antigonon leptopus | 0.87 | (0.99) | 1.40 | (0.26) | 1.09 | (0.06) |
| Quisqualis indica | 0.94 | (0.45) | 0.63 | (0.01) | 0.95 | (0.06) |
| Quercus roburpedunculiflora | 1.51 | (0.54) | 1.45 | (2.04) | 3.22 | (1.83) |
| Diospyros virginiana | 2.00 | (0.36) | 1.90 | (0.70) | 1.40 | (0.12) |
| Psidium cattleianum | 5.04 | (2.82) | 1.99 | (0.55) | 1.52 | (0.77) |
| Ficus carica L. | 1.36 | (0.12) | 2.12 | (0.04) | ||
| Average | 2.07 | (1.72) | 1.45 | (0.48) | 1.72 | (0.84) |
| Evergreen | ||||||
| Citrus madurensis | 1.36 | (0.27) | 1.56 | (0.35) | 2.91 | (1.86) |
| Citrus maxima | 0.77 | (0.05) | 1.32 | (0.13) | 1.94 | (0.87) |
| Diospyros digyna | 3.60 | (0.47) | 2.91 | (0.98) | 0.73 | (0.02) |
| Ficus neriifolia | 2.22 | (0.14) | 1.65 | (0.94) | 1.72 | (1.26) |
| Macadamia | 1.92 | (0.55) | 1.19 | 1.46 | (0.95) | |
| Passiflora edulis | 1.84 | (0.68) | 1.68 | (0.70) | ||
| Persea | 0.56 | (0.24) | 0.94 | (0.86) | 1.23 | (0.15) |
| Average | 1.74 | (1.11) | 1.63 | (0.64) | 1.67 | (0.67) |
| Nontrees | ||||||
| Grasses | ||||||
| Agapanthus africanus | 2.12 | (0.13) | 1.76 | (0.65) | 2.90 | (0.79) |
| Belamcanda chinensis | 1.34 | (0.49) | 1.11 | (0.27) | ||
| Crocosmia × crocosmiiflora [aurea × pottsii] | 1.82 | (0.80) | 1.91 | (0.67) | 1.15 | (0.35) |
| Average | 1.97 | (0.21) | 1.67 | (0.30) | 1.72 | (1.02) |
| Shrubs | ||||||
| Abutilon pictum | 0.92 | (0.98) | 1.01 | (0.40) | 1.00 | (0.35) |
| Cestrum nocturnum | 1.20 | (0.12) | 1.93 | (0.79) | ||
| Jasminum sambac | 1.59 | (1.01) | 1.81 | (1.56) | 1.49 | (0.79) |
| Limonium perezii | 1.22 | (0.10) | 1.11 | (0.08) | 0.71 | (0.09) |
| Passiflora edulis | 1.84 | (0.68) | 1.68 | (0.70) | ||
| Viburnum tinus | 0.85 | (0.05) | 0.88 | (0.13) | 0.75 | (0.15) |
| Average | 1.15 | (0.34) | 1.31 | (0.42) | 1.26 | (0.51) |
| Total average | 1.76 | (0.44) | 1.51 | (0.44) | 1.60 | (0.64) |
Figure 3.
Mean LRU ratios across 22 plant species, grouped into vegetation types, measured under three levels of light intensity (low, medium, and high refer to 179, 352, and 1,889 μmol photons m−2 s−1, respectively). The number of species sampled for each group is indicated in parentheses. The overall mean value for all plants was 1.61 ± 0.26 (indicated by black and dashed lines). Conditions during measurements are as indicated in Figure 1.
Does the CA-Mediated gs Response Involve H2S?
Stimler et al. (2010a) hypothesized that the apparent enhancement of gs by COS could be a stomatal response to H2S, which is quantitatively produced from COS in its reaction with water and CA (Liu et al., 2010). The product, H2S, can lead to the synthesis of Cys (De Kok et al., 1998; Stuiver and De Kok, 2001) and can be oxidized to SO32−S2O32−and eventually sulfate. These compounds are not likely to have major signaling effects. H2S, however, is a reactive gas with a wide range of activities, including effects on membrane ion channels, and was suggested to be a third biosignaling compound together with nitric oxide and carbon monoxide (for a recent review, see Wang, 2010). It was recently argued that H2S could cause both opening (Lisjak et al., 2010) and closing (García-Mata and Lamattina, 2010) of stomata (Coyne and Bingham, 1978; Unsworth and Black, 1981; Gonzales, 1983). Note, however, that these studies rely on chemical compounds that are expected to produce intracellular H2S, with limited controls on its concentrations, or on application of external concentrations of H2S that are difficult to relate to concentrations inside the leaf. For example, the studies of Coyne and Bingham (1978) used parts per million levels of H2S. At such high concentrations, H2S is toxic to plants (Thompson and Kats, 1978; De Kok et al., 1998, 2002). It is difficult to estimate what concentrations may have occurred inside the leaves, but it seems likely that these concentrations are well above the expected levels around leaves in nature. Background atmospheric concentrations of H2S are only approximately 7 to 14 pmol mol−1 (parts per trillion level; Watts, 2000). Therefore, it may be more relevant to consider internal sources of H2S. H2S can be produced, de novo, in the leaves, but fluxes into sulfur-free air (i.e. enhanced fluxes) measured from untreated spruce (Picea sp.) leaves were only in the range of 0.2 to 0.5 pmol m−2 s−1 (Rennenberg et al., 1990). Under steady-state conditions, the COS inflow into leaves must be nearly balanced by H2S outflow (assuming that the metabolic consumption of H2S is negligible). Given a flux of, say, 20 pmol m−2 s−1 (a modest rate observed by Stimler et al. [2010a, 2011]), the rate of production of H2S from COS may be 1 order of magnitude larger than the observed rate of endogenous synthesis. Furthermore, H2S produced in the mesophyll must diffuse out through the stomata, and the intercellular concentration must be well above the ambient level. We calculate that the internal H2S concentration in a leaf during steady-state photosynthesis might be 100 to 300 pmol mol−1 higher than ambient concentrations.
The possible mechanism of the H2S effect on gs is not known at present. But, as noted above, H2S is an active gas and is known to activate specific anion channels in mammalian cells and to specifically influence the flow of calcium ions across the cell membranes (Wang, 2010) as well as stimulate K+ channels (Zhao et al., 2001; Jiang et al., 2010). It is possible that similar effects also exist in plants. It is also not yet clear why the effect is so variable among plant species. But this may reflect variable sensitivity to H2S, internal gradients in H2S concentrations that would depend on internal conductance to COS and H2S, as well as the type and location of CA involved and its activity (Fabre et al., 2007; Furne et al., 2008). The plants might also differ in their capacity to consume H2S in sulfur metabolism (Rennenberg, 1984).
The Role of CA
Using a different line of research, CA was also implicated as a “sensoenzyme” through influencing the production of HCO3− (Frommer, 2010; Hu et al., 2010 ). However, under natural conditions, CA action on CO2 and COS, to produce HCO3−H2S, respectively, cannot be separated without control of the COS concentration. Furthermore, the COS effect on gs reported here and by Stimler et al. (2010a) was observed at concentrations likely to occur under natural or experimental conditions. The studies reported here clearly implicate CA as a plausible source of H2S within the leaf. This report is, to our knowledge, the first to demonstrate a possible alternative mechanism whereby CA could function as a sensoenzyme.
In this study, we examined the stimulation of conductance by COS in a range of species and show that there is a large variation, with some species showing almost no response while others are highly responsive. Using C3 and C4 plants with antisense constructs to the enzyme CA, we show that the activity of this enzyme is essential for both the uptake of COS and the enhancement of gs by COS. Since CA catalyzes the conversion of COS to CO2 and H2S, it seems likely that H2S produced in the mesophyll is involved in the stomatal response. In all plant species examined, the uptake of COS and CO2 was highly correlated, and there was no relationship between the sensitivity of stomata and the rate of COS uptake (or, by inference, H2S production). The basis for the stomatal sensitivity and the variation in sensitivity is still to be determined, but the results evoke a possible new role for CA in plant response to the environment.
MATERIALS AND METHODS
Plant Material
To cover a diverse range of plant species and functional types, we used plants of 22 species that include six deciduous trees, seven evergreen trees, three grasses, and six shrubs. All plants were purchased in local nurseries and were grown under standard screenhouse conditions. Seeds of antisense lines and wild-type Nicotiana tabacum (C3) and Flaveria bidentis (C4) were contributed by Susanne von Caemmerer (Australian National University) and grown in pots in the greenhouse. Various levels of CA activity were achieved in each plant using suppression methods as described (Price et al., 1994; Cousins et al., 2006). Plants were kept under ambient light and temperature during the experimental period.
Gas-Exchange Measurements
The experimental system consisted of a flow-through leaf cuvette made of Teflon-coated stainless steel with a magnetically operated fan and a glass window at the top. A whole leaf was sealed in the cuvette (O-ring seal except around the petiole, which was sealed with high-vacuum putty). Measurements on intact leaves sealed into the leaf chamber were performed under a relative humidity (RH) of approximately 70% and an air temperature of approximately 24°C. Two types of measurements were conducted for each species: first, exposing the plants to three light intensities (135, 352, and 1,889 μmol photons m−2 s−1), regulated with layers of Miracloth and filtered through 5 cm of water; second, conducting COS response curves by mixing purified synthetic air that contains approximately 500 μmol mol−1 CO2 with compressed air from a calibrated high-concentration COS tank (550 nmol mol−1). Outflow from the leaf cuvette was split into two streams for COS and CO2/water analysis. All flow rates were regulated and measured with mass-flow controllers (MKS Instruments).
CO2 and COS Analysis
CO2 and water vapor concentrations in the air entering and leaving the leaf cuvette were measured with an infrared gas analyzer (Li-6262; Li-Cor) at precision better than 0.5 μmol mol−1 for CO2 and 0.1 mmol mol−1 for water vapor.
COS concentration was measured using a mid-infrared dual-quantum cascade laser at a wavelength of 2,056 cm−1 using an LN2-cooled HgCdTe detector (Kolmar Technologies) as described by Stimler et al. (2010b). Briefly, the measurement method is direct detection of the absorption spectrum followed by quantitative spectral fitting combined with the measured pressure, temperature, and path length of the absorption cell and the laser spectral line width using TDL WINTEL software, as described by Nelson et al. (2004). The concentrations of COS and the laser line widths are real-time determined from the spectra through a nonlinear least-squares fittings algorithm that uses spectral parameters from HITRAN (Rothman et al., 2003). The data analysis procedure includes pulse normalization reduction of the sample and automatic background correction (N2). Pulse normalization corrects for variations in pulse-to-pulse amplitude in pulsed laser systems by normalizing the signal pulse train to a reference pulse train. The automatic background correction uses the dry nitrogen spectrum and divides the sample spectra by it. Corrections were carried out every 300 s. Maximum precision of the COS measurements was ±10 pmol mol−1 in a 138-s integration time, reducing to 50 pmol mol−1 in fast 1-Hz measurements (Stimler et al., 2010b).
As for CO2, COS uptake rates were calculated based on the concentration difference between the inlet and outlet of the leaf cuvette, the flow rate, and the leaf area. gs was estimated from conventional gas-exchange measurements (von Caemmerer and Farquhar, 1981).
References
- Blake NJ, Campbell JE, Vay SA, Fuelberg HE, Huey LG, Sachse G, Meinardi S, Rowland FS, Blake DR. (2008) Carbonyl sulfide (OCS): large scale distributions over North America during INTEX-NA and relationship to CO2. J Geophys Res Atmos 113: D09S90 [Google Scholar]
- Campbell JE, Carmichael GR, Chai T, Mena-Carrasco M, Tang Y, Blake DR, Blake NJ, Vay SA, Collatz GJ, Baker I, et al. (2008) Photosynthetic control of atmospheric carbonyl sulfide during the growing season. Science 322: 1085–1088 [DOI] [PubMed] [Google Scholar]
- Cousins AB, Badger MR, von Caemmerer S. (2006) A transgenic approach to understanding the influence of carbonic anhydrase on C18OO discrimination during C4 photosynthesis. Plant Physiol 142: 662–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne PI, Bingham GE. (1978) Photosynthesis and stomatal light responses in snap beans exposed to hydrogen sulfide and ozone. J Air Pollut Control Assoc 28: 1119–1123 [Google Scholar]
- De Kok LJ, Stuiver CEE, Stulen I. (1998) The impact of elevated levels of atmospheric H2S on plants. De Kok LJ, Stulen I, , Responses of Plant Metabolism to Air Pollution and Global Change. Backhuys Publishers, Leiden, The Netherlands, pp 51–63 [Google Scholar]
- De Kok LJ, Stuiver CEE, Westerman S, Stulen I. (2002) Elevated levels of hydrogen sulfide in the plant environment: nutrient or toxin. Omasa K, Saji H, Youssefian S, Kondon N, , Air Pollution and Biotechnology in Plants. Springer-Verlag, Tokyo, pp 201–213 [Google Scholar]
- Fabre N, Reiter IM, Becuwe-linka N, Genty B, Rumeau D. (2007) Characterization and expression analysis of genes encoding α and β carbonic anhydrases in Arabidopsis. Plant Cell Environ 30: 617–629 [DOI] [PubMed] [Google Scholar]
- Frommer WB. (2010) Biochemistry: CO2mmon sense. Science 327: 275–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furne J, Saeed A, Levitt MD. (2008) Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am J Physiol Regul Integr Comp Physiol 295: R1479–R1485 [DOI] [PubMed] [Google Scholar]
- García-Mata C, Lamattina L. (2010) Hydrogen sulphide, a novel gasotransmitter involved in guard cell signalling. New Phytol 188: 977–984 [DOI] [PubMed] [Google Scholar]
- Geng C, Mu Y. (2004) Carbonyl sulfide and dimethyl sulfide exchange between lawn and the atmosphere. J Geophys Res 109: 1–9 [Google Scholar]
- Goldan PD, Fall R, Kuster WC, Feshenfeld FC. (1988) Uptake of COS by growing: a major trophospheric sink. J Geophys Res 93: 14186–14192 [Google Scholar]
- Gonzales GJ. (1983) Potential effects of hydrogen sulfide gas from geothermal energy conversion on two plant species native to northern New Mexico. PhD thesis. New Mexico State University, Los Alamos [Google Scholar]
- Hu H, Boisson-Dernier A, Israelsson-Nordström M, Böhmer M, Xue S, Ries A, Godoski J, Kuhn JM, Schroeder JI. (2010) Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells. Nat Cell Biol 12: 87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Tang G, Cao K, Wu L, Wang R. (2010) Molecular mechanism for H2S-induced activation of KATP channels. Antioxid Redox Signal 12: 1167–1178 [DOI] [PubMed] [Google Scholar]
- Kesselmeier J, Merk L. (1993) Exchange of carbonyl sulfide (COS) between agricultural plants and the atmosphere: studies on the deposition of COS to peas, corn and rapeseeds. Biogeochemistry 23: 47–59 [Google Scholar]
- Kesselmeier J, Teusch N, Kuhn U. (1999) Controlling variables for the uptake of atmospheric carbonyl sulfide by soil. J Geophys Res 104: 11577–11584 [Google Scholar]
- Kluczewski SM, Brown KW, Bell JNB. (1985) Deposition of [35S]-carbonyl sulphide to vegetable crops. Radiat Prot Dosimetry 11: 173–177 [Google Scholar]
- Lisjak M, Srivastava N, Teklic T, Civale L, Lewandowski K, Wilson I, Wood ME, Whiteman M, Hancock JT. (2010) A novel hydrogen sulfide donor causes stomatal opening and reduces nitric oxide accumulation. Plant Physiol Biochem 48: 931–935 [DOI] [PubMed] [Google Scholar]
- Liu Y, Ma J, He H. (2010) Heterogeneous reactions of carbonyl sulfide on mineral oxides: mechanism and kinetics study. Atmos Chem Phys 10: 10335–10344 [Google Scholar]
- Montzka S, Calvert P, Hall BD, Elkins JW, Conway TJ, Tans PP, Sweeny C. (2007) On the global distribution, seasonality and budget of atmospheric carbonyl sulfide (COS) and some similarities to CO2. J Geophys Res 112: D09302 [Google Scholar]
- Montzka SA, Aydin M, Battle M, Butler JH, Saltzman ES, Hall BD, Clarke AD, Mondeel D, Elkins JW. (2004) A 350-year atmospheric history for carbonyl sulfide inferred from Antarctic firn air and air trapped in ice. J Geophys Res 109: D22302 [Google Scholar]
- Nelson DD, McManus B, Urbanski S, Herndon S, Zahniser MS. (2004) High precision measurements of atmospheric nitrous oxide and methane using thermoelectrically cooled mid-infrared quantum cascade lasers and detectors. Spectrochim Acta A Mol Biomol Spectrosc 60: 3325–3335 [DOI] [PubMed] [Google Scholar]
- Price GD, von Caemmerer S, Evans JR, Yu JW, Lloyd J, Oja V, Kell P, Harrison K, Gallagher A, Badger MR. (1994) Specific reduction of chloroplast carbonic anhydrase activity by antisense RNA in transgenic tobacco plants has a minor effect on photosynthetic CO2 assimilation. Planta 193: 331–340 [Google Scholar]
- Protoschill-Krebs G, Wilhelm C, Kesselmeier J. (1996) Consumption of carbonyl sulfide (COS) by higher plant carbonic anhydrase (CA). Atmos Environ 30: 3151–3156 [Google Scholar]
- Rennenberg H. (1984) The fate of excess sulfur in higher plants. Annu Rev Plant Physiol 35: 121–153 [Google Scholar]
- Rennenberg H, Huber B, Schröder P, Stahl K, Haunold W, Georgii HW, Slovik S, Pfanz H. (1990) Emission of volatile sulfur compounds from spruce trees. Plant Physiol 92: 560–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman LS, Barbe A, Benner DC, Brown L, Camy-Payret C, Carleer MR, Chance K, Clerbaux C, Dana V, Devi VM, et al. (2003) The HITRAN molecular spectroscopic database: edition of 2000 including updates through 2001. J Quant Spectrosc Radiat Transfer 82: 5–44 [Google Scholar]
- Sandoval-Soto L, Stanimirov M, Von Hobe M, Schmitt V, Valdes J, Wild A, Kesselmeier J. (2005) Global uptake of carbonyl sulfide (COS) by terrestrial vegetation: estimates corrected by deposition velocities normalized to the uptake of carbon dioxide (CO2). Biogeoscience 2: 183–201 [Google Scholar]
- Stimler K, Berry JA, Montzka SA, Yakir D. (2011) Association between carbonyl sulfide uptake and 18Δ during gas exchange in C3 and C4 leaves. Plant Physiol 157: 509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimler K, Montzka SA, Berry JA, Rudich Y, Yakir D. (2010a) Relationships between carbonyl sulfide (COS) and CO2 during leaf gas exchange. New Phytol 186: 869–878 [DOI] [PubMed] [Google Scholar]
- Stimler K, Nelson D, Yakir D. (2010b) High precision measurements of atmospheric concentrations and plant exchange rates of carbonyl sulfide (COS) using mid-IR quantum cascade laser. Global Change Biol 16: 2496–2503 [Google Scholar]
- Stuiver CEE, De Kok LJ. (2001) Atmospheric H(2)S as sulfur source for Brassica oleracea: kinetics of H(2)S uptake and activity of O-acetylserine (thiol)lyase as affected by sulfur nutrition. Environ Exp Bot 46: 29–36 [DOI] [PubMed] [Google Scholar]
- Taylor GE, Mclaughlin JSB, Shriner JDS, Selvidge WJ. (1983) The flux of sulfur-containing gases to vegetation. Atmos Environ 17: 789–796 [Google Scholar]
- Thompson CR, Kats G. (1978) Effects of continuous H2S fumigation crop and forest plants. Environ Sci Technol 12: 550–553 [Google Scholar]
- Unsworth MH, Black VJ. (1981) Response to pollutants. Jarvis PG, Mansfield TA, , Stomatal Physiology. Cambridge University Press, New York, pp 187–203 [Google Scholar]
- von Caemmerer S, Farquhar GD. (1981) Some relationship between biochemistry of photosynthesis and the gas exchange of leaves. Planta 153: 376–387 [DOI] [PubMed] [Google Scholar]
- Wang R. (2010) Toxic gas, lifesaver. Sci Am 302: 66–71 [DOI] [PubMed] [Google Scholar]
- Watts SF. (2000) The mass budget of carbonyl sulfide, dimethylsulfide, carbon disulfide and hydrogen sulfide. Atmos Environ 34: 761–779 [Google Scholar]
- Yonemura S, Sandoval-Soto L, Kesselemeier J, Kuhn U, Von Hobe M, Yakir D, Kawashima S. (2005) Uptake of carbonyl sulfide (COS) and emission of dimethyl sulfide (DMS) by plants. Phyton 45: 17–24 [Google Scholar]
- Zhao W, Zhang J, Lu Y, Wang R. (2001) The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J 20: 6008–6016 [DOI] [PMC free article] [PubMed] [Google Scholar]