Abstract
Transcription factors activate or repress target gene expression or switch between activation and repression. In animals and yeast, Groucho/Tup1 corepressor proteins are recruited by diverse transcription factors to induce context-specific transcriptional repression. Two groups of Groucho/Tup1-like corepressors have been described in plants. LEUNIG and LEUNIG_HOMOLOG constitute one group and TOPLESS (TPL) and the four TPL-related (TPR) corepressors form the other. To discover the processes in which TPL and the TPR corepressors operate, high-throughput yeast two-hybrid approaches were used to identify interacting proteins. We found that TPL/TPR corepressors predominantly interact directly with specific transcription factors, many of which were previously implicated in transcriptional repression. The interacting transcription factors reveal that the TPL/TPR family has been coopted multiple times to modulate gene expression in diverse processes, including hormone signaling, stress responses, and the control of flowering time, for which we also show biological validation. The interaction data suggest novel mechanisms for the involvement of TPL/TPR corepressors in auxin and jasmonic acid signaling. A number of short repression domain (RD) sequences have previously been identified in Arabidopsis (Arabidopsis thaliana) transcription factors. All known RD sequences were enriched among the TPL/TPR interactors, and novel TPL-RD interactions were identified. We show that the presence of RD sequences is essential for TPL/TPR recruitment. These data provide a framework for TPL/TPR-dependent transcriptional repression. They allow for predictions about new repressive transcription factors, corepressor interactions, and repression mechanisms and identify a wide range of plant processes that utilize TPL/TPR-mediated gene repression.
Differential gene expression is fundamental to an organism’s ability to respond to its environment and underpins the processes of development and differentiation. The majority of genes that have been associated with the regulation of plant development encode transcription factors that control the expression of downstream target genes (Kaufmann et al., 2010). These factors often act as positive regulators of gene expression by activating the expression of downstream target genes. However, it is increasingly being recognized that repression or context-dependent switching between activation and repression plays a vital role in gene regulatory networks (Krogan and Long, 2009; Kagale and Rozwadowski, 2011). Corepressors are transcriptional regulators that are incapable of independent DNA binding, being recruited directly or indirectly by DNA-binding transcription factors to repress target gene expression. The Groucho (Gro)/Tup1 family, first identified in Drosophila and Saccharomyces, respectively, represents an archetypal class of corepressors that are recruited by a range of DNA-binding transcription factors to elicit a repressed chromosomal state, thereby shutting off gene expression (Liu and Karmarkar, 2008).
The first Gro/Tup1 family member to be isolated in plants was LEUNIG (LUG; Conner and Liu, 2000). LUG was identified due to its repressive activity, restricting the expression domain of the floral homeotic gene AGAMOUS (Conner and Liu, 2000). LUG and its close homolog LEUNIG_HOMOLOG (LUH) share the characteristic C-terminal WD-40 and N-terminal Gln-rich motifs of the Gro/Tup1 family but have an additional N-terminal domain, known as LUFS, that includes a previously defined LisH (for Lis1-homologous) motif. LisH domains have been shown to promote protein-protein interaction (Cerna and Wilson, 2005). The LUFS domain is required for interaction with the SEUSS protein that serves as an adaptor to link LUG and LUH to a range of transcription factors (Pfluger and Zambryski 2004; Sridhar et al., 2004, 2006; Gregis et al., 2006; Stahle et al., 2009). As well as directly interacting with YABBY transcription factors (Stahle et al., 2009), LUG/LUH corepressors indirectly interact with transcription factors via the SEUSS adaptor protein or related SEUSS-LIKE proteins (Franks et al., 2002; Sridhar et al., 2004). Another Gro/Tup1 corepressor family, TOPLESS (TPL; including TPL and TPL-related [TPR]), has recently been described in plants that interacts both directly and indirectly with transcription factors (Kieffer et al., 2006, Long et al., 2006). TPL/TPR corepressors have been shown to interact with transcription complexes involved in auxin and jasmonate signal transduction, meristem maintenance, and defense responses (Kieffer et al., 2006; Szemenyei et al., 2008; Gallavotti et al., 2010; Pauwels et al., 2010; Zhu et al., 2010; Arabidopsis Interactome Mapping Consortium, 2011).
TPL/TPR corepressors were first described as direct interactors of the Arabidopsis (Arabidopsis thaliana) homeodomain transcription factor WUSCHEL (WUS; Laux et al., 1996; Kieffer et al., 2006). WUS is expressed in the organizing center at the base of the apical meristem, where it signals to the overlying stem cells to maintain their meristematic fate as part of a well-studied feedback loop that controls meristem homeostasis (Brand et al., 2000; Schoof et al., 2000; Sablowski, 2007). Within this network, WUS represses the expression of type A Arabidopsis response regulator genes (Leibfried et al., 2005). Two members of the TPL/TPR family of corepressors were identified as interactors of WUS (TPL and TPR4, previously known as WSIP1 and WSIP2), and the interaction was shown to require the C-terminal domain of WUS, which contains three short conserved protein sequences, and the N-terminal region of TPL/TPR, which contains a LisH domain as well as a “C-terminal to LiSH” (CTLH) domain (Kieffer et al., 2006). By analogy to Gro/Tup1, TPL/TPR-mediated repression by WUS was hypothesized to act via histone deacetylation, a conclusion supported by the WUS-like meristem defects observed in plants grown on histone deacetylase inhibitor (Kieffer et al., 2006). More recently, it has been shown that in maize (Zea mays), the zinc (Zn)-finger transcription factor RAMOSA1 interacts with the TPL/TPR factor REL2 to repress indeterminate meristem fate (Gallavotti et al., 2010), thus demonstrating another involvement of TPL/TPR corepressors in meristem maintenance, although acting with a different transcription factor and with an opposite developmental outcome.
TPL was identified as the gene affected in the temperature-sensitive, semidominant tpl-1 embryo development mutant of Arabidopsis. tpl-1 plants show severe polarity defects, ranging from fused cotyledons to the complete replacement of the shoot with a second root (Long et al., 2002, 2006). TPL defines a family of five genes in Arabidopsis (TPL and TPR1 to -4; Long et al., 2006). The dominant negative nature of the tpl-1 mutation was demonstrated by the fact that a quintuple loss of function, in which all five TPL/TPR genes were inactivated by mutation or RNA interference, is required to phenocopy the original tpl-1 phenotype, suggesting that the five members of the TPL/TPR family act redundantly (Long et al., 2006). Consistent with a histone deacetylation repression mechanism, tpl-1 is enhanced by a histone deacetylase mutant and suppressed by a histone acetyl transferase mutant (Long et al., 2006).
The auxin/indole-3-acetic acid (AUX/IAA) protein BODENLOS (BDL) functions in root development and was recently shown to interact with TPL. AUX/IAA proteins, including BDL, interact with activating AUXIN RESPONSE FACTOR (ARF) transcription factors, thereby converting them to transcriptional repressors (Szemenyei et al., 2008). In the presence of auxin, the AUX/IAA proteins are ubiquitinated and degraded, freeing the ARFs to activate the expression of their auxin-induced target genes. A similar mechanism has also been demonstrated for jasmonic acid (JA) signaling, where the activating MYC2 transcription factors recruit JAZ proteins, which, in turn, recruit TPL/TPR corepressors via an accessory protein known as NINJA (Pauwels et al., 2010). In this system, JA promotes the ubiquitination and degradation of the JAZ proteins, preventing the recruitment of TPL/TPR and activating JA-responsive gene expression (Pauwels et al., 2010).
The roles of TPL/TPR corepressors are not confined to development. Recently, a genetic suppressor of a constitutively active disease resistance gene SUPPRESSOR OF NPR1-1, CONSTITUTIVE1 (SNC1) was identified as TPR1, and the two proteins were shown to interact to repress downstream target gene expression during the response to pathogen infection (Zhu et al., 2010). Taken together, these findings suggest that TPL/TPR corepressors are involved in a wide range of processes and that they interact directly or indirectly with DNA-binding transcription factors to repress the expression of downstream target genes.
Interaction between the TPL/TPR corepressors and WUS, AUX/IAA, NINJA, and RAMOSA1 is mediated by a small conserved protein motif known as the ethylene response factor (ERF)-associated amphiphilic repression (EAR) domain (Ohta et al., 2001), with the consensus sequence (L/F)DLN(L/F)xP, which has also been identified in several other transcription factors with repressive activity (Ohta et al., 2001; Hiratsu et al., 2004; Hill et al., 2008). EAR repression domains (RDs) have been shown to convert activating transcription factors into dominant repressors even when as few as six amino acids (DLELRL) are added and can overcome strong transactivating motifs such as VP16 from Herpes simplex virus (Ohta et al., 2001; Hiratsu et al., 2002). A recent bioinformatic study attempted to predict the complete repertoire of the EAR repressome in Arabidopsis, identifying 219 candidate proteins belonging to 21 transcription regulator families (Kagale et al., 2010).
To discover the extent of the processes in which the TPL/TPR family of corepressors operates in plants, we used high-throughput yeast two-hybrid approaches to establish the framework of TPL/TPR protein-protein interactions, with the aim of providing a resource that will inform future studies. TPL/TPR proteins interact overwhelmingly with specific transcription factor families, many of which have previously been implicated in transcriptional repression. The framework of TPL/TPR interactions reveals the widespread use of TPL/TPR corepressors in diverse processes, including hormone signaling, stress responses, and the control of flowering time, and makes new predictions about the mechanism of repression in both auxin and JA signal transduction. We show that all known plant RD sequences are enriched among the TPL/TPR interactome and that these domains are necessary for TPL/TPR recruitment. These data constitute an experimentally derived framework for the repressive control of gene expression in Arabidopsis and facilitate the identification of further regulatory factors that repress transcription in plants. Finally, to demonstrate the biological relevance of the TPL/TPR interactions, we show that TPL contributes to WUS function and is required for the repression of FLOWERING LOCUS T (FT) expression by TARGET OF EAT1 (TOE1).
RESULTS
TPL/TPR Corepressors Interact with Specific Transcription Factor Families
Each of the five TPL/TPR proteins was individually tested for protein interaction partners in two-hybrid library screens, testing a total of 43 million clones. Lists of interactors are presented in Figure 1 and Supplemental Table S1. Most of the detected interacting proteins were transcription factors (Supplemental Table S2). The Arabidopsis Information Resource 9 lists 27,379 proteins encoded by the Arabidopsis genome, of which approximately 1,922 (approximately 7%) are transcription factors or regulators of transcription (based on information from the Database of Arabidopsis Transcription Factors [DATF]; Guo et al., 2005). Transcription factors were significantly enriched in all screens, representing between 88% and 51% of the interactors (Supplemental Table S2).
Figure 1.
A summary of the TPL/TPR protein-protein interactions. A, Interactions detected in the whole plant yeast two-hybrid experiments. B, Interactions identified from the arrayed transcription factor library. In each case, the first column provides the Arabidopsis Genome Initiative (AGI) number for each factor. The second column indicates the identity of the factor, if known. Factors highlighted in gray in this column represent those that are known to act as transcriptional repressors. The third column indicates the transcription factor family to which each factor belongs, where dark green represents the AP2/ERF family, purple the MYB family, red the AUX/IAAs, pink the ARFs, light blue the C2H2 Zn fingers, dark blue the homeodomain family, brown the NAC proteins, orange the MADS box family, and yellow the TCP factors. Interactions between these factors and the TPL/TPR proteins are represented by dark gray boxes in the appropriate columns. For the whole plant yeast two-hybrid experiment, numbers represent the frequency (%) at which each factor was isolated. The RD column shows the sequence of any known functional RD found in the TPL/TPR-interacting factors. Black boxes to the right side of each table indicate those factors isolated in both the whole plant library and arrayed transcription factor screens. Factors and RDs highlighted in pale green in the arrayed transcription factor library table represent those proteins in which mutagenesis of the RD was performed to evaluate its requirement for interaction with the TPL protein.
Since the majority of all TPL/TPR interactions were with transcription factors, we attempted to determine the range of transcription factor interactions by screening an arrayed yeast two-hybrid library of 1,296 Arabidopsis transcription factors (Paz-Ares and The REGIA Consortium, 2002; Castrillo et al., 2011) with each TPL/TPR protein, making a total of 6,480 individual interaction tests. Screens were conducted in triplicate, with only interactions seen in at least two replicates being considered bona fide. The combination of two screening approaches, using different yeast and vector systems, provides a robust interaction framework. The screens show that TPL/TPR proteins interact with transcriptional regulators, representing at least 17 distinct families (Fig. 1; Table I). AP2/ERF, Zn finger, AUX/IAA, and MYB factors were found to interact with multiple TPL/TPR proteins and were highly represented in the yeast two-hybrid screens (Fig. 1; Supplemental Fig. S1). Proteins from the ARF, JAZ, class II LBD, MADS box, TCP, NAC domain, WOX, and other families were also found to interact with the TPL/TPR proteins (Fig. 1; Table I), suggesting that TPL/TPRs are recruited as corepressors by diverse transcription factors.
Table I. Comparison of the major transcription factor families isolated in the yeast two-hybrid experiments.
Transcription factor families are as defined in the DATF. Seventeen families in total were identified in the large-scale yeast two-hybrid experiments. Five additional proteins (AT2G31720, AT3G63180, AT4G03250, AT5G55040, and AT5G63470) were also isolated that did not belong to any of the DATF families. Factors in parentheses represent putative weak interactions.
| Transcription Factor Family | Whole Plant Library Screen | Arrayed Transcription Factor Screen |
| Class II LBDa | LBD37 | |
| LBD41 | ||
| AP2/ERF | ||
| AP2 family | TOE1 | TOE1 |
| TOE2 | TOE2 | |
| AP2 | ||
| ANT family | BBM1 | |
| ERF family | ERF3 | ERF3 |
| ERF4 | ERF4 | |
| ERF7 | ERF7 | |
| ERF8 | ERF8 | |
| ERF11 | ERF10 | |
| ERF11 | ||
| ERF12 | ||
| (ERF6) | ||
| (ERF13) | ||
| RAV family | TEM1 | (TEM1) |
| (TEM2) | ||
| RAV1 | ||
| NGA2 | ||
| AT1G13260 | ||
| AT1G50680 | ||
| AT1G51120 | ||
| AT5G06250 | ||
| DREB family | CBF4 | |
| (RAP2.1) | ||
| (TINY) | ||
| Zn finger | ||
| C2H2 | ZAT5 | |
| ZAT6 | ZFP4 | |
| STZ | ZFP8 | |
| ZFP11 | ZFP10 | |
| WIP3 | AT4G35610 | |
| GATA | GATA9 | |
| GATA18 | GATA16 | |
| Others | ||
| FRS3 | AT2G44410 | |
| AT1G30810 | AT3G21890 | |
| AUX/IAA | IAA1 | |
| IAA2 | IAA2 | |
| IAA7 | IAA3/SHY2 | |
| IAA8 | IAA4 | |
| IAA16 | IAA7 | |
| IAA17/AXR3 | IAA10 | |
| IAA18 | IAA11 | |
| IAA27 | IAA13 | |
| (IAA18) | ||
| IAA19 | ||
| IAA26 | ||
| IAA28 | ||
| (IAA34) | ||
| ARF | ARF2 | |
| ARF9 | ARF17 | |
| ARF18 | (ARF1) | |
| (ARF3) | ||
| (ARF4) | ||
| (ARF19) | ||
| JAZa | JAZ5 | |
| JAZ6 | ||
| TCP | AT1G58100 | AT1G58100 |
| TCP2 | ||
| TCP3 | ||
| TCP4 | ||
| TCP14 | ||
| TCP16 | ||
| AT1G35560 | ||
| HSF | HSF4 | |
| HSFB2A | ||
| HSFB2B | ||
| bZIP | bZIP9 | |
| bZIP45 | ||
| bZIP67 | ||
| bZIP-A | ||
| bZIP-B | ||
| OBF5 | ||
| NAC | NAC1/52 | |
| NAC3 | ||
| NAC50 | ||
| NAC052 | ||
| NAC96 | ||
| MYB | AS1 | |
| EPR1 | EPR1-LIKE | |
| EPR1-LIKE | MYB15 | |
| MYB7 | MYB20 | |
| MYB32 | MYB73 | |
| MYB44 | MYB97 | |
| MYB77 | MYB98 | |
| AT3G52250 | KAN | |
| MYBL2 | ||
| MYB3R2 | ||
| GT-2 | ||
| AT1G25550 | ||
| AT1G68670 | ||
| AT2G38300 | ||
| AT3G12730 | ||
| AT3G16350 | ||
| HD WUS/WOX | WOX4 | |
| WOX2 | ||
| (WOX3) | ||
| (WOX4) | ||
| (WOX6) | ||
| (WOX7) | ||
| MADS | SOC1 | |
| AGL15 | ||
| (AGL18) | ||
| (AGL21) | ||
| (AGL24) | ||
| (AGL43) | ||
| (AGL78) | ||
| (MAF5) | ||
| (SEP1) | ||
| (SEP4) | ||
| (SHP2) | ||
| WRKY | WRKY6 | |
| WRKY30 | ||
| WRKY32 | ||
| WRKY61 | ||
| BES1 | BEH4 | |
| ABI3-VP1 | AT2G36080 | |
| SBP | SPL1 | SPL1 |
Class II LBD and JAZ proteins are not present in the arrayed transcription factor library.
Current evidence suggests that the TPL/TPR family mediates transcriptional repression by acting with histone deacetylases to induce a repressive chromatin state at the target locus (Kieffer et al., 2006; Long et al., 2006; Zhu et al., 2010). In addition, Gro/Tup1 proteins from animals and yeast, and LUG from Arabidopsis, also interact with components of the mediator complex, disrupting its function (Courey and Jia, 2001; Malavé and Dent, 2006; Gonzalez et al., 2007). We did not detect direct interactions between TPL and the histone deacetylase HDA19 or the mediator complex component HEN3 (Supplemental Fig. S2), suggesting that additional factors might mediate such associations. An alternative mechanism for TPL-mediated gene silencing was suggested by the interaction between TPL/TPRs and the SET domain protein SDG19 (Fig. 1; Supplemental Table S3), which is a histone methyltransferase that induces a repressive chromatin state.
Gro/TLE proteins form homotetramers or heterotetramers (for review, see Chen and Courey, 2000). We see evidence for the formation of oligomers between the TPL/TPR proteins (Fig. 1). Furthermore, TPL has previously been reported to interact with itself (Szemenyei et al., 2008; Arabidopsis Interactome Mapping Consortium, 2011). Together, the interaction data indicate that the formation of TPL/TPR complexes is also important for gene silencing mediated by plant Gro/TLE corepressors.
TPL/TPR Interacting Transcription Factors Are Enriched for RDs
Of the 1,922 Arabidopsis transcription factors listed in DATF, only 2.2% are associated with the Gene Ontology (GO) term “transcription repressor activity” (GO:0016564 and children; Gene Ontology Consortium [www.geneontology.org]), whereas 9% of the transcriptional regulators that interact with TPL/TPR are associated with this GO term. When DATF GO data are combined with knowledge of repressive transcription factors gathered from the literature, functional repressors are further enriched among the TPL/TPR interactors (41% of interactors annotated as repressors; Fig. 1). Known repressive transcription factors, therefore, are highly represented among the TPL/TPR interactors. As more functional information becomes available for the remaining interactors, it seems likely that further enrichment will be seen, suggesting that the TPL/TPR family has the potential to be involved in much of the transcription repression in plants.
Several RDs have been identified in Arabidopsis transcription factors, including the EAR domain [defined as (L/F)DLN(L/F)xP, including LxLxL, DLNxP, and DLNxxP, which also includes FDLNI] and the (R/K)LFGV and TLxLF sequences (Ohta et al., 2001; Matsui et al., 2008; Ikeda and Ohme-Takagi, 2009; Ikeda et al., 2009; Kagale et al., 2010). All of these RDs are present among the TPL/TPR interactors (Fig. 1), and they are enriched among the “gene regulator” class of interactors (Supplemental Table S4). Approximately 65% of interactors belonging to the gene regulator class have a complete, known RD and a further 29% have a partial RD (such as LxL and FxLxF, which is related to the FDLNI EAR motif). Only 5% of the gene regulator class of interactors appears to contain no known RD sequence (Supplemental Table S4). Characterized RDs, therefore, are highly overrepresented among the TPL/TPR interactors. The LxLxL motif is found in 22% of transcription factors in the DATF database but is present in 48% of the 130 interactors (Supplemental Table S5), confirming significant enrichment for this domain among TPL/TPR interacting factors. Enrichment for many of the other RD motifs was also observed among the TPL/TPR interactors (Supplemental Table S5).
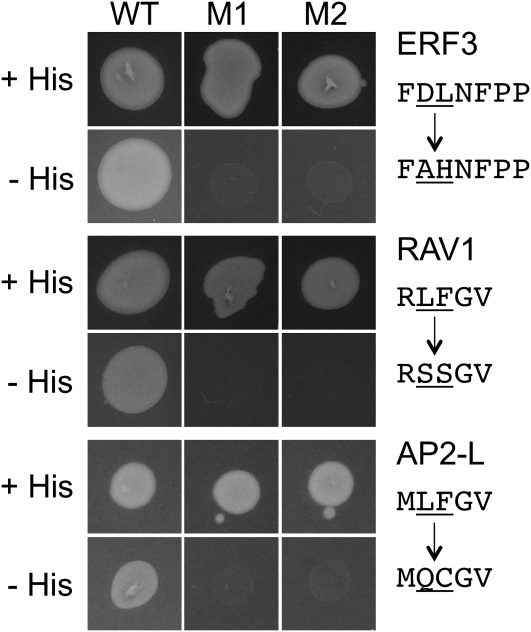
Previous data have demonstrated that the LxLxL motif of BDL is necessary for interaction with TPL (Szemenyei et al., 2008). To test whether the DLNxxP and RLFGV RD sequences are also necessary for TPL interaction, we mutated these motifs in ERF3 and RAV1, respectively, and tested interaction with TPL. Conversion of the DLNFPP motif to AHNFPP in ERF3 (and ERF7) abolished the interaction (Fig. 2). Similarly, mutation of the RLFGV motif to RSSGV in RAV1 prevented interaction with TPL (Fig. 2). The TPL/TPR library screens also identified interacting proteins with variations of the R/KLFGV RD sequence, including MLFGV, RLFGI, and RLFGF (Fig. 1). To determine whether such variants are necessary for TPL interaction, we mutated the MLFGV sequence to MQCGV in the AP2/B3 factor AT1G51120 and found that the mutated protein was unable to interact with TPL (Fig. 2). These findings indicate that all the RD motifs identified in the TPL/TPR screens are necessary for the observed interactions. This confirms that TPL is able to interact via diverse peptide sequences, allowing for the prediction of novel RDs and thus new factors that can function as transcriptional repressors.
Figure 2.
Short RD sequences are necessary for interaction with TPL. The predicted RDs of ERF3 (FDLNFPP to FAHNFPP), RAV1 (RLFGV to RSSGV), and AP2-L (MLFGV to MQCGV) were mutagenized in the context of the full-length prey proteins and tested for interaction with the TPL bait in a yeast two-hybrid assay. Transformed yeast were spotted onto medium that selects for protein-protein interactions (−His) or control medium without selection (+His). Wild-type prey proteins (WT) were compared with mutant prey proteins (M1 and M2) for interaction with TPL.
While the direct interactions between transcription factors and the TPL/TPR proteins are mediated by the presence of a conserved RD in the transcription factor, interactions between TPL/TPR factors and other factors involved in the repression mechanism might involve very different motifs. Consistent with this, presumed chromatin-remodeling factors isolated in the screens, such as SDG19, EMF1, VRN5, FVE, and the TPRs themselves, lack any known repression motifs (Fig. 1).
Validation of TPL Interactions
False positives and false negatives can be reported in yeast two-hybrid screens. Although we used a normalized whole plant library to increase the sensitivity of the assay and to minimize the presence of false positives, heat shock factor (HSF) and ribosomal proteins, both of which have previously been reported as false positives in yeast two-hybrid screens (for review, see Causier and Davies, 2002), were isolated in each screen. However, the overwhelming predominance of transcriptional regulators among the positives was an unusual and striking feature of all TPL/TPR screens (Supplemental Table S2).
To test further the specificity of the two-hybrid screens, we took a protein closely related to the TPL/TPR family and tested it for interactions against the arrayed transcription factor library. The protein, herein designated TPR-like (AT2G25420), is shorter than other family members; it has fewer WD-40 domains at its C terminus and a repeat of the LisH/CTLH domains at its N terminus. Phylogenetic and synteny studies reveal that TPR-like is the closest protein to the TPL/TPR family of corepressors in the Arabidopsis genome. No interactions were detected between the TPR-like bait and the 1,296 transcription factors. This further suggests that our yeast two-hybrid approaches are specific and that this relative of the TPL/TPR corepressors does not act as a transcriptional corepressor by interacting directly with transcription factors. We cannot rule out an indirect linkage to transcription via an as yet unidentified adaptor protein.
To provide further validation for the detected TPL/TPR interactions with specific transcription factor families, two example interactions were chosen to determine whether they are of biological significance: the interaction between TPL and the AP2 protein TOE1 and the interaction between TPL and the WOX protein WUS.
TPL/TPR-AP2 Factor Interactions in Floral Induction
AP2/ERF transcription factors fall into two general classes, and proteins from each of these groups interact with members of the TPL/TPR family (Fig. 1). Interesting among these were several known repressors of the flowering-time gene FT, including TOE1, TOE2, and TEM1 (Jung et al., 2007; Castillejo and Pelaz, 2008). While TOE1 and TOE2 were recently shown to interact with TPL and TPR3 (Arabidopsis Interactome Mapping Consortium, 2011), our data extend the interactions seen between the TOE proteins and the TPL family, as both TOE1 and TOE2 interact with all five TPL/TPR proteins.
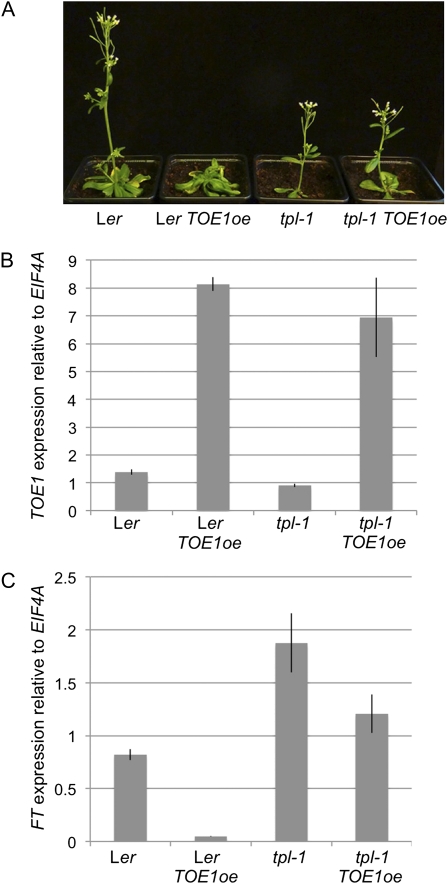
To determine the biological significance of the TPL/TPR-TOE1 interaction, we examined the dependence of TOE1 on TPL activity. Consistent with a previous report (Jung et al., 2007), constitutive expression of the TOE1 protein (35S::TOE1; n = 15 independent lines) delays flowering by approximately 6 d compared with wild-type Landsberg erecta (Ler) control plants (n = 14; Fig. 3A). However, this delay in flowering is abolished in a tpl-1 mutant background, where 35S::TOE1 plants (n = 22 independent lines) flower at the same time as nontransgenic tpl-1 plants (n = 16; Fig. 3A). In wild-type plants, TOE1 represses the transcription of FT to prevent precocious flowering (Jung et al., 2007). To test whether this repression requires TPL, we compared FT expression in the leaves of Ler, Ler 35S::TOE1, tpl-1, and tpl-1 35S::TOE1 lines. As expected, high levels of TOE1 in the leaves of Ler 35S::TOE1 plants (Fig. 3B) resulted in a 16.4-fold reduction in the abundance of the FT transcript compared with wild-type Ler control plants (Fig. 3C), correlating with the late flowering phenotype. In contrast, despite high levels of TOE1 expression in the leaves of tpl-1 35S::TOE1 lines (Fig. 3B), relative FT expression levels were approximately 64% of those found in nontransgenic tpl-1 plants (Fig. 3C), indicating that at least part of the repression of FT by TOE1 requires TPL. The slight but statistically significant (P ≤ 0.05; Welch’s t test) differences in FT expression between tpl-1 and tpl-1 35S::TOE1 probably reflects residual TPL/TPR activity in tpl-1, but it is also possible that TOE1 can repress FT through a TPL/TPR-independent mechanism. Interestingly, FT levels were found to be consistently higher in transgenic and nontransgenic tpl-1 lines compared with Ler controls (Fig. 3C). The TPL/TPR interactome data identified a number of other interactors involved in FT repression, including TEM1, AP2, and AGL15 (Fig. 1), suggesting that loss of TPL disrupts the function of several pathways leading to FT repression. Comparison of flowering time between Ler and tpl-1 lines was not possible due to the developmental defects of tpl-1 plants. Together, these data suggest that TOE1 recruits TPL to repress FT in the leaves of wild-type plants and provide a role for TPL/TPR corepressors in the floral transition.
Figure 3.
Comparison of flowering-time phenotypes and FT expression in wild-type and tpl-1 plants constitutively expressing TOE1. A, Left to right: wild-type (Ler) plants, Ler plants overexpressing TOE1 (TOE1oe), tpl-1 mutant, and tpl-1 overexpressing TOE1. For Ler lines, photographs were taken 30 d after germination; for tpl-1 lines, photographs were taken 27 d after germination. B, qRT-PCR analysis of TOE1 expression in Ler, Ler TOE1oe, tpl-1, and tpl-1 TOE1oe leaves relative to the EIF4A control. C, qRT-PCR analysis of FT expression in Ler, Ler TOE1oe, tpl-1, and tpl-1 TOE1oe leaves relative to the EIF4A control. Values represent means of three independent biological replicates.
TPL/TPR-WOX Interactions
We originally isolated TPL using the WUS protein as bait in a yeast two-hybrid library screen (Kieffer et al., 2006; Supplemental Fig. S2). In the screens employed in this study, we found further interactions between these two families. Using TPL as bait, we recovered WOX4 and WOX2 interactions as well as potential interactions between a number of other WOX proteins and the TPL/TPRs (Fig. 1; Supplemental Table S3). To analyze the biological significance of TPL/TPR-WOX interactions, we carried out complementation assays in a wus-1 mutant background using full-length WUS and variants thereof under the control of the WUS promoter (Bäurle and Laux, 2005; Fig. 4A), which gives an appropriate expression pattern in GUS assays (Fig. 4B). Full-length WUS (pWUS::WUS) rescued essentially all aspects of the wus-1 mutant phenotype (Fig. 4C). wus-1 mutants flower rarely and produce flowers with reduced reproductive organ number, often terminating after the development of a single stamen and never producing carpels (Laux et al., 1996; Table II). pWUS::WUS wus-1 inflorescences produced numerous flowers, similar to wild-type plants. Approximately 50% of the lines analyzed produced flowers with a full complement of organs (strong rescue), including fully fertile carpels that later set viable seed. In the remaining lines, flowers terminated after the development of several stamens and did not produce carpels (weak rescue; Fig. 4C; Table II). The pWUS::WUSΔ construct, in which the conserved TPL/TPR interaction domain of WUS has been deleted (Kieffer et al., 2006), was unable to rescue any aspect of the mutant phenotype (Fig. 4C), indirectly suggesting that the loss of the WUS-TPL interaction impairs WUS function.
Figure 4.
Complementation of wus mutants. A, WUS constructs used in the complementation experiments. B, GUS staining patterns for plants expressing GUS from the WUS promoter (3,351 bp) used to prepare the constructs for the complementation experiment. The left panel shows staining in seedlings, and the right panel shows staining in an early flower and the anthers of a later flower. C, Complementation of the strong wus-1 allele. The first column shows mature wild-type plants, the second column shows wus-1 plants with the pWUS::WUS construct, the third column shows wus-1 plants with the pWUS::WUSΔTPL construct, the fourth column shows wus-1 plants with the pWUS::WUSΔ construct, and the fifth column shows wus-1 mutant plants. In all cases, the top panel shows mature plants, the middle panel shows typical inflorescences, and the bottom panel shows typical flowers. For wus-1 pWUS::WUS, the bottom panel shows a typical weak rescue flower on the left with a strong rescue flower on the right. D, Complementation of the weak wus-3 allele. The left panel shows a typical wus-3 mutant flower, the center panel shows a wus-3 flower with the pWUS::WUSΔ construct, and the right panel shows a wus-3 flower with the pWUS::WUSΔTPL construct.
Table II. Analysis of floral organ number in complemented wus-1 and wus-3 flowers.
| Plant | na | Organ No. ± sd |
|||
| Sepals | Petals | Stamens | Carpels | ||
| Wild type | 15 | 4.0 ± 0.0 | 4.0 ± 0.0 | 6.0 ± 0.0 | 2.0 ± 0.0 |
| wus-1 pWUS::WUS FL (strong) | 42 | 4.0 ± 0.0 | 4.0 ± 0.0 | 5.6 ± 0.71 | 1.7 ± 0.2 |
| wus-1 pWUS::WUS FL (weak) | 54 | 4.0 ± 0.0 | 4.0 ± 0.0 | 4.5 ± 0.54 | 0.75 ± 0.3 |
| wus-1 pWUS::WUSΔ-TPL | 96 | 4.0 ± 0.0 | 4.0 ± 0.0 | 3.9 ± 0.56 | 0.0 ± 0.0 |
| wus-1 | 14 | 3.7 ± 0.62 | 3.4 ± 0.71 | 1.4 ± 0.36 | 0.0 ± 0.0 |
| wus-3 | 16 | n.d.b | n.d. | 2.8 ± 0.45 | 0.0 ± 0.0 |
| wus-3 pWUSΔ | 18 | n.d. | n.d. | 2.8 ± 0.38 | 0.0 ± 0.0 |
| wus-3 pWUSΔ-TPL (weak) | 30 | n.d. | n.d. | 4.0 ± 0.56 | 0.0 ± 0.0 |
| wus-3 pWUSΔ-TPL (strong) | 37 | n.d. | n.d. | 4.3 ± 0.79 | 0.6 ± 0.93 |
n = total number of flowers analyzed from the following numbers of plants (independent lines): pWUS::WUS FL (strong), seven lines; WUS FL (weak), nine lines; WUSΔ-TPL, 12 lines; wus-1, seven plants; wus-3, four plants; wus-3 pWUS::WUSΔ , three lines; wus-3 pWUS::WUSΔ-TPL (weak), five lines; wus-3 pWUS::WUSΔ-TPL (strong), two lines. Note that, like wus-1 mutants, wus-1 pWUS::WUSΔ plants rarely flowered. Any flowers that were produced were essentially identical to those of the wus-1 mutant.
n.d., Not determined.
To test this, we generated a third WUS construct expressing a WUSΔ-TPL hybrid protein (pWUS::WUSΔ-TPL), recapitulating the WUS-TPL interaction. As with the pWUS::WUSΔ construct, pWUS::WUSΔ-TPL was unable to rescue any aspect of the vegetative phenotype of wus-1 (Fig. 4C), perhaps indicating that TPL does not play a role in WUS function during vegetative stages of development. However, during the reproductive phase, partial rescue of wus-1 by the hybrid construct was observed. Inflorescences of wus-1 bear very few flowers before the inflorescence meristem terminates, while in pWUS::WUSΔ-TPL wus-1 plants, inflorescences are indeterminate, producing many flowers (Fig. 4C). In contrast to wus-1 flowers, pWUS::WUSΔ-TPL wus-1 flowers produce between three and five stamens (Fig. 4C; Table II) but never produce carpels. Thus, the phenotype of pWUS::WUSΔ-TPL wus-1 flowers closely resembles that of wus-1 flowers expressing full-length WUS, in which weak rescue was observed (Fig. 4C; Table II). The pWUS::WUSΔ-TPL wus-1 phenotype is also similar to that of the weak wus-3 mutant allele, the inflorescences of which produce many flowers that terminate after the production of approximately three stamens (Mayer et al., 1998; Fig. 4D). Thus, wus-3 reproductive meristems are maintained longer than those of wus-1 mutants. We predicted that complementation of wus-3 with the pWUS::WUSΔ-TPL construct would maintain floral meristems long enough for carpels to develop. Transgenic wus-3 plants expressing WUSΔ from the native WUS promoter were not rescued and resembled the wus-3 mutant (Fig. 4D). In contrast, pWUS::WUSΔ-TPL wus-3 flowers showed a range of phenotypes. In a small proportion of lines (less than 10% of primary transformants), no obvious rescue was observed. In the majority of lines (approximately 80%), stamen number increased to an average of four per flower (Table II). In the remaining lines (approximately 10%), not only was stamen number increased, but approximately 30% of flowers also developed carpels in the fourth whorl (Fig. 4D; Table II).
In summary, the C-terminal domain of the WUS protein that is required for TPL interaction is essential for WUS function. By fusing TPL to an inactive, truncated WUS protein, both inflorescence and floral meristems are maintained for longer than in wus mutants, suggesting that TPL is required for WUS function during the reproductive stages of development.
DISCUSSION
The Specificity and Sensitivity of the Yeast Two-Hybrid Approaches
Several factors indicate the robustness of the interaction framework. First, we found an abundance of repressive transcription factors among the identified TPL/TPR interactors. Our screens support most of the previously reported interactions by identifying TPL/TPR interactions with WOX, AUX/IAA, Zn-finger, AP2/ERF, JAZ, AFP, and TIR-NB-LRR proteins (Kieffer et al., 2006; Szemenyei et al., 2008; Gallavotti et al., 2010; Pauwels et al., 2010; Zhu et al., 2010; Arabidopsis Interactome Mapping Consortium, 2011). The recent Arabidopsis interactome lists 20 non-AUX/IAA interactors for TPL and two for TPR3 (Arabidopsis Interactome Mapping Consortium, 2011). We confirm both of the TPR3 interactors and seven of the TPL interactors, with other family members being identified for a further four interactors. Second, there was an overrepresentation of functional RDs among the isolated transcription factors. Finally, we chose two examples of TPL-transcription factor interactions to show the importance of TPL in the biological function of those transcription factors. Although the results suggest that false positives are likely to be limited in these screens, there is evidence that false negatives are more common. The relatively small degree of overlap between the library screens and the transcription factor array screens (35% overlap; Fig. 1), which is common when comparing experimentally distinct screens (Vidal and Legrain, 1999; Rajagopala et al., 2009), highlights the fact that no single screen can detect the full range of interactions. However, the combination of yeast two-hybrid approaches used in this study also enhances the sensitivity of the screens by reporting more interactions than are obtained in a single screen. Although the overlap between TPL/TPRs and individual transcription factors in the different screens was only 35%, at the family level, the overlap was much greater (60%; Table I).
TPL/TPR Act as Corepressors in Many Developmental Programs
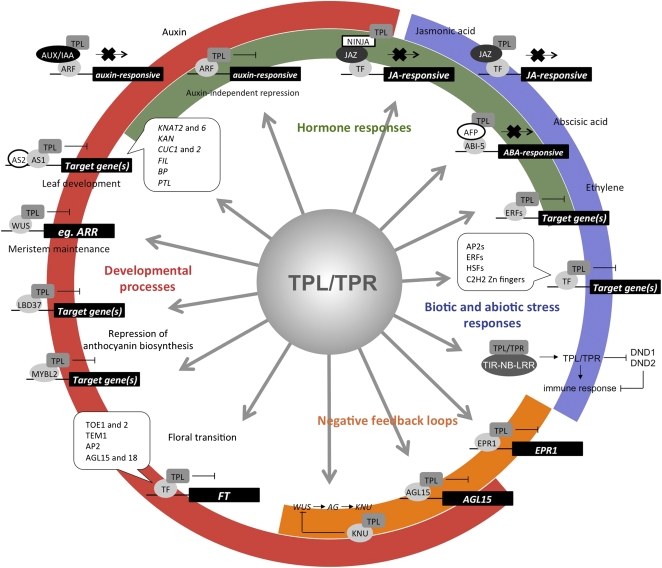
The TPL proteins appear to have been coopted multiple times in evolution to cause transcriptional repression. Database analyses indicate that the expression of TPL/TPR genes is largely constitutive; therefore, our data suggest that this family of proteins act as an “always-on” hub through which repression can be utilized in diverse pathways and in response to environmental cues (Fig. 5). The framework of TPL/TPR interaction allows us to propose a number of additional developmental mechanisms and programs in which these corepressors function and to predict novel aspects of the auxin and JA signaling pathways.
Figure 5.
The TPL family acts as general corepressors in diverse biological pathways. The interactome data place the TPL/TPR family of corepressors at the center of many biological processes. TPL is implicated in hormone responses, with the interaction data suggesting that the TPL/TPR family is involved in novel mechanisms of auxin and JA signaling. TPL/TPRs appear to be involved in a broad range of stress and plant immune responses in addition to numerous developmental pathways, such as floral transition and leaf and flower development. Colored arcs represent the different processes in which the TPL family may act and show how aspects of these pathways overlap. TF, Transcription factor.
Hormone and Stress Responses
TPL is recruited by AUX/IAA proteins to suppress the expression of auxin-responsive genes in the absence of auxin (Szemenyei et al., 2008). Our data, together with those of two earlier studies (Szemenyei et al., 2008; Arabidopsis Interactome Mapping Consortium, 2011), identify 20 of the 29 Arabidopsis AUX/IAA proteins as interaction partners of the TPL/TPRs (AUX/IAA1, -2, -3, -4, -6, -7, -8, -9, -10, -11, -12, -13, -14, -16, -17, -18, -19, -26, -27, and -28). The AUX/IAA-TPL complex is tethered to the promoters of auxin-responsive genes through interaction between AUX/IAA proteins and activating ARF transcription factors. However, a distinct class of ARFs represses transcription without interacting with AUX/IAA proteins. The mechanism of repression by this class has not been determined. Here, we show that repressive ARF proteins, such as ARF2 and ARF9, interact directly with TPL/TPR proteins (Fig. 1), suggesting a mechanism for repression and implicating TPL/TPR corepressors in both forms of ARF-mediated repression (Fig. 5).
Our data, together with other recent findings, also provide compelling evidence for the involvement of TPL in JA signaling (Pauwels et al., 2010; Arabidopsis Interactome Mapping Consortium, 2011; Fig. 5). It has been reported that the adaptor protein NINJA links TPL to JAZ proteins recruited to the promoters of JA-responsive genes (Pauwels et al., 2010). Although we did not identify NINJA as an interactor in our screens, like Pauwels et al. (2010) we identified AFP proteins interacting with the TPL/TPRs, which suggests that many combinations of interactions between the TPL and AFP families may be possible. Consistent with recent data (Arabidopsis Interactome Mapping Consortium, 2011), we also found direct interactions between TPL/TPRs and JAZ proteins (Fig. 1), which may reveal a novel way to achieve target gene repression in JA signaling.
JA is important for plant responses to wounding and pathogen attack. The hormone ethylene is also involved in biotic and abiotic stress responses, in which ERFs play a role. For example, Arabidopsis ERF3 and ERF4, both of which interact with TPL/TPR corepressors, are induced by ethylene, high-salt conditions, drought stress, and pathogen attack. The TPL/TPR interaction framework also indicates corepressor interaction with several other stress response transcription factors. The Zn-finger protein STZ (ZAT10) plays a role in salt tolerance and other stress responses, while ZAT6 is involved in nutrient stress responses (Sakamoto et al., 2004; Mittler et al., 2006; Devaiah et al., 2007). Many MYB factors, including MYB32 and MYB44, are induced in response to wounding or abscisic acid (ABA) treatment (Preston et al., 2004; Jung et al., 2008), while the MYB protein AS1 has been shown to be involved in phytopathogen response (Nurmberg et al., 2007). From our data, we predict that aspects of the function of these and other repressive MYB proteins will require TPL/TPR. ABA also plays a key role in stress responses, seed germination, and development (for review, see Hubbard et al., 2010). ABI5-related bZIP transcription factor proteins regulate ABA signaling and are found to interact with AFP proteins to mediate the ABA response (Lopez-Molina et al., 2003; Garcia et al., 2008). Since interactions between the TPL family and the Arabidopsis AFP proteins have been identified in independent studies (Pauwels et al., 2010; this study), it is tempting to speculate that AFPs may link TPL to the ABA signaling pathway (Fig. 5). TPL/TPRs were recently shown to be involved in the SNC1-mediated immune response (Zhu et al., 2010), and we also show interaction between TPR3 and a TIR-NB-LRR protein related to SNC1 (Fig. 1). Thus, the interaction framework points to a widespread involvement of the TPL/TPR family in biotic and abiotic stress responses (Fig. 5).
Transition to Flowering
The TPL/TPR genes are expressed in apical tissues, particularly during the transition to flowering, and in floral tissues (data from the Arabidopsis eFP Browser at bbc.botany.utoronto.ca/efp/cgi-bin/efpWeb.cgi; Winter et al., 2007), suggesting that these proteins may have roles to play during flowering. TPL/TPR corepressors interact with AP2 family transcription factors, including AP2, TEM1, TOE1, and TOE2, all of which have been implicated in flowering-time control through the repression of FT. For example, Arabidopsis plants overexpressing TOE1 show significantly delayed flowering (Jung et al., 2007; Fig. 3). We show that this late-flowering phenotype requires TPL/TPR activity (Fig. 3), providing evidence to support the role in floral induction that was predicted by the interaction framework (Fig. 5). Our data also reveal interactions between TPL/TPRs and proteins such as SOC1, EMF1, VRN5, and FVE (Fig. 1), suggesting that TPL/TPRs could be involved in the regulation of flowering at multiple control points.
TPL/TPR Interact via Short Protein Motifs
Short, specific peptide motifs of certain transcription factors are required for the recruitment of Gro/TLE corepressors (Courey and Jia, 2001; Swingler et al., 2004). Similarly, the EAR RD conserved in the AUX/IAA, NINJA, and WUS proteins is required for interaction with plant TPL/TPR corepressors (Kieffer et al., 2006; Szemenyei et al., 2008; Pauwels et al., 2010). EAR motifs were significantly enriched among the transcriptional regulators found to interact with the TPL/TPR proteins (Supplemental Table S5). In addition to the EAR motif, the factors found in the TPL/TPR interaction framework contain a number of different RDs with quite diverse sequences (Supplemental Table S5), which we demonstrate are also required to recruit TPL (Fig. 2). Together, these data demonstrate that all known functional RDs described in Arabidopsis are able to recruit the TPL/TPR proteins and that all such transcriptional repression might be mediated, at least in part, by this family of corepressors. A significant number of proteins isolated in the protein interaction screens contained only a partial, or no known, RD, suggesting that other RD sequences remain to be discovered. For example, we identified the MLFGV motif in an AP2/B3 factor (AT1G51120) and showed that it is necessary for TPL interaction (Fig. 2). Our data already indicate that TPL/TPR-mediated transcriptional repression is a widespread mechanism, but the discovery of new RD sequences found in the interaction partners of the TPL/TPR corepressors allows for predictions about new repressive pathways.
Recently, Kagale et al. (2010) defined the Arabidopsis EAR repressome using a bioinformatic approach to identify transcription regulators containing EAR (LxLxL and/or DLNxxP) domains. They identified 219 proteins belonging to 21 families of regulatory factors. By analyzing our yeast two-hybrid data of interacting proteins containing LxLxL and/or DLNxxP (73 in total), we find 48 in common with the data set of Kagale et al. (2010), representing approximately 22% of the 219 factors. While the overlap was modest for individuals, at the family level, we found factors belonging to 17 of the 21 families identified by Kagale et al. (2010). This indicates that these two very different approaches provide similar broad frameworks for EAR-mediated transcriptional repression in Arabidopsis and suggests that family-level data can be used to infer possible interactions.
TPL/TPR proteins are recruited by LxLxL-containing proteins, but there is at least one other unrelated Arabidopsis corepressor that also utilizes this motif. AtSAP18 is the ortholog of human SAP18, which interacts with LxLxL-containing proteins, including ERFs and MADS box factors, involved in flowering (Song and Galbraith, 2006; Hill et al., 2008; Liu et al., 2009), suggesting that AtSAP18 repression may overlap with repression mediated by TPL/TPR. SAP18 is conserved in higher eukaryotes and suppresses transcription through interaction with histone deacetylases. Thus, SAP18 may be part of a conserved repression mechanism, while TPL/TPR, and the related factors LUG and LUH (Conner and Liu, 2000; Sitaraman et al., 2008), may represent gene repression systems that evolved specifically in plants. Our data extend the repressome beyond EAR to incorporate proteins with other RDs such as (R/K)LFGV and TLxLF, providing a means of identifying whether uncharacterized factors may act as transcriptional repressors.
Mechanisms of Repression
The Gro/Tup1 group of corepressors induces transcriptional repression using several mechanisms. In particular, they induce local chromatin compaction at target sites through an association with chromatin remodelers such as histone deacetylases. Arabidopsis Gro/Tup1 proteins belonging to the LUG and TPL families have also been shown to function together with histone deacetylases (Sridhar et al., 2004; Long et al., 2006; Gonzalez et al., 2007), indicating that the recruitment of these corepressors to target genes results in histone deacetylation, chromatin condensation, and gene silencing. In the case of LUG, repression is mediated by direct association between the LUG and HDA19 proteins (Gonzalez et al., 2007). However, we and others have failed to detect an association between TPL and HDA19 (Supplemental Fig. S2; Kagale and Rozwadowski, 2011). Genetic evidence suggests that TPL acts through HDA19 (Long et al., 2006), and interactions between TPR1 and HDA19 were observed in pull-down experiments from plant extracts (Zhu et al., 2010), which might suggest that routes to histone modification require additional factors to bridge between TPL/TPR proteins and histone deacetylases.
Chromatin modifications such as methylation of histone H3 Lys residue 9, which is catalyzed by SUV39H histone methyltransferases, also result in nucleosome compaction and gene silencing (for review, see Zhao and Shen, 2004; Pontvianne et al., 2010). Interestingly, we identified a SUV39H-like protein, SUVH3, as a TPL/TPR interactor (Fig. 1; Supplemental Table S3). Furthermore, we also identified a protein related to the PICKLE (PKL) CHD3/Mi-2-like chromatin remodeler (Ogas et al., 1999), PKR1, as an interaction partner of TPR2 (Fig. 1). PKL acts to repress the expression of seed-associated genes during germination by promoting the methylation of histone H3 Lys residue 27 (Zhang et al., 2008). The protein interaction data, therefore, imply that the TPL/TPR proteins can use multiple chromatin-remodeling mechanisms to induce transcriptional repression, and it will be interesting to discover whether different mechanisms are utilized depending on the developmental context.
MATERIALS AND METHODS
Yeast Two-Hybrid Whole Plant Library Screens
A normalized, random-primed whole plant Arabidopsis (Arabidopsis thaliana) yeast two-hybrid library, prepared in the pGADT7-Rec plasmid and transformed into yeast strain AH109 (Clontech), was used for each screen. TPL/TPR coding sequences were cloned into bait vectors pGBKT7 or pGBT9, which were transformed to yeast strain Y187 (Clontech), using standard protocols (Gietz and Woods, 2002). Library screens were performed by yeast mating between the bait and library strains according to the manufacturer’s instructions (Clontech Matchmaker3 system). In each case, over 1 million diploids were screened for interactions (Supplemental Table S2), and putative positives were isolated on minimal medium plates lacking His but containing 2.5 mm 3-amino-1,2,4-triazole (3-AT). Interactions were validated by the use of the ADE2 and/or MEL1 reporters.
Yeast Two-Hybrid Transcription Factor Library Screens
TPL, TPR2, TPR3, and TPR4 coding sequences were cloned into the bait vector pDEST32 (Invitrogen). The TPR1 bait construct was the same as that used in the whole plant library screen. Baits were transformed to yeast strain PJ69-4α. Each bait was mated against the arrayed yeast two-hybrid library (in vector pDEST22 and yeast strain PJ69-4a) of Arabidopsis transcription factors (Paz-Ares and The REGIA Consortium, 2002; Castrillo et al., 2011) in triplicate on yeast peptone dextrose plates. Diploids were selected on minimal medium lacking Trp and Leu, and interactions were detected by patching the diploids onto minimal medium lacking His but containing 2.5 mm 3-AT.
Yeast Two-Hybrid Analysis of Putative RDs
RD sequences were mutated using the Phusion Site-Directed Mutagenesis Kit (Finnzymes) according to the manufacturer’s instructions. Point mutations were introduced by PCR using primers E3mF (5′-GTTTCAATTCGCTCATAATTTTCCACCGTTGG-3′) and E3R (5′-GGCGGATTCCGTCGCCGTGAAGACGATGCGATATC-3′) for the ERF3 gene (AT1G50640); RAVmF (5′-GGTTTTGAGATCGTCCGGAGTTAACATTTCACC-3′) and RAVR (5′-CGACCCGCATCTAAATCTGACCCGGATCTCGAC-3′) for the RAV1 gene (AT1G13260); and A2mF (5′-GAGGGTTTATGCAGTGTGGTGTTAGGATCCAATAG-3′) and A2R (5′-CTTTCTTCTCCTCTGATTTGGTTTCTTCTTCTACC-3′) for the AP2-like gene (AT1G51120). Ligated PCR products were propagated in Escherichia coli, and plasmid DNA was isolated from at least five independent colonies. Of these, at least four verified constructs for each prey were transformed into the pDEST32-TPL bait yeast strain. As a control, the TPL bait strain was also transformed with the appropriate wild-type prey construct. Interactions were assessed by growth on minimal medium lacking His but containing 2.5 mm 3-AT.
Bioinformatics
Putative RD sequences (LxLxL, DLNxxP/FDLNI, K/RLFGV, and TLxLF) were identified manually among the sequences of TPL/TPR interactors. To identify all Arabidopsis proteins (The Arabidopsis Information Resource 9) containing these RDs, we used the Patmatch algorithm at www.arabidopsis.org (default settings). To identify the complement of Arabidopsis transcription factors containing the RDs, we compared the DATF (datf.cbi.pku.edu.cn; Guo et al., 2005) with the Patmatch list of Arabidopsis proteins containing the same motifs using the COUNTIF function in Microsoft Excel. Arabidopsis factors previously reported to act as transcriptional repressors were identified using the Gene Ontology Consortium’s annotation and ontology toolkit AmiGO (amigo.geneontology.org; Carbon et al., 2009) and through literature surveys.
In Planta Analyses
All constructs were transformed into Agrobacterium tumefaciens strain GV3101, and Arabidopsis plants were transformed using the floral dip method (Clough and Bent, 1998). All plants were grown in glasshouses at a constant temperature of 21°C with a 16/8-h photoperiod.
35S::TOE1 Transgenic Lines
The TOE1 coding sequence was amplified from Arabidopsis cDNA using primers TOE1-F (5′-ggggacaagtttgtacaaaaaagcaggctTCGCTAGATTTGTAATTTTCAGAG-3′; lowercase bases represent the Gateway sequence) and TOE1-R (5′-ggggaccactttgtacaagaaagctgggtTTAAGGGTGTGGATAAAAGTAACC-3′) and cloned into pALLIGATOR III plasmid (Gateway-modified pFP101; Bensmihen et al., 2004) to generate the 35S::TOE1 construct. 35S::TOE1 was transformed to wild-type (Ler) and tpl-1 plant lines, and flowering time was monitored under long-day conditions.
Expression levels of FT and TOE1 in leaves of transgenic and nontransgenic plants were measured by quantitative reverse transcription (qRT)-PCR. cDNA was synthesized from 2 μg of total RNA extracted from leaves using SuperScript II Reverse Transcriptase (Invitrogen). qPCRs were run in triplicate on the Bio-Rad CFX96 Real-Time PCR System, using SsoFast EvaGreen supermix (Bio-Rad), as follows: 30 s at 95°C, 39 cycles of 3 s at 95°C, 5 s at 60°C, and plate read, followed by a melt curve of 65°C to 95°C, with a plate read at every 0.2°C increase. FT amplifications were performed using primers described by Jang et al. (2009). TOE1 amplifications were performed using primers qTOE-F (5′-GCTGAAGGGATGATGAGTAACTGG-3′) and qTOE-R (5′-ACTGAGAACAATGGTGGTGGTTG-3′). For each genotype, expression levels of TOE and FT were calculated relative to the EIF4A control and amplified using primers SR_elf4A_i_F (5′-GGTCATGCGTGCCCTTGGTGA-3′) and SR_elf4A_i_R (5′-ACCAGCCTGGAGAATGCGCTG-3′).
Complementation of wus Mutants
Plants heterozygous for the wus-1 or wus-3 mutation were transformed with a number of WUS constructs, each under the control of 3.3 kb of the WUS promoter, prepared in the pALLIGATOR V vector (Gateway-converted pFP100; Bensmihen et al., 2004). Full-length WUS was amplified from genomic DNA using Gateway-compatible primers WUS-F (5′-ggggacaagtttgtacaaaaaagcaggctATGGCTTTTTGGCAAGACGGATC-3′; lowercase bases represent the Gateway sequence) and WUS-R (5′-ggggaccactttgtacaagaaagctgggtCTAGTTCAGACGTAGCTCAAGAG-3′). C-terminally truncated WUS (WUSΔ) was amplified from genomic DNA using primers WUS-F and WUSD*-R (5′-ggggaccactttgtacaagaaagctgggtCTAATGACCTTCTAGACCAAACAGAGG-3′). The WUSΔ-TPL construct was generated using MultiSite Gateway, to generate a fusion between WUSΔ and the TPL coding sequence. The WUSΔ sequence was amplified from genomic DNA using WUS-F and WUSD-R (5′-ggggacaacttttgtatacaaagttgtATGACCTTCTAGACCAAACAGAGG-3′). The TPL sequence was amplified from inflorescence first-strand cDNA using primers TPL-F (5′-ggggacaactttgtatacaaaagttgtgTCTTCTCTTAGTAGAGAGCTCG-3′) and TPL-R (5′-ggggaccactttgtacaagaaagctgggtTCATCTCTGAGGCTGATCAGATG-3′). The genotype of the wus-1 allele in all transgenics was confirmed by PCR amplification of the endogenous WUS gene using primers OL95 (5′-GATCTTGATTGGGGCAAACC-3′) and WUS-UTR (5′-CTAGCGAAGCATAGTTGTGAACATACG-3′) and DNA sequencing. The genotype of the wus-3 allele was confirmed by PCR using primers wus3F (5′-ATGGAGCCGCCACAGCATCAGCATC-3′) and WUS-UTR and DNA sequencing.
To ensure that the 3.3 kb of WUS promoter gave the appropriate expression pattern, it was cloned upstream of the GUS reporter gene in the pJawohl11-GW-GUS vector using Gateway (Ulker et al., 2007).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Breakdown of transcription factors isolated in library screens.
Supplemental Figure S2. Additional TPL interactions.
Supplemental Table S1. Non-TF TPL/TPR interactors.
Supplemental Table S2. Summary of TPL/TPR Y2H screens.
Supplemental Table S3. Putative weak TPL/TPR interactors.
Supplemental Table S4. Analysis of RDs among the TPL/TPR interactors.
Supplemental Table S5. Comparison of Arabidopsis RDs from various sources.
Acknowledgments
We thank George Coupland and Franziska Turck at the Max Planck Institute (Cologne, Germany) and Gerco Angenent and Richard Immink at Plant Research International (Wageningen, The Netherlands) for the arrayed yeast two-hybrid library of Arabidopsis transcription factors. The normalized Arabidopsis whole plant yeast two-hybrid library was the generous gift of Hans Sommer and Simona Masiero (Max Planck Institue, Cologne, Germany). Seed for the tpl-1 allele was kindly provided by Kathy Barton (Department of Plant Biology, Carnegie Institution, Stanford, CA), and wus-3 seed was the generous gift of Thomas Laux (University of Freiburg, Freiburg, Germany). We are also grateful to Stefan Kepinski and James Lloyd (Centre for Plant Sciences, University of Leeds, UK) for comments on the manuscript, Sam Rayson for her assistance with qRT-PCR, and Katie Curnock, Amie Blinkhorn, and Lauren Hill (University of Leeds, UK) for help with the yeast two-hybrid library screens.
References
- Arabidopsis Interactome Mapping Consortium (2011) Evidence for network evolution in an Arabidopsis interactome map. Science 333: 601–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäurle I, Laux T. (2005) Regulation of WUSCHEL transcription in the stem cell niche of the Arabidopsis shoot meristem. Plant Cell 17: 2271–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensmihen S, To A, Lambert G, Kroj T, Giraudat J, Parcy F. (2004) Analysis of an activated ABI5 allele using a new selection method for transgenic Arabidopsis seeds. FEBS Lett 561: 127–131 [DOI] [PubMed] [Google Scholar]
- Brand U, Fletcher JC, Hobe M, Meyerowitz EM, Simon R. (2000) Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science 289: 617–619 [DOI] [PubMed] [Google Scholar]
- Carbon S, Ireland A, Mungall CJ, Shu S, Marshall B, Lewis S, AmiGO Hub, Web Presence Working Group (2009) AmiGO: online access to ontology and annotation data. Bioinformatics 25: 288–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillejo C, Pelaz S. (2008) The balance between CONSTANS and TEMPRANILLO activities determines FT expression to trigger flowering. Curr Biol 18: 1338–1343 [DOI] [PubMed] [Google Scholar]
- Castrillo G, Turck F, Leveugle M, Lecharny A, Carbonero P, Coupland G, Paz-Ares J, Oñate-Sánchez L. (2011) Speeding cis-trans regulation discovery by phylogenomic analyses coupled with screenings of an arrayed library of Arabidopsis transcription factors. PLoS ONE 6: e21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causier B, Davies B. (2002) Analysing protein-protein interactions with the yeast two-hybrid system. Plant Mol Biol 50: 855–870 [DOI] [PubMed] [Google Scholar]
- Cerna D, Wilson DK. (2005) The structure of Sif2p, a WD repeat protein functioning in the SET3 corepressor complex. J Mol Biol 351: 923–935 [DOI] [PubMed] [Google Scholar]
- Chen G, Courey AJ. (2000) Groucho/TLE family proteins and transcriptional repression. Gene 249: 1–16 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Conner J, Liu Z. (2000) LEUNIG, a putative transcriptional corepressor that regulates AGAMOUS expression during flower development. Proc Natl Acad Sci USA 97: 12902–12907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courey AJ, Jia S. (2001) Transcriptional repression: the long and the short of it. Genes Dev 15: 2786–2796 [DOI] [PubMed] [Google Scholar]
- Devaiah BN, Nagarajan VK, Raghothama KG. (2007) Phosphate homeostasis and root development in Arabidopsis are synchronized by the zinc finger transcription factor ZAT6. Plant Physiol 145: 147–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks RG, Wang C, Levin JZ, Liu Z. (2002) SEUSS, a member of a novel family of plant regulatory proteins, represses floral homeotic gene expression with LEUNIG. Development 129: 253–263 [DOI] [PubMed] [Google Scholar]
- Gallavotti A, Long JA, Stanfield S, Yang X, Jackson D, Vollbrecht E, Schmidt RJ. (2010) The control of axillary meristem fate in the maize ramosa pathway. Development 137: 2849–2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia ME, Lynch T, Peeters J, Snowden C, Finkelstein R. (2008) A small plant-specific protein family of ABI five binding proteins (AFPs) regulates stress response in germinating Arabidopsis seeds and seedlings. Plant Mol Biol 67: 643–658 [DOI] [PubMed] [Google Scholar]
- Gietz RD, Woods RA. (2002) Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol 350: 87–96 [DOI] [PubMed] [Google Scholar]
- Gonzalez D, Bowen AJ, Carroll TS, Conlan RS. (2007) The transcription corepressor LEUNIG interacts with the histone deacetylase HDA19 and mediator components MED14 (SWP) and CDK8 (HEN3) to repress transcription. Mol Cell Biol 27: 5306–5315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregis V, Sessa A, Colombo L, Kater MM. (2006) AGL24, SHORT VEGETATIVE PHASE, and APETALA1 redundantly control AGAMOUS during early stages of flower development in Arabidopsis. Plant Cell 18: 1373–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo A, He K, Liu D, Bai S, Gu X, Wei L, Luo J. (2005) DATF: a database of Arabidopsis transcription factors. Bioinformatics 21: 2568–2569 [DOI] [PubMed] [Google Scholar]
- Hill K, Wang H, Perry SE. (2008) A transcriptional repression motif in the MADS factor AGL15 is involved in recruitment of histone deacetylase complex components. Plant J 53: 172–185 [DOI] [PubMed] [Google Scholar]
- Hiratsu K, Ohta M, Matsui K, Ohme-Takagi M. (2002) The SUPERMAN protein is an active repressor whose carboxy-terminal repression domain is required for the development of normal flowers. FEBS Lett 514: 351–354 [DOI] [PubMed] [Google Scholar]
- Hiratsu K, Mitsuda N, Matsui K, Ohme-Takagi M. (2004) Identification of the minimal repression domain of SUPERMAN shows that the DLELRL hexapeptide is both necessary and sufficient for repression of transcription in Arabidopsis. Biochem Biophys Res Commun 321: 172–178 [DOI] [PubMed] [Google Scholar]
- Hubbard KE, Nishimura N, Hitomi K, Getzoff ED, Schroeder JI. (2010) Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Genes Dev 24: 1695–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Mitsuda N, Ohme-Takagi M. (2009) Arabidopsis WUSCHEL is a bifunctional transcription factor that acts as a repressor in stem cell regulation and as an activator in floral patterning. Plant Cell 21: 3493–3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Ohme-Takagi M. (2009) A novel group of transcriptional repressors in Arabidopsis. Plant Cell Physiol 50: 970–975 [DOI] [PubMed] [Google Scholar]
- Jang S, Torti S, Coupland G. (2009) Genetic and spatial interactions between FT, TSF and SVP during the early stages of floral induction in Arabidopsis. Plant J 60: 614–625 [DOI] [PubMed] [Google Scholar]
- Jung C, Seo JS, Han SW, Koo YJ, Kim CH, Song SI, Nahm BH, Choi YD, Cheong JJ. (2008) Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis. Plant Physiol 146: 623–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JH, Seo YH, Seo PJ, Reyes JL, Yun J, Chua NH, Park CM. (2007) The GIGANTEA-regulated microRNA172 mediates photoperiodic flowering independent of CONSTANS in Arabidopsis. Plant Cell 19: 2736–2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagale S, Links MG, Rozwadowski K. (2010) Genome-wide analysis of ethylene-responsive element binding factor-associated amphiphilic repression motif-containing transcriptional regulators in Arabidopsis. Plant Physiol 152: 1109–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagale S, Rozwadowski K. (2011) EAR motif-mediated transcriptional repression in plants: an underlying mechanism for epigenetic regulation of gene expression. Epigenetics 6: 141–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann K, Pajoro A, Angenent GC. (2010) Regulation of transcription in plants: mechanisms controlling developmental switches. Nat Rev Genet 11: 830–842 [DOI] [PubMed] [Google Scholar]
- Kieffer M, Stern Y, Cook H, Clerici E, Maulbetsch C, Laux T, Davies B. (2006) Analysis of the transcription factor WUSCHEL and its functional homologue in Antirrhinum reveals a potential mechanism for their roles in meristem maintenance. Plant Cell 18: 560–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogan NT, Long JA. (2009) Why so repressed? Turning off transcription during plant growth and development. Curr Opin Plant Biol 12: 628–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux T, Mayer KF, Berger J, Jürgens G. (1996) The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122: 87–96 [DOI] [PubMed] [Google Scholar]
- Leibfried A, To JP, Busch W, Stehling S, Kehle A, Demar M, Kieber JJ, Lohmann JU. (2005) WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature 438: 1172–1175 [DOI] [PubMed] [Google Scholar]
- Liu C, Xi W, Shen L, Tan C, Yu H. (2009) Regulation of floral patterning by flowering time genes. Dev Cell 16: 711–722 [DOI] [PubMed] [Google Scholar]
- Liu Z, Karmarkar V. (2008) Groucho/Tup1 family co-repressors in plant development. Trends Plant Sci 13: 137–144 [DOI] [PubMed] [Google Scholar]
- Long JA, Ohno C, Smith ZR, Meyerowitz EM. (2006) TOPLESS regulates apical embryonic fate in Arabidopsis. Science 312: 1520–1523 [DOI] [PubMed] [Google Scholar]
- Long JA, Woody S, Poethig S, Meyerowitz EM, Barton MK. (2002) Transformation of shoots into roots in Arabidopsis embryos mutant at the TOPLESS locus. Development 129: 2797–2806 [DOI] [PubMed] [Google Scholar]
- Lopez-Molina L, Mongrand S, Kinoshita N, Chua NH. (2003) AFP is a novel negative regulator of ABA signaling that promotes ABI5 protein degradation. Genes Dev 17: 410–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malavé TM, Dent SY. (2006) Transcriptional repression by Tup1-Ssn6. Biochem Cell Biol 84: 437–443 [DOI] [PubMed] [Google Scholar]
- Matsui K, Umemura Y, Ohme-Takagi M. (2008) AtMYBL2, a protein with a single MYB domain, acts as a negative regulator of anthocyanin biosynthesis in Arabidopsis. Plant J 55: 954–967 [DOI] [PubMed] [Google Scholar]
- Mayer KF, Schoof H, Haecker A, Lenhard M, Jürgens G, Laux T. (1998) Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95: 805–815 [DOI] [PubMed] [Google Scholar]
- Mittler R, Kim Y, Song L, Coutu J, Coutu A, Ciftci-Yilmaz S, Lee H, Stevenson B, Zhu JK. (2006) Gain- and loss-of-function mutations in Zat10 enhance the tolerance of plants to abiotic stress. FEBS Lett 580: 6537–6542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurmberg PL, Knox KA, Yun BW, Morris PC, Shafiei R, Hudson A, Loake GJ. (2007) The developmental selector AS1 is an evolutionarily conserved regulator of the plant immune response. Proc Natl Acad Sci USA 104: 18795–18800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogas J, Kaufmann S, Henderson J, Somerville C. (1999) PICKLE is a CHD3 chromatin-remodeling factor that regulates the transition from embryonic to vegetative development in Arabidopsis. Proc Natl Acad Sci USA 96: 13839–13844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta M, Matsui K, Hiratsu K, Shinshi H, Ohme-Takagi M. (2001) Repression domains of class II ERF transcriptional repressors share an essential motif for active repression. Plant Cell 13: 1959–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels L, Barbero GF, Geerinck J, Tilleman S, Grunewald W, Pérez AC, Chico JM, Bossche RV, Sewell J, Gil E, et al. (2010) NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature 464: 788–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Ares J, The Regia Consortium (2002) REGIA, an EU project on functional genomics of transcription factors from Arabidopsis thaliana. Comp Funct Genomics 3: 102–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfluger J, Zambryski P. (2004) The role of SEUSS in auxin response and floral organ patterning. Development 131: 4697–4707 [DOI] [PubMed] [Google Scholar]
- Pontvianne F, Blevins T, Pikaard CS. (2010) Arabidopsis histone lysine methyltransferases. Adv Bot Res 53: 1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston J, Wheeler J, Heazlewood J, Li SF, Parish RW. (2004) AtMYB32 is required for normal pollen development in Arabidopsis thaliana. Plant J 40: 979–995 [DOI] [PubMed] [Google Scholar]
- Rajagopala SV, Hughes KT, Uetz P. (2009) Benchmarking yeast two-hybrid systems using the interactions of bacterial motility proteins. Proteomics 9: 5296–5302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablowski R. (2007) The dynamic plant stem cell niches. Curr Opin Plant Biol 10: 639–644 [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Maruyama K, Sakuma Y, Meshi T, Iwabuchi M, Shinozaki K, Yamaguchi-Shinozaki K. (2004) Arabidopsis Cys2/His2-type zinc-finger proteins function as transcription repressors under drought, cold, and high-salinity stress conditions. Plant Physiol 136: 2734–2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoof H, Lenhard M, Haecker A, Mayer KF, Jürgens G, Laux T. (2000) The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100: 635–644 [DOI] [PubMed] [Google Scholar]
- Sitaraman J, Bui M, Liu Z. (2008) LEUNIG_HOMOLOG and LEUNIG perform partially redundant functions during Arabidopsis embryo and floral development. Plant Physiol 147: 672–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CP, Galbraith DW. (2006) AtSAP18, an orthologue of human SAP18, is involved in the regulation of salt stress and mediates transcriptional repression in Arabidopsis. Plant Mol Biol 60: 241–257 [DOI] [PubMed] [Google Scholar]
- Sridhar VV, Surendrarao A, Gonzalez D, Conlan RS, Liu Z. (2004) Transcriptional repression of target genes by LEUNIG and SEUSS, two interacting regulatory proteins for Arabidopsis flower development. Proc Natl Acad Sci USA 101: 11494–11499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar VV, Surendrarao A, Liu Z. (2006) APETALA1 and SEPALLATA3 interact with SEUSS to mediate transcription repression during flower development. Development 133: 3159–3166 [DOI] [PubMed] [Google Scholar]
- Stahle MI, Kuehlich J, Staron L, von Arnim AG, Golz JF. (2009) YABBYs and the transcriptional corepressors LEUNIG and LEUNIG_HOMOLOG maintain leaf polarity and meristem activity in Arabidopsis. Plant Cell 21: 3105–3118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swingler TE, Bess KL, Yao J, Stifani S, Jayaraman PS. (2004) The proline-rich homeodomain protein recruits members of the Groucho/Transducin-like enhancer of split protein family to co-repress transcription in hematopoietic cells. J Biol Chem 279: 34938–34947 [DOI] [PubMed] [Google Scholar]
- Szemenyei H, Hannon M, Long JA. (2008) TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science 319: 1384–1386 [DOI] [PubMed] [Google Scholar]
- Ulker B, Shahid Mukhtar M, Somssich IE. (2007) The WRKY70 transcription factor of Arabidopsis influences both the plant senescence and defense signaling pathways. Planta 226: 125–137 [DOI] [PubMed] [Google Scholar]
- Vidal M, Legrain P. (1999) Yeast forward and reverse ‘n’-hybrid systems. Nucleic Acids Res 27: 919–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ. (2007) An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2: e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Rider SD, Jr, Henderson JT, Fountain M, Chuang K, Kandachar V, Simons A, Edenberg HJ, Romero-Severson J, Muir WM, et al. (2008) The CHD3 remodeler PICKLE promotes trimethylation of histone H3 lysine 27. J Biol Chem 283: 22637–22648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Shen WH. (2004) Plants contain a high number of proteins showing sequence similarity to the animal SUV39H family of histone methyltransferases. Ann N Y Acad Sci 1030: 661–669 [DOI] [PubMed] [Google Scholar]
- Zhu Z, Xu F, Zhang Y, Cheng YT, Wiermer M, Li X, Zhang Y. (2010) Arabidopsis resistance protein SNC1 activates immune responses through association with a transcriptional corepressor. Proc Natl Acad Sci USA 107: 13960–13965 [DOI] [PMC free article] [PubMed] [Google Scholar]