Abstract
Recent studies suggest that intercellular transport via plasmodesmata (PD) is regulated by cellular redox state. Until now, this relationship has been unclear, as increased production of reactive oxygen species (ROS) has been associated with both increased and decreased intercellular transport via PD. Here, we show that silencing two genes that both increase transport via PD, INCREASED SIZE EXCLUSION LIMIT1 (ISE1) and ISE2, alters organelle redox state. Using redox-sensitive green fluorescent proteins targeted to the mitochondria or plastids, we show that, relative to wild-type leaves, plastids are more reduced in both ISE1- and ISE2-silenced leaves, whereas mitochondria are more oxidized in ISE1-silenced leaves. We further show that PD transport is positively regulated by ROS production in mitochondria following treatment with salicylhydroxamic acid but negatively regulated by an oxidative shift in both chloroplasts and mitochondria following treatment with paraquat. Thus, oxidative shifts in the mitochondrial redox state positively regulate intercellular transport in leaves, but oxidative shifts in the plastid redox state counteract this effect and negatively regulate intercellular transport. This proposed model reconciles previous contradictory evidence relating ROS production to PD transport and supports accumulating evidence that mitochondria and plastids are crucial regulators of PD function.
Cellulosic cell walls prevent contact between plant cell membranes. Plants have evolved cell wall spanning, plasma membrane-lined channels called plasmodesmata (PD) that enable cell-to-cell transport. PD mediate transport of micro- and macromolecules, including metabolites, proteins, RNA, and plant viruses. PD transport is a vital function in plants, and mutants lacking PD have not been isolated. PD transport is tightly regulated throughout growth and development and in response to environmental stimuli. In general, developing tissues, such as young embryos, root tips, and sink leaves, permit larger molecules to traffic between cells, whereas in mature leaves, intercellular transport of large molecules is restricted. PD transport capacity is correlated with PD ultrastructure; most PD in young tissues are composed of a single channel, whereas PD in older tissues are frequently modified or branched (for review, see Burch-Smith et al., 2011b).
Recent genetic studies suggest that alterations in the redox state of plant cells may regulate cell-to-cell trafficking via PD. A plastid-localized thioredoxin was identified in a mutant screen for decreased unloading of GFP from seedling root phloem. This mutant, gfp arrested trafficking1 (gat1), which encodes THIOREDOXIN M-TYPE3 (TRX-m3), also showed elevated production of reactive oxygen species (ROS; Benitez-Alfonso et al., 2009). In fact, the decreased phloem unloading phenotype could be replicated by treating seedlings with either paraquat or alloxan, compounds that lead to overproduction of ROS. We identified a mutant, increased size exclusion limit1 (ise1), which exhibits increased transport via PD during embryogenesis (Stonebloom et al., 2009). ISE1 encodes a mitochondria-localized putative RNA helicase. Silencing ISE1 in Nicotiana benthamiana leaves also shows an increased intercellular transport phenotype. Both ise1 mutant and ISE1-silenced tissues exhibit increased production of ROS. The transmembrane proton gradient is defective in ise1 mitochondria and likely leads to the observed increase in ROS production (Stonebloom et al., 2009). Thus, genetic studies have identified plastid and mitochondrial mutants that both induce the production of ROS, but with opposite effects on intercellular transport via PD.
Numerous conditions that limit plant growth and development also alter cellular redox state. The best studied redox changes are those inducing oxidative stress such as high light, cold, heat, drought, flood, and pathogen attack. ROS also are produced during normal growth and development and have important signaling functions. Because different forms of reactive oxygen, such as superoxide anion, singlet oxygen, hydroxyl radicle, or hydrogen peroxide (H2O2), are produced in specific subcellular locations by distinct stimuli and then interact with distinct ROS sensors and signal transduction pathway components in each subcellular compartment, changes in plant cellular redox state are well poised to modulate cellular responses to changing environmental conditions (Foyer and Noctor, 2009) as well as endogenous developmental processes (Bashandy et al., 2010; Tsukagoshi et al., 2010). Ascorbic acid/dehydroascorbic acid and glutathione (GSH)/glutathione disulfide act as redox buffers to regulate cellular responses to changes in environmental conditions (Potters et al., 2010). The status of the GSH and ascorbic acid pools is generally regarded as a reliable proxy for the overall redox state of a particular tissue, cell, or cellular compartment.
Here, we test whether changes in plastid or mitochondrial redox state participate in the regulation of intercellular transport via PD. Silencing ISE1 or ISE2 results in increased intercellular transport via PD and increased production of secondary PD (Burch-Smith and Zambryski, 2010). We demonstrate that the redox state of plastids is reduced in leaves after silencing either gene and that the redox state of mitochondria is oxidized in ISE1-silenced leaves. To further test whether changes in organelle redox state are sufficient to affect PD function, Arabidopsis (Arabidopsis thaliana) leaves were treated with moderate levels of inhibitors of mitochondrial or plastid metabolism known to induce ROS production in the organelles, and then cell-to-cell transport via PD was measured. ROS production in mitochondria leads to increased cell-to-cell transport, and ROS production in plastids leads to decreased cell-to-cell transport. The effects and specificity of metabolic inhibitor treatments or gene silencing on organelle redox states were evaluated and confirmed by examining leaves expressing redox-sensitive GFP (roGFP) targeted to the mitochondria (mito-roGFP1) or plastids (plastid-roGFP2).
RESULTS
Silencing Genes That Affect PD Transport Alters the Cellular Redox State
ise1 and ise2 mutants and virus-induced gene silencing (VIGS) of ISE1 and ISE2 increase intercellular transport and induce the production of secondary PD (Stonebloom et al., 2009; Burch-Smith and Zambryski, 2010). ISE1 localizes to mitochondria, and both ise1 mutants and ISE1-silenced tissue exhibit increased production of ROS (Stonebloom et al., 2009). The specific effects of loss of ISE1 function on redox state in subcellular compartments, however, are unknown. ISE2 localizes to plastids, as has been recently demonstrated using bioinformatics, proteomics, and microscopy (Ichikawa et al., 2006; Olinares et al., 2010; Burch-Smith et al., 2011a), and its effect on cellular redox state has not been tested. To monitor specific redox states in subcellular compartments, we utilized roGFPs that target to the cytoplasm, mitochondria, or plastids.
roGFPs are a set of GFP variants in which two Cys residues have been introduced near the fluorophore. Formation of a disulfide bridge between the two introduced cysteines induces a structural rearrangement of amino acid side chains contacting the chromophore, favoring a neutral rather than an anionic chromophore (Cannon and James Remington, 2009). The neutral and anionic forms of the chromophore possess distinct peak fluorescence excitation wavelengths at 400 and 495 nm, respectively. Measurement of roGFP fluorescence intensity following excitation at each excitation maximum permits an evaluation of the relative proportion of roGFP in a reduced or oxidized state. In vitro and in vivo studies have shown that roGFPs are in equilibrium with the redox potential of the GSH pool (Schwarzländer et al., 2008). Therefore, changes in the proportion of oxidized roGFP reflect changes in the ratio of GSH to GSH disulfide in the subcellular compartment where roGFP is targeted. roGFPs have been used in Arabidopsis to examine the cellular redox state during root development (Jiang et al., 2006), drought stress (Jubany-Mari et al., 2010), dark treatment (Rosenwasser et al., 2011), and response to treatment with metabolic inhibitors (Lehmann et al., 2009; Schwarzländer et al., 2009).
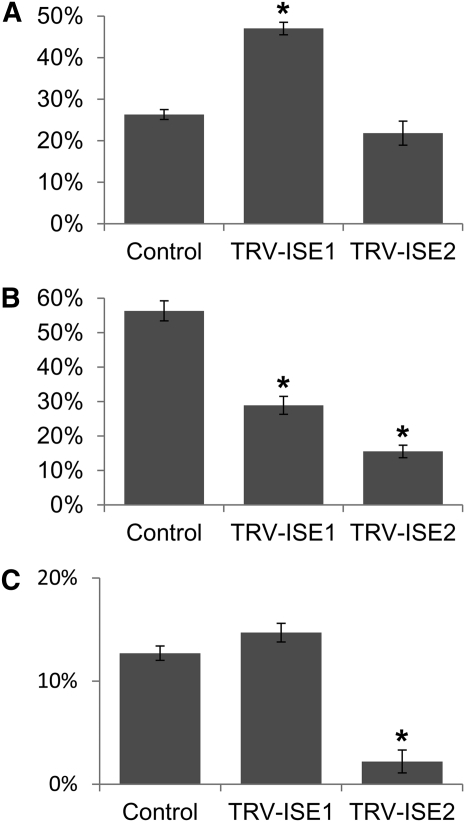
We first silenced ISE1 and ISE2 via VIGS in N. benthamiana plants (as in Burch-Smith and Zambryski, 2010). Then, cytoplasmic (cyto)-roGFP1, mito-roGFP1, or plastid-roGFP2 were transiently expressed in silenced leaves, and the oxidation states of six regions from at least eight leaves were measured using ratiometric fluorescence microscopy. Silencing ISE1 induced oxidation of mito-roGFP1, reduction of plastid-roGFP2, and did not significantly alter the redox state of cyto-roGFP1 (Fig. 1). Silencing ISE2 induced reduction of plastid-roGFP2 and cyto-roGFP1 but did not affect the oxidation state of mito-roGFP1 (Fig. 1). These results indicate that the net increases in ROS accumulation seen in ise1 mutants and ISE1-silenced plants, which had been observed using techniques that cannot resolve the subcellular location of ROS accumulation (Stonebloom et al., 2009), are actually due to increases in ROS production specifically within the mitochondria. Unexpectedly, silencing either ISE1 or ISE2 induces reductive shifts in plastids, although ISE1 localizes exclusively to mitochondria. The data together reveal that oxidative shifts in mitochondria and reductive shifts in plastids lead to increased cell-to-cell transport. The data further suggest interorganelle signaling between mitochondria and plastids in the absence of ISE1 function.
Figure 1.
VIGS of ISE1 and ISE2 in N. benthamiana alters organelle redox state. The proportion of oxidized mito-roGFP1 (A), plastid roGFP2 (B), or cyto-roGFP1 (C) following tobacco rattle VIGS of ISE1, ISE2, or a control construct. *P < 0.001.
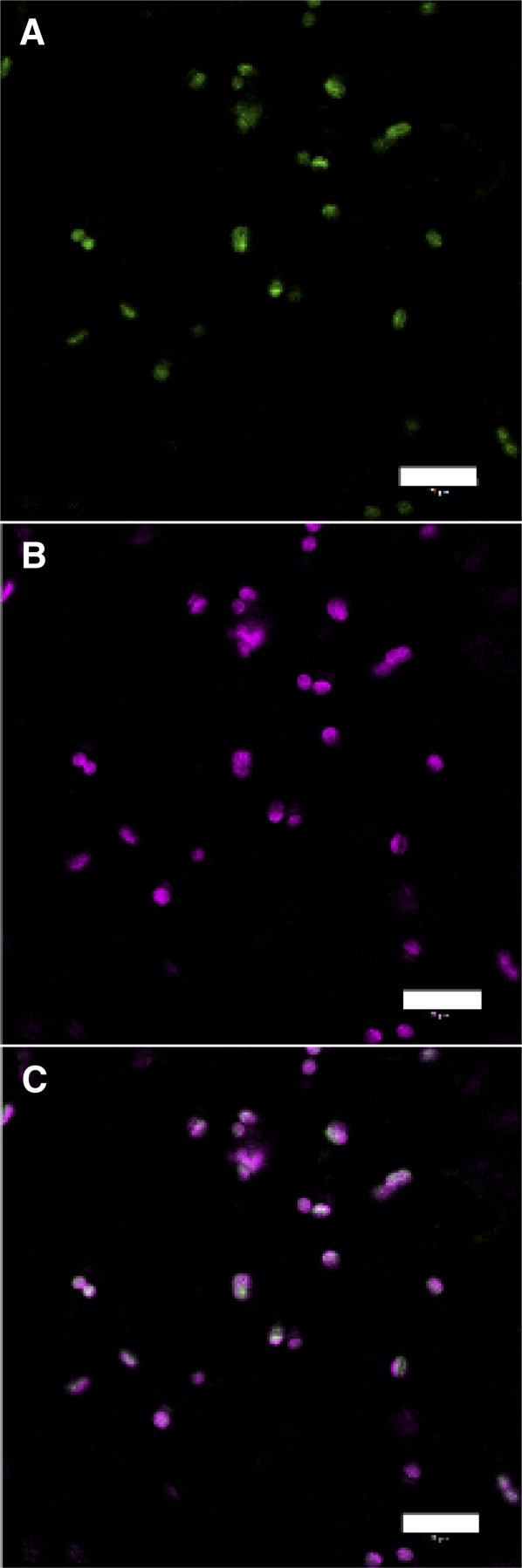
The localization of mito-roGFP1 and cyto-roGFP1 has been documented previously (Jiang et al., 2006). Figure 2 documents the plastid-specific localization of the plastid-roGFP2 constructed herein.
Figure 2.
Plastid-roGFP2 localizes to plastids. Chloroplast autofluorescence, shown in green (A), and plastid-roGFP2 fluorescence, shown in magenta (B), colocalize, shown in white in the merged image (C). Scale bars, 20 μm.
Effects of Metabolic Inhibitor Treatment on Intercellular Transport via PD
To determine whether changes in individual organelle redox state are capable of regulating PD function, we selected metabolic inhibitors predicted to specifically alter the redox state of chloroplasts or mitochondria and then determined their impact on PD-mediated intercellular transport. Paraquat (also called methyl viologen dichloride) is a herbicide that primarily affects plants by acting as a terminal oxidant for PSI and by direct oxidation of plastid reductants, including NADP+ and ferredoxin (Farrington et al., 1973; Babbs et al., 1989; Foyer and Noctor, 2009). Reduced paraquat interacts with molecular oxygen to yield superoxide, and then paraquat serves as an oxidant again, creating a redox cycle that rapidly increases ROS levels and oxidizes the plastids. Salicylhydroxamic acid inhibits the mitochondrial alternative oxidase, an enzyme that allows electrons to bypass the mitochondrial electron transport chain by transferring electrons from ubiquinol to oxygen, thus limiting the translocation of protons and decreasing the efficiency of ATP generation (Schwarzländer et al., 2009). The alternative oxidase pathway is induced in response to cold and other stresses and serves to stabilize cellular redox state (Armstrong et al., 2008). Inhibition of the alternative oxidase pathway leads to production of ROS, specifically in the mitochondria (Maxwell et al., 1999).
To evaluate the effects of metabolic inhibitors on PD transport, we developed a particle bombardment-based, cell-to-cell movement assay for Arabidopsis leaves following metabolic inhibitor treatment. Expanded true leaves of 11-d-old Arabidopsis seedlings were removed and placed on agar media with varied concentrations of paraquat or salicylhydroxamic acid. We initially applied relatively high concentrations of metabolic inhibitors (2 mm salicylhydroxamic acid and 100 μm paraquat) comparable to the amounts used by other investigators studying oxidative stress (Schwarzländer et al., 2009). These high concentrations induced severe damage to leaf tissues within 24 h of application. We therefore serially reduced the concentrations of the compounds to find optimal levels that did not affect leaf viability after 24 h of treatment but still likely affected the cellular redox state. One micromolar paraquat and 200 μm salicylhydroxamic acid treatments were used for the first set of experiments.
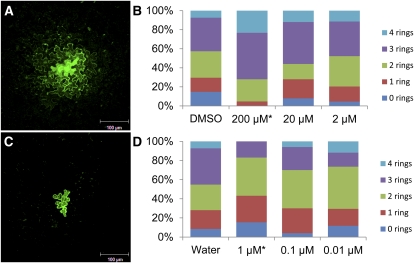
Our assay for the effects of metabolic inhibitors on cell-to-cell transport via PD involves four steps. First, true leaves from 11-d-old Arabidopsis plants were isolated and placed on growth medium plates. Second, low-pressure particle bombardment was used to introduce plasmid DNA containing a 35S::sGFP expression cassette into isolated epidermal cells. To ensure efficient and random transfection of DNA, the abaxial epidermis was bombarded twice in rapid succession. Third, leaves were transferred to one-half-strength Murashige and Skoog plates supplemented with 1% Suc and metabolic inhibitors or a comparable volume of the relevant solvent. Treated leaves were incubated for 24 h under constant light. Fourth, GFP-expressing foci were imaged using confocal laser scanning microscopy. Images were captured as stacks of confocal sections through the epidermal layer in the region of each GFP-expressing cell. Examples of reconstructed projections of stacked images for typical GFP foci after treatment with each metabolic inhibitor are presented in Figure 3. Control leaves (water treatment for paraquat experiments, dimethyl sulfoxide [DMSO] treatment for salicylhydroxamic acid experiments) show visible movement of GFP away from the initial transfected cell. Leaves treated with salicylhydroxamic acid show significantly more intercellular transport of GFP than control leaves (Fig. 3A), whereas leaves treated with paraquat show significantly less intercellular transport of GFP than untreated leaves (Fig. 3C).
Figure 3.
Effects of salicylhydroxamic acid and paraquat on cell-to-cell movement of GFP. GFP often moves to at least three rings of cells in leaves treated with 200 μm salicylhydroxamic acid (A) or is restricted to one or fewer rings in leaves treated with 1 μm paraquat (C), as shown in these representative images from the intercellular movement assay. Intercellular movement was determined by size of fluorescent foci, measured as numbers of rings of cells containing GFP in the nuclei of cells surrounding a single transfected cell 24 h after particle bombardment in leaves treated with several concentrations of salicylhydroxamic acid in DMSO (B) or paraquat in water (D). *P < 0.001.
Figure 3 and Tables I and II present a quantitative study of cell-to-cell movement in approximately 50 independent foci for each treatment, where the number of contiguous rings of cells with detectable levels of GFP away from each primary transfected cell was counted. Free GFP (27 kD) is below the size limit of the nuclear pore, so GFP movement into cells surrounding the initial transfected cell is visible by GFP fluorescence in nuclei, where GFP can accumulate. Nuclear localization is an easily scorable marker for quantifying cell-to-cell movement in the leaf epidermis, as shown previously (Stonebloom et al., 2009; Burch-Smith and Zambryski, 2010). In control leaves, GFP was confined to two or fewer rings of cells in 55% of expression foci and could travel as far as four cells away from the initial transfected cell. Treatment with 200 μm salicylhydroxamic acid treatment dramatically induced cell-to-cell movement of GFP in leaves: Only 28% of foci exhibited movement of GFP to two or fewer rings of cells, and 72% exhibited movement to three or more rings of cells (Fig. 3, A and B). In contrast, following treatment with 1 μm paraquat, GFP was restricted to two or fewer rings of cells in 83% of foci and was never observed in cells more than three rings away from the initial transfected cell (Fig. 3, C and D). Thus, 1 μm paraquat treatment markedly decreased the cell-to-cell movement of GFP. Treatment with salicylhydroxamic acid or paraquat significantly affected the measured intercellular transport in this assay (n > 43, P < 0.001).
Table I. Effects of reduced concentrations of salicylhydroxamic acid (SH) on cell-to-cell movement of GFPa.
| Treatment | Size of Foci (No. of Rings) | ||||
| 0 | 1 | 2 | 3 | 4 | |
| % | |||||
| Control (DMSO) | 14.8 | 14.8 | 27.8 | 35.2 | 7.4 |
| 200 μm SH | 0.0 | 4.7 | 23.3 | 48.8 | 23.3 |
| 20 μm SH | 8.0 | 20.0 | 16.0 | 44.0 | 12.0 |
| 2 μm SH | 4.5 | 15.9 | 31.8 | 36.4 | 11.4 |
Size of foci measured as number of rings of cells surrounding transfected cells containing GFP following treatment with several concentrations of SH.
Table II. Effects of reduced concentrations of paraquat on cell-to-cell movement of GFPa.
| Treatment | Size of Foci (No. of Rings) | ||||
| 0 | 1 | 2 | 3 | 4 | |
| % | |||||
| Control (water) | 8.5 | 19.5 | 26.8 | 37.8 | 7.3 |
| 1 μm Paraquat | 15.4 | 27.7 | 40.0 | 16.9 | 0.0 |
| 0.1 μm Paraquat | 4.0 | 26.0 | 40.0 | 24.0 | 6.0 |
| 0.01 μm Paraquat | 11.8 | 17.6 | 44.1 | 14.7 | 11.8 |
Size of foci measured as number of rings of cells surrounding transfected cells containing GFP following treatment with several concentrations of paraquat.
To determine whether a threshold exists for the oxidative shifts necessary to alter the transport abilities of leaf epidermal PD, we serially reduced the concentrations of metabolic inhibitors in our movement assay. The cell-to-cell movement assay was repeated with 20 and 2 μm salicylhydroxamic acid treatments and 0.1 and 0.01 μm paraquat treatments. Treatment with these lower concentrations of salicylhydroxamic acid did not significantly alter cell-to-cell movement of GFP (P > 0.05; Table I; Fig. 3B). In control leaves, GFP trafficked to three or more rings of cells in 42.6% of foci, and 20 or 2 μm salicylhydroxamic acid treatment similarly induced movement to three or more rings of cells in 56% or 47% of foci, respectively, whereas treatment with 200 μm salicylhydroxamic acid induced movement to three or more rings of cells in 72% of foci. Similarly, treatment of Arabidopsis leaves with concentrations of paraquat < 1 μm did not significantly inhibit cell-to-cell movement (P > 0.05), and GFP moved up to four rings of cells as in control leaves (Fig. 3D). GFP was restricted to one or fewer rings of cells in 28% (control), 30% (0.1 μm paraquat treatment), and 29.4% (0.01 μm paraquat treatment) of foci (Table II). In contrast, in leaves treated with 1 μm paraquat, GFP was restricted to one or fewer rings of cells in 43% of foci (Table II). In summary, treatment with lowered concentrations of paraquat and salicylhydroxamic acid did not notably affect cell-to-cell movement of GFP. Thus, the higher concentrations of each compound used for the results presented in Figures 3 and 4 approach the minimum concentrations necessary to induce statistically significant changes in cell-to-cell movement.
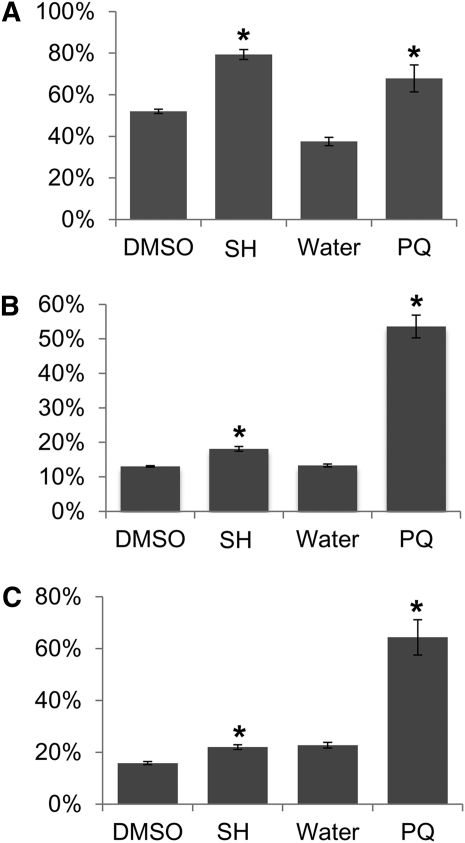
Figure 4.
Salicylhydroxamic acid (SH) and paraquat treatment affect the organelle redox state. Proportion of oxidized mito-roGFP1 (A), plastid roGFP2 (B), or cyto-roGFP1 in Arabidopsis leaves following treatment with 200 μm SH in DMSO or 1 μm paraquat (PQ) in water. *P < 0.001.
Effects of Metabolic Inhibitor Treatment on Cellular Redox State
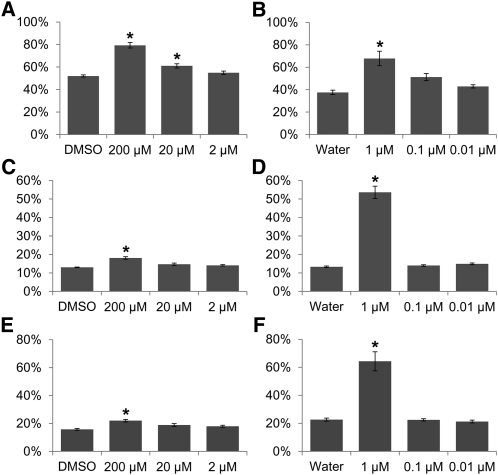
To evaluate redox state in different cellular compartments following metabolic inhibitor treatment, we measured the oxidation state of roGFP in transgenic Arabidopsis seedlings expressing cyto-roGFP1, mito-roGFP1, or plastid-roGFP2. As in the cell-to-cell movement assay, expanded true leaves were removed from 11-d-old seedlings and placed on agar media containing inhibitors. Leaves treated for 24 h with 200 μm salicylhydroxamic acid or 1 μm paraquat were imaged by ratiometric fluorescence excitation microscopy. Fluorescence intensity ratios in about 50 foci distributed over at least eight leaves were then used to calculate the proportion of oxidized roGFP (Fig. 4).
As expected, treatment with 200 μm salicylhydroxamic acid induced ROS production specifically in the mitochondria, while inducing a very minor oxidative shift in plastids and the cytoplasm. On the other hand, treatment with paraquat, although believed to have little direct impact on mitochondrial redox state in plants (Vicente et al., 2001), showed strong induction of ROS production in plastids, mitochondria, and cytoplasm. These data imply that an oxidative shift in the plastid leads to secondary effects in the cytoplasm and mitochondria, further demonstrating cross talk between the organelles, as shown above when VIGS of mitochondria-localized ISE1 led to reductive shifts in plastids (Fig. 1).
Because treatment with reduced concentrations of paraquat or salicylhydroxamic acid did not significantly affect transport via PD, we tested whether these lower concentrations have a significant effect on subcellular redox states. Leaves expressing transgenic cyto-roGFP1, mito-roGFP1, or plastid-roGFP2 were treated with the lowered concentrations of each metabolic inhibitor and examined using ratiometric fluorescence microscopy. Treatment with lowered concentrations of salicylhydroxamic acid did not significantly affect the subcellular redox states as reported by roGFP (Fig. 5, A, C, and E). As a slight exception, treatment with 20 μm salicylhydroxamic acid led to minor oxidation of mito-roGFP1, but this small change was insufficient to induce detectable changes in intercellular transport using the cell-to-cell movement assays. Treatment with 0.1 or 0.01 μm paraquat did not induce significant changes in the redox state of cyto-roGFP1, mito-roGFP1, or plastid-roGFP2 (Fig. 5, B, D, and F). We conclude that the changes in intercellular transport with 1 μm paraquat or 200 μm salicylhydroxamic acid (Fig. 3) reflect the lowest concentration capable of also inducing changes in redox state.
Figure 5.
The effects of reduced concentrations of metabolic inhibitors on organelle redox state. The proportion of oxidized mito-roGFP1 (A and B), plastid roGFP2 (C and D), or cyto-roGFP1 (E and F) after treatment with varying concentrations of salicylhydroxamic acid in DMSO (A, C, and E) or paraquat in water (B, D, and E). *P < 0.001.
DISCUSSION
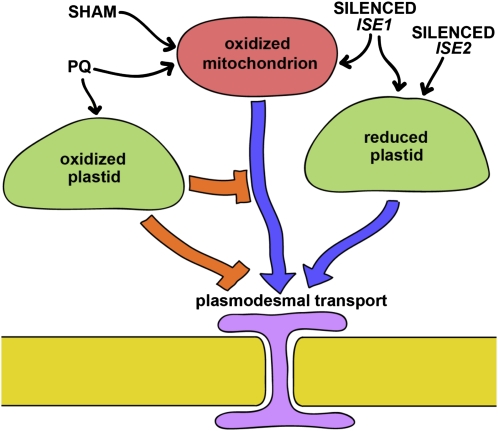
Here, we show that oxidative shifts in both chloroplasts and mitochondria of Arabidopsis leaves, induced by treatment with paraquat, result in decreased transport via PD. Oxidation of only mitochondria, induced by treatment with salicylhydroxamic acid, instead leads to increased cell-to-cell transport via PD. In accordance with these results, silencing ISE1 (which encodes a mitochondrial RNA helicase; Stonebloom et al., 2009) induces an oxidative shift in only the mitochondria and a reductive shift in plastids and causes increased intercellular transport via PD. Silencing ISE2 (which encodes a plastid RNA helicase; Burch-Smith et al., 2011a) does not affect the mitochondrial redox state but does induce a reductive shift in plastids and also causes increased transport via PD. The results imply that plastids and mitochondria differentially alter PD in response to their redox states. We therefore propose that oxidative shifts in mitochondria positively regulate PD-mediated transport, whereas oxidative shifts in plastids override this mitochondrial regulation to negatively regulate PD-mediated transport. Furthermore, based on the reductive shift in plastids observed in ISE2-silenced leaves that occurs without alteration to the mitochondrial redox state, reduction of plastids promotes intercellular transport via PD (Fig. 6).
Figure 6.
Organelle redox states differentially regulate PD. VIGS of ISE1 or ISE2 or treatment with salicylhydroxamic acid or paraquat (PQ) alter (increase, blue arrow; decrease, orange bar) PD-mediated transport and induce shifts in the redox states of plastids (green) and/or mitochondria (pink). See text for details. Purple, Endoplasmic reticulum; yellow, cell wall.
The data provide two examples of cross talk between plastids and mitochondria. First, silencing mitochondrial ISE1 reduces the redox state of plastids. Second, paraquat induces an oxidative shift in both chloroplasts and mitochondria, which negatively regulates intercellular transport via PD. Because salicylhydroxamic acid induces oxidative shifts in mitochondria, leading to increased PD transport, the paraquat-induced oxidative shift in chloroplasts must override mitochondrial oxidative shifts. These data are supported by independent microarray studies of ise1 and ise2 mutants, demonstrating that loss of either ISE1 or ISE2 function has numerous common effects in plastid-targeted gene expression (including down-regulation of ISE2 transcription) but little common impact on mitochondria-targeted gene expression (Burch-Smith et al., 2011a). Note further that plastid and mitochondrial retrograde signaling to the nucleus can both be initiated by changes in the redox state of these organelles (Rhoads, 2011).
We recently proposed a model of organelle-nucleus-PD signaling that connects plastid development, especially the induction of photosynthesis-related nuclear genes during the midtorpedo stage of embryogenesis, to PD structure, function, and biogenesis (Burch-Smith et al., 2011a). Besides plastid-targeted gene products, Burch-Smith et al. (2011a) further demonstrate that genes for products that localize to PD or to plant cell walls are also altered during mitochondrial (ise1 mutant) or plastid (ise2 mutant) dysfunction. Potentially, alterations in mitochondrial or plastid redox states also utilize organelle-nucleus-PD signaling to signal alterations in expression of genes that encode products that alter PD formation or function.
Independent studies demonstrate that a mutant for the plastid thioredoxin gat1/trx-m3 exhibits decreased intercellular transport via PD and increased ROS production, and paraquat treatment phenocopies this result (Benitez-Alfonso et al., 2009). Biochemical studies have argued that paraquat predominantly acts to oxidize chloroplasts in plant cells (Vicente et al., 2001), although paraquat does function as an oxidant in animal mitochondria by accepting electrons from complex I of the mitochondrial electron transport chain (Cochemé and Murphy, 2008). The data presented here suggest that mitochondria, chloroplasts, and cytoplasm are all profoundly oxidized following treatment with minimal concentrations of paraquat. These results further demonstrate substantial cross talk among subcellular compartments because the oxidative shift in plastids induced by paraquat leads to broad oxidation of the cell. Similarly, loss of GAT1/TRX-m3 function likely results in a local oxidative shift in plastids, which in turn leads to oxidation of other subcellular compartments.
Beyond organelle-nucleus signaling initiated by changes in organelle redox state due to aberrant ROS production within the organelles, there are likely two distinct types of pathways where ROS may function directly or as upstream signals to modify PD. First, ROS may act directly as oxidants at or near PD to alter their formation or function. For example, de novo biogenesis of secondary PD necessarily entails modification of the cell wall to allow for the membrane-bound channel to form. Several studies indicate that hydroxyl radicals can directly cause cell wall loosening or breakage through oxidation of cell wall polysaccharides and that this process occurs in vivo through controlled release of hydroxyl radicals (Cosgrove, 2005; Müller et al., 2009; Tsukagoshi et al., 2010). Cytological and proteomic studies further indicate that several class III peroxidases may localize to PD, where they could produce H2O2 to rapidly induce changes to cell wall polysaccharides around PD to dynamically regulate PD structure and function (Ehlers and van Bel, 2010; Fernandez-Calvino et al., 2011). Thus, ROS may participate directly in promoting the formation of secondary PD or in cell wall modifications near PD.
Second, ROS may act as upstream signaling molecules to facilitate intracellular communication, resulting in alterations of PD either through regulation of protein activity or transcriptional regulation of genes associated with PD. The deposition of callose (β-1,3-glucan), a cell wall polysaccharide, is positively regulated by ROS produced in response to aluminum uptake and pathogen response in a pathway mediated by Ca2+ signaling (Jones et al., 2006; Luna et al., 2011). Deposition of callose at PD occludes intercellular transport, and increased callose synthesis has been suggested to contribute to the decreased intercellular transport phenotype observed in gat1/trx-m3 mutants (Benitez-Alfonso et al., 2009). Thus, ROS may function in the cytosol to promote callose-mediated down-regulation of PD transport.
Besides site of production, a recent, elegant study measuring changes in permeability of Arabidopsis roots (Rutschow et al., 2011) supports that ROS quantity regulates PD function. After only 2 h of treatment, application of low concentrations of H2O2 (0.6 mm) to root tips increases PD permeability, but application of high concentrations of H2O2 (6 mm) to root tips nearly abolished PD permeability. The authors speculate that plant cells may interpret low concentrations of ROS as a stress that might be ameliorated by increased cellular connectivity, while high concentrations of ROS signal a more extreme state where cellular isolation is beneficial. Treatment with similarly high concentrations of H2O2 leads to rapid and virtually complete oxidation of GSH pools in the plastids, mitochondria, and cytoplasm in Arabidopsis roots and leaves (Jiang et al., 2006; Rosenwasser et al., 2010).
The connection between mitochondrial physiology and PD transport has been previously characterized in studies linking abiotic stress to intercellular transport. Roots often encounter hypoxic or anoxic conditions during flooding (Gibbs and Greenway, 2003), and anoxia in turn induces profound metabolic changes in mitochondria (Blokhina et al., 2001; Blokhina and Fagerstedt, 2010). In correlation, anaerobic or sodium azide treatments of wheat (Triticum aestivum) seedling roots induce an increase in the PD size exclusion limit (Cleland et al., 1994), and treatment of Arabidopsis seedling roots with the respiratory inhibitor cyanide m-chlorophenylhydrazone induces symplastic unloading of carboxyfluorescein from the transport phloem in the treated region (Wright and Oparka, 1997). The present study indicates that the oxidative shift associated with these mitochondrial stresses is likely the upstream signal inducing increased intercellular transport via PD. These physiological examples also indicate one possible explanation for the observed relationship between oxidized mitochondria and increased PD-mediated transport: During anoxia, greater intercellular connectivity may allow end products of carbohydrate catabolism to flow from anoxic zones to regions capable of supporting oxidative phosphorylation, thus promoting efficient use of metabolic resources (Gibbs and Greenway, 2003).
Plastids coordinate many aspects of plant physiology because they are the sites of photosynthesis and many secondary metabolic processes. Moreover, plastids are crucial sensors of both abiotic and biotic stresses and then initiate and regulate cellular stress responses (Piñas Fernández and Strand, 2008). Signaling from the plastids to other cellular compartments, especially the nucleus, is likely achieved through multiple pathways, and the plastid redox state has been established as a central upstream signal in intracellular coordination of stress responses (Foyer and Noctor, 2005; Potters et al., 2010). Previous studies have hinted that intercellular transport via PD is regulated by plastid metabolic processes (Botha et al., 2000; Provencher et al., 2001), and a recent study argues that inhibition of photosynthesis during chilling stress is related to restriction of intercellular transport via PD, further connecting plastid physiology to regulation of PD (Bilska and Sowinski, 2010). As mentioned above, microarray studies of ise1 and ise2 mutants suggest that plastid development and photosynthesis are also essential for regulating intercellular transport via PD during embryogenesis (Burch-Smith et al., 2011a).
Here, we show that oxidized or reduced plastids differentially regulate intercellular transport via PD. Plastid GSH pools remain highly reduced under typical, unstressed conditions (Law et al., 1983); thus, a strongly oxidized plastid (whether induced by paraquat, mutation, or stress) is a very unusual state. Plant cells may have evolved to reduce PD transport to prevent loss of resources to neighboring cells when coping with metabolic stress indicated by strongly oxidized plastids. Symplasmic isolation of oxidatively damaged or dying cells prevents widespread harm, and indeed occurs during programmed cell death or pathogen response (Roberts and Oparka, 2003). Intracellular accumulation of photosynthates and other metabolites may then initiate feedback loops to regulate metabolic processes, including expression of photosynthesis genes (Paul and Foyer, 2001). On the other hand, plastids with reduced redox states promote intercellular transport, allowing for increased symplasmic communication and exchange of resources when the plant is not experiencing oxidative stresses.
CONCLUSION
Silencing two genes whose products localize to mitochondria (ISE1) or plastids (ISE2) increases ROS production in mitochondria (in the case of silenced ISE1) and decreases ROS production in plastids (in both cases), and loss of either gene function leads to increased PD transport. Two compounds that differentially alter the redox state of mitochondria and plastids have opposite effects on cell-to-cell transport via PD: Salicylhydroxamic acid induces ROS production in mitochondria and leads to increased intercellular transport, whereas paraquat induces oxidative redox shifts in both plastids and mitochondria, leading to decreased intercellular transport. Thus, ROS production in plant organelles triggers intracellular communication pathways that alter intercellular communication via PD, providing strong evidence that PD function is intimately linked to the physiological state of plant cells.
MATERIALS AND METHODS
Plant Material
Arabidopsis (Arabidopsis thaliana Columbia-0) ecotype seedlings expressing roGFP1 targeted to the cytoplasm and mitochondria were described by Jiang et al. (2006), and Arabidopsis Columbia-0 ecotype seedlings expressing roGFP2 targeted to the plastids were described by Schwarzländer et al. (2008). Nicotiana benthamiana plants were grown as previously reported (Stonebloom et al., 2009).
Plant Growth Conditions
For particle bombardment and metabolic inhibitor treatments, Arabidopsis seeds were surface sterilized and grown on agar plates containing 0.8% Bacto Agar, 0.5× Murashige and Skoog medium, and 1% Suc, pH 5.6. Seedlings were grown in a Conviron plant growth chamber under long-day conditions (16 h of light, 8 h of dark) at 22°C. For movement assays and metabolic inhibitor treatment, expanded true leaves were excised from 11-d-old seedlings with a razor blade. Following bombardment and transplanting of leaves to plates containing metabolic inhibitors, leaves were placed in a plant growth room under constant light.
Cell-to-Cell Movement Assay
Microprojectile bombardment was conducted as in Crawford and Zambryski (2000), with slight modifications. A total of 5 μg of pRTL2-sGFP plasmid was precipitated onto 750 μg of 1.0-μ gold particles (Bio-Rad). Gold particles were resuspended in 100% ethanol and then dried onto rupture discs prior to bombardment. Leaves were placed on growth medium plates and covered with plastic mesh to stabilize the leaves on the plate during bombardment. Bombarded leaves were transferred to agar plates containing metabolic inhibitors or a comparable quantity of the control solvent (salicylhydroxamic acid was dissolved in DMSO, whereas paraquat was dissolved in water). Imaging of expression foci was conducted with a Zeiss 510 Meta UV-vis microscope equipped with argon ion (458/488 nm) and helium/neon (543 nm) lasers. GFP was excited with the 488-nm laser band at 49% laser power, and emitted light was collected between 505 and 530 nm. Foci were imaged with a 25× oil immersion objective. Z-stacks were collected through the epidermis in the region of each primary expression focus.
Ratiometric Measurement of roGFP
roGFP imaging and analysis of roGFP excitation ratios was conducted as in Jiang et al. (2006). Leaves were imaged on a Nikon Diaphot Nikon FN600 microscope (Nikon) fitted with Plan Fluor 10×/0.3 numerical aperture dry objectives and a Chroma Technology Phluorin filter set (exciters D410/30 and D470/20, dichroic mirror 500DCXR, emitter HQ535/50). Images were acquired with a Hamamatsu Orca-100 cooled CCD camera (Hamamatsu). Images were processed, and the relative fluorescence intensity was measured using MetaFluor 6.1 image analysis software (Molecular Devices), which controlled both filters and the data collection. The 410:474 fluorescence ratios were normalized using maximal reduced, and oxidized values were obtained after adding 100 mm H2O2 or 200 mm dithiothreitol; the ratio under maximally oxidized conditions was set equal to 1.0, and the minimum ratio measured during fully reduced conditions was set to 0. The relative fluorescence intensities in the 410- and 474-nm images were measured, and the relative proportion of roGFP in the oxidized or reduced state was calculated for leaves expressing each roGFP construct as in Jubany-Mari et al. (2010). For metabolic inhibitor treatments, fluorescence intensity measurements were made for six regions in eight leaves expressing each roGFP construct.
VIGS
VIGS was performed as in Burch-Smith and Zambryski (2010).
Transient Expression of roGFPs
roGFP1 and roGFP2 differ in that roGFP2 contains a S65T substitution in addition to the introduced cysteines at positions 147 and 204. The redox potentials at which equal amounts of roGFP1 and roGFP2 are in reduced and oxidized states (the midpoint potentials) are distinct: −288 mV (roGFP1) and −272 mV (roGFP2), respectively (Schwarzländer et al., 2008). Therefore, roGFP1 is more appropriate for measurements of the more reduced cytoplasm and mitochondria, whereas roGFP2 is better suited for measurement of changes in the redox state of more oxidizing environments such as chloroplasts. To produce plastid-targeted roGFP2 for transient expression in N. benthamiana, the sequence coding for the mitochondrial transit peptide in the mito-roGFP2 expression plasmid (Jiang et al., 2006) was replaced with that coding for the Rubisco small subunit transit peptide. The Rubisco small subunit transit peptide sequence was amplified from Arabidopsis cDNA using primers rbcsTPF_Nco1 5′-CCATGGCTTCCTCTATGCTCTC-3′ and rbcsTPR_spe1 5′-ACTAGTGGAATCGGTAAGGTCAGGAAG-3′. The mitochondrial transit peptide coding sequence was excised from a mito-roGFP2 plasmid and replaced with the Rubisco small subunit chloroplast transit peptide using Nco1 and Spe1 restriction sites. Agrobacterium tumefaciens-containing binary vectors inducing expression of cyto-roGFP1, mito-roGFP1, or plastid-roGFP2 were induced for vir gene expression and then infiltrated into N. benthamiana leaves using standard protocols (Stonebloom et al., 2009). Leaves were imaged 48 h after agroinfiltration. The plastid localization of plastid-roGFP2 was confirmed using confocal microscopy (Fig. 2).
Statistical Analyses
The Mann-Whitney U test was used to determine the significance of results in Figures 1, 3, 4, and 5 and Tables I and II.
Acknowledgments
We thank Steve Ruzin (University of California Berkeley College of Natural Resources Biological Imaging Center) and Andreas Meyer (University of Heidelberg) for sending the plastid targeted redox sensitive GFP transgenic line. K.J. acknowledges the generous support of L.F.
References
- Armstrong AF, Badger MR, Day DA, Barthet MM, Smith PM, Millar AH, Whelan J, Atkin OK. (2008) Dynamic changes in the mitochondrial electron transport chain underpinning cold acclimation of leaf respiration. Plant Cell Environ 31: 1156–1169 [DOI] [PubMed] [Google Scholar]
- Babbs CF, Pham JA, Coolbaugh RC. (1989) Lethal hydroxyl radical production in paraquat-treated plants. Plant Physiol 90: 1267–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashandy T, Guilleminot J, Vernoux T, Caparrós-Ruiz D, Ljung K, Meyer Y, Reichheld JP. (2010) Interplay between the NADP-linked thioredoxin and glutathione systems in Arabidopsis auxin signaling. Plant Cell 22: 376–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez-Alfonso Y, Cilia M, San Roman A, Thomas C, Maule A, Hearn S, Jackson D. (2009) Control of Arabidopsis meristem development by thioredoxin-dependent regulation of intercellular transport. Proc Natl Acad Sci USA 106: 3615–3620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilska A, Sowinski P. (2010) Closure of plasmodesmata in maize (Zea mays) at low temperature: a new mechanism for inhibition of photosynthesis. Ann Bot (Lond) 106: 675–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokhina O, Fagerstedt KV. (2010) Oxidative metabolism, ROS and NO under oxygen deprivation. Plant Physiol Biochem 48: 359–373 [DOI] [PubMed] [Google Scholar]
- Blokhina OB, Chirkova TV, Fagerstedt KV. (2001) Anoxic stress leads to hydrogen peroxide formation in plant cells. J Exp Bot 52: 1179–1190 [PubMed] [Google Scholar]
- Botha CEJ, Cross RHM, van Bel AJE, Peter CI. (2000) Phloem loading in the sucrose-export-defective (SXD-1) mutant maize is limited by callose deposition at plasmodesmata in bundle sheath-vacular parenchyma interface. Protoplasma 214: 65–72 [Google Scholar]
- Burch-Smith TM, Brunkard JO, Choi YG, Zambryski PC. (2011a) Organelle-nucleus crosstalk regulates plant intercellular communication via plasmodesmata. PNAS Plus (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch-Smith TM, Stonebloom S, Xu M, Zambryski PC. (2011b) Plasmodesmata during development: re-examination of the importance of primary, secondary, and branched plasmodesmata structure versus function. Protoplasma 248: 61–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch-Smith TM, Zambryski PC. (2010) Loss of INCREASED SIZE EXCLUSION LIMIT (ISE)1 or ISE2 increases the formation of secondary plasmodesmata. Curr Biol 20: 989–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon MB, James Remington S. (2009) Redox-sensitive green fluorescent protein: probes for dynamic intracellular redox responses: a review. Methods Mol Biol 476: 50–64 [DOI] [PubMed] [Google Scholar]
- Cleland RE, Fujiwara T, Lucas WJ. (1994) Plasmodesmal-mediated cell-to-cell transport in wheat roots is modulated by anaerobic stress. Protoplasma 178: 81–85 [DOI] [PubMed] [Google Scholar]
- Cochemé HM, Murphy MP. (2008) Complex I is the major site of mitochondrial superoxide production by paraquat. J Biol Chem 283: 1786–1798 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6: 850–861 [DOI] [PubMed] [Google Scholar]
- Crawford KM, Zambryski PC. (2000) Subcellular localization determines the availability of non-targeted proteins to plasmodesmatal transport. Curr Biol 10: 1032–1040 [DOI] [PubMed] [Google Scholar]
- Ehlers K, van Bel AJE. (2010) Dynamics of plasmodesmal connectivity in successive interfaces of the cambial zone. Planta 231: 371–385 [DOI] [PubMed] [Google Scholar]
- Farrington JA, Ebert M, Land EJ, Fletcher K. (1973) Bipyridylium quaternary salts and related compounds. V. Pulse radiolysis studies of the reaction of paraquat radical with oxygen: implications for the mode of action of bipyridyl herbicides. Biochim Biophys Acta 314: 372–381 [DOI] [PubMed] [Google Scholar]
- Fernandez-Calvino L, Faulkner C, Walshaw J, Saalbach G, Bayer E, Benitez-Alfonso Y, Maule A. (2011) Arabidopsis plasmodesmal proteome. PLoS ONE 6: e18880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. (2005) Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ 28: 1056–1071 [Google Scholar]
- Foyer CH, Noctor G. (2009) Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid Redox Signal 11: 861–905 [DOI] [PubMed] [Google Scholar]
- Gibbs J, Greenway H. (2003) Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct Plant Biol 30: 1–47 [DOI] [PubMed] [Google Scholar]
- Ichikawa T, Nakazawa M, Kawashima M, Iizumi H, Kuroda H, Kondou Y, Tsuhara Y, Suzuki K, Ishikawa A, Seki M, et al. (2006) The FOX hunting system: an alternative gain-of-function gene hunting technique. Plant J 48: 974–985 [DOI] [PubMed] [Google Scholar]
- Jiang K, Schwarzer C, Lally E, Zhang S, Ruzin S, Machen T, Remington SJ, Feldman L. (2006) Expression and characterization of a redox-sensing green fluorescent protein (reduction-oxidation-sensitive green fluorescent protein) in Arabidopsis. Plant Physiol 141: 397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL, Blancaflor EB, Kochian LV, Gilroy S. (2006) Spatial coordination of aluminium uptake, production of reactive oxygen species, callose production and wall rigidification in maize roots. Plant Cell Environ 29: 1309–1318 [DOI] [PubMed] [Google Scholar]
- Jubany-Mari T, Alegre-Batlle L, Jiang K, Feldman LJ. (2010) Use of a redox-sensing GFP (c-roGFP1) for real-time monitoring of cytosol redox status in Arabidopsis thaliana water-stressed plants. FEBS Lett 584: 889–897 [DOI] [PubMed] [Google Scholar]
- Law MY, Charles SA, Halliwell B. (1983) Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts: the effect of hydrogen peroxide and of paraquat. Biochem J 210: 899–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Schwarzländer M, Obata T, Sirikantaramas S, Burow M, Olsen CE, Tohge T, Fricker MD, Møller BL, Fernie AR, et al. (2009) The metabolic response of Arabidopsis roots to oxidative stress is distinct from that of heterotrophic cells in culture and highlights a complex relationship between the levels of transcripts, metabolites, and flux. Mol Plant 2: 390–406 [DOI] [PubMed] [Google Scholar]
- Luna E, Pastor V, Robert J, Flors V, Mauch-Mani B, Ton J. (2011) Callose deposition: a multifaceted plant defense response. Mol Plant Microbe Interact 24: 183–193 [DOI] [PubMed] [Google Scholar]
- Maxwell DP, Wang Y, McIntosh L. (1999) The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA 96: 8271–8276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K, Linkies A, Vreeburg RAM, Fry SC, Krieger-Liszkay A, Leubner-Metzger G. (2009) In vivo cell wall loosening by hydroxyl radicals during cress seed germination and elongation growth. Plant Physiol 150: 1855–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olinares PD, Ponnala L, van Wijk KJ. (2010) Megadalton complexes in the chloroplast stroma of Arabidopsis thaliana characterized by size exclusion chromatography, mass spectrometry, and hierarchical clustering. Mol Cell Proteomics 9: 1594–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MJ, Foyer CH. (2001) Sink regulation of photosynthesis. J Exp Bot 52: 1383–1400 [DOI] [PubMed] [Google Scholar]
- Piñas Fernández AP, Strand Å. (2008) Retrograde signaling and plant stress: plastid signals initiate cellular stress responses. Curr Opin Plant Biol 11: 509–513 [DOI] [PubMed] [Google Scholar]
- Potters G, Horemans N, Jansen MA. (2010) The cellular redox state in plant stress biology: charging concept. Plant Physiol Biochem 48: 292–300 [DOI] [PubMed] [Google Scholar]
- Provencher LM, Miao L, Sinha N, Lucas WJ. (2001) Sucrose export defective1 encodes a novel protein implicated in chloroplast-to-nucleus signaling. Plant Cell 13: 1127–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads DM. (2011) Plant mitochondrial retrograde regulation. Kempken F, , Plant Mitochondria, Vol 1. Springer, New York, pp 411–437 [Google Scholar]
- Roberts AG, Oparka KJ. (2003) Plasmodesmata and the control of symplastic transport. Plant Cell Environ 26: 103–124 [Google Scholar]
- Rosenwasser S, Rot I, Meyer AJ, Feldman L, Jiang K, Friedman H. (2010) A fluorometer-based method for monitoring oxidation of redox-sensitive GFP (roGFP) during development and extended dark stress. Physiol Plant 138: 493–502 [DOI] [PubMed] [Google Scholar]
- Rosenwasser S, Rot I, Sollner E, Meyer AJ, Smith Y, Leviatan N, Fluhr R, Friedman H. (2011) Organelles contribute differentially to reactive oxygen species-related events during extended darkness. Plant Physiol 156: 185–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutschow HL, Baskin TI, Kramer EM. (2011) Regulation of solute flux through plasmodesmata in the root meristem. Plant Physiol 155: 1817–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzländer M, Fricker MD, Müller C, Marty L, Brach T, Novak J, Sweetlove LJ, Hell R, Meyer AJ. (2008) Confocal imaging of glutathione redox potential in living plant cells. J Microsc 231: 299–316 [DOI] [PubMed] [Google Scholar]
- Schwarzländer M, Fricker MD, Sweetlove LJ. (2009) Monitoring the in vivo redox state of plant mitochondria: effect of respiratory inhibitors, abiotic stress and assessment of recovery from oxidative challenge. Biochim Biophys Acta 1787: 468–475 [DOI] [PubMed] [Google Scholar]
- Stonebloom S, Burch-Smith T, Kim I, Meinke D, Mindrinos M, Zambryski P. (2009) Loss of the plant DEAD-box protein ISE1 leads to defective mitochondria and increased cell-to-cell transport via plasmodesmata. Proc Natl Acad Sci USA 106: 17229–17234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukagoshi H, Busch W, Benfey PN. (2010) Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 143: 606–616 [DOI] [PubMed] [Google Scholar]
- Vicente JA, Peixoto F, Lopes ML, Madeira VM. (2001) Differential sensitivities of plant and animal mitochondria to the herbicide paraquat. J Biochem Mol Toxicol 15: 322–330 [DOI] [PubMed] [Google Scholar]
- Wright KM, Oparka KJ. (1997) Metabolic inhibitors induce symplastic movement of solutes from the transport phloem of Arabidopsis roots. J Exp Bot 48: 1807–1814 [Google Scholar]