Abstract
A simple, sensitive, and reliable analytical method is developed for the rapid determination of fumonisin B1 and fumonisin B2 in corn by high-performance liquid chromatography–positive electrospray ionization tandem mass spectrometry (LC–ESI-MS–MS). Fumonisin B1 and fumonisin B2 are extracted from corn with methanol–water (3:1, v/v) by means of ultrasonic extraction, and directly injected into an LC–MS–MS system after centrifugation. Fumonisin B1 and fumonisin B2 are separated on a Zorbax Eclipse XDB-C18 column with a solution of methanol–water–formic acid as the mobile phase. The method is validated with respect to linearity, accuracy, precision, specificity, and stability. Moreover, the method was applied to real samples and demonstrated to be suitable for the determination of fumonisin B1 and fumonisin B2 in corn. The total time required for the analysis of one sample was ∼30 min.
Introduction
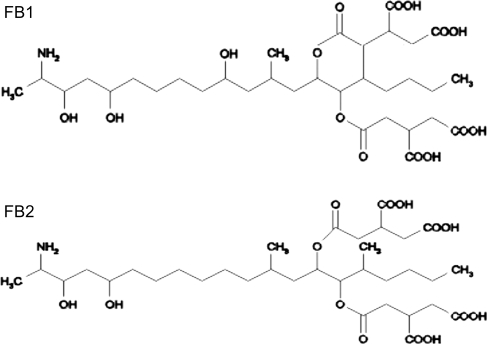
Fumonisins are a group of mycotoxins produced principally by Fusarium moniliforme, which is a worldwide contaminant of corn and corn products consumed by humans and animals (1–3). There are many naturally produced forms of fumonisins, of which B-series fumonisins are the most abundant analogs (4). Among B-series fumonisins, fumonisin B1 (FB1) and fumonisin B2 (FB2) appear to be biologically significant (5). The chemical structure of FB1 is 2S-amino-12S,16R-dimethyl-3S,5R,10R,14S, 15R-pentahydroxyeicosane with the C-14 and C-15 hydroxy groups esterified by a terminal carboxyl group of propane-1,2,3-tricarboxylic acid; FB2 is 10-deoxy FB1 (Figure 1). Fumonisins are animal carcinogens, and long-term exposure to fumonisins induces liver and kidney tumors in rats (6–7). Human consumption of fumonisin-contaminated corn has been associated with increased risk for esophageal cancer in humans (8–10). The International Agency for Research on Cancer (IARC) classified FB1 as a probable human carcinogen (class 2B) (11). To protect human health, the European Union (EU) has set a maximum level of 1000 µg/kg for the sum of FB1 and FB2 in corn and corn-based foods intended for direct human consumption (12). Therefore, rapid, specific, and sensitive methods for the identification and quantification of FB1 and FB2 in corn are required.
Figure 1.
Chemical structures of FB1 and FB2.
To detect FB1 and FB2 in food and feed samples, many liquid chromatography methods with fluorescence detection (LC–FD) (13–31) and liquid chromatography–mass spectrometry (LC–MS and LC–MS–MS) (23, 32–41) have been developed. However, all of the LC–FD methods require a derivatization step, and false results occur frequently because of retention time shifts or interfering peaks. LC–MS–MS makes a shorter pre-treatment sample preparation possible due to its higher selectivity and sensitivity for the analysis of fumonisins than the LC–MS and LC–FD methods. Recently, several rapid LC–MS–MS methods for the analysis of fumonisins in biological samples have been developed without a solid-phase extraction (SPE) procedure (32, 40, 42). Among them, the method developed by D'Arco et al. was the fastest; the total time for the analysis of one sample is ∼ 45 min, including 12 min for the sample extraction by means of pressurized liquid extraction (32) and 8 min for the LC–MS–MS analysis. However, an expensive accelerated solvent extraction (ASE) system must be used in the method. Moreover, recoveries only ranged from 68% to 83% at fortification levels of 200 µg/kg because the extract must be concentrated to dryness by a complicated concentration procedure. Ultrasonic extraction (USE) is a fast and economic method for extraction. Until recently, there have not been methods for extracting fumonisins in biological samples using USE, with only a 4 min LC–MS–MS analysis. At the same time, a method without concentration procedure will be beneficial for improved recovery.
The purpose of this study was to develop a simple, rapid, and sensitive method for the simultaneous determination of FB1 and FB2 in corn by LC–MS–MS. The pretreatment procedure is very simple without purification, and the total time for the analysis of one sample is ∼ 30 min.
Experimental
Materials and reagents
Acetonitrile, methanol, and formic acid were of LC grade. FB1 and FB2 were purchased from Sigma (St. Louis, MO). Water was purified with a Milli-Q reverse osmosis system (Millipore, Milford, MA).
Standard solutions
The individual stock solutions of FB1 and FB2 (100 µg/mL) were prepared in acetonitrile–water (50:50, v/v) and stored at 4°C. Three working standard solutions of FB1 and FB2 (10, 20 and 30 µg/mL) and one working standard solution (0.226 µg/mL for FB1 and 0.174 µg/mL for FB2) were prepared by diluting and mixing each stock standard solution with methanol–water (3:1, v/v). Mixed matrix-matched standard solutions (0–400 µg/L) were prepared by diluting and mixing each stock solution with the extract obtained from a blank corn sample.
Chromatographic conditions
A Waters 2695 LC instrument was used in this study. Separation was carried out on a Zorbax Eclipse XDB-C18 column (150 mm × 2.1 mm, 3.5 µm) maintained at 30°C. The LC mobile phase consisted of methanol–water–formic acid (75:25:0.2, v/v/v). The flow rate was 0.2 mL/min. The injection volume was 10 µL, and the runtime was 4 min.
Mass spectrometry conditions
Detection was carried out by a Waters Quattro MicroTM API triple-quadrupole MS fitted with an electrospray ionization (ESI) probe and operated in the positive ion mode. The following parameters were optimal: capillary voltage, 3500 V; ion source temperature, 120°C; desolvation gas temperature, 350°C; and desolvation gas flow rate, 600 L/h. Detection was carried out in multiple reaction monitoring (MRM) mode. Argon was used as the collision gas, and the collision cell pressure was 3.8 mBar. Other parameters are shown in Table I.
Table I.
LC–ESI–MS–MS Parameters for FB1 and FB2
| Parent Ion | Daughter | Dwell Time | Collision Energy | Cone Voltage | |
|---|---|---|---|---|---|
| Analyte | (m/z) | Ion (m/z) | (s) | (eV) | (V) |
| FB1 | 722.4 | 352.4*-[M + H-2TCA-H2O] + | 0.5 | 38 | 50 |
| 334.4-[M + H-2TCA-2H2O] + | |||||
| FB2 | 706.4 | 336.4*-[M + H-2TCA-H2O] + | 0.5 | 34 | 50 |
| 318.4-[M + H-2TCA-2H2O] + |
*Ion for quantification, TCA was tricarboxylic acid.
Sample preparation
The fresh corn samples were bought from three local supermarkets. After being dried and grounded to a fine meal in a laboratory mill, the blank corn (5 g) was fortified with FB1 and FB2 by adding 0.25 mL fortifying standard solution in polypropylene tube; it was then vortex-mixed for 15 s and allowed to stand at room temperature for 30 min. The fortified samples contained FB1 and FB2 at a limit of quantitation of (LOQ, 11.7 µg/kg for FB1 and 8.3 µg/kg for FB2) 500, 1000, and 1500 µg/kg.
Ultrasonic extraction
Extraction was performed in an ultrasonic clean bath (300 × 150 × 150 mm, Kunshan Ultrasonic Instrument Co. Ltd., Jiangshu, China). Methanol–water (25 mL, 3:1, v/v) was added to 5 g of the fortified corn in polypropylene tube. After the tube was placed in the clean bath, the extraction process was conducted for 10 min at room temperature, and the output powder was set at 120 W. After ultrasonication, the tube was centrifuged at 5000 rpm for 5 min, followed by filtration with a 0.22-µm filter prior to injection into the LC system.
Confirmation
For confirmation of FB in corn, the following three criteria had to be met: (i) the retention time should be within ± 2.5 % of the external standard solution; (ii) the signal-to-noise ratio (S/N) for each diagnostic ion should be ≥ 3:1; and (iii) the relative abundance of the two reaction product ions of the samples should be within an acceptable range relative to the average external standards according to the Commission Decision 2002/657/EC for LC–MS methods (43).
Method Validation
An “in house” validation was applied in accordance with an internal procedure as well as the Commission Decision 2002/657/EC (43).
Specificity
To verify the absence of interfering substances around the retention time of analytes, 20 blank corn samples were analyzed.
Linearity
Calibration curves were constructed using matrix-matched standard solutions by plotting the peak area of the quantitative ion of each analyte versus concentrations of 0, 2, 5, 20, 50, 100, and 400 µg/L.
Limit of detection and quantitation
The limits of detection (LODs), defined as the lowest concentration that the analytical process can reliably differentiate from background levels, were estimated for those concentrations that provide a signal-to-noise ratio of 3:1. The LOQs were estimated at a signal-to-noise ratio of 10:1.
Accuracy and precision
The accuracy and precision (inter-day and intra-day) of the method were determined using a blank corn sample that was fortified at MRL level (1000 µg/kg), at half of the MRL level (500 µg/kg), at one and a half of the MRL level (1500 µg/kg), and at the LOQ (11.7 µg/kg for FB1 and 8.3 µg/kg for FB2). The intra-day precision was assessed by performing six runs of each level during a single day, and the inter-day precision was assessed by performing six runs of each level per day over three different days.
Stability
The stability was determined in two different ways: (A) in solvent (stock solutions), and (B) in a matrix (fortified corn at 1000 µg/kg).
Results and Discussion
Optimization of extraction conditions
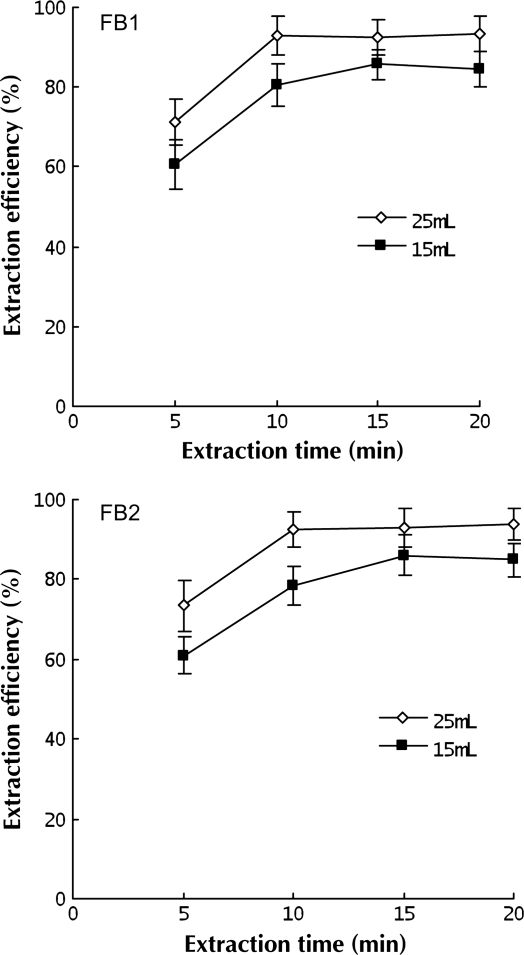
Many methods for the determination of FB1 and FB2 use methanol, acetonitrile, or mixtures of both solvents with water as an extraction solution. Among them, methanol–water (3:1 v/v) and acetonitrile–water (1:1, v/v) were used most frequently (44) because they all can provide good extraction efficiency for FB1 and FB2. In this study, the extraction efficiency was studied with methanol–water (3:1, v/v) as an extraction solution. As shown in Figure 2, extraction efficiencies of FB1 and FB2 reached nearly 71.3% and 73.4%, using 25 mL of the extraction solution in the first 5 min. When the extraction lasted for 10 min, ∼ 93.0% of FB1 and 92.6% of FB2 were extracted. A complete extraction of FB1 and FB2 was achieved at 10 min with 25 mL of the extraction solution or 15 min with 10 mL of extraction solution. Thus, the optimized ultrasonication time was 10 min, and 25 mL of the extraction solution was used.
Figure 2.
Time dependence of the extraction efficiency for FB1 and FB2 by means of ultrasonic extraction.
Instrument conditions
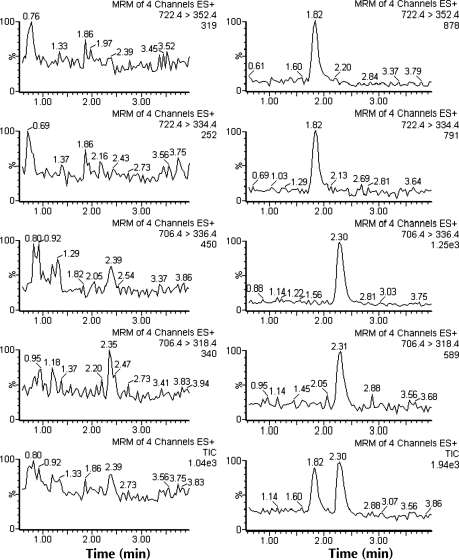
Most of methods used gradient conditions to separate fumonisins (23, 32, 34–35, 37–38, 42). Faberi et al. used an Alltima C18 column to successfully separate fumonisins under isocratic condition at a flow of 0.3 mL/min. The mobile phase was acetonitrile–water (60:40, v/v) containing 0.3% formic acid. The runtime was 15 min. A successful separation of FB1 and FB2 was also obtained using a Zorbax Eclipse XDB-C18 column under isocratic conditions with methanol–water–formic acid (75:25:0.2, v/v/v) as the mobile phase. Moreover, the runtime was only 4 min. The capacity factor (k′) was 2.4 and 3.0 for FB1 and FB2, respectively. The chromatogram obtained from corn fortified at LOQ is depicted in Figure 3, demonstrating a good chromatography separation and satisfactory sensitivity.
Figure 3.
Left: The MRM chromatograms of blank corn. Right: The MRM chromatograms of blank corn fortified with FB1 and FB2 at LOQ (11.7 µg/kg for FB1 and 8.3 µg/kg for FB2).
For the mycotoxins included in Group B of the Annex I of Council Directive 96/23/EC (45), a minimum of three identification points are required. In this experiment, four identification points, one parent (1 point) and two transitions (each 1.5 points), were monitored. Most of the previously developed LC–MS–MS methods of fumonisins are exclusively analyzed with ESI (43). The precursor ion [M + H]+ was chosen because the ion was the most abundant peak in the mass spectra when the mobile phase consisted of methanol–water–formic acid (75:25:0.2, v/v/v).
Confirmation
The retention time of each FB in the fortified samples was within ± 2.5% of the external standards. The signal-to-noise for all the daughter ions listed in Table I was ≥ 3:1. The ratios of the daughter ions of the fortified samples were always within 20% of the ratios of the concurrently analyzed standards.
Method Validation
Linearity
One significant drawback in ESI-MS quantitative analysis is matrix effect. In this study, the matrix effect was evaluated comparing the detector responses from the standard solutions of the FBs in solvent with those from different matrix extracts added of FBs at different concentration levels. From the calculated matrix effect results, it could be concluded that there are ∼1.5 times enhancement effect for both FBs, and it did not vary significantly between the individual matrix samples. So, the external calibration using matrix-matched samples was obtained by plotting the peak area of the quantitative ion pair of each analyte versus concentrations in 0–400 µg/L. The results of the linearity are reported in Table II. The correlation coefficients (r) of the calibration curves were 0.9970 and 0.9991.
Table II.
The Linear Equations and Correlation Coefficients of Matrix-Matched Standard Curves of FB1 and FB2 by LC–MS–MS
| Analyte | Linear equations | R |
|---|---|---|
| FB1 | y = 37.8x–37.5 | 0.9970 |
| FB2 | y = 84.0x–104 | 0.9991 |
Specificity
The specificity was evaluated by analyzing 20 blank corn samples. Figure 3 indicate that there were no interfering peaks from endogenous compounds at the retention times of FB1 and FB2.
Accuracy and precision
The recovery and reproducibility of the method were measured by analyzing seventy-two blank samples fortified with FB1 and FB2 at LOQ (11.7 µg/kg for FB1 and 8.3 µg/kg for FB2), 500, 1000, and 1500 µg/kg on three separate occasions. The results are shown in Table III. The mean recoveries were between 82.6% and 94.4%. The intra-day and inter-day RSDs ranged from 3.08% to 7.91% and 5.28% to 8.62%, respectively.
Table III.
Mean Recoveries (MR) and Relative Standard Deviation (RSD) of FB1 and FB2 from Corn by LC–MS–MS
| Fortified | Intra-assay | Inter-assay | ||
|---|---|---|---|---|
| conc. | Mean recovery | RSD | RSD | |
| Type | (µg/kg) | (%, n = 6) | (%) | (%) |
| FB1 | 11.7 | 82.6, 85.1, 92.0 | 6.08, 4.27, 5.77 | 8.62 |
| 500 | 88.5, 89.9, 94.4 | 4.77, 5.03, 5.91 | 8.50 | |
| 1000 | 87.7, 91.0, 92.2 | 3.77, 4.82, 4.62 | 6.69 | |
| 1500 | 92.3, 90.1, 93.6 | 4.05, 3.98, 4.74 | 7.01 | |
| FB2 | 8.3 | 84.5, 87.8, 90.3 | 5.02, 6.24, 7.91 | 8.31 |
| 500 | 89.4, 89.3, 90.7 | 5.06, 4.71, 6.22 | 5.28 | |
| 1000 | 93.2, 91.1, 90.0 | 4.07, 5.12, 4.69 | 5.93 | |
| 1500 | 89.0, 91.9, 91.5 | 6.05, 4.91, 3.08 | 6.99 |
LOD and LOQ
The LODs were 3.5 µg/kg and 2.5 µg/kg for FB1 and FB2, respectively. The LOQs were 11.7 µg/kg and 8.3 µg/kg for FB1 and FB2, respectively. The LOD of this method is better or similar to some of the previously published LC–MS and LC–MS–MS methods for analyzing FB1 and FB2 in corn or corn-based food (23, 36, 39, 41).
Stability
The stock standard solutions in acetonitrile/water were stored at 4°C for at least 6 months. The stock solutions were analyzed every month, and the instrumental responses were compared with the peak areas obtained at the moment of solution preparation (t = 0). The acceptance criterion was a response comprised between 95 and 105% of the initial one. Fortified corn samples at 1000 µg/kg were stored at –20°C and were analyzed after 7, 14, and 28 days. It was found that the recoveries of FB1 and FB2 had no significant change.
Applications of the method
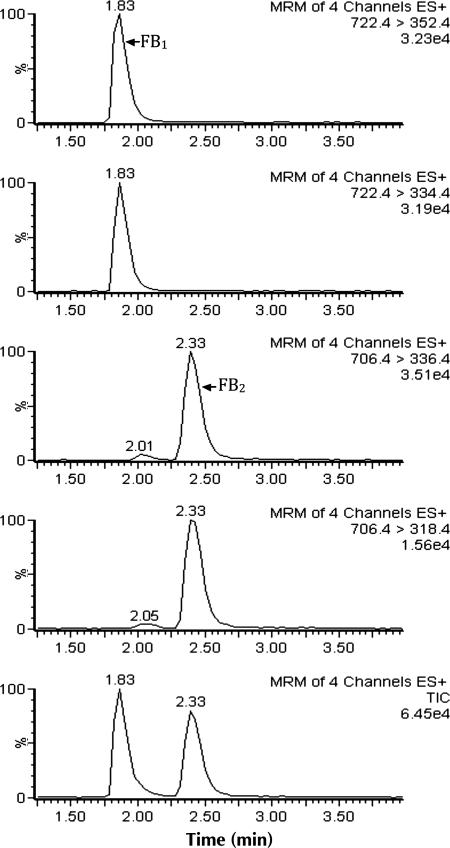
The applicability of the method for routine analysis was evaluated by analyzing 62 corn samples obtained from three local supermarkets. Positive results were simultaneously quantified and identified by the acquisition of two transitions for each compound and confirmed based on the Council Directive 96/23/EC (45). None of the samples exceeded the limits established by European Union. The highest concentration was 769 µg/kg with 475 µg kg for FB1 and 294 µg/kg for FB2, respectively. In the other three detected samples, the concentration of FB1 and FB2 were between 37.6 µg/kg (FB1 + FB2) and 133 µg/kg (FB1 + FB2). Figure 4 shows a chromatogram of a positive corn sample.
Figure 4.
LC–MS–MS chromatograms of a contaminated corn sample with 475 µg/kg for FB1 and 294 µg/kg for FB2.
Conclusion
In the present study, a rapid and sensitive method for the simultaneous determination of FB1 and FB2 in corn by LC–MS–MS with USE and without SPE cleanup procedure was developed. This method was validated with fortified corn samples and good recoveries with excellent RSDs were obtained. The LODs were found to be sufficiently low to determine the residues of FB1 and FB2 in corn.
Acknowledgements
The Breakthrough Project in Agriculture of Ningbo (No. 2010C10029) provided financial supports for this work.
References
- 1.Dutton M.F. Fumonisins, mycotoxins of increasing importance: their nature and their effects. Pharmacol. Ther. 1996;70:137–161. doi: 10.1016/0163-7258(96)00006-x. [DOI] [PubMed] [Google Scholar]
- 2.Nelson P.E., Plattner R.D., Shackelford D.D., Desjardins A.E. Production of fumonisins by Fusarium moniliforme strains from various substrates and geographic areas. Appl. Environ. Microbiol. 1991;57:2410–2412. doi: 10.1128/aem.57.8.2410-2412.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riley R.T., Richard J.L. Fumonisins: a current perspective and view to the future. Mycopathologia. 1992;117:1–124. [PubMed] [Google Scholar]
- 4.Nelson P.E., Desjardins A.E., Plattner R.D. Fumonisins, mycotoxins produced by Fusarium species: biology, chemistry, and significance. Annu. Rev. Phytopathol. 1993;31:233–252. doi: 10.1146/annurev.py.31.090193.001313. [DOI] [PubMed] [Google Scholar]
- 5.WHO. Environmental health criteria 219: Fumonisin B1. Geneva. (2000) [Google Scholar]
- 6.Gelderblom W.C., Marasas W.F., Lebepe-Mazur S., Swanevelder S. Interaction of fumonisin B(1) and aflatoxin B(1) in a short-term carcinogenesis model in rat liver. Toxicol. 2002;171:161–173. doi: 10.1016/s0300-483x(01)00573-x. [DOI] [PubMed] [Google Scholar]
- 7.Gelderblom W.C., Snyman S.D., Lebepe-Mazur S., van der Westhuizen L. The cancer-promoting potential of fumonisins B1 in rat liver using diethylnitrosamine as a cancer initiator. Cancer Lett. 1996;109:101–108. doi: 10.1016/s0304-3835(96)04431-x. [DOI] [PubMed] [Google Scholar]
- 8.Doko M.B., Visconti A. Occurrence of fumonisin-B(l) and fumonisin-B(2) in corn and corn-based human foodstuffs in Italy. Food Addit. Contam. 1994;11:433–439. doi: 10.1080/02652039409374245. [DOI] [PubMed] [Google Scholar]
- 9.Marasas W.F.O. Discovery and occurrence of the fumonisins: a historical perspective. Environ. Health Perspect. 2001;109:239–243. doi: 10.1289/ehp.01109s2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitt J.I. Toxigenic fungi: which are important? Med. Mycol. 2000;38:17–22. [PubMed] [Google Scholar]
- 11.International Agency for Research on Cancer (IARC) pp. 301–366. Monographs on the Evaluation of Carcinogenic Risks of Chemicals to Human, vol. 82, Some Traditional Herbal Medicines, Some Mycotoxins, Naphtalene and Styrene. Lyons, France: IARC Press 2002. [PMC free article] [PubMed]
- 12.European Commission. Off J Eur Union. L. 2007;255:14–17. Regulation (EC) No 1126/2007 of 28 September 2007 amending Regulation (EC) No. 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in maize and maize products. [Google Scholar]
- 13.Sharma M. Detection of hydrolyzed fumonisins B1 and B2 by use of high performance liquid chromatography in sorghum. Asian J. Chem. 2007;19:499–504. [Google Scholar]
- 14.Tardieu D., Auby A., Bluteau C., Bailly J.D., Guerre P. Determination of Fumonisin B1 in animal tissues with immunoaffinity purification. J. Chromatogr. B. 2008;870:140–144. doi: 10.1016/j.jchromb.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 15.Dasko L., Rauova D., Belajoba E., Kovac M. Determination of fumonisins B1 and B2 in beer. Czech J. Food Sci. 2005;23:20–26. [Google Scholar]
- 16.Lino C.M., Silva L.J.G., Pena A.L.S., Silveira M.I. Determination of fumonisins B1 and B2 in Portuguese maize and maize-based samples by HPLC with fluorescence detection. Anal. Bioanal. Chem. 2006;384:1214–1220. doi: 10.1007/s00216-005-0295-z. [DOI] [PubMed] [Google Scholar]
- 17.Muscarella M., Magro S.L., Nardiello D., Palermo C., Centonze D. Development of a new analytical method for the determination of fumonisins B1 and B2 in food products based on high performance liquid chromatography and fluorimetric detection with post-column derivatization. J. Chromatogr. A. 2008;1203:88–93. doi: 10.1016/j.chroma.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 18.Mateo J.J., Mateo R., Hinojo M.J., Llorens A., Jiménez M. Liquid chromatographic determination of toxigenic secondary metabolites produced by Fusarium strains. J. Chromatogr. A. 2002;955:245–256. doi: 10.1016/s0021-9673(02)00214-5. [DOI] [PubMed] [Google Scholar]
- 19.Scott P.M., Lawrence G.A. Liquid chromatographic determination of fumonisin with 4-fluoro-7-nitrobenzofurazan. J. AOAC Int. 1992;75:829–834. [Google Scholar]
- 20.Holcomb M., Jr., Thomson H.C., Hankins L.J. Analysis of fumonisin B1 in rodent feed by gradient elution HPLC using precolumn derivatization with FMOC and fluorescence detection. J. Agric. Food Chem. 1993;41:764–767. [Google Scholar]
- 21.Velázquez C., Llovera M., Plana J., Canela R. Effect of solvents on the fumonisin analysis by high-performance liquid chromatography with AccQ. Fluor as derivatizing reagent. J. Chromatogr. A. 2000;870:469–472. doi: 10.1016/s0021-9673(99)00908-5. [DOI] [PubMed] [Google Scholar]
- 22.Lombaert G.A., Pellaers P., Roscoe V., Mankotia M., Neil R., Scott P.M. Mycotoxins in infant cereal foods from the Canadian retail market. Food Addit. Contam. 2003;20:494–504. doi: 10.1080/0265203031000094645. [DOI] [PubMed] [Google Scholar]
- 23.Silva L.J.G., Lino C.M., Pena A., Moltó J.C. Occurrence of fumonisins B1 and B2 in Portuguese maize and maize-based foods intended for human consumption. Food Addit. Contam. 2007;24:381–390. doi: 10.1080/02652030601003811. [DOI] [PubMed] [Google Scholar]
- 24.Bittencourt A.B.F., Oliveira C.A.F., Dilkin P., Corrêa B. Mycotoxin occurrence in corn meal and flour traded in São Paulo, Brazil. Food Control. 2005;16:117–120. [Google Scholar]
- 25.Dilkin P., Mallmann C.A., deAlmeida C.A.A., Corrêa B. Robotic automated clean-up for detection of fumonisins B1 and B2 in corn and corn-based feed by high-performance liquid chromatography. J. Chromatogr. A. 2001;925:151–157. doi: 10.1016/s0021-9673(01)01037-8. [DOI] [PubMed] [Google Scholar]
- 26.Samapundo S., De Meulenaer B., De Muer N., Debevere J., Devlieghere F. Influence of experimental parameters on the fluorescence response and recovery of the high-performance liquid chromatography analysis of fumonisin B1. J. Chromatogr. A. 2006;1109:312–316. doi: 10.1016/j.chroma.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Sydenham E.W., Shephard G.S., Thiel P.G., Stockenström S. Liquid chromatographic determination of fumonisins B1, B2, and Bs in corn: AOAC-nJPAC collaborative study. J. AOAC Int. 1996;79:688–696. [PubMed] [Google Scholar]
- 28.Akiyama H., Miyahara M., Toyoda M., Saito Y. Liquid chromatographic determination of fumonisins B1 and B2 in corn by precolumn derivatization with 4-(N,N-Dimethylaminosulfonyl)-7-fluoro-2,1,3-benzoxadiazole (DBD-F) J. Food Hyg. Soc. 1995;36:77–81. [Google Scholar]
- 29.Kim E.K., Maragos C.M., Kendra D.F. Liquid chromatographic determination of fumonisins B1, B2, and B3 in corn silage. J. Agric. Food Chem. 2004;52:196–200. doi: 10.1021/jf034934t. [DOI] [PubMed] [Google Scholar]
- 30.Kushiro M., Zheng Y., Sagou Y., Tanaka K., Nagata T. Liquid chromatographic determination of fumonisins B1, B2, and B3 in rice. Mycotoxins. 2007;57:95–104. [Google Scholar]
- 31.Ofitserova M., Nerkar S., Pickering M., Torma L., Thiex N. Multiresidue mycotoxin analysis in corn grain by column high-performance liquid chromatography with postcolumn photochemical and chemical derivatization: single-laboratory validation. J. AOAC Int. 2009;92:15–25. [PubMed] [Google Scholar]
- 32.D'Arco G., Fernández-Franzón M., Font G., Damiani P., Mañes J. Analysis of fumonisins B1 B2 and B3 in corn-based baby food by pressurized liquid extraction and liquid chromatography/tandem mass spectrometry. J. Chromatogr. A. 2008;1209:188–194. doi: 10.1016/j.chroma.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 33.Berthiller F., Sulyok M., Krska R., Schuhmacher R. Chromatographic methods for the simultaneous determination of mycotoxins and their conjugates in cereals. Int. J. Food Microbiol. 2007;119:33–37. doi: 10.1016/j.ijfoodmicro.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 34.Senyuva H.Z., Ozcan S., Cimen D., Gilbert J. Determination of fumonisins B1 and B2 in corn by liquid chromatography/mass spectrometry with immunoaffinity column cleanup: single-laboratory method validation. J. AOAC Int. 2008;91:598–606. [PubMed] [Google Scholar]
- 35.Faberi A., Foglia P., Pastorini E., Samperi R., Laganà A. Determination of type B fumonisin mycotoxins in maize and maize-based products by liquid chromatography/tandem mass spectrometry using a QqQ linear ion trap mass spectrometer. Rapid Commun. Mass Spectrom. 2005;19:275–282. doi: 10.1002/rcm.1778. [DOI] [PubMed] [Google Scholar]
- 36.Paepens C., De Saeger S., Van Poucke C., Dumoulin F., Van Calenbergh S., Van Peteghem C. Development of a liquid chromatography/tandem massspectrometry method for the quantification of fumonisin B1, B2 and B3 in cornflakes. Rapid Commun. Mass Spectrom. 2005;19:2021–2029. doi: 10.1002/rcm.2022. [DOI] [PubMed] [Google Scholar]
- 37.Monbaliu S., Van Poucke C., Van Peteghem C., Van Poucke K., Heungens K., De Saeger S. Development of a multi-mycotoxin liquid chromatography/tandem mass spectrometry method for sweet pepper analysis. Rapid Commun. Mass Spectrom. 2009;23:3–11. doi: 10.1002/rcm.3833. [DOI] [PubMed] [Google Scholar]
- 38.Cavaliere C., Foglia P., Pastorini E., Samperi R., Laganà A. Development of a multiresidue method for analysis of major Fusarium mycotoxins in corn meal using liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2005;19:2085–2093. doi: 10.1002/rcm.2030. [DOI] [PubMed] [Google Scholar]
- 39.Senyuva H.Z., Gilbert J. Identification of fumonisin B2, HT-2 toxin, patulin, and zearalenone in dried figs by liquid chromatography-time of flight mass spectrometry and liquid chromatography mass spectrometry. J. Food Protect. 2008;71:1500–1504. doi: 10.4315/0362-028x-71.7.1500. [DOI] [PubMed] [Google Scholar]
- 40.Spanjer M.C., Rensen P.M., Scholten J.M. LC–MS–MS multi-method for mycotoxins after single extraction, with validation data for peanut, pistachio, wheat, maize, cornflakes, raisins and figs. Food Addit. Contam. 2008;25:472–489. doi: 10.1080/02652030701552964. [DOI] [PubMed] [Google Scholar]
- 41.Dohnal V., Ježková A., Polišenská K.I., Kuca K. Determination of Fumonisins in milled corn grains using HPLC-MS. J. Chromatogr. Sci. 2010;48(8):680–684. doi: 10.1093/chromsci/48.8.680. [DOI] [PubMed] [Google Scholar]
- 42.Zitomer N.C., Glenn A.E., Bacon C.W., Riley R.T. A single extraction method for the analysis by liquid chromatography/tandem mass spectrometry of fumonisins and biomarkers of disrupted sphingolipid metabolism in tissues of maize seedlings. Anal. Bioanal. Chem. 2008;391:2257–2263. doi: 10.1007/s00216-008-2166-x. [DOI] [PubMed] [Google Scholar]
- 43.European Commission. Commission Decision 2002/657/EC of 12 August 2002 implementing council directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off J. Eur. Comm. L. 2002;221:8–36. [Google Scholar]
- 44.Zöllner P., Mayer-Helm B. Trace mycotoxin analysis in complex biological and food matrices by liquid chromatography-atmospheric pressure ionisation mass spectrometry. J. Chromatogr. A. 2006;1136:123–169. doi: 10.1016/j.chroma.2006.09.055. [DOI] [PubMed] [Google Scholar]
- 45.European Commission. Council Directive 96/23/EC of 29 April 1996 on measures to monitor certain substances and residues thereof in live animals and animal products and repealing Directives 85/358/EEC and 86/469/EEC and Decision 89/187/EEC and 91/664/EEC (1996) [Google Scholar]