Abstract
RNA PROCESSING FACTOR1 (RPF1) and RPF2 are pentatricopeptide repeat (PPR) proteins involved in 5′ processing of different mitochondrial mRNAs in Arabidopsis (Arabidopsis thaliana). Both factors are highly similar to RESTORERS OF FERTILITY (RF), which are part of cytoplasmic male sterility/restoration systems in various plant species. These findings suggest a predominant role of RF-like PPR proteins in posttranscriptional 5′ processing. To further explore the functions of this group of proteins, we examined a number of T-DNA lines carrying insertions in the corresponding PPR genes. This screening identified a nearly complete absence of mature ccmC transcripts in an At1g62930 T-DNA insertion line, a phenotype that could be restored by the introduction of the intact At1g62930 gene into the mutant. The insertion in this nuclear gene, which we now call RPF3, also leads to a severe reduction of the CcmC protein in mitochondria. The analysis of C24/rpf3-1 F2 hybrids lacking functional RPF3 genes revealed that this gene has less influence on the generation of the mature ccmC 5′ transcript end derived from a distinct ccmC 5′ upstream configuration found in mitochondrial DNAs from C24 and other accessions. These data show that a particular function of an RF-like protein is required only in connection with a distinct mtDNA configuration. Our new results further substantiate the fundamental role of RF-like PPR proteins in the posttranscriptional generation of plant mitochondrial 5′ transcript termini.
Plant mitochondria contain a complex genetic framework to realize the genetic information encoded in their DNA (Kubo and Newton, 2008). These systems include many different proteins required for the posttranscriptional processing of the organellar transcripts. Among these factors, pentatricopeptide repeat (PPR) proteins play a crucial role (Delannoy et al., 2007; Schmitz-Linneweber and Small, 2008). In higher plants, PPR proteins form one of the largest protein families, comprising more than 400 members in Arabidopsis (Arabidopsis thaliana). PPR proteins, which are characterized by degenerate 35-amino acid repeats arranged in tandem, predominantly localize to chloroplasts or/and mitochondria and take part in virtually all processes affecting RNA (Small and Peeters, 2000; Lurin et al., 2004). This includes processes that link the formation of RNA extremities to RNA stability. In chloroplasts, this is achieved by specific binding of the PPR proteins to the target RNA (Loiselay et al., 2008; Pfalz et al., 2009; Johnson et al., 2010). The binding leads to the formation of so-called caps, which define the RNA ends by building barriers to 5′ or/and 3′ exonucleases. This relatively simple mechanism, which has been found for PPR10 from maize (Zea mays) and similarly for two other proteins from Chlamydomonas, might account for the formation and stabilization of the majority of chloroplast RNAs. But PPR proteins affect endonucleolytic processing reactions as well. For instance binding of PPR5 protects a distinct tRNAGly precursor transcript from endonucleolytic cleavage in maize chloroplasts (Beick et al., 2008).
In mitochondria, nuclear-encoded PPR proteins also influence processing, accumulation, and stability of RNAs. Of the investigated proteins most function as RESTORERS OF FERTILITY (RF) in various cytoplasmic male sterility (CMS)/restoration systems described in different species. The majority of RF proteins reconstitutes male fertility by altering transcript levels and/or patterns of CMS-related mitochondrial genes, which reduces expression of the corresponding CMS-associated proteins (Budar and Pelletier, 2001; Bentolila et al., 2002; Brown et al., 2003; Desloire et al., 2003; Koizuka et al., 2003; Wang et al., 2006; Schmitz-Linneweber and Small, 2008). Up to now, the exact mechanisms influencing the steady-state levels of the CMS-specific transcripts are mostly unknown. The sole exception is the Boro II CMS/restoration system in rice (Oryza sativa). Here RF1A stimulates the cleavage of the CMS-related B-atp6/orf79 dicistronic transcript probably by directing an unknown endonuclease to a specific cleavage site (Wang et al., 2006; Kazama et al., 2008). Analogous functions have been suggested for RNA PROCESSING FACTOR1 (RPF1) and 2. These PPR proteins, which are highly similar to RFs, participate in 5′ processing of the major transcripts of the genes encoding subunits 4 and 9 of NADH dehydrogenase (nad4 and nad9) and subunit 3 of cytochrome c oxidase (cox3) in mitochondria of the autogamous species Arabidopsis, where no CMS lines have been described (Jonietz et al., 2010; Hölzle et al., 2011).
Here, we report the identification of RPF3, which is required for formation of the mature mRNA of the gene encoding cytochrome c maturation protein C (ccmC) in mitochondria of the Arabidopsis accession Columbia (Col). The gene was identified by screening mutants with T-DNA insertions in RF-like PPR genes. The presence of the T-DNA in the RPF3 gene provokes a nearly complete absence of the mature ccmC transcripts, accompanied by an increase of corresponding precursor RNAs. The recovery of ccmC transcript accumulation by the introduction of the intact RPF3 gene into the mutant unambiguously demonstrates its function in 5′ processing of ccmC transcripts. In contrast to Col, RPF3 is less important for the formation of a mature ccmC 5′ transcript end, which is generated from a specific mtDNA configuration found in C24 and other accessions (Forner et al., 2008). In the rpf3-1 mutant, the extremely reduced amount of the CcmC protein, a potential component of the cytochrome c biogenesis system, has no negative influence on the activity of the mitochondrial respiratory chain or on plant fitness.
RESULTS
At1g62930 Is Required for the Accumulation of ccmC Transcripts
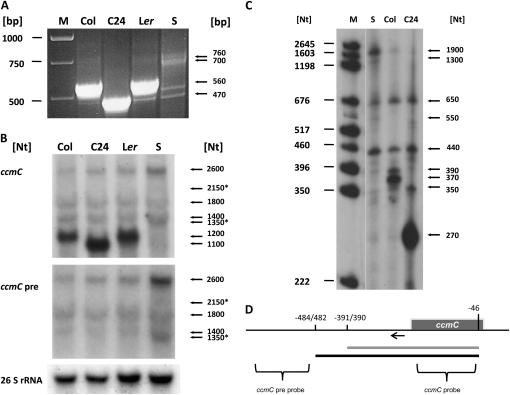
We have recently identified two genes involved in the generation of 5′ ends of nad4, nad9, and cox3 mRNAs in mitochondria of Arabidopsis (Jonietz et al., 2010; Hölzle et al., 2011). Both genes, RPF1 (At1g12700) and RPF2 (At1g62670) encode PPR proteins with high similarity to RF active in CMS-restoration systems in various plant species (Budar and Pelletier, 2001; Bentolila et al., 2002; Brown et al., 2003; Desloire et al., 2003; Koizuka et al., 2003; Wang et al., 2006). This suggests an important role of RF-like PPR proteins in 5′-end formation of mitochondrial mRNAs in the autogamous species Arabidopsis. Therefore, a circularized RNA (CR)-reverse transcription (RT)-PCR approach was used to analyze major mRNA extremities in various lines with T-DNA insertions in RF-like PPR genes (data not shown). In almost all mutants, no aberrant PCR product patterns were found when compared to wild type. This indicates that these PPR genes do not play an important role in the generation of main 5′ and 3′ ends of mitochondrial transcripts. However, in the SAIL line 18E04, we could not amplify a CR-RT-PCR product that would be consistent with the previous results obtained for the ccmC mRNAs in accessions Col, Landsburg erecta (Ler), and C24 (560 and 470 bp, respectively; Forner et al., 2008). Instead, comparatively weak products of about 700 and 760 bp were generated in the mutant (Fig. 1A). These results suggested the absence of the mature ccmC mRNA in this mutant.
Figure 1.
Analysis of ccmC transcripts. RNAs derived from the ccmC gene in accession Col, C24, and Ler and the mutant line SAIL 18E04 (S) were analyzed by CR-RT-PCR (A), northern-blot hybridization (B), and primer extension analysis (C). Lengths of size markers are given on the left-hand sides of the images. Relevant products or signals are indicated by small arrows and corresponding sizes on the right-hand sides. Asterisks indicate mRNA species predominantly detected in the mutant (the 2.15-kb mRNA is difficult to see in the image, but can be seen on the x-ray film). D, Schema of the ccmC gene (dark-gray box) and the major transcripts (gray [C24] and black [Col] bold lines). Mature 5′ (relative to the translation start codon NATG, n = −1) and 3′ ends (relative to the translation stop codon, TAAN, n = +1) are indicated. Probes used for the northern analysis are depicted in the bottom part of the schema and indicated on the left-hand side of the images in B. The position of the oligonucleotide (Atccb3Mega5′.nah) used for the primer extension experiments is indicated by an arrow. Schema not drawn to scale.
To examine the ccmC transcripts by an alternative approach, a northern-blot analysis was performed with a probe covering the ccmC reading frame (from position 208 to 607 with respect to the ATG). In Col, Ler, and C24 total RNA, ccmC transcripts of about 1,200 (Col and Ler) and 1,100 (C24) nucleotides were detected, which is consistent with previous mapping results (Fig. 1B; Forner et al., 2007, 2008). In addition, weak signals corresponding to RNAs with sizes of about 1.4, 1.8, and 2.6 kb were seen in all RNA samples. In contrast, almost no mature ccmC mRNA was detected in the SAIL 18E04 mutant. Instead, the approximately 2.6-kb RNA was found to be increased. In addition, approximately 1.35- and 2.15-kb ccmC RNAs were detected, which were not seen in the wild-type RNAs of the different accessions (Fig. 1B). The elevated accumulation of the mutant-specific larger ccmC RNAs was confirmed by a probe corresponding to regions upstream of the ccmC reading frame (−513 to −734 with respect to the ATG). This hybridization experiment identifies the 1.35-, 2.15-, and 2.6-kb transcripts as ccmC precursor RNAs. To the contrary, no increase was observed for the signals corresponding to the 1.4- and 1.8-kb RNAs.
In addition, a primer extension analysis of total mitochondrial RNA (mtRNA) isolated from cell suspension cultures established from Col, C24, and the SAIL 18E04 line was performed (Fig. 1C). In Col wild type, strong extension products of about 390 and 370 nucleotides and a relatively weak 350-nucleotide cDNA, respectively, correspond to 5′ ends at −500, −484/482, and −465, all of which correlate with mature ccmC transcripts of approximately 1,200 nucleotides. In the mutant and C24, the 390- and 370-nucleotide extension products are barely detectable, which is consistent with the almost complete absence of the mature 1,200-nucleotide ccmC transcripts in these lines (Forner et al., 2008). Only the 350-nucleotide extension product is detectable in the mutant or even slightly increased in C24. In this accession, a very strong 270-nucleotide cDNA corresponds to the −391/390 5′ termini of the mature ccmC mRNA of about 1,100 nucleotides. These ends are also present in Col and the mutant, but only in trace amounts. Apart from that extension products of 440 and 650 nucleotides are present in similar amounts in all investigated lines or are overrepresented in the mutant (550, 1,300, and 1,900 nucleotides). The 5′ end indicated by the 1,900-nucleotide product very likely correlates with the 2.6-kb mRNA, which is also overrepresented in the SAIL 18E04 line.
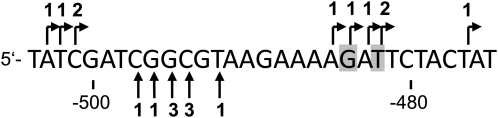
Since the primer extension experiment indicated a complex 5′ end pattern for the mature ccmC transcript in Col, we reexamined this end by sequencing of 10 clones obtained from the 560-bp CR-RT-PCR product. This revealed two clusters of ends (Fig. 2). One group of termini found in five clones surrounds the previously described ends at −484/−482, which had been determined by direct sequencing of a PCR product (Forner et al., 2007). The second cluster comprising four clones is found at positions −501 to −503. Both clusters correspond to the primer extension products of 370 and 390 bp, respectively, suggesting that the mature ccmC transcripts from Col have indeed two 5′ ends located about 20 nucleotides apart from each other. An additional 5′ end at position −476 was found represented only in single clone.
Figure 2.
RNA extremities of ccmC RNAs. The 5′ termini found in 10 clones obtained after cloning of CR-RT-PCR products of Col ccmC transcripts (shown in Fig. 1A) are given as bent arrows above the sequence. Previously mapped 5′ ends are indicated by gray boxes (Forner et al., 2007). The 3′ ends of ccmC leader RNAs determined by sequence analysis of nine individual clones derived from the 3′-RACE analysis of the ccmC 5′ leader molecules (see also Fig. 4B) are indicated by vertical arrows below the sequence.
At1g62930 Rescues the Formation of Mature ccmC mRNAs in the SAIL 18E04 Mutant
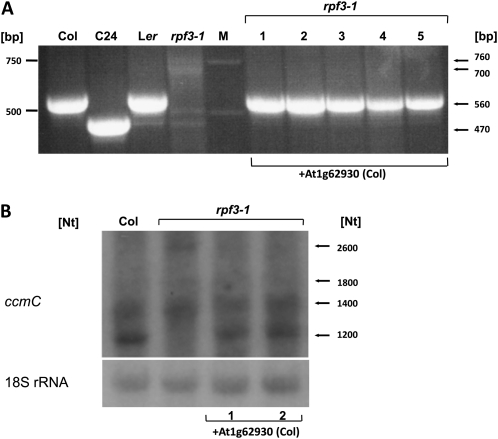
The T-DNA in the SAIL 18E04 mutant is located 31 nucleotides downstream of the predicted translation start codon. An RT-PCR analysis showed that sequences downstream of the insertion are transcribed (Supplemental Fig. S1). To test whether the absence of mature ccmC mRNAs is indeed linked to the T-DNA insertion in At1g62930, a 2.6-kb DNA fragment containing the At1g62930 gene and 0.2 kb of the 5′ and 0.5 kb of the 3′ flanking sequences from Col wild type was stably introduced into the SAIL 18E04 mutant. After selection of transformants, the presence of the transgene was confirmed by PCR with T-DNA-specific primers. The accumulation of the mature ccmC mRNAs was then examined by CR-RT-PCR and northern-blot analyses (Fig. 3).
Figure 3.
Complementation of rpf3-1 with At1g62930 rescues the formation of mature ccmC mRNA. A, CR-RT-PCR analysis of five rpf3-1 plants containing the wild-type allele of At1g62930. Five hundred and sixty base pair products were amplified from total RNA from all of these plants and from Col and Ler wild-type plants, while no such product was amplified from the untransformed rpf3-1 mutant. A 470-bp product was generated in C24. B, A northern analysis confirmed the generation of the 1,200-nucleotide ccmC in two complemented plants. The size of the ccmC transcripts is identical in the complemented plants and in Col wild type. No mature ccmC mRNA was detected in the untransformed mutant. Lengths of size markers are given in the left margin, relevant products are indicated in the right margin.
In five independent transformants, a CR-RT-PCR analysis showed that the presence of At1g62930 rescues the formation of the mature ccmC mRNA. Using total cDNA as template, 560-bp PCR products were amplified in the transformed mutant as in Col and Ler wild-type plants. No such product is seen in the untransformed mutant (Fig. 3A). Additionally, a northern-blot analysis of two complemented plants confirmed that mature ccmC mRNAs with sizes identical to wild type accumulate only in the presence of At1g62930, while no such transcript was seen in the untransformed mutant (1,200 nucleotides; Fig. 3B). These results unambiguously demonstrate that an intact At1g62930 gene is required for the formation of the mature ccmC mRNA in Arabidopsis accession Col. We designate this gene RPF3 and the corresponding mutant rpf3-1.
RPF3 Is a Factor Involved in the Posttranscriptional Generation of ccmC Transcripts
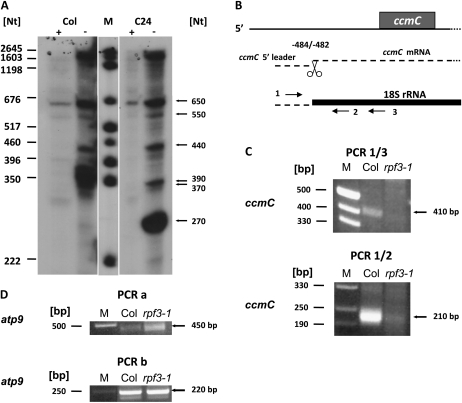
The failure to generate mature ccmC mRNAs in the rpf3-1 mutant can be attributed to a defect in transcription initiation or to a disturbance of posttranscriptional processing. To discriminate between both possibilities, we investigated whether the mature ccmC mRNAs carry 5′ triphosphate or 5′ monophosphate groups by primer extension analysis of terminator exonuclease-treated mtRNA. This exonuclease specifically degrades RNA containing 5′ monophosphate groups, which are generated by posttranscriptional cleavage. Primary RNAs having 5′ triphosphate groups, which derive from transcription initiation, are not degraded by this enzyme. As shown in Figure 4A, strong extension products of approximately 270 and 370/390 nucleotides were obtained from untreated mtRNA preparations. These indicate the mature −391/390 in C24 and −484/482 and −501 to −503 5′ termini in Col. Again further signals indicated 5′ termini of precursor molecules, consistent with the results shown in Figure 1. In contrast, no signals corresponding to the mature ccmC 5′ ends have been detected with terminator exonuclease-treated mtRNA from both accessions. Only the less-abundant extension products were seen after terminator exonuclease treatment. In both accessions, the strongest signals correspond to extension products of about 650 nucleotides. Additionally, relatively weak extension products of about 1 kb and larger cDNAs up to 1.9 kb are seen after exonuclease treatment. Although the exact sizes of these signals cannot be determined in our experiment, they might well correspond to different primary 5′ ends found around positions −1,800 and −1,600 in respect to the translation initiation codon (Kühn et al., 2009). Taken together, these results strongly suggest that the −484/482 and −501 to −503 5′ termini in Col are generated posttranscriptionally, which makes a function of RPF3 in transcription initiation unlikely. Similarly, the mature 5′ ends of the ccmC transcripts in C24 are also formed posttranscriptionally.
Figure 4.
The mature ccmC 5′ ends are generated by posttranscriptional processing. A, Primer extension analysis of untreated (−) and terminator exonuclease-treated (+) mtRNA from Col and C24. B, Schema explaining the analysis of 3′ ends of potential ccmC 5′ leader molecules. The ccmC reading frame is indicated by a gray box, ccmC RNAs are given as dashed lines. The 18S rRNA, which was used as anchor molecule, is depicted as a black bold line. Primers (horizontal arrows) used for this analysis are Atccb3QPCR.3 (1), At18S-Adapter.R4 (2), and At18S-Adapter.R3 (3). C, PCR products amplified with primer pair 1/3 (top section) and 1/2 (bottom section) from Col wild type and from rpf3-1 total mtRNA. Lengths of size markers are given on the left, relevant products are indicated on the right-hand side of the images (A and C). D, Control reactions performed with primer pairs Atatp9Mega-Endo.A/At18S-Adapter.R3 (PCR a) and Atatp9-11/At18S-Adapter.R4 (PCR b) amplified atp9 leader RNA molecules as described previously (Forner et al., 2007). These reactions were performed with identical volumes of the same cDNA preparation used in the PCRs shown in C. The results of these amplifications demonstrate that in rpf3-1 the reduced amount of PCR product derived of ccmC leader molecules is not due to the use of lower amounts or insufficient quality of the cDNA.
To support the posttranscriptional origin of the major 5′ termini in Col and to obtain information whether these ends could be derived from endonucleolytic processing, total mtRNA was searched for the presence of ccmC 5′ leader RNAs that might result from a cleavage at positions −484/482 or −501 to −503. To this end, the mitochondrial 18S rRNA was used as anchor, which was linked to the 3′ end of such potential leader molecules in a bulk RNA ligation reaction. After cDNA synthesis initiated from an oligonucleotide annealing to the 18S rRNA the applied PCR strategy selected for ccmC 5′ leader-18S rRNA ligation products (Fig. 4B). After a single PCR, a product with a size of about 410 nucleotides was generated from Col mtRNA (Fig. 4C), while a much weaker product of the same size was amplified from rpf3-1 mtRNA. A half-nested approach using an alternative primer annealing to 18S rRNA amplified a strong product of approximately 210 nucleotides. Almost no cDNA product of this size was seen in the mutant. The 210-bp product was inserted into pCR 2.1-TOPO vectors and nine individual clones were sequenced. These sequences identified 5′ termini at positions −492 to −497 (Fig. 2). Taken together these results demonstrate that ccmC 5′ leader molecules exist in Arabidopsis mitochondria. The 3′ ends of these molecules are located 10 to 14 nucleotides upstream of the −484/482 and around eight nucleotides downstream of the −503 to −501 5′ ends of the mature ccmC mRNA. However, the preferential amplification of these leader molecules from wild-type mtRNA demonstrates that their accumulation depends on a functional RPF3 gene. This correlation strongly suggests a function of RPF3 in endonucleolytic cleavage.
A CcmC Protein Is Detectable in the rpf3-1 Mutant
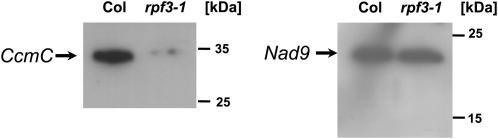
The above-described northern-blot hybridization revealed (Fig. 1) that the mature ccmC transcript is almost completely absent in the mutant, which might be at least partially compensated by increased amounts of ccmC precursor molecules. To determine the abundance of transcripts containing the coding sequence (CDS) in the mutant, real-time quantitative RT-PCR was performed. Amplification of a product spanning the ccmC CDS from positions 273 to 368 relative to the ATG (A = +1) revealed no difference in the amount of ccmC RNAs containing the reading frame between rpf3-1 and Col wild type. In contrast, an amplification reaction covering ccmC precursor molecules from −510 to −607 revealed about 6-times more ccmC precursor RNA species in the mutant, confirming a defect in ccmC 5′ processing in the rpf3-1 mutant (Supplemental Fig. S2). These data suggested that a CcmC protein might still be translated in the mutant. To test this assumption, an immunodetection analysis was performed with total mitochondrial protein from rpf3-1 and Col wild-type cell suspension cultures, respectively. Using an antiserum against the CcmC polypeptide, a strong signal corresponding to a protein with the apparent molecular mass of about 34 kD detected the CcmC protein in Col wild type (Fig. 5). To the contrary, a much weaker signal shows a substantially reduced accumulation of the CcmC polypeptide in rpf3-1 but confirmed that still minor amounts of this protein can be synthesized in this mutant. To test whether this severe reduction of the CcmC protein has any consequences on the activity of the respiratory chain, activities of complexes I, III, and IV were measured with mitochondrial protein isolated from the rpf3-1 mutant and Col wild-type cell suspension cultures. However, no differences were observed between the activities of these complexes from the different lines (Supplemental Fig. S3).
Figure 5.
The steady-state level of the CcmC polypeptide is substantially reduced in the rpf3-1 mutant. Immunodetection analyses of total mitochondrial protein isolated from cell suspension cultures from Col wild type (Col) and the rpf3-1 mutant (rpf3-1). While in both lines a Nad9 antiserum detects the target protein in similar amounts, an antiserum raised against an oligopeptide representing the core region of the CcmC protein (Kitazaki et al., 2009) revealed a substantially reduced level of CcmC polypeptide in the mutant. Sizes of marker proteins are indicated on the right-hand side of the images.
RPF3 Is a Mitochondrial Protein
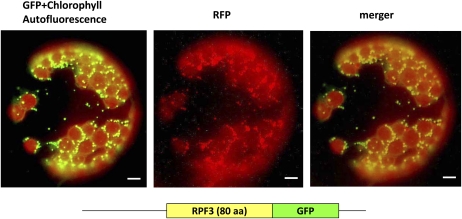
RPF3 is nuclear-encoded protein involved in a processing event that occurs in mitochondria. Accordingly this protein is expected to be imported into theses organelles. To test this assumption, a DNA fragment encoding 80 amino acids at the N terminus of RPF3 was fused in frame upstream of the gene encoding the GFP (Davis and Vierstra, 1998). After transient transformation of this construct into transgenic tobacco (Nicotiana tabacum) protoplasts, which express a isovaleryl-CoA dehydrogenase:red fluorescence fusion protein as mitochondrial marker (Forner and Binder, 2007), the subcellular sorting of the fusion proteins was inspected by fluorescence microscopy. Green fluorescence was exclusively observed in mitochondria, as demonstrated by the nearly identical pattern of the green and red fluorescence (Fig. 6). This result strongly suggests that RPF3 is also targeted to mitochondria in vivo.
Figure 6.
Subcellular targeting of RPF3. A hybrid protein consisting of the RPF3 mitochondrial targeting sequence (80 amino acids) and the GFP was transiently expressed in transgenic tobacco protoplasts. Fluorescence of GFP and chlorophyll and the red fluorescent protein from the sea anemone Entacmaea quadricolor (eqFP611) were visualized through different filter sets. eqFP611, which is C-terminally fused to the N-terminal part of the mitochondrial isovaleryl-CoA dehydrogenase had been established as mitochondrial marker in transgenic tobacco (Forner and Binder, 2007). A merger (GFP + eqFP611) of both images demonstrated the nearly perfect match of both patterns. Small differences were due to the rapid movement of mitochondria in the protoplast. Size bars correspond to 10 μm.
RPF3 Has a Minor Influence on the Generation of the Mature ccmC Transcript in C24
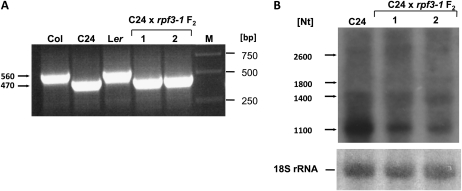
In a previous analysis of mitochondrial transcript extremities in different Arabidopsis accessions we identified a ccmC 5′-end polymorphism between accessions Col and C24 (Forner et al., 2008). The analysis of reciprocal Col/C24 F1 hybrids clearly demonstrated that this polymorphism is linked to differences in the mitochondrial DNA sequences, most likely to a small segment located upstream of both accession-specific 5′ ends (Supplemental Fig. S4). Since RPF3 is crucial for the generation of the mature 5′ end of the ccmC transcript in Col, we wanted to know whether this protein might also be required for 5′ processing of the mature ccmC transcript derived from the C24 mtDNA configuration. Thus C24 (egg cell donor) was crossed with the rpf3-1 mutant (pollen donor). Since in Arabidopsis mtDNA is maternally inherited, the offspring of this cross contains the C24 ccmC upstream configuration, which was confirmed by PCR. In the F2 generation, plants homozygous for the rpf3-1 T-DNA allele were selected by PCR and investigated for their ability to generate mature ccmC transcripts. As shown in Figure 7, CR-RT-PCR products with sizes identical to the C24 wild-type control could be amplified from two F2 plants. This result was confirmed by a northern-blot hybridization, which confirmed identical sizes of mature ccmC mRNAs in these F2 and C24 wild-type plants. But the northern analysis also revealed a reduced level of the mature ccmC transcripts in the F2 hybrids. These results demonstrate that the function of the RPF3 gene is per se not required for the generation of substantial amounts of the mature C24-type ccmC mRNA in these F2 hybrids and probably also in C24. These observations suggest that a distinct mtDNA configuration, i.e. in the ccmC upstream region, might be associated with a particular version of the RPF3 reading frame. Thus we examined the RPF3 coding region in C24. A PCR analysis of the CDS sequence generated products of identical sizes in Col and C24 (data not shown). Sequencing of the product from C24 identified 35 amino acid polymorphisms in comparison to Col (Supplemental Table S1). None of the nucleotide exchanges interrupts the coding region, suggesting that C24 retained a functional RPF3 reading frame.
Figure 7.
RPF3 is less important for the generation of the −391/390 5′ termini in C24. A, CR-RT-PCR analysis of total RNA from two F2 hybrids obtained from a cross of C24 ($ ) with the rpf3-1 mutant (# ) and from Col, C24, and Ler wild type. Products of 470 bp were generated from total RNA of both F2 plants lacking an intact RPF3 gene and from C24. Products of 560 bp were amplified from Col and Ler. B, F2 hybrids were also analyzed by northern-blot hybridization. Relevant signals are indicted in the left. The northern probe against the CDS is shown in Figure 1D. A hybridization with an oligonucleotide complementary to the cytosolic 18S rRNA showed equal loading of the RNA gel (bottom part).
Taken together these results demonstrated that in both C24 and Col RPF3 is involved in ccmC transcript processing, but that the gene is of different importance for the generation of the mature 5′ ends of ccmC mRNAs in these accessions.
DISCUSSION
RPF3 Is Required for the Generation of Mature 5′ Ends of ccmC mRNAs in Arabidopsis Accession Col
In Arabidopsis mitochondria, RPF1 and RPF2, two PPR proteins with high similarity to RF, are involved in the generation of the mature 5′ ends of nad9, cox3, and nad4 transcripts. This observation suggests that RF-like PPR proteins play a predominant role in 5′ processing. In accordance with this hypothesis, several lines of evidence now demonstrate an analogous function of the RF-like PPR protein RPF3 in the formation of mature 5′ end of ccmC mRNAs in Arabidopsis accession Col.
First, although the T-DNA insertion in the rpf3-1 mutant does not interfere with the generation of RPF3 transcripts corresponding to regions downstream of the insertion, it prevents the accumulation of the mature ccmC mRNA in the accession Col. Second, the generation of this mRNA could be restored by the introduction of the functional RPF3 gene into rpf3-1. Third, as demonstrated by real-time quantitative RT-PCR, northern-blot hybridization, and primer extension analysis, the severely impaired generation of the mature ccmC mRNA is accompanied by an increase of different 5′-extended precursor molecules strongly suggesting that RPF3 functions in posttranscriptional generation of the mature 5′ end. Fourth, this function is corroborated by the analysis of terminator exonuclease-treated RNA, which shows the investigated mature ccmC 5′ ends in Col but also the mature 5′ termini in C24 to be derived from processing rather than from transcription initiation, although the 5′-CRTA-3′ promoter motif is found at these transcript ends (Forner et al., 2007; Kühn et al., 2009). Fifth, the preferential detection of ccmC 5′ leader molecules in Col wild type indicates that the accumulation of these by products depends on an intact RPF3 gene. This observation strongly suggests a function of RPF3 in endonucleolytic cleavage, although the identified 3′ ends of these leader molecules slightly deviate from the detected 5′ ends. This pattern of ends indicates that after the endonucleolytic cuts, a few nucleotides are removed most likely by 3′ exonucleases. Alternatively, the few nucleotides might be lost by two endonucleolytic cuts in close vicinity to each other (e.g. −484/−482 mRNA 5′ ends and −492 to −497 3′ leader ends).
In summary, our data unambiguously demonstrate a function of RPF3 in the generation of the mature 5′ end of the mitochondrial ccmC transcript in the accession Col. Thus three RF-like PPR proteins are now shown to be required for the posttranscriptional generation of 5′ transcript termini corroborating the predominant role of this group of proteins for this process (Jonietz et al., 2010; Hölzle et al., 2011). In addition, RPF1 to RPF3 as well as RF1A from rice (Wang et al., 2006; Kazama et al., 2008) are involved in endonucleolytic cleavage while no evidence has been found that would support a function of these proteins as barriers to exonucleolytic degradation as it has been seen for some PPR proteins from chloroplasts (Loiselay et al., 2008; Pfalz et al., 2009; Johnson et al., 2010).
Function of RPF3 in Mitochondria of Arabidopsis Accession C24
In Col, the function of RPF3 is very important for the generation of the mature 5′ ends since almost no mature ccmC transcripts are detectable in the corresponding rpf3-1 mutant. In contrast, the analysis of C24 X rpf3-1 F2 plants without functional RPF3 indicated that this gene is per se not necessary for the generation of the −391/−390 5′ termini of ccmC RNAs derived from the mtDNA configuration present in C24 and other accessions (Fig. 7; Forner et al., 2008). In these plants still substantial amounts of mature ccmC mRNAs are detectable by northern-blot analysis. However, the level of these transcripts is reduced to about 50% (Fig. 7). This indicates that RPF3 has a function in RNA processing of ccmC transcripts originating from two different upstream configurations. While in Col the absence of an intact RPF3 almost completely prevents accumulation of mature ccmC mRNAs, in C24 RPF3 has only a moderate stimulatory effect on the processing event that results in the −391/−390 5′ termini.
The RPF3 protein from C24 shows 35 changes of amino acid identities in comparison to Col, most of them located in the N-terminal half. But all 15 repeats retain the characteristic degenerative motif, even repeat 6, where 12 amino acid exchanges were seen (Supplemental Table S1). These observations suggest that RPF3 might have a slightly different function in C24. However, we cannot exclude that the reduction of ccmC mRNA level in the F2 plants is due to other factors in the nuclear genome, which is combined and mixed up from C24 and Col. Thus further analyses are required to elucidate the exact function of the RPF3 gene in C24.
The Mature ccmC mRNA Is Almost Completely Absent in rpf3-1
In the previously analyzed rpf2-1 mutant still substantial amounts of mature nad9 and cox3 mRNA accumulate and accordingly no changes were seen in the steady-state levels of the corresponding proteins (Jonietz et al., 2010). Likewise, Ler plants lacking a functional RPF1 gene accumulate an approximately 170-nucleotide-longer precursor RNA to amounts comparable to the normal mature nad4 transcript found in Col with the intact RPF1 (Hölzle et al., 2011). In contrast to these observations, the northern analysis shows that the presence of the T-DNA insertion in RPF3 results in a nearly complete loss of the mature ccmC mRNA and accordingly only small amounts of this protein are detectable in the rpf3-1 mutant. On the one hand there might be still enough mature ccmC mRNA to be translated. On the other hand it is also possible that one or the other species of the precursor molecules can be translated. It is rather unlikely though that all of the ccmC pre-RNA species are used for protein biosynthesis. In this case more CcmC protein would be expected, since quantitative RT-PCR revealed no difference of transcripts containing the ccmC reading frame (Supplemental Fig. S2).
To generate functional c-type cytochromes, the prosthetic heme group has to be covalently attached to the corresponding apoproteins. For this biochemical process, three different systems have been described (Giegé et al., 2008; Hamel et al., 2009; Kranz et al., 2009; Sanders et al., 2010). The cytochrome c maturation (ccm) system (also called system I) is found in mitochondria of plants and red algae, in archaea, and in different subgroups of bacteria. In the latter, 12 proteins are involved in the heme delivery and attachment process, most of them being conserved in plants. In recent years, many of the plant mitochondrial Ccm proteins, either encoded in the mitochondrial or nuclear DNA, have been analyzed and partially characterized (Spielewoy et al., 2001; Meyer et al., 2005; Raczynska et al., 2006; Rayapuram et al., 2007, 2008). In Arabidopsis mitochondria, the CcmC polypeptide is translated from mRNAs that lack the predicted translation stop codon. As its counterpart in bacteria, the protein from Arabidopsis contains the WWD motif and an important His residue. In bacteria, the CcmC protein is an essential component of the cytochrome c biogenesis pathway where it is involved in the translocation of heme from the cytoplasm into the periplasm and for the ligation of heme to CcmE (Kranz et al., 2009). Likewise, plant ccmC might be involved in the delivery of heme from the mitochondrial matrix into the intermembrane space, however, experimental evidence for this potential function has not been obtained yet (Giegé et al., 2008). If mitochondrial CcmC also has a crucial function in plants, one might expect a reduction of the respiratory activity in the rpf3-1 mutant. However, the activities of complex I, III, and IV have been found to be identical in the mutant and in wild-type mitochondria (Supplemental Fig. S3). In addition, no changes were seen in the macroscopic phenotype or in the development of the mutant under normal growth conditions (C. Jonietz and S. Binder, unpublished data). Thus mitochondria of rpf3-1 seem to have retained normal activity, which can be explained by two scenarios. First, the residual amount of CcmC protein in the mutant still supports maturation of c-type cytochromes without negative consequences for the respiratory potential of mitochondria. It might be possible that levels of c-type cytochromes are reduced in the mutant (maybe not as much as the CcmC protein itself), but these lower amounts still support full function of the mitochondrial respiratory chain. Unfortunately no data are available whether plants would overcome reductions in c-type cytochromes as they do for instance with impaired complex I activity. Second, the plant mitochondrial CcmC protein has no essential function in cytochrome c biogenesis in plants. This scenario is consistent with the fact that the enzymes required for protoporphyrin IX biosynthesis localize to chloroplasts. Moreover, the mitochondrial localization of ferrochelatase, which catalyzes the insertion of iron into the protoporphyrin IX, has not yet been unambiguously demonstrated. Thus it is speculated that heme is delivered from the outside directly to the mitochondrial intermembrane space so that there is no need for a transport of heme across the inner mitochondrial membrane (Giegé et al., 2008). In this scenario, CcmC might fulfill an alternative function, which is not essential for cytochrome c biogenesis and for the function of the respiratory chain.
Presently there is no experimental evidence for these scenarios, but the rpf3-1 mutant might be a valuable system for further studies of CcmC and its role in ccm in mitochondria of higher plants.
MATERIALS AND METHODS
Plants and Plant Cultivation
Arabidopsis (Arabidopsis thaliana) plants were grown and checked for correct genotypes as described (Forner et al., 2008). Cell suspension cultures were established and cultivated as outlined previously (Köhler et al., 2010). The Arabidopsis SAIL line 18E 04 was obtained from the Nottingham Arabidopsis Stock Centre (http://arabidopsis.info/). Crosses to generate F1 and F2 hybrid plants were done following standard procedures (Weigel and Glazebrook, 2002).
Analysis of RNA
The 5′ ends of mitochondrial ccmC mRNA were determined by CR-RT-PCR analysis (Kuhn and Binder, 2002; Forner et al., 2007, 2008; Jonietz et al., 2010) using primers Atccb3Mega5‘.nah and Atccb3Mega3‘.nah. The 3′ termini of 5′ leader RNA molecules were determined by an established alternative 3′-RACE approach (Forner et al., 2007). First-strand cDNA synthesis was initiated from primer At18S-Adapter.R3 followed by the first PCR with primer pair Atccb3QPCR.3/At18S-Adapter.R3 and a second half-nested PCR with oligonucleotides Atccb3QPCR.3/At18S-Adapter.R4. PCR products were directly sequenced and cloned into pCR 2.1-TOPO (Invitrogen), respectively. ccmC 5′ ends were determined by sequence analysis of 10 clones. Total RNA and total mtRNA were isolated using RNeasy plant mini kits (Qiagen, http://www1.qiagen.com). Northern-blot hybridization, terminator exonuclease treatment, and primer extension analyses were done as described formerly (Jonietz et al., 2010). Extension was initiated at oligonucleotide Atccb3Mega5‘.nah, the probes for the northern analyses were amplified using primer pairs Atccb3-21/Atccb3-22 (CDS) and Atccb3-Mega5′-fern/Atccb3-12 (upstream probe). PCR products were labeled using a random-primed DNA labeling kit and [α32P]dCTP following the recommendations given by the manufacturer (GE Healthcare).
Real-time quantitative RT-PCR was done using transcriptor high-fidelity cDNA synthesis and light-cycler 480 SYBR green I master kits on a light-cycler 480 real-time PCR system according to instructions given by the manufacturer (Roche). Data were evaluated using the light-cycler 480 software (1.5). Relative transcript levels were measured in respect to the validated reference genes encoding ubiquitin conjugating enzyme 9 (UBC9, At4g27960) and protein phosphatase 2A subunit 3 (PP2A, At1g13320) with established primer pairs (Czechowski et al., 2005). Oligonucleotides for quantitative ccmC transcript analysis (Atccb3QPCR1–4) were selected using the primer design tool at the National Center for Biotechnology Information home page (http://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi?LINK_LOC=BlastHomeAd).
Complementation Assays
A complementation construct containing the RPF3 gene as well as 184- and 480-bp upstream and downstream regions (amplified with primers At1g62930-Kompl.H and At1g62930-Kompl.R) was established in pMDC123 using standard procedures (Sambrook and Russel, 2001; Curtis and Grossniklaus, 2003). To circumvent potential effects by PCR errors a mixture of 20 different clones was used for each construct. Plant transformation was done by floral dip (Clough and Bent, 1998). The presence of the transgene was tested with a PCR with primers KAN1 and KAN2.
Miscellaneous Methods
DNA sequencing was commercially performed (4baselab, http://www.4base-lab.de/index_4.html). Immunodetection analysis of mitochondrial protein was performed as described (Jonietz et al., 2010). Basic methods of molecular biology followed standard protocols (Sambrook and Russel, 2001). Protoplast transformation and fluorescence microscopy were done following an established protocol (Matthes et al., 2007). In silico sequence analyses were done at the National Center for Biotechnology Information server applying various blast tools (McGinnis and Madden, 2004). Oligonucleotide sequences are listed in Supplemental Table S2.
Respiratory chain activity tests were performed at room temperature in 25 mm potassium phosphate buffer pH 7.4 using standard protocols for complex I (Singer, 1974) and complexes III and IV (Birch-Machin et al., 1994).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number HE 585245.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. RT-PCR analysis of RPF3 transcripts in the SAIL 18E04 mutant.
Supplemental Figure S2. Real-time quantitative RT-PCR of ccmC transcripts in rpf3-1.
Supplemental Figure S3. Activities of respiratory chain complexes I, III, and IV in mitochondria from rpf3-1 and Col wild type.
Supplemental Figure S4. Sequences of the ccmC upstream regions in the mitochondrial DNAs from Arabidopsis accessions Col and C24.
Supplemental Table S1. Amino acid exchanges between RPF3 proteins from Col and C24.
Supplemental Table S2. Oligonucleotide sequences.
Acknowledgments
We thank Conny Guha and Uli Tengler (Ulm University) for the excellent technical assistance. We thank Geraldine Bonnard and Philippe Giegé (Institut de Biologie Moléculaire des Plantes) and Kazuyoshi Kitazaki and Tomohiko Kubo (Hokkaido University) for the kind gift of Nad9 and CcmC antisera. We also thank Hans-Peter Braun (Leibniz University) for contribution to the activity tests and Katrin and Birgit Stoll (Ulm University) for brilliant technical support.
References
- Beick S, Schmitz-Linneweber C, Williams-Carrier R, Jensen B, Barkan A. (2008) The pentatricopeptide repeat protein PPR5 stabilizes a specific tRNA precursor in maize chloroplasts. Mol Cell Biol 28: 5337–5347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentolila S, Alfonso AA, Hanson MR. (2002) A pentatricopeptide repeat-containing gene restores fertility to cytoplasmic male-sterile plants. Proc Natl Acad Sci USA 99: 10887–10892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch-Machin MA, Briggs HL, Saborido AA, Bindoff LA, Turnbull DM. (1994) An evaluation of the measurement of the activities of complexes I-IV in the respiratory chain of human skeletal muscle mitochondria. Biochem Med Metab Biol 51: 35–42 [DOI] [PubMed] [Google Scholar]
- Brown GG, Formanová N, Jin H, Wargachuk R, Dendy C, Patil P, Laforest M, Zhang J, Cheung WY, Landry BS. (2003) The radish Rfo restorer gene of Ogura cytoplasmic male sterility encodes a protein with multiple pentatricopeptide repeats. Plant J 35: 262–272 [DOI] [PubMed] [Google Scholar]
- Budar F, Pelletier G. (2001) Male sterility in plants: occurrence, determinism, significance and use. C R Acad Sci III 324: 543–550 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U. (2003) A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol 133: 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139: 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SJ, Vierstra RD. (1998) Soluble, highly fluorescent variants of green fluorescent protein (GFP) for use in higher plants. Plant Mol Biol 36: 521–528 [DOI] [PubMed] [Google Scholar]
- Delannoy E, Stanley WA, Bond CS, Small ID. (2007) Pentatricopeptide repeat (PPR) proteins as sequence-specificity factors in post-transcriptional processes in organelles. Biochem Soc Trans 35: 1643–1647 [DOI] [PubMed] [Google Scholar]
- Desloire S, Gherbi H, Laloui W, Marhadour S, Clouet V, Cattolico L, Falentin C, Giancola S, Renard M, Budar F, et al. (2003) Identification of the fertility restoration locus, Rfo, in radish, as a member of the pentatricopeptide-repeat protein family. EMBO Rep 4: 588–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forner J, Binder S. (2007) The red fluorescent protein eqFP611: application in subcellular localization studies in higher plants. BMC Plant Biol 7: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forner J, Hölzle A, Jonietz C, Thuss S, Schwarzländer M, Weber B, Meyer RC, Binder S. (2008) Mitochondrial mRNA polymorphisms in different Arabidopsis accessions. Plant Physiol 148: 1106–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forner J, Weber B, Thuss S, Wildum S, Binder S. (2007) Mapping of mitochondrial mRNA termini in Arabidopsis thaliana: t-elements contribute to 5′ and 3′ end formation. Nucleic Acids Res 35: 3676–3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giegé P, Grienenberger JM, Bonnard G. (2008) Cytochrome c biogenesis in mitochondria. Mitochondrion 8: 61–73 [DOI] [PubMed] [Google Scholar]
- Hamel P, Corvest V, Giegé P, Bonnard G. (2009) Biochemical requirements for the maturation of mitochondrial c-type cytochromes. Biochim Biophys Acta 1793: 125–138 [DOI] [PubMed] [Google Scholar]
- Hölzle A, Jonietz C, Törjek O, Altmann T, Binder S, Forner J. (2011) A RESTORER OF FERTILITY-like PPR gene is required for 5′-end processing of the nad4 mRNA in mitochondria of Arabidopsis thaliana. Plant J 65: 737–744 [DOI] [PubMed] [Google Scholar]
- Johnson X, Wostrikoff K, Finazzi G, Kuras R, Schwarz C, Bujaldon S, Nickelsen J, Stern DB, Wollman FA, Vallon O. (2010) MRL1, a conserved pentatricopeptide repeat protein, is required for stabilization of rbcL mRNA in Chlamydomonas and Arabidopsis. Plant Cell 22: 234–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonietz C, Forner J, Hölzle A, Thuss S, Binder S. (2010) RNA PROCESSING FACTOR2 is required for 5′ end processing of nad9 and cox3 mRNAs in mitochondria of Arabidopsis thaliana. Plant Cell 22: 443–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama T, Nakamura T, Watanabe M, Sugita M, Toriyama K. (2008) Suppression mechanism of mitochondrial ORF79 accumulation by Rf1 protein in BT-type cytoplasmic male sterile rice. Plant J 55: 619–628 [DOI] [PubMed] [Google Scholar]
- Kitazaki K, Nomoto Y, Aoshima A, Mikami T, Kubo T. (2009) A mitochondrial gene involved in cytochrome c maturation (ccmC) is expressed as a precursor with a long NH2-terminal extension in sugar beet. J Plant Physiol 166: 775–780 [DOI] [PubMed] [Google Scholar]
- Köhler D, Schmidt-Gattung S, Binder S. (2010) The DEAD-box protein PMH2 is required for efficient group II intron splicing in mitochondria of Arabidopsis thaliana. Plant Mol Biol 72: 459–467 [DOI] [PubMed] [Google Scholar]
- Koizuka N, Imai R, Fujimoto H, Hayakawa T, Kimura Y, Kohno-Murase J, Sakai T, Kawasaki S, Imamura J. (2003) Genetic characterization of a pentatricopeptide repeat protein gene, orf687, that restores fertility in the cytoplasmic male-sterile Kosena radish. Plant J 34: 407–415 [DOI] [PubMed] [Google Scholar]
- Kranz RG, Richard-Fogal C, Taylor JS, Frawley ER. (2009) Cytochrome c biogenesis: mechanisms for covalent modifications and trafficking of heme and for heme-iron redox control. Microbiol Mol Biol Rev 73: 510–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T, Newton KJ. (2008) Angiosperm mitochondrial genomes and mutations. Mitochondrion 8: 5–14 [DOI] [PubMed] [Google Scholar]
- Kuhn J, Binder S. (2002) RT-PCR analysis of 5′ to 3′-end-ligated mRNAs identifies the extremities of cox2 transcripts in pea mitochondria. Nucleic Acids Res 30: 439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn K, Richter U, Meyer EH, Delannoy E, de Longevialle AF, O’Toole N, Börner T, Millar AH, Small ID, Whelan J. (2009) Phage-type RNA polymerase RPOTmp performs gene-specific transcription in mitochondria of Arabidopsis thaliana. Plant Cell 21: 2762–2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loiselay C, Gumpel NJ, Girard-Bascou J, Watson AT, Purton S, Wollman FA, Choquet Y. (2008) Molecular identification and function of cis- and trans-acting determinants for petA transcript stability in Chlamydomonas reinhardtii chloroplasts. Mol Cell Biol 28: 5529–5542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurin C, Andrés C, Aubourg S, Bellaoui M, Bitton F, Bruyère C, Caboche M, Debast C, Gualberto J, Hoffmann B, et al. (2004) Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell 16: 2089–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes A, Schmidt-Gattung S, Köhler D, Forner J, Wildum S, Raabe M, Urlaub H, Binder S. (2007) Two DEAD-box proteins may be part of RNA-dependent high-molecular-mass protein complexes in Arabidopsis mitochondria. Plant Physiol 145: 1637–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis S, Madden TL. (2004) BLAST: at the core of a powerful and diverse set of sequence analysis tools. Nucleic Acids Res (Web Server issue) 32: W20–W25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EH, Giegé P, Gelhaye E, Rayapuram N, Ahuja U, Thöny-Meyer L, Grienenberger JM, Bonnard G. (2005) AtCCMH, an essential component of the c-type cytochrome maturation pathway in Arabidopsis mitochondria, interacts with apocytochrome c. Proc Natl Acad Sci USA 102: 16113–16118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfalz J, Bayraktar OA, Prikryl J, Barkan A. (2009) Site-specific binding of a PPR protein defines and stabilizes 5′ and 3′ mRNA termini in chloroplasts. EMBO J 28: 2042–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raczynska KD, Le Ret M, Rurek M, Bonnard G, Augustyniak H, Gualberto JM. (2006) Plant mitochondrial genes can be expressed from mRNAs lacking stop codons. FEBS Lett 580: 5641–5646 [DOI] [PubMed] [Google Scholar]
- Rayapuram N, Hagenmuller J, Grienenberger JM, Bonnard G, Giegé P. (2008) The three mitochondrial encoded CcmF proteins form a complex that interacts with CCMH and c-type apocytochromes in Arabidopsis. J Biol Chem 283: 25200–25208 [DOI] [PubMed] [Google Scholar]
- Rayapuram N, Hagenmuller J, Grienenberger JM, Giegé P, Bonnard G. (2007) AtCCMA interacts with AtCcmB to form a novel mitochondrial ABC transporter involved in cytochrome c maturation in Arabidopsis. J Biol Chem 282: 21015–21023 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russel DW. (2001) Molecular Cloning: A Laboratory Manual, Ed 3. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Sanders C, Turkarslan S, Lee D-W, Daldal F. (2010) Cytochrome c biogenesis: the Ccm system. Trends Microbiol 18: 266–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz-Linneweber C, Small I. (2008) Pentatricopeptide repeat proteins: a socket set for organelle gene expression. Trends Plant Sci 13: 663–670 [DOI] [PubMed] [Google Scholar]
- Singer TP. (1974) Determination of the activity of succinate, NADH, choline, and alpha-glycerophosphate dehydrogenases. Methods Biochem Anal 22: 123–175 [DOI] [PubMed] [Google Scholar]
- Small ID, Peeters N. (2000) The PPR motif—a TPR-related motif prevalent in plant organellar proteins. Trends Biochem Sci 25: 46–47 [DOI] [PubMed] [Google Scholar]
- Spielewoy N, Schulz H, Grienenberger JM, Thony-Meyer L, Bonnard G. (2001) CCME, a nuclear-encoded heme-binding protein involved in cytochrome c maturation in plant mitochondria. J Biol Chem 276: 5491–5497 [DOI] [PubMed] [Google Scholar]
- Wang Z, Zou Y, Li X, Zhang Q, Chen L, Wu H, Su D, Chen Y, Guo J, Luo D, et al. (2006) Cytoplasmic male sterility of rice with boro II cytoplasm is caused by a cytotoxic peptide and is restored by two related PPR motif genes via distinct modes of mRNA silencing. Plant Cell 18: 676–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D, Glazebrook J. (2002) Arabidopsis, a Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York [Google Scholar]