Abstract
Pathogenic and symbiotic bacteria rely on quorum sensing to coordinate the collective behavior during the interactions with their eukaryotic hosts. Many Gram-negative bacteria use N-acyl-homoserine lactones (AHLs) as signals in such communication. Here we show that plants have evolved means to perceive AHLs and that the length of acyl moiety and the functional group at the γ position specify the plant’s response. Root treatment with the N-3-oxo-tetradecanoyl-L-homoserine lactone (oxo-C14-HSL) reinforced the systemic resistance to the obligate biotrophic fungi Golovinomyces orontii in Arabidopsis (Arabidopsis thaliana) and Blumeria graminis f. sp. hordei in barley (Hordeum vulgare) plants. In addition, oxo-C14-HSL-treated Arabidopsis plants were more resistant toward the hemibiotrophic bacterial pathogen Pseudomonas syringae pv tomato DC3000. Oxo-C14-HSL promoted a stronger activation of mitogen-activated protein kinases AtMPK3 and AtMPK6 when challenged with flg22, followed by a higher expression of the defense-related transcription factors WRKY22 and WRKY29, as well as the PATHOGENESIS-RELATED1 gene. In contrast to wild-type Arabidopsis and mpk3 mutant, the mpk6 mutant is compromised in the AHL effect, suggesting that AtMPK6 is required for AHL-induced resistance. Results of this study show that AHLs commonly produced in the rhizosphere are crucial factors in plant pathology and could be an agronomic issue whose full impact has to be elucidated in future analyses.
Rhizosphere communication is based on a complex exchange and perception of molecules originating from interacting organisms. Many bacteria use N-acyl-homo-Ser lactones (AHLs) to coordinate the behavior of individual cells in a population. This communication phenomenon is called quorum sensing (QS), and was first described in Vibrio fisheri (Engebrecht and Silverman, 1984). V. fisheri secretes N-3-oxo-hexanoyl-L-homo-Ser lactone (oxo-C6-HSL), which is synthesized by the enzyme LuxI from S-adenosyl Met and an acyl chain carrier protein (Schaefer et al., 1996) and sensed by a LuxR-type receptor (Hanzelka and Greenberg, 1995). In many Gram-negative bacteria, the control of the population density relies on production of diverse AHLs that influence gene transcription once detected by a companion. Numerous AHL derivatives varying in the length of the acyl chain (from 4–18 carbons) and in the substitution at the γ position of the chain with hydroxyl (OH) or oxo (O) groups have been so far identified (for review, see Williams, 2007).
AHLs also have an impact on eukaryotic cells. N-3-oxo-dodecanoyl-L-homo-Ser lactone (oxo-C12-HSL), produced by the opportunistic plant and human pathogen Pseudomonas aeruginosa, induces distension of mitochondria and endoplasmic reticulum (Kravchenko et al., 2006). In addition, it increases phosphorylation of the mitogen-activated protein kinase (MAPK) p38 and the translation initiation factor elF2α. In human NCI-H292 cells, treatment with oxo-C12-HSL enhances the phosphorylation of the negative regulator of NF-κB, the I-κB, and the ERK1/2 MAP kinases (Imamura et al., 2004). Moreover, oxo-C12-HSL modulates the NFκB signaling and abolishes the TOLL-LIKE RECEPTOR4-dependent immune response in mice (Kravchenko et al., 2008). It was suggested that mammalian cells perceive oxo-C12-HSL in a TOLL-LIKE RECEPTOR-independent manner (Kravchenko et al., 2006). Jahoor et al. (2008) proposed the peroxisome proliferator-activated receptors PPARγ and PPARβ, members of the nuclear hormone receptor family as potential candidates for AHLs receptors in animals. On the other hand, AHLs were shown to interact directly with different cellular and artificial membranes, which suggest another possible membrane-located AHL-reception system (Davis et al., 2010).
In the plant-interacting Agrobacterium tumefaciens, N-3-oxo-octoanoyl-L-homo-Ser lactone is produced by the TraI enzyme and perceived by the LuxR-homologous receptor TraR (Fuqua and Winans, 1994). Both traI and traR genes are located on the Ti plasmid and are induced by the tumor-released octopine; TraR transcriptionally induces all genes required for conjugation and intrabacterial transfer of the Ti plasmid. N-3-oxo-octoanoyl-L-homo-Ser lactone also induces the repABC operon, obligatory for stable vegetative replication of the octopine-type Ti plasmid (Fuqua and Winans, 1994; Pappas and Winans, 2003). On the other hand, plants may interact with the Agrobacterium QS. Different plant signals induce the transcription of bacterial attM and aiiB genes coding for two lactonases, AttM and AiiB. They cleave the γ-butyrolactone ring of AHLs and might in this way modulate the QS-dependent virulence of Agrobacterium (Haudecoeur et al., 2009a, 2009b).
In recent years, several reports have shown that plants evolved means to perceive AHLs and respond to them with changes in gene expression or modifications in development (Mathesius et al., 2003; Schuhegger et al., 2006; Götz et al., 2007; Ortíz-Castro et al., 2008; von Rad et al., 2008; Pang et al., 2009). Assessment of AHLs’ impact on root development revealed that C6-HSL promotes root growth (von Rad et al., 2008), C10-HSL alters root architecture similarly to auxin, but in an auxin-independent way, and C12-HSL strongly induces root hair formation (Ortíz-Castro et al., 2008). Several reports provided indirect evidences that AHLs play a role in plant immunity. Serratia liquefaciens MG1 increases systemic resistance of tomato (Solanum lycopersicum) plants against the fungal leaf pathogen Alternaria alternata. However, the AHL negative mutant S. liquefaciens MG44 is less effective (Schuhegger et al., 2006). Similarly, colonization with the Serratia plymuthica protects cucumber (Cucumis sativus) plants from the damping-off disease caused by Pythium aphanidermatum, as well as tomato and bean (Phaseolus vulgaris) plants from infection with the gray mold fungus Botrytis cinerea (Pang et al., 2009). The splI− mutant of S. plymuthica, impaired in the production of AHLs, could not provide this protection (Pang et al., 2009). However, the molecular basis of AHLs’ influence on the plant immune system is still unknown.
In this report we addressed the question how AHLs affect the plant immune system. We tested AHL-induced resistance toward different pathogens, using a monocotyledonous (barley [Hordeum vulgare]) and a dicotyledonous (Arabidopsis) host. Our results show that even though the long-chained AHLs cannot be transported within the plant, their perception in roots enhances the systemic resistance toward biotrophic and hemibiotrophic pathogens. Furthermore, in Arabidopsis plants pretreated with N-3-oxo-tetradecanoyl-L-homo-Ser lactone (oxo-C14-HSL), we observed prolonged activities of the MAPKs AtMPK3 and AtMPK6 after induction with the bacterial pathogen-associated molecular pattern (PAMP) flg22. The modified activities of MAPKs preceded enhanced transcription of the defense-related WRKY22 and WRKR29 transcription factors, as well as the PATHOGENESIS-RELATED1 (PR1) gene. Taken together, results presented here suggest that perception of long-chained AHLs has a positive impact on plant resistance.
RESULTS
Long-Chain AHLs Have No Impact on Plant Growth
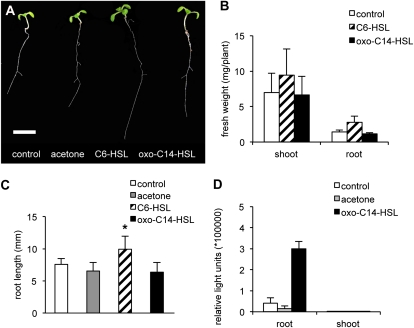
Several AHLs have been reported to modify root development and promote plant growth (Ortíz-Castro et al., 2008; von Rad et al., 2008). Therefore, to study how oxo-C14-HSL modulates plant physiology, we first monitored the impact of oxo-C14-HSL on growth and development of Arabidopsis Columbia-0 (Col-0). Ten-day-old seedlings grown on standard one-half Murashige and Skoog (MS) medium were transferred to medium supplied with 6 μm oxo-C14-HSL, and cultivated for additional 4 or 8 d. No alteration in the shoot or root development was observed (Fig. 1A). However, in accordance with previous reports (von Rad et al., 2008), the short-chained C6-HSL, used here as a positive control, promoted shoot and root biomass accumulation (Fig. 1B), as well as the root length (Fig. 1, A and C).
Figure 1.
Long-chained oxo-C14-HSL has no impact on plant growth and is not transported within the plant. A to C, Col-0 plants were grown for 10 d on one-half MS medium and afterward transferred to one-half MS medium supplied with 6 μm oxo-C14-HSL, or 6 μm C6-HSL. A, Col-0 plants grown for 4 d on C6-HSL or oxo-C14-HSL, bar = 1 cm. B, Fresh weights (mg/plant) of plants treated with different AHLs, n ≥ 80. C, Root length of Arabidopsis plants on media supplied with different AHLs for 4 d, n ≥ 40, *P ≤ 0.05 (Student’s t test). D, Oxo-C14-HSL is not transported systemically throughout the plant. Six micromolar oxo-C14-HSL was added to the root medium for 3 d. Subsequently, fresh plant material was extracted in acetone and detection assays were performed by using the reporter bacterium E. coli Top10 pSB403 lxR+luxI::luxCDABE. [See online article for color version of this figure.]
Application of Oxo-C14-HSL to Roots Does Not Result in AHL Detection in Leaves
Next, we addressed the question whether AHLs can be transported within the plant. To this end, we recorded the presence of AHL exogenously applied to the root medium, in different plant tissues. We used reporter bacteria carrying either the GFP gene or the lux operon from V. fisheri under AHL-inducible promoters (Supplemental Fig. S1, A and D). Cleared acetone extracts from either roots or shoots were prepared and applied to lawns of reporter bacteria (Supplemental Fig. S1B). GFP or luminescence signals were recorded 2 h after application. The most sensitive reporter bacterium was Pseudomonas putida F117 pKR C12 GFP (Steidle et al., 2001; carrying the lasR+lasB::gfp construct) with a detection limit of approximately 60 nm for oxo-C14-HSL (Supplemental Fig. S1A). In accord with previous reports (von Rad et al., 2008), if Arabidopsis roots were treated with C6-HSL, the AHL was detectable in extracts from roots and shoots of the treated plants (Supplemental Fig. S1B). In contrast, the long-chained oxo-C14-HSL was not detected in leaf extracts even though it was detectable in extracts from the roots of oxo-C14-HSL-treated plants. Neither the P. putida F117 pKR C12 GFP strain, nor the Escherichia coli strain pSB403 (Winson et al., 1998) carrying the lux operon from V. fisheri, were able to detect oxo-C14-HSL in the leaf extracts (Fig. 1D; Supplemental Fig. S1B). These results support the notion that long-chain AHLs are not transported systemically throughout the plant organism.
AHLs Confer Resistance against Hemibiotrophic and Biotrophic Pathogens
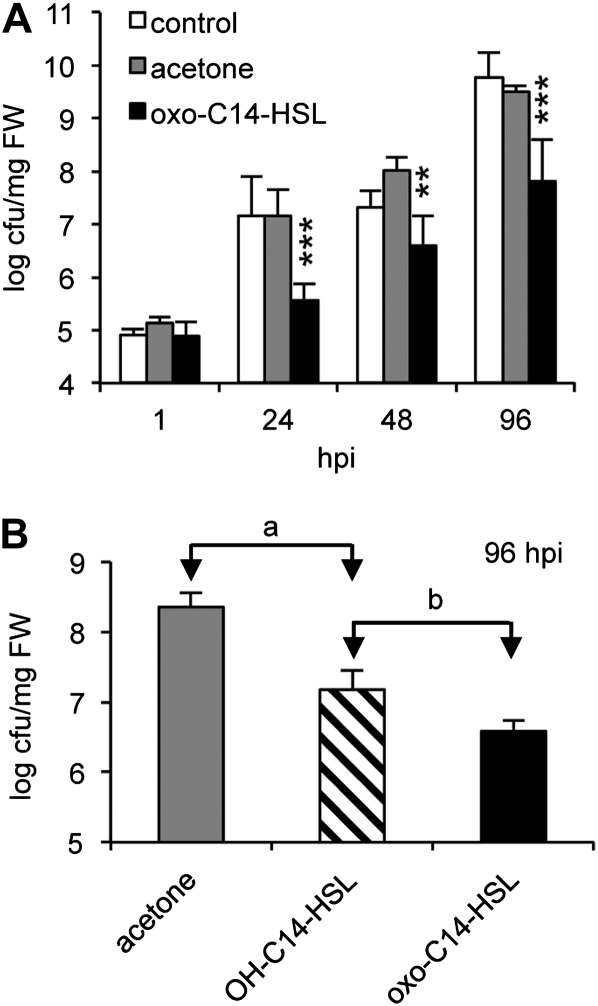
Based on the strong effect of AHLs on the mammalian immune system (Kravchenko et al., 2008), we followed the working hypothesis that AHLs may induce plant resistance to microbial pathogens. Arabidopsis plants were grown for 5 weeks in sterile hydroponics culture, and then the growing medium was supplied with 6 μm oxo-C14-HSL for 3 d. Subsequently plants were spray inoculated with Pseudomonas syringae pv tomato DC3000 (Pst). Bacterial population on leaves was monitored during 96 h post inoculation (hpi). Plants pretreated with oxo-C14-HSL developed significantly smaller populations of Pst bacteria then the control plants (Fig. 2A).
Figure 2.
C14-HSL derivatives induce resistance against P. syringae. A, Proliferation of Pst on 5-week-old Arabidopsis was analyzed at hpi as indicated. Plants were untreated (control) or pretreated with acetone or 6 μm oxo-C14-HSL, 3 d prior to infection with Pst bacteria. **P ≤ 0.005; ***P ≤ 0.0005. B, Colony forming units (cfu) of Pst harvested from 5-week-old Arabidopsis plants pretreated for 3 d with acetone, 6 μm oxo-C14-HSL, or 6 μm OH-C14-HSL, and subsequently inoculated with Pst bacteria for 96 h. a = P < 5.2E-6, b = P < 9.7E-11 (Student’s t test).
To exclude the possibility that oxo-C14-HSL has a direct effect on bacterial fitness, we cultivated bacteria in the presence of 6 μm oxo-C14-HSL and monitored population density over 48 h. No change in bacterial duplication time was observed (Supplemental Fig. S2A). In addition, we examined the virulence of bacteria grown in the presence of oxo-C14-HSL and subsequently sprayed onto Arabidopsis leaves (Supplemental Fig. S2B), or grown in standard Kings B medium and sprayed onto leaves together with the AHL (Supplemental Fig. S2C). None of these tests showed that oxo-C14-HSL lowers bacterial fitness or the virulence. Hence, results presented above suggest that oxo-C14-HSL enhances resistance of Arabidopsis toward Pst bacteria. Interestingly, not only oxo-C14-HSL, but also OH-C14-HSL and oxo-C12-HSL reduce Pst proliferation, though to lesser extent than oxo-C14-HSL (Fig. 2B; Supplemental Fig. S7A).
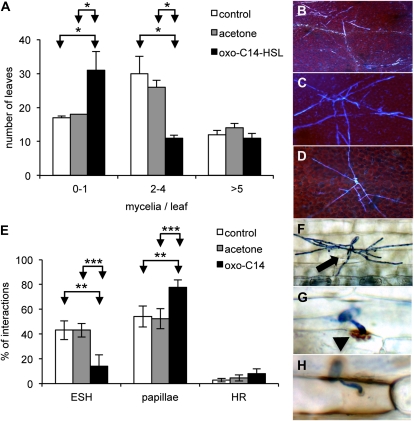
To expand our observations to another pathogen, we analyzed the impact of oxo-C14-HSL on the development of the fungus Golovinomyces orontii, the causal agent of powdery mildew in Arabidopsis. Hydroponically grown Arabidopsis plants were pretreated with 6 μm oxo-C14-HSL and leaves were subsequently inoculated with G. orontii (560 conidia/cm2 leaf). The number of mycelia per leaf was calculated 5 d after inoculation. We found that the number of mycelia developing on leaves was significantly reduced in plants pretreated with oxo-C14-HSL (Fig. 3A). On the other hand, the morphology of G. orontii mycelium was not affected by the AHL treatment (Fig. 3, B–D). These results support the concept that long-chained AHL exhibits a positive effect on the resistance toward hemibiotrophic and biotrophic pathogens.
Figure 3.
Oxo-C14-HSL induces resistance against G. orontii and B. graminis. Five-week-old Arabidopsis or 5-d-old barley plants were pretreated for 3 d with 6 μm oxo-C14-HSL in hydroponics cultures and then fungal conidia suspension was sprayed on their leaves. A to d, Proliferation of G. orontii on Arabidopsis leaves. A, Number of mycelia per leaf at 5 d post inoculation (dpi). Student’s t test performed on the raw data resulted in P = 0.33 (control versus acetone); P = 0.04 (control versus oxo); and P = 0.01 (acetone versus oxo). B to D, Mycelia formation on untreated Arabidopsis leaves (control; B), pretreated with acetone (C), or oxo-C14-HSL (D), shows no alternation in fungal development. E to H, Proliferation of B. graminis on barley. E, Percent of interaction sites with ESH, papillae, and HR, on first leaves at 5 dpi. F, ESH formation, haustorium (arrow). G, Papillae formation (arrowhead). H, HR. *P ≤ 0.05; **P ≤ 0.005; ***P ≤ 0.0005 (Student’s t test).
Oxo-C14-HSL Induces Resistance against the Biotrophic Powdery Mildew Fungus in Barley Plants
In addition to experiments with Arabidopsis, we assessed oxo-C14-HSL-induced resistance in the monocotyledonous crop plant barley. Barley seedlings were grown for 5 d and then pretreated with 6 μm oxo-C14-HSL for 3 d in hydroponics culture. Subsequently first leaves were inoculated with the casual agent of barley powdery mildew Blumeria graminis f. sp. hordei (450 conidia/cm2 of leaf). As shown by the increased rate of fungal penetration failure (Fig. 3E) the effect of oxo-C14-HSL on barley is similar to that on Arabidopsis. The frequency of elongating secondary hyphae (ESH) shown in Figure 3F, indicative of successful infection, decreased from 43% of all interactions in control plants to 14% in oxo-C14-HSL-treated plants, while the frequency of papillae (Fig. 3G), indicative of reduced host cell accessibility, increased from about 54% in control to over 77% of all interactions in AHL-treated plants (Fig. 3E). The third possibility to respond is the hypersensitivity response (HR; Fig. 3H), also this resistance mechanism was enhanced in AHL-treated plants (Fig. 3E). In conclusion, oxo-C14-HSL affects the basal immune system of Arabidopsis and barley.
Oxo-C14-HSL Does Not Induce Resistance against Necrotrophic Pathogens
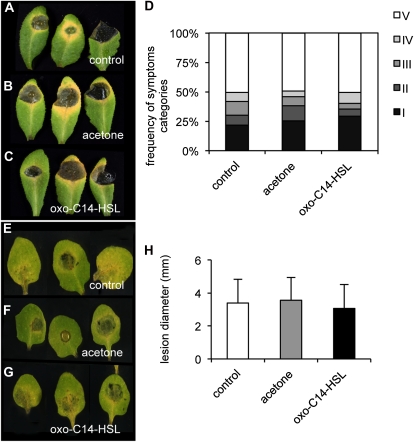
To examine the hypothesis that oxo-C14-HSL induces resistance particularly against hemibiotrophic and biotrophic pathogens, we performed a series of tests with necrotrophic fungal pathogens. Arabidopsis plants were pretreated as above for 3 d with 6 μm oxo-C14-HSL, and detached leaves were subsequently inoculated with B. cinerea or Plectosphaerella cucumerina BMM. Disease symptoms were analyzed after 5 d. Both pathogens caused maceration of leaf tissue, which can be classified into different symptom categories (B. cinerea; Fig. 4, A–D) or measured by lesion diameter (P. cucumerina BMM; Fig. 4, E–H). No significant differences in symptoms caused by B. cinerea or P. cucumerina BMM, as compared with water and acetone controls were observed (Fig. 4). To evaluate the possibility that resistance against necrotrophic fungi may be induced by different AHL concentration, we conducted a dose-response experiment with B. cinerea, using various concentrations of oxo-C14-HSL (0.6–12 μm). Yet, none of the tested concentration significantly enhanced resistance toward this fungus (Supplemental Fig. S3).
Figure 4.
Oxo-C14-HSL does not induce resistance against B. cinerea or P. cucumerina BMM. Five-week-old Arabidopsis plants were untreated (A and E), pretreated with acetone (B and F), or 6 μm oxo-C14-HSL (C and G) for 3 d. Conidial suspensions were dropped onto detached leaves. Necrotic symptoms or lesion diameters were analyzed at 5 dpi. Classification of symptoms: I, healthy leaf; II, leaf necrotic at 25%; III, 50% of leaf surface show necrosis; IV, 75% necrotic surface; V, 100% of the leaf surface is necrotic. D, Necrotic symptoms caused by B. cinerea. H, Lesions caused by P. cucumerina BMM.
AHL Modulates the Response to the Bacterial Pathogen-Associated Molecular Pattern flg22
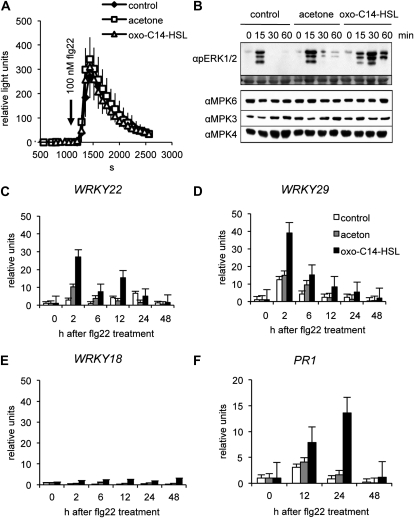
To study the mechanism by which AHL induces resistance in more detail, we examined the response of AHL-treated Arabidopsis plants to flg22. First, we monitored the accumulation of reactive oxygen species (ROS) after solely treatment with oxo-C14-HSL. For this, leaves of soil-grown plants were treated for 3 d with 6 μm oxo-C14-HSL in floating conditions and stained with diaminobenzidine (DAB). DAB staining revealed no changes in the steady-state level of hydrogen peroxide (H2O2; Supplemental Fig. S4). Next, we analyzed the production of ROS (oxidative burst) in those leaves after elicitation with 100 nm flg22. Likewise, ROS production induced by flg22 in Arabidopsis leaves was neither enhanced nor inhibited by pretreatment with AHL (Fig. 5A).
Figure 5.
Response to flg22 in plants pretreated with AHL. A, Oxidative burst in response to 100 nm flg22. Production of H2O2 was measured using the luminol-based assay in detached leaf discs from soil-grown plants. Leaves were untreated (control), or pretreated with acetone or oxo-C14-HSL for 3 d before treatment with flg22. Arrow shows the time of flg22 addition. B, Time course of phosphorylation status of AtMPK6 and AtMPK3. Whole 2-week-old Arabidopsis Col-0 seedlings were pretreated with 6 μm oxo-C14-HSL for 3 d. Responses were analyzed in seedlings during minutes after treatment with 100 nm flg22. Top section shows an immunoblot with αpERK1/2 antibody, the bottom section shows Coomassie stain of the membrane. Sections below show immunoblots with the specific αMAPKs antibodies. C to F, Transcriptional regulation of WRKY and PR1 genes after pretreatment with oxo-C14-HSL. Total mRNA was extracted from 2-week-old Arabidopsis Col-0 seedlings, pretreated for 3 d with 6 μm AHL plants, and subsequent treatment with 100 nm flg22 at hour as indicated. qRT-PCR analysis was performed using WRKY- and PR1-specific primers (Supplemental Table S1).
In the following step, we analyzed the activation of the MAPKs AtMPK3 and AtMPK6. Both kinases have a high degree of functional redundancy and are involved in plant defense mechanisms. Total protein extract was prepared from 2-week-old seedlings grown on agar plates and pretreated with oxo-C14-HSL in liquid one-half MS medium for additional 3 d. MAPKs were activated by treatment with 100 nm flg22. Phosphorylated forms of AtMPK3 and AtMPK6 were visualized using the αpERK1/2 antibody (Hamel et al., 2005). Consistent with earlier reports, flg22 triggered a short transient activation of AtMPK3 and AtMPK6 at 15 min after application (Fig. 5B). Intriguingly, AHL-pretreated seedlings showed stronger activation of both kinases, which lasted until 60 min after treatment (Fig. 5B). Immunoblots with respective αAtMPK3 and αAtMPK6 antibodies showed no changes in AtMPK3 and AtMPK6 quantities during 1 h after treatment (Fig. 5B). Similarly, quantitative reverse transcription (qRT-PCR) analysis detected no changes in AtMPK3 and AtMPK6 transcript levels after pretreatment with AHLs (Supplemental Fig. S5A), and only slightly changes after the subsequent treatment with flg22 (Supplemental Fig. S5, B and C). Although we cannot exclude a short transcriptional induction of AtMPK3/6 between 1 and 2 h after flg22 treatment (those intermediate time points were not examined), the results obtained in our tests indicate rather that oxo-C14-HSL modulates the flg22-induced phosphorylation status of AtMPK3 and AtMPK6 and not the amount of the kinases.
Owing to the fact that numerous WRKY transcription factors are induced upon pathogen attack and some WRKY are direct targets of activated MAPKs, we assessed whether oxo-C14-HSL-mediated enhanced activation of MAPKs influences the expression levels of defense-related WRKYs. We examined the relative expression levels of WRKY18, WKRY22, and WRKY29 whose activation is pathogenesis associated or induced by flg22 (Asai et al., 2002; Xu et al., 2006; Zheng et al., 2006). Two-week-old Arabidopsis seedlings were pretreated with 6 μm oxo-C14-HSL as described above and subsequently elicited with 100 nm flg22. Total RNA was extracted and transcript levels of WRKYs normalized to the expression of UBQ (At5g25760). Pretreatment with the AHL alone did not induce the expression of either WRKY (Supplemental Fig. S6, A–C). However, a subsequent treatment with 100 nm flg22 resulted in different expression patterns. Both WRKY22 and WRKY29 show induction already 30 min after treatment with elicitor (Libault et al., 2007). Here we show a prolonged and stronger transcriptional activation of WRKY22 and WRKY29 in plants pretreated with oxo-C14-HSL even at 2, 6, and 12 h after flg22 treatment (Fig. 5, C and D). WRKY18 expression did not change after the subsequent flg22 treatment (Fig. 5E).
PR genes such as PR1, are prominent example of defense-associated gene in Arabidopsis. Consequently, we examined the expression level of PR1 after treatment with oxo-C14-HSL. Similar to the expression of WRKY22 and WRKY29, AHL treatment alone does not induce the expression of PR1 (Supplemental Fig. S6D). However, expression of PR1 is induced after treatment with 100 nm flg22, and pretreatment with oxo-C14-HSL enhances it even more (Fig. 5F).
Active Kinases Are Necessary for AHL-Induced Resistance
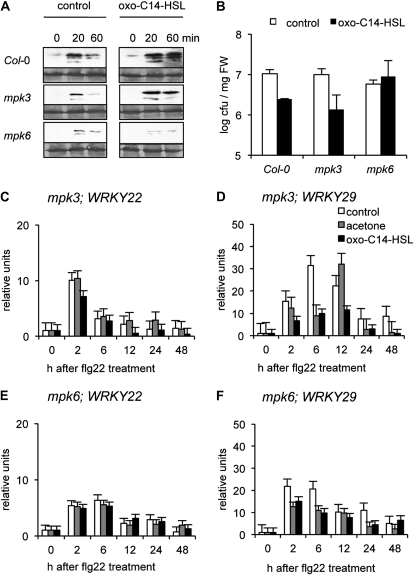
To test the assumption that AtMPK3 and/or AtMPK6 activations are required for AHL-induced resistance we analyzed the mpk3 and mpk6 mutants. First we monitored the phosphorylation status of the kinases in both mutants. Two-week-old Col-0 wild-type and mutant seedlings were pretreated for 3 d with 6 μm oxo-C14-HSL in liquid medium and MAPK’s activation was triggered by addition of 100 nm flg22. In the mpk3 mutant the phosphorylation pattern of AtMPK6 is similar to that in Col-0 plants: The 45-kD band representing the double-phosphorylated form of AtMPK6 is stronger in AHL-treated mpk3 plants (Fig. 6A). Interestingly, in the mpk6 mutant, the pAtMPK3 signal is much weaker if compared to pAtMPK3 in the Col-0 plants (Fig. 6A). To investigate this in further detail, we tested AHL-induced resistance toward Pst in the mpk3 and the mpk6 mutants. Mutant plants were grown for 5 weeks in hydroponics culture and pretreated for 3 d with 6 μm oxo-C14-HSL prior to a subsequent inoculation with Pst. In clear contrast to Col-0 wild-type and mpk3 mutant plants, the mpk6 mutant was compromised in the induced resistance toward Pst, suggesting that AtMPK6 plays a critical role in the AHL-induced resistance (Fig. 6B).
Figure 6.
AHL fails to modulate the response to flg22 in MAPK mutants. A, Time course of phosphorylation status of AtMPK3 and AtMPK6 in Col-0, mpk3, and mpk6. Whole 2-week-old mpk3, mpk6, or Col-0 seedlings were pretreated with 6 μm oxo-C14-HSL for 3 d. Responses were analyzed during minutes after treatment with 100 nm flg22. Top sections show an immunoblot with αpERK1/2 antibody, bottom sections show Coomassie stain of the membrane. B, Pst proliferation on mpk3 and mpk6 plants. Plants were pretreated as indicated for 3 d and spray inoculated with bacteria. Cfu numbers were analyzed after 48 h. Col-0 plants were used as a control. C to F, Transcriptional regulation of WRKY transcription factors after pretreatment with oxo-C14-HSL. Total mRNA was extracted from 2-week-old Arabidopsis mpk3 or mpk6 seedlings, pretreated for 3 d with 6 μm AHL plants, and subsequent treatment with 100 nm flg22 at hour as indicated. qRT-PCR analysis was performed using WRKY-specific primers (Supplemental Table S1). The fold induction was normalized to 0 h after flg22 treatment, note that mpk3 mutant has approximately 4 and 144 times lower basal level of WRKY22 and WRKY29 than Col-0 plants, respectively. The basal level of WRKY22 expression in mpk6 plants is slightly higher; the WRKY29 is 3 times lower than in Col-0 plants.
Next, we analyzed the expression pattern of WRKY22 and WRKY29 in the mpk3 and mpk6 mutants. In wild-type Col-0 plants, expression of both WRKYs is up-regulated upon treatment with flg22, and could be even more induced by pretreatment with AHL (Fig. 5, C and D). mpk3 and mpk6 mutants were grown for 2 weeks on standard medium and transferred to liquid one-half MS medium supplied with AHL 3 d before the treatment with flg22. In both the mpk3 and the mpk6 mutant, expression of WRKYs is induced after treatment with flg22, yet it is not further modified by the pretreatment with oxo-C14-HSL (Fig. 6, C–F).
AHL Pretreatment Enhances Effector-Triggered Immunity-Based Defense Mechanism in Arabidopsis
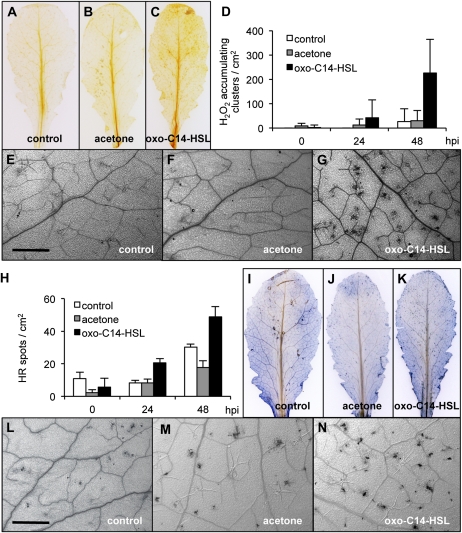
In plants, recognition of a pathogen occurs at two levels: The pattern recognition receptors detect PAMPs and induce the PAMP-triggered immunity; the host-encoded R proteins, on the other hand, recognize (directly or indirectly) microbial effectors and initiate the effector-triggered immunity (ETI). We wondered whether AHL might influence also the second level of defense: the ETI. An increased frequency of HR is often associated with ETI as the consequence of the recognition of bacterial effectors via the R proteins. Therefore, we used the Pst strain expressing the AvrRpt2 gene, whose product is recognized in Arabidopsis by the RPS2 receptor (Kunkel et al., 1993; Yu et al., 1993). Arabidopsis Col-0 plants were grown in soil for 4 weeks and detached leaves were pretreated for 3 d with 6 μm oxo-C14-HSL in floating conditions. Leaves were then spray inoculated with the Pst AvrRpt2 strain and the accumulation of H2O2, as well as the cell death rate were analyzed during 48 h. Pretreatment with oxo-C14-HSL resulted in a strong accumulation of H2O2 after Pst AvrRpt2 inoculation, as visualized by the DAB staining (Fig. 7, A–C), and evidenced by the increased number of DAB-positive clusters per leaf area (Fig. 7D). Cell death was visualized with trypan blue (TB) stain. Similar to the DAB stain, the positively stained clusters were counted in the 48-h time range following infection (Fig. 7H). Like H2O2 production, the number of dead cell clusters was enhanced in leaves pretreated with AHL as indicated by the increased numbers of blue-stained clusters in those leaves (Fig. 7, I–N). In conclusion, this result supports the notion that the AHL affects defense mechanisms on different levels. Moreover, the systemic action (Figs. 2 and 3) might be accompanied with local effects (Fig. 7).
Figure 7.
Oxo-C14-HSL promotes accumulation of ROS and induces HR rate after challenge with Pst in Arabidopsis plant. Arabidopsis plants were grown on soil and detached leaves were pretreated for 3 d with water (control; A, E, I, L), acetone (B, F, J, M), or 6 μm oxo-C14-HSL (C, G, K, N). Pretreated leaves were inoculated with Pst strain AvrRpt2 (avirulent) by spraying. Leaves were stained with DAB to visualize the accumulation of H2O2 (A–G), or with TB to visualize the dead cells (H–N). A to C, DAB stain of Arabidopsis leaves inoculated with Pst AvrRpt2 for 48 h. E to G show magnifications of respective A to C. Bar = 0.1 cm. D, Representation of DAB-positive stained clusters in Pst AvrRpt2-inoculated leaves. Pretreated leaves were infected with bacteria and stained with DAB at hpi as indicated. Number of stained clusters was calculated per cm2, 60 clusters were analyzed in three independent experiments. H, Numbers of TB-positive stained clusters in Pst AvrRpt2-inoculated leaves. Pretreated leaves were inoculated with bacteria and stained with TB at hpi as indicated. Clusters presence was calculated per cm2, 60 clusters were analyzed in three independent experiments. I to K, TB stain of Arabidopsis leaves inoculated with Pst AvrRpt2 for 48 h. L to N show respective magnifications of I to K. [See online article for color version of this figure.]
DISCUSSION
In this report we show that AHL modulates plant-pathogen interactions. Until now AHLs were identified in over 70 species of Gram-negative bacteria. Among the numerous QS systems, the AHL-based mechanism is best understood (Whitehead et al., 2001; Zhang and Dong, 2004; Waters and Bassler, 2005; Dong et al., 2007). Several studies show that also eukaryotes (plants as well as animals) respond to the presence of AHLs (Mathesius et al., 2003; Jahoor et al., 2008; Kravchenko et al., 2008; Ortíz-Castro et al., 2008; von Rad et al., 2008). Nevertheless, a direct evidence for their involvement in plant defense reactions has been missing. In this report we show that perception of oxo-C14-HSL significantly increases the resistance toward hemibiotrophic bacteria and biotrophic fungi, while it appears rather ineffective to microbes with a necrotrophic life style. Furthermore, our data suggest that activation of MAPKs, especially AtMPK6, is crucial for AHL-induced resistance.
Plants React to Bacterial QS Molecules
AHLs trigger diverse plant reactions: C6-HSL promotes growth (von Rad et al., 2008), while C10-HSL and C12-HSL modulate root development and root hair formation, respectively (Ortíz-Castro et al., 2008). Here we show that the long-chained AHL (oxo-C14-HSL) increased Arabidopsis resistance toward Pst and powdery mildew fungi in Arabidopsis and barley (Figs. 2 and 3). Diverse bacteria such as Acidithiobacillus ferrooxidans, Burkholderia pseudomallei, Rhodobacter spheroides, Sinorhizobium meliloti, and Yersinia enterocolitica produce C14-HSL derivatives (Williams, 2007). In biofilms of the opportunistic plant and human pathogen P. aeruginosa, oxo-C14-HSL can reach concentration of 40 μm (Charlton et al., 2000). Interestingly, the length of the fatty acid chain is not the only determinant for induced resistance. Modifications at the γ positions in the fatty acid chain are also critical. Substitution by a OH or O group at this position has impact on the plant response; in our tests the oxo group appeared to be more efficient in resistance induction (Fig. 2B). The strongest effect on resistance was observed in plants pretreated with C14 or C12 derivatives of AHLs (Figs. 2 and 3; Supplemental Fig. S7A). In contrast, similarly to the previous report by von Rad et al. (2008), the growth-promoting effect was present only in plants pretreated with the short-chained C6-HSL (Fig. 1; Supplemental Fig. S7B).
Homo-Ser Lactones Induce Systemic Reaction
The transport of AHLs within plants has been addressed in several previous reports. Götz et al. (2007) demonstrated move of both C6-HSL and C8-HSL from roots into barley shoots, whereas C10-HSL was not transported. Similarly, C6-HSL but not the C10-HSL was translocated from roots to shoots in Arabidopsis plants (von Rad et al., 2008). Using different marker bacteria with sensitivity as low as 60 nm AHL, we confirmed that C6-HSL is systemically transported in Arabidopsis plants from root to shoot. However, we were unable to detect oxo-C14-HSL in shoots after root treatment (Fig. 1; Supplemental Fig. S1B), showing that increased chain length reduces the motility of AHLs within the plant. Accordingly, when roots were treated with long-chained AHL, the resulting change of the resistance status in leaves toward leaf pathogens is consistent with an inducible, systemic disease resistance.
Active AtMPK6 Is Required for AHL-Induced Resistance
Induction of different MAPK cascades is one of the first steps in pathogen perception. The involvement of AtMPK6 and upstream compounds of the MAPK cascade in prompt response to pathogen attack is well documented (Asai et al., 2002). AtMPK6 together with its closest homolog AtMPK3, plays an important role in signaling cascades downstream of several pattern recognition receptors being crucial in induction of defense mechanisms, reviewed by Pitzschke et al. (2009). Nonetheless, little is known about the role of MAPK cascades in systemic resistance mechanisms such as systemic acquired resistance (SAR) or induced systemic resistance (Vlot et al., 2008; Shah, 2009). Activation of the MAP kinase kinase 7 (MKK7) is required for SAR against P. syringae pv maculicola ES4326 and Hyaloperonospora parasitica Noco2 (syn. Hyaloperonospora arabidopsidis) in Arabidopsis (Zhang et al., 2007). The authors demonstrated that ectopic expression of MKK7 is sufficient to generate a SAR-inducing signal. By showing that the edr1 mutant constitutively expresses SAR, the EDR1, a CRT1-like MAPK kinase kinase, was suggested to be a negative regulator of SAR (Frye and Innes, 1998; Frye et al., 2001). Here we show that already local pretreatment of Arabidopsis with AHL modulates the inductions of AtMPK6 and AtMPK3. Moreover, the AHL-induced systemic resistance to Pst is compromised in mpk6 but not in the mpk3, supporting the assumption that the AtMPK6 is predominantly required for resistance reinforcement caused by bacterial QS molecules. This is further supported by the transcriptional induction of PR1 gene in oxo-C14-HSL-pretreated Col-0 and mpk3, but not in mpk6 mutant, after subsequent flg22 induction (Supplemental Fig. S8).
Interestingly, in direct response to oxo-C14-HSL, Arabidopsis plants did not accumulate ROS, nor was SAR-associated PR1 gene induced (Supplemental Figs. S4 and S6). Nevertheless, if oxo-C14-HSL-pretreated plants are challenged with biotrophic or hemibiotrophic pathogens they show higher resistance if compared to control plants. This phenomenon is comparable to the priming response induced in many plants by chemical resistance inducers such as benzo-(1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester (BTH; Conrath et al., 2006). Recently it was suggested that AtMPK3 is the molecular base of priming responses (Beckers et al., 2009). The authors showed that AtMPK3 initially accumulates in an inactive form in the response to BTH, and that, upon inoculation with Pst DC3000, the kinase is activated and shows a prolonged activity. Results presented in this report are very similar to those presented by Beckers et al. (2009), suggesting that also AHL may induce priming-like state in Arabidopsis and barley. However, we did observe some differences. The lack of enhanced accumulation of transcripts or inactive forms of either AtMPK3 or AtMPK6 upon AHL treatments or the fact that AtMPK6 seems to play the major role in AHL-induced resistance, whereas AtMPK3 appears to be the major player in BTH priming (Beckers et al., 2009) suggest that AHL-induced resistance may differ from the BTH-induced priming mechanism.
An interesting possibility to explain the mechanism of AHL-enforced activation of MAPKs may be the inhibition of phosphatases. In Arabidopsis, several dual-specificity phosphatases and at least one of protein phosphatases 2C interact with AtMPK6 or AtMPK3 (Schweighofer et al., 2007; Bartels et al., 2009; Lumbreras et al., 2010). The Arabidopsis mkp2 mutant exhibits delayed wilting symptoms after infection with the biotrophic bacterium Ralstonia solanacearum, albeit stronger disease symptoms were observed after infection with the necrotrophic B. cinerea (Lumbreras et al., 2010).
CONCLUSION
Together with other reports, the results presented here provide strong evidence that plants evolved potent ways to take advantage of the bacterial QS. The data show that the specificity of the plant’s response to AHLs depends on the length of the acyl moiety and on the functional group at the γ position. We suggest that long-chained AHLs have the ability to induce resistance against microbial pathogens. Finally we demonstrate that the mechanism of systemic resistance induced by oxo-C14-HSL relies on the presence of AtMPK6, and is most probably different from other chemical-induced priming effects.
MATERIALS AND METHODS
Plant Growth
For pathogenicity assays Arabidopsis (Arabidopsis thaliana) Col-0, mpk3, and mpk6 plants were grown in a sterile hydroponics system. Surface-sterilized seeds (3 min with 50% ethanol/0.5%Tritron X-100 mix and briefly rinsed with 95% ethanol) were germinated and grown for 5 weeks at 22°C with 150 μmol m−2 s−1 light in 8/16 h day/night photoperiod. Seeds were directly planted into 96-well plates with one-half MS medium (Murashige and Skoog, 1962) supplemented with vitamins, 0.7% agar, and 1% Suc. Plates were placed on 200 mL liquid one-half MS in a sterile box. Medium was exchanged every second week. AHLs were added directly into the medium.
For transcriptional and biochemical analyses, Arabidopsis seeds were surface sterilized and germinated on sterile one-half MS with 0.4% gelrite and 1% Suc for 2 weeks. Seedlings were then transferred into six-well plates with 5 mL liquid one-half MS medium, prior to the pretreatment with AHLs.
For oxidative burst, ROS accumulation, and HR rate assays Arabidopsis plants were grown on soil in short-day condition (8/16 h day/night photoperiod) at 21°C for 4 weeks. Detached leaves were floated on sterile medium (10 mm phosphate buffer pH 8.0), supplied with AHLs for 3 d, then treated with flg22 or spray inoculated with Pst bacteria.
Barley (Hordeum vulgare ‘Golden Promise’) seeds were surface sterilized with 70% ethanol and 6% NaClO and germinated for 2 d in the dark. Seedlings were transferred for 5 d on plant nutrient medium (Schäfer et al., 2009) supplied with 0.4% gelrite, and then for 3 additional days to liquid plant nutrient medium for AHL treatment.
AHL Treatment
AHLs (Sigma-Aldrich) were solved in acetone as 60 mm stock. AHL pretreatment occurred either directly in the hydroponics system, or AHLs were added into liquid or solid (0.4% gelrite) one-half MS medium. Plants were pretreated for 3 d. All experiments were performed with two controls: untreated plants (control) and solvent control (acetone).
AHL Detection
The detection of AHLs was done using transgenic bacterial strains: Pseudomonas putida (F117 pKR C12 GFP; Steidle et al., 2001), Serratia liquefaciens (MG44 pBAH9 GFP; Li, 2010), Escherichia coli (MT102 GFP pJBA89; Andersen et al., 2001), and the E. coli strain (Top10 pSB403; Winson et al., 1998) expressing luxR+luxI::luxCDABE from Vibrio fisheri, detecting a range of AHL from C6-HSL to oxo-C14-HSL (Supplemental Fig. S2). Reporter bacteria were grown on Luria-Bertani medium with specific antibiotics. Leaves (70 mg) or roots (30 mg) were homogenized in acetone. Ten microliters of cleared acetone extract was dropped on the bacteria lawn. Fluorescence was observed 2 h after incubation using an ex: 480/40 nm, em: 510 nm filter.
Pathogenicity Tests
Pst and Pst expressing AvrRpt2 were cultured overnight in Kings B medium, washed in 10 mm MgSO4. Inoculation solution was adjusted to OD600 = 0.1. Bacterial solution with 0.02% Silwet was sprayed uniformly over the plants. After: 1, 24, 48, and 96 h 100-mg leaves were homogenized in 10 mm MgSO4. Samples were diluted and plated for colony forming units counting.
Golovinomyces orontii spore suspension (40,000 spores/mL) was sprayed uniformly onto leaves from plants grown in hydroponics culture and incubated for 5 d. Infected leaves were stained with calcofluor, and mycelia formation was counted in fluorescence Axioplan2 (Zeiss) microscope using an ex: 360/40 nm, em: 460/50 nm filter.
Five microliters of spore suspension of Plectosphaerella cucumerina BMM (for isolate Brigitte Mauch-Mani; 20,000 spores/mL) or Botrytis cinerea (20,000 spores/mL) were dropped directly onto the leaf surface. Conidia of Blumeria graminis f. sp. hordei race A6 (450 conidia/cm2) were directly sprayed onto barley leaves.
Oxidative Burst Assay
Oxidative burst was monitored after treatment with 100 nm flg22 using luminol-based assay. Leaves from soil-grown, 4-week-old plants were floated for 3 d on AHL-supplied medium and used to cut out segments, which were incubated overnight in sterile water in 96-well plates. Water was then replaced with 180 μL of luminol working solution (1 mL luminol stock, 50 μL peroxidase, 50 mL distillated water), and 5 μL of 200 mm phosphate buffer pH 8.0. Luminol stock: 1.77 mg luminol (Sigma-Aldich) dissolved in 1 mL 10 mm NaOH and 9 mL water. The background was measured for 10 min, and then 20 μL of 0.25 μm flg22 were added into each well and measured for additional 60 min.
Immunodetection of Phosphorylated MAPKs
Arabidopsis seedlings pretreated with AHLs (Col-0, mpk3, and mpk6) were treated as indicated with 100 nm flg22. Total proteins were extracted and separated on 12% SDS-polyacrylamide gel. Western-blot analyses were done using αMPK3, αMPK6, αMPK4 (Sigma-Aldrich), and the αpERK1/2 (Cell Signaling) antibodies.
Transcriptional Analysis
Seedlings were treated with 100 nm flg22 and harvested as indicated. Fifty to one hundred milligrams of plant material was homogenized and RNA extracted using the Trizol system. Two micrograms of total RNA was used for DNAse digestion. cDNA synthesis was done according to the qScript cDNA synthesis kit from Quanta BioScience Inc. RT efficiency was verified with semiquantitative amplification of the actin 2 transcript. qRT-PCR was done using primers listed in Supplemental Table S1. All expression values were normalized to expression of UBQ4 (At5g25760). The fold induction was normalized to 0 h after flg22 treatment, note that mpk3 mutant has approximately 4 and 144 times lower basal level of WRKY22 and WRKY29 then Col-0 plants, respectively. The basal level of WRKY22 expression in mpk6 plants is slightly higher; the WRKY29 is 3 times lower then in Col-0 plants.
TB and DAB Staining
Leaves of 4-week-old plants were floated for 3 d on AHL-supplied medium and afterward spray inoculated with Pst AvrRpt2. Samples were harvested after 0, 24, and 48 hpi. Leaves were either: (1) incubated in TB solution for 1 min at 60°C followed by 30 min at 22°C and destained in 25 mm chloral hydrate; or (2) incubated in DAB solution (1 mg/mL 3,3′-DAB in water) overnight at 22°C and destained in ethanol/chloroform/trichloroacetic acid (4:1:0.15) for 24 h.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Detection of AHLs using reporter bacteria.
Supplemental Figure S2. AHL has no impact on bacterial virulence.
Supplemental Figure S3. Different concentrations of oxo-C14-HSL have no effect on the resistance against B. cinerea.
Supplemental Figure S4. Oxo-C14-HSL has no effect on the accumulation of ROS or the spontaneous cell death.
Supplemental Figure S5. Transcriptional regulation of the MAP kinases AtMPK6 and AtMPK3 after pretreatment with oxo-C14-HSL.
Supplemental Figure S6. Pretreatment with oxo-C14-HSL is not sufficient to induce the transcription of WRKY or PR1 genes.
Supplemental Figure S7. Long-chained AHLs impact on plants resistance and development.
Supplemental Figure S8. Transcriptional analysis of PR1 gene in mpk3/6 mutants.
Supplemental Table S1. List of primers used in quantitative RT-PCR.
Acknowledgments
We thank Prof. Anton Hartmann (Hemholtz Zentrum München, Germany) for the kind gift of reporter bacteria used in the study, Prof. Brigitte Mauch-Mani (University of Neuchatel, Switzerland) for the kind gift of P. cucumrina BMM, and Christina Neumann (Justus Liebig University Giessen, Germany) for help with expression analysis.
References
- Andersen JB, Heydorn A, Hentzer M, Eberl L, Geisenberger O, Christensen BB, Molin S, Givskov M. (2001) gfp-based N-acyl homoserine-lactone sensor systems for detection of bacterial communication. Appl Environ Microbiol 67: 575–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983 [DOI] [PubMed] [Google Scholar]
- Bartels S, Anderson JC, González Besteiro MA, Carreri A, Hirt H, Buchala A, Métraux JP, Peck SC, Ulm R. (2009) MAP kinase phosphatase1 and protein tyrosine phosphatase1 are repressors of salicylic acid synthesis and SNC1-mediated responses in Arabidopsis. Plant Cell 21: 2884–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers GJ, Jaskiewicz M, Liu Y, Underwood WR, He SY, Zhang S, Conrath U. (2009) Mitogen-activated protein kinases 3 and 6 are required for full priming of stress responses in Arabidopsis thaliana. Plant Cell 21: 944–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton TS, de Nys R, Netting A, Kumar N, Hentzer M, Givskov M, Kjelleberg S. (2000) A novel and sensitive method for the quantification of N-3-oxoacyl homoserine lactones using gas chromatography-mass spectrometry: application to a model bacterial biofilm. Environ Microbiol 2: 530–541 [DOI] [PubMed] [Google Scholar]
- Conrath U, Beckers GJ, Flors V, García-Agustín P, Jakab G, Mauch F, Newman MA, Pieterse CM, Poinssot B, Pozo MJ, et al. (2006) Priming: getting ready for battle. Mol Plant Microbe Interact 19: 1062–1071 [DOI] [PubMed] [Google Scholar]
- Davis BM, Jensen R, Williams P, O’Shea P. (2010) The interaction of N-acylhomoserine lactone quorum sensing signaling molecules with biological membranes: implications for inter-kingdom signaling. PLoS ONE 5: e13522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong YH, Wang LY, Zhang LH. (2007) Quorum-quenching microbial infections: mechanisms and implications. Philos Trans R Soc Lond B Biol Sci 362: 1201–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebrecht J, Silverman M. (1984) Identification of genes and gene products necessary for bacterial bioluminescence. Proc Natl Acad Sci USA 81: 4154–4158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Innes RW. (1998) An Arabidopsis mutant with enhanced resistance to powdery mildew. Plant Cell 10: 947–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Tang D, Innes RW. (2001) Negative regulation of defense responses in plants by a conserved MAPKK kinase. Proc Natl Acad Sci USA 98: 373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuqua WC, Winans SC. (1994) A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J Bacteriol 176: 2796–2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz C, Fekete A, Gebefuegi I, Forczek ST, Fuksová K, Li X, Englmann M, Gryndler M, Hartmann A, Matucha M, et al. (2007) Uptake, degradation and chiral discrimination of N-acyl-D/L-homoserine lactones by barley (Hordeum vulgare) and yam bean (Pachyrhizus erosus) plants. Anal Bioanal Chem 389: 1447–1457 [DOI] [PubMed] [Google Scholar]
- Hamel L-P, Miles GP, Samuel MA, Ellis BE, Séguin A, Beaudoin N. (2005) Activation of stress-responsive mitogen-activated protein kinase pathways in hybrid poplar (Populus trichocarpa x Populus deltoides). Tree Physiol 25: 277–288 [DOI] [PubMed] [Google Scholar]
- Hanzelka BL, Greenberg EP. (1995) Evidence that the N-terminal region of the Vibrio fischeri LuxR protein constitutes an autoinducer-binding domain. J Bacteriol 177: 815–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haudecoeur E, Planamente S, Cirou A, Tannières M, Shelp BJ, Moréra S, Faure D. (2009a) Proline antagonizes GABA-induced quenching of quorum-sensing in Agrobacterium tumefaciens. Proc Natl Acad Sci USA 106: 14587–14592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haudecoeur E, Tannières M, Cirou A, Raffoux A, Dessaux Y, Faure D. (2009b) Different regulation and roles of lactonases AiiB and AttM in Agrobacterium tumefaciens C58. Mol Plant Microbe Interact 22: 529–537 [DOI] [PubMed] [Google Scholar]
- Imamura Y, Yanagihara K, Mizuta Y, Seki M, Ohno H, Higashiyama Y, Miyazaki Y, Tsukamoto K, Hirakata Y, Tomono K, et al. (2004) Azithromycin inhibits MUC5AC production induced by the Pseudomonas aeruginosa autoinducer N-(3-oxododecanoyl) homoserine lactone in NCI-H292 cells. Antimicrob Agents Chemother 48: 3457–3461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahoor A, Patel R, Bryan A, Do C, Krier J, Watters C, Wahli W, Li G, Williams SC, Rumbaugh KP. (2008) Peroxisome proliferator-activated receptors mediate host cell proinflammatory responses to Pseudomonas aeruginosa autoinducer. J Bacteriol 190: 4408–4415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravchenko VV, Kaufmann GF, Mathison JC, Scott DA, Katz AZ, Grauer DC, Lehmann M, Meijler MM, Janda KD, Ulevitch RJ. (2008) Modulation of gene expression via disruption of NF-kappaB signaling by a bacterial small molecule. Science 321: 259–263 [DOI] [PubMed] [Google Scholar]
- Kravchenko VV, Kaufmann GF, Mathison JC, Scott DA, Katz AZ, Wood MR, Brogan AP, Lehmann M, Mee JM, Iwata K, et al. (2006) N-(3-oxo-acyl)homoserine lactones signal cell activation through a mechanism distinct from the canonical pathogen-associated molecular pattern recognition receptor pathways. J Biol Chem 281: 28822–28830 [DOI] [PubMed] [Google Scholar]
- Kunkel BN, Bent AF, Dahlbeck D, Innes RW, Staskawicz BJ. (1993) RPS2, an Arabidopsis disease resistance locus specifying recognition of Pseudomonas syringae strains expressing the avirulence gene avrRpt2. Plant Cell 5: 865–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D. (2010) Phenotypic variation and molecular signaling in the interaction of the rhizosphere bacteria Acidovorax sp. N35 and Rhizobium radiobacter F4 with roots. PhD thesis. Ludwig Maximilian University, Munich [Google Scholar]
- Libault M, Wan J, Czechowski T, Udvardi M, Stacey G. (2007) Identification of 118 Arabidopsis transcription factor and 30 ubiquitin-ligase genes responding to chitin, a plant-defense elicitor. Mol Plant Microbe Interact 20: 900–911 [DOI] [PubMed] [Google Scholar]
- Lumbreras V, Vilela B, Irar S, Solé M, Capellades M, Valls M, Coca M, Pagès M. (2010) MAPK phosphatase MKP2 mediates disease responses in Arabidopsis and functionally interacts with MPK3 and MPK6. Plant J 63: 1017–1030 [DOI] [PubMed] [Google Scholar]
- Mathesius U, Mulders S, Gao M, Teplitski M, Caetano-Anolles G, Rolfe BG, Bauer WD. (2003) Extensive and specific responses of a eukaryote to bacterial quorum-sensing signals. Proc Natl Acad Sci USA 100: 1444–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Ortíz-Castro R, Martínez-Trujillo M, López-Bucio J. (2008) N-acyl-L-homoserine lactones: a class of bacterial quorum-sensing signals alter post-embryonic root development in Arabidopsis thaliana. Plant Cell Environ 31: 1497–1509 [DOI] [PubMed] [Google Scholar]
- Pang Y, Liu X, Ma Y, Chernin L, Berg G, Gao K. (2009) Induction of systemic resistance, root colonisation and biocontrol activities of the rhizospheric strain of Serratia plymuthica are dependent on N-acyl homoserine lactones. Eur J Plant Pathol 124: 261–268 [Google Scholar]
- Pappas KM, Winans SC. (2003) A LuxR-type regulator from Agrobacterium tumefaciens elevates Ti plasmid copy number by activating transcription of plasmid replication genes. Mol Microbiol 48: 1059–1073 [DOI] [PubMed] [Google Scholar]
- Pitzschke A, Schikora A, Hirt H. (2009) MAPK cascade signalling networks in plant defence. Curr Opin Plant Biol 12: 421–426 [DOI] [PubMed] [Google Scholar]
- Schaefer AL, Val DL, Hanzelka BL, Cronan JE, Jr, Greenberg EP. (1996) Generation of cell-to-cell signals in quorum sensing: acyl homoserine lactone synthase activity of a purified Vibrio fischeri LuxI protein. Proc Natl Acad Sci USA 93: 9505–9509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer P, Pfiffi S, Voll LM, Zajic D, Chandler PM, Waller F, Scholz U, Pons-Kühnemann J, Sonnewald S, Sonnewald U, et al. (2009) Manipulation of plant innate immunity and gibberellin as factor of compatibility in the mutualistic association of barley roots with Piriformospora indica. Plant J 59: 461–474 [DOI] [PubMed] [Google Scholar]
- Schuhegger R, Ihring A, Gantner S, Bahnweg G, Knappe C, Vogg G, Hutzler P, Schmid M, Van Breusegem F, Eberl L, et al. (2006) Induction of systemic resistance in tomato by N-acyl-L-homoserine lactone-producing rhizosphere bacteria. Plant Cell Environ 29: 909–918 [DOI] [PubMed] [Google Scholar]
- Schweighofer A, Kazanaviciute V, Scheikl E, Teige M, Doczi R, Hirt H, Schwanninger M, Kant M, Schuurink R, Mauch F, et al. (2007) The PP2C-type phosphatase AP2C1, which negatively regulates MPK4 and MPK6, modulates innate immunity, jasmonic acid, and ethylene levels in Arabidopsis. Plant Cell 19: 2213–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J. (2009) Plants under attack: systemic signals in defence. Curr Opin Plant Biol 12: 459–464 [DOI] [PubMed] [Google Scholar]
- Steidle A, Sigl K, Schuhegger R, Ihring A, Schmid M, Gantner S, Stoffels M, Riedel K, Givskov M, Hartmann A, et al. (2001) Visualization of N-acylhomoserine lactone-mediated cell-cell communication between bacteria colonizing the tomato rhizosphere. Appl Environ Microbiol 67: 5761–5770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlot AC, Klessig DF, Park SW. (2008) Systemic acquired resistance: the elusive signal(s). Curr Opin Plant Biol 11: 436–442 [DOI] [PubMed] [Google Scholar]
- von Rad U, Klein I, Dobrev PI, Kottova J, Zazimalova E, Fekete A, Hartmann A, Schmitt-Kopplin P, Durner J. (2008) Response of Arabidopsis thaliana to N-hexanoyl-DL-homoserine-lactone, a bacterial quorum sensing molecule produced in the rhizosphere. Planta 229: 73–85 [DOI] [PubMed] [Google Scholar]
- Waters CM, Bassler BL. (2005) Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol 21: 319–346 [DOI] [PubMed] [Google Scholar]
- Whitehead NA, Barnard AM, Slater H, Simpson NJ, Salmond GP. (2001) Quorum-sensing in Gram-negative bacteria. FEMS Microbiol Rev 25: 365–404 [DOI] [PubMed] [Google Scholar]
- Williams P. (2007) Quorum sensing, communication and cross-kingdom signalling in the bacterial world. Microbiology 153: 3923–3938 [DOI] [PubMed] [Google Scholar]
- Winson MK, Swift S, Fish L, Throup JP, Jørgensen F, Chhabra SR, Bycroft BW, Williams P, Stewart GS. (1998) Construction and analysis of luxCDABE-based plasmid sensors for investigating N-acyl homoserine lactone-mediated quorum sensing. FEMS Microbiol Lett 163: 185–192 [DOI] [PubMed] [Google Scholar]
- Xu X, Chen C, Fan B, Chen Z. (2006) Physical and functional interactions between pathogen-induced Arabidopsis WRKY18, WRKY40, and WRKY60 transcription factors. Plant Cell 18: 1310–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu GL, Katagiri F, Ausubel FM. (1993) Arabidopsis mutations at the RPS2 locus result in loss of resistance to Pseudomonas syringae strains expressing the avirulence gene avrRpt2. Mol Plant Microbe Interact 6: 434–443 [DOI] [PubMed] [Google Scholar]
- Zhang LH, Dong YH. (2004) Quorum sensing and signal interference: diverse implications. Mol Microbiol 53: 1563–1571 [DOI] [PubMed] [Google Scholar]
- Zhang X, Dai Y, Xiong Y, DeFraia C, Li J, Dong X, Mou Z. (2007) Overexpression of Arabidopsis MAP kinase kinase 7 leads to activation of plant basal and systemic acquired resistance. Plant J 52: 1066–1079 [DOI] [PubMed] [Google Scholar]
- Zheng Z, Qamar SA, Chen Z, Mengiste T. (2006) Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J 48: 592–605 [DOI] [PubMed] [Google Scholar]