Abstract
Phytochromes mediate the photoperiodic control of flowering in rice (Oryza sativa), a short-day plant. Recent molecular genetics studies have revealed a genetic network that enables the critical daylength response of florigen gene expression. Analyses using a rice phytochrome chromophore-deficient mutant, photoperiod sensitivity5, have so far revealed that within this network, phytochromes are required for expression of Grain number, plant height and heading date7 (Ghd7), a floral repressor gene in rice. There are three phytochrome genes in rice, but the roles of each phytochrome family member in daylength response have not previously been defined. Here, we revealed multiple action points for each phytochrome in the critical daylength response of florigen expression by using single and double phytochrome mutant lines of rice. Our results show that either phyA alone or a genetic combination of phyB and phyC can induce Ghd7 mRNA, whereas phyB alone causes some reduction in levels of Ghd7 mRNA. Moreover, phyB and phyA can affect Ghd7 activity and Early heading date1 (a floral inducer) activity in the network, respectively. Therefore, each phytochrome gene of rice has distinct roles, and all of the phytochrome actions coordinately control the critical daylength response of florigen expression in rice.
In many plants, flowering time is controlled by daylength, which helps to ensure reproductive success (Song et al., 2010). The seasonal change in daylength (photoperiod) is an important environmental cue for many plants, as it is associated with upcoming seasonal change. Long-day (LD) and short-day (sd) plants accelerate flowering when daylength become longer and shorter, respectively.
Several plant photoreceptors play roles in measuring daylength. Molecular genetics studies in Arabidopsis (Arabidopsis thaliana), an LD plant, revealed that both a cryptochrome blue-light receptor, encoded by the CRY2 gene, and another blue-light receptor, a LOV domain protein encoded by the FLAVIN-BINDING, KELCH REPEAT, F BOX1 (FKF1) gene, function to produce the LD response (Imaizumi et al., 2003; Valverde et al., 2004; Sawa et al., 2007; Liu et al., 2008; Zuo et al., 2011). Phytochrome is one such photoreceptor, which is converted to the biologically active Pfr (far-red-absorbing) form by absorbing red light or to the inactive Pr (red-absorbing) form by absorbing far-red light (Borthwick, 1964). The active Pfr form mediates light signals to control various physiological traits. Although phytochromes play a supporting role in regulating floral transitions such as entrainment of circadian clocks and stability control of a key regulator, CONSTANS (CO), in Arabidopsis (Franklin and Quail, 2010), blue-light signals seem to be even more important in the LD response in this species. Nevertheless, it has been long thought that phytochromes are essential for daylength measurement in many other plants, based on numerous physiological analyses (Hanumappa et al., 1999; Izawa et al., 2000). It is also known that phytochromes control the photoperiodic control of flowering in rice (Oryza sativa), a model SD plant, because flowering time in photoperiod sensitivity5 (se5), a phytochrome chromophore mutant of phytochromes in rice, exhibits extremely early flowering and is completely insensitive to daylength (often, slightly earlier under LD conditions than SD; Izawa et al., 2000).
Rice is a typical SD plant (Izawa et al., 2003; Izawa, 2007), and its florigen gene expression has a critical daylength threshold of around 13.5 h (Itoh et al., 2010). Rice has three phytochrome genes: PHYA, PHYB, and PHYC (Takano et al., 2005). The differences in flowering time between various combinations of single and double phytochrome mutants suggest that each phytochrome makes distinct contributions to the control of flowering time (Takano et al., 2005).
In the photoperiodic control of flowering, rice has both evolutionarily conserved and unique pathways compared with Arabidopsis, a well-studied LD plant (Izawa et al., 2003; Izawa, 2007). Heading date 3a (Hd3a) and RICE FLOWERING LOCUS T1 (RFT1) are two highly conserved genes in rice that function as florigens (Kojima et al., 2002; Tamaki et al., 2007; Komiya et al., 2008). These florigen genes are strongly induced in leaf blades under inductive SD conditions; the florigen proteins then move to shoot apex regions and trigger floral induction. Hd1 is an ortholog of the Arabidopsis CO gene (Yano et al., 2000). Although CO functions only as a promoter of flowering under LD conditions, Hd1 functions as both a promoter of flowering under SD conditions and a repressor of flowering under LD conditions. In contrast, both Grain number, plant height and heading date7 (Ghd7), whose only conserved motif is a CCT domain, and Early heading date1 (Ehd1), a B-type response regulator, are unique to rice flowering pathways and not evolutionarily conserved in Arabidopsis (Doi et al., 2004; Xue et al., 2008). Recently, our laboratory reported that Ghd7 and Ehd1 are required for the critical daylength recognition, leading to Hd3a transcription (Itoh et al., 2010), in which Hd3a is turned on under photoperiods of less than 13.5 h. Ghd7 mRNA is induced by phytochrome signals that are gated by circadian-clock action in rice. Under LD conditions, the Ghd7 gate is open in the early morning when phytochromes perceive light signals, whereas under SD conditions the Ghd7 gate opens at midnight when light signals are normally absent. Note that, here, the increase in Ghd7 expression in the morning was only 2- to 3-fold when the daylength was extended from 10 to 16 h.
The Ghd7 expression induced in a morning under LD conditions (or after night-break treatments under SD conditions) can repress Ehd1 transcription the following morning (Itoh et al., 2010); in turn, Hd3a expression is also repressed since Ehd1 functions as an activator of Hd3a. In this floral pathway, Ghd7 mRNA expression varies up to 3-fold in response to daylength, whereas the changes of Ehd1 and Hd3a mRNA are up to 100-fold (Itoh et al., 2010). The molecular mechanisms to amplify the activity of Ghd7 and Ehd1 remain unknown.
Although analyses using the se5 chromophore-less mutant have provided many insights into the measurement of daylength in rice (Izawa et al., 2000, 2002; Itoh et al., 2010), it is impossible to evaluate the molecular contributions of each individual phytochrome in rice photoperiodic flowering by using the se5 mutant. Under LD conditions, some single and double phytochrome mutants of rice flower earlier than wild-type cv Nipponbare but significantly later than the se5 mutant (Takano et al., 2005). Therefore, by using single and double phytochrome mutants, the details of molecular function of each phytochrome can be dissected in the critical daylength recognition controlling florigen mRNA expression. In this work, we analyzed the expression of flowering-time genes such as Ghd7, Ehd1, Hd3a, and RFT1 in all six single and double phytochrome mutants under various light conditions. Our results clearly demonstrate that each rice phytochrome has a distinct role in controlling florigen gene expression and reveal multiple action points in the critical daylength recognition controlling expression of the florigen genes.
RESULTS
Role of Each Phytochrome Family Member in Ghd7 Expression
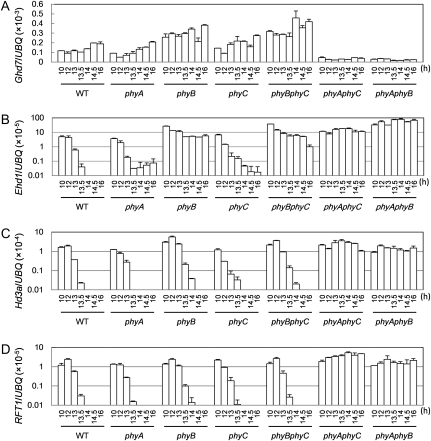
We previously performed gene expression analysis of flowering-time genes under various daylength conditions by using the se5 mutant and the parental wild-type cultivar, Norin 8 (Itoh et al., 2010). In that study, we found that the Hd3a florigen gene is toggled on under photoperiods of less than 13.5 h and that Ghd7 expression is gradually increased under longer photoperiods (Fig. 1; Itoh et al., 2010). Here, we performed very similar experiments using all six single and double phytochrome mutants and their parental wild-type cultivar, Nipponbare, under photoperiods from 10 to 16 h (Fig. 2). Refer their flowering-time phenotypes under the same growth conditions to Supplemental Figure S1. In wild type (Nipponbare), Hd3a mRNA was toggled on under photoperiods less than 13.5 h, as was previously shown for Norin 8 (Fig. 2C; Itoh et al., 2010). In wild type, Ghd7 mRNA levels gradually increased as photoperiods increased, which was also consistent with the previous results (Fig. 2A; Itoh et al., 2010). The transcription pattern of Ghd7 in phyA and phyC was comparable to that of wild type. In phyB and phyB phyC, Ghd7 mRNA was expressed to higher levels than in wild type, phyA, or phyC under all daylength conditions examined, although all of these lines responded to the extension of daylength (Fig. 2A). These results indicate that wild-type PHYB represses Ghd7 mRNA levels. In phyA phyC and phyA phyB, Ghd7 mRNA was not induced, as was also previously seen with the se5 mutant (Itoh et al., 2010).
Figure 1.
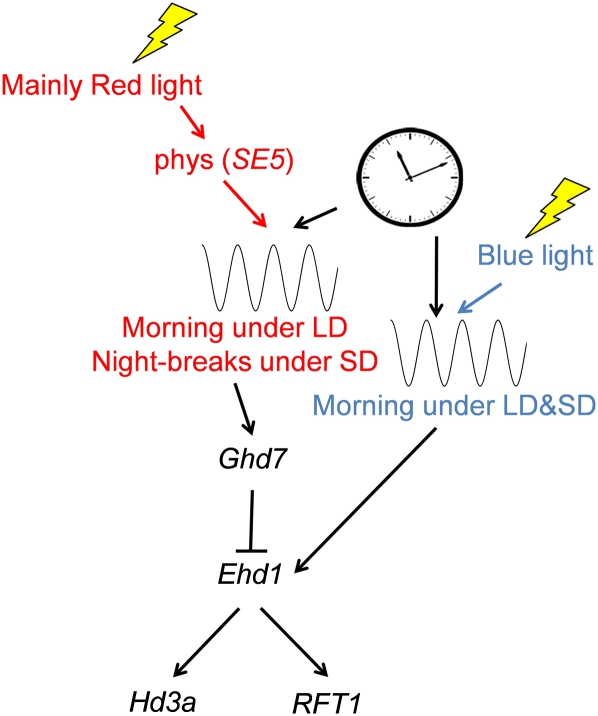
Previous model of daylength measurement in rice. Red and blue lines indicate red- and blue-light-mediated responses, respectively. According to this model, there are two gate mechanisms in rice: Ghd7 induction by red light both in morning under LD and at midnight with night break under SD and Ehd1 induction by blue light around dawn. In this model, phytochromes are required only for Ghd7 induction. This model was based on data reported in Itoh et al. (2010).
Figure 2.
Expression of Ghd7, Ehd1, Hd3a, and RFT1 in all possible single and double phytochrome mutants of rice under various daylength conditions. A to D, Quantitative reverse transcription (RT)-PCR analysis of Ghd7 (A), Ehd1 (B), Hd3a (C), and RFT1 (D). After plants were grown for 2 weeks under LD conditions, they were entrained under various daylengths for 5 d prior to sampling. The aboveground parts of the plants were collected at 3 h after dawn and used for RNA preparation. The x axis indicates daylength. The y axis indicates the expression levels relative to UBIQUITIN (UBQ), on a standard scale for A and a log scale for B to D. Bars and error bars indicate average values and sds, respectively, based on three biological repeats. Data are representative of at least three independent experiments.
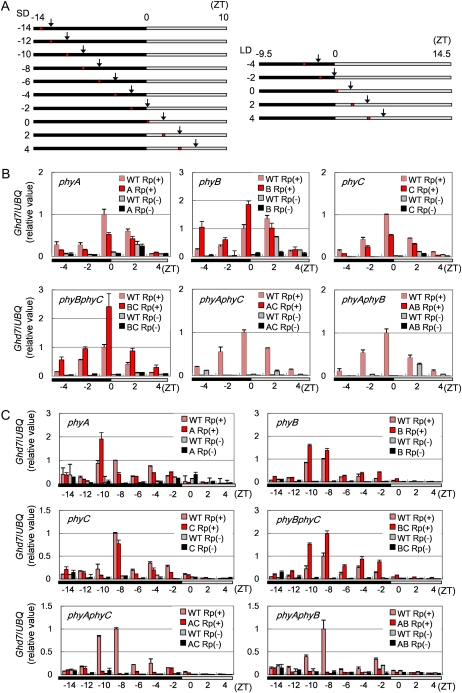
Since Ghd7 expression is gated by the circadian clock, there might be at least two possible actions of a phytochrome that affect the expression levels of Ghd7: an action involving the light signals mediated by phytochromes, or an action involving the gate phasing of Ghd7 through the phytochrome effect of entrainment of the circadian clock. In Arabidopsis, phytochromes function in clock input and various output pathways (Pruneda-Paz and Kay, 2010). In rice, however, phytochromes may not be necessary for clock input pathways in rice since loss of all active phytochromes did not disrupt the cyclic expression of a clock gene and clock-regulated genes under both light-dark cycles and constant conditions (Izawa et al., 2002). To examine which type of action altered Ghd7 expression in phytochrome mutants (Fig. 2A), we analyzed the effects on Ghd7 gate phasing in all single and double phytochrome mutants (Fig. 3). Plants grown under LD or SD conditions were transferred to continuous dark conditions and samples were collected at 2 h after irradiation with red light (Fig. 3A). In phyA phyC and phyA phyB, Ghd7 mRNA was not induced within any of the time zones tested under either LD or SD conditions (Fig. 3, B and C). Therefore, the effects of phytochrome on the Ghd7 gate could not be evaluated in these lines. In the other phytochrome mutants (phyA, phyB, phyC, and phyB phyC) Ghd7 expression was induced at similar times as wild type under both LD and SD conditions (Fig. 3, B and C). These data strongly suggest that phytochromes do not alter the timing of the light-sensitive phase for Ghd7 expression.
Figure 3.
Ghd7 induction by light signals at distinct phases of the circadian clock. A, Schematic diagrams of light treatments for the expression analysis. After entrainment under SD (right) and LD (left) conditions for 14 d, plants were transferred to continuous dark conditions at dusk. Plants were then exposed to a 10-min red-light pulse, and aboveground plant parts were collected 2 h after irradiation for RNA preparation. Black, gray, and red bars represent dark condition, subjective light condition, and red-light irradiation, respectively. Black arrows show when samples were collected. B to C, Quantitative RT-PCR analysis of Ghd7 mRNA in phytochrome mutants entrained under LD (B) or SD (C) conditions. Values on the x axis of each section correspond to the treatment and sampling patterns in A. The labels A, B, C, BC, AC, and AB in the figure keys indicate phyA, phyB, phyC, phyB phyC, phyA phyC, and phyA phyB, respectively. Rp(+) and Rp(−) indicate treatments with and without red-light pulse, respectively. Bars and error bars indicate average values and sds, respectively, based on three biological repeats. Data are representative of three or more independent experiments.
The fact that Ghd7 expression could be induced in phyB phyC (in which only PHYA is functional), but not in either phyA phyC (in which only PHYB is functional) or phyA phyB (in which only PHYC is functional) indicated that PHYA alone is sufficient to induce Ghd7 expression (Figs. 2A and 3). Furthermore, the fact that Ghd7 was expressed in the phyA single mutant, but not in either phyA phyC or phyA phyB, indicated that PHYB and PHYC together can induce Ghd7 expression. In Arabidopsis, phytochromes function as both homodimers and heterodimers (Sharrock and Clack, 2004; Clack et al., 2009); the same may be true of rice. Therefore, the observations reported in Figures 2A and 3 suggest that light signals mediated by both phyA/phyA homodimer and phyB/phyC heterodimer can induce Ghd7 expression but that phytochromes do not set up the Ghd7 gate in rice. The lack of induction of Ghd7 in phyA phyC and phyA phyB can be explained by the lack of both phyA/phyA and phyB/phyC dimers in these mutant lines. It is worth noting here again that phyB appears to function as a weak repressor of Ghd7 expression.
Role of Each Phytochrome Family Member in Ehd1 Expression
Next, we examined Ehd1 mRNA levels in all of the single and double phytochrome mutant lines under various daylength conditions (Fig. 2B). In wild type, phyA, and phyC, Ehd1 was highly expressed when daylength was shorter than 13.5 h, but severely repressed under photoperiods longer than 13.5 h, indicating a critical daylength recognition. In phyB, phyB phyC, phyA phyC, and phyA phyB, Ehd1 expression was derepressed under photoperiods longer 13.5 h, so Ehd1 expression levels were similar in these phytochrome mutants among all daylength conditions examined (Fig. 2B). We previously demonstrated that Ehd1 expression was induced by blue light and derepressed in a ghd7-deficient near-isogenic line, and that artificially induced Ghd7 expression resulted in severe repression of the Ehd1 mRNA expression normally induced by morning light (Itoh et al., 2010). Therefore, Ghd7 is a major repressor of Ehd1. Consistent with this model, the marked derepression of Ehd1 in phyA phyC and phyA phyB can be explained by loss of Ghd7 expression (Fig. 2, A and B). The derepression of Ehd1 under photoperiods longer than 13.5 h in phyB and phyB phyC, but not in the phyA and phyC single mutants, however, suggested that phyB enhances Ghd7 repressor activity under long photoperiods. OsCOL4, a CO-like gene, can repress Ehd1 mRNA, and the OsCOL4 gene is induced by phyB (Lee et al., 2010), so here we monitored OsCOL4 mRNA under various daylength conditions (Fig. 1; Supplemental Fig. S2). In the Nipponbare background, OsCOL4 mRNA was not down-regulated compared to wild type even in phyB and phyB phyC; therefore, no correlation between OsCOL4 and up-regulation of Ehd1 was observed. OsCOL4 is highly expressed in adult plants but not young plants (Lee et al., 2010), so in our experiments, the tested plants might have been too young for OsCOL4 to function. The OsMADS51 and Ehd2/OsID1/RID1 genes (Kim et al., 2007; Matsubara et al., 2008; Park et al., 2008; Wu et al., 2008), both of which regulate levels of Ehd1 mRNA, were also not significantly changed compared to wild type in phyB and phyB phyC (data not shown).
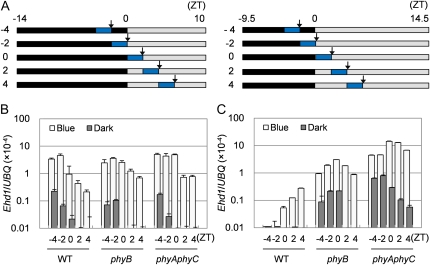
To evaluate the effects of phyB on the Ghd7-dependent repression of Ehd1 in more detail, we next examined Ehd1 induction under LD and SD conditions, when Ghd7 mRNA abundance is high and low, respectively. We have previously shown that Ehd1 is induced by blue light in the se5 mutant (Itoh et al., 2010). This blue-light induction was gated by the circadian clock and open mainly in the morning regardless of the photoperiod applied. Therefore, we examined Ehd1 mRNA induced by 2-h blue-light treatments at different timings administered to plants grown under SD and LD conditions and then transferred to continuous dark conditions (Fig. 4A). In plants grown under SD conditions, blue-light irradiation strongly induced Ehd1 mRNA to similar levels in wild type, phyB, and phyA phyC (Fig. 4B). As expected, the timing of blue-light treatment acutely affected the induced levels of Ehd1 mRNA, especially in wild type. In contrast, Ehd1 mRNA levels were very low under continuous dark conditions, although they appeared to have been affected somewhat by the circadian clock. In plants entrained under LD conditions, in which Ghd7 should be expressed in the morning, however, Ehd1 mRNA was differentially induced among the tested lines and also depended on the timing of blue-light irradiation; the expression levels were phyA phyC > phyB > wild type (Fig. 4C). Even in darkness, Ehd1 mRNA was derepressed in phyB and in phyA phyC compared to wild type; the derepression was stronger in phyA phyC than in phyB. These results imply that Ehd1 is not repressed in phyA phyC under LD conditions because Ghd7 expression is not induced in this mutant (Figs. 2A and 3, B and C). Since Ghd7 mRNA was induced to a higher level in phyB than in wild type (Fig. 2A), the ability of Ghd7 to repress Ehd1 is significantly weaker in phyB than in other genotypes. Furthermore, the observed derepression of Ehd1 mRNA under dark conditions after LD entrainment in phyB and phyA phyC (Fig. 4) indicates that the Ghd7 repressor function is likely to be independent of the blue-light-gated induction of Ehd1. Derepression of Ehd1 at 0, 2, and 4 h with/without blue-light pulses in phyAphyC were observed after LD entrainments compared with them after SD entrainments (Fig. 4, B and C). Although we could not explain all the results on these derepressions obtained in Figure 4, one main reason would be photoperiod-dependent variations of shapes of the Ehd1 gate between SD and LD conditions.
Figure 4.
Ehd1 induction by blue-light signals at different phases of the circadian clock. A, Schematic diagrams of light treatment for the expression analysis. After entrainment under (right) SD or (left) LD conditions for 14 d, plants were transferred to continuous dark conditions at dusk. Plants were exposed to 2 h of blue light at various timings. Aboveground plant parts were collected at the end of blue-light irradiation for RNA preparation. Black, gray, and blue bars represent dark condition, subjective light condition, and blue-light irradiation, respectively. Black arrows show when samples were collected. B to C, Quantitative RT-PCR analysis of Ehd1 mRNA in plants entrained under SD (B) or LD (C) conditions. Values on the x axis of each section correspond to the treatment/sampling patterns in A. Blue and Dark indicate treatments with and without blue-light pulse, respectively. Bars and error bars indicate average values and sds, respectively, based on three biological repeats. Data shown are representative of more than two independent experiments.
Roles of Each Phytochrome Family Member in Hd3a and RFT1 Expression
Morning expression of Hd3a and RFT1 under SD conditions is mainly regulated by Ehd1 (Doi et al., 2004). In addition, Ehd1 induces Hd3a mRNA in the morning after blue-light treatments (Itoh et al., 2010). Furthermore, Hd3a expression is strongly associated with Ehd1 expression in se5, an se5 circadian clock gene double mutant, and in a ghd7-deficient line (Itoh et al., 2010). Based on those findings, we proposed a simple model in which Ehd1 controls Hd3a and RFT1 expression (Fig. 1; Itoh et al., 2010).
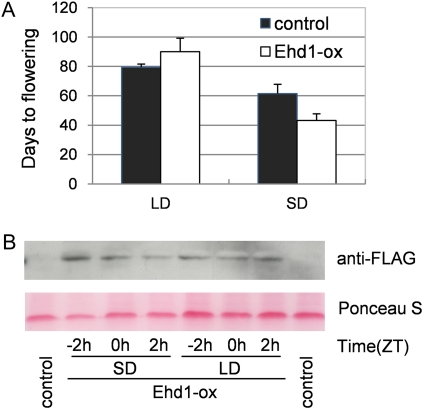
Here, we examined Hd3a and RFT1 mRNA levels in all possible single and double phytochrome mutants under various daylength conditions (Fig. 2, C and D). In phyA, phyB, phyC, and phyB phyC, Hd3a, and RFT1 mRNA exhibited weakened critical daylength recognition compared to wild type (Itoh et al., 2010), although phyA had a clear critical daylength around 13.5 h, like wild type. In contrast, both Hd3a and RFT1 were strongly derepressed in phyA phyC and phyA phyB, as predicted by a previous model (Fig. 1; Itoh et al., 2010). In addition, Ehd1 was expressed in phyB and phyB phyC under all daylength conditions (Fig. 2B). These findings indicate that the derepressed Ehd1 expression observed in phyB and phyB phyC did not promote Hd3a and RFT1 expression under longer daylengths, whereas the derepressed Ehd1 expression observed in phyA phyB and phyA phyC did promote Hd3a and RFT1 expression, suggesting that phyA can reduce the ability of Ehd1 to induce Hd3a and RFT1 under longer photoperiods. To further address this possibility, we generated transgenic plants overexpressing Ehd1. The rice cultivar Taichung 65, which lacks both Ehd1 and Hd1 (another Hd3a/RFT1 activator under SD conditions) was used as a host to eliminate the effects of Hd1 (Doi et al., 2004). The Ehd1 overexpressors flowered significantly earlier than nontransgenic Taichung 65 under SD conditions, but the flowering times were not significantly earlier under LD conditions (Fig. 5A). Overexpressing Ehd1 protein levels were not affected by different photoperiod conditions (Fig. 5B). Therefore, the activity of the ectopic Ehd1 was reduced under LD conditions. Taking this finding together with the data in Figure 2, C and D, we conclude that phyA reduces the ability of Ehd1 to promote Hd3a and RFT1 expression in a daylength-dependent manner. Alternatively, the Ghd7 expressed in phyB and phyB phyC may be involved in the critical daylength responses of Hd3a and RFT1 mRNA regardless of Ehd1 expression.
Figure 5.
Overexpression of Ehd1 can promote Hd3a and RFT1 expression only under SD (10 h L, 14 h D) conditions. A, Flowering time was measured in transgenic plants overexpressing Ehd1 (Ehd1-ox). The average value and sd are based on more than five individuals. Data from a representative transgenic line are shown. B, Top section, immunoblots of transgenic plants around dawn. Overexpressing Ehd1 protein was detected using anti-FLAG antibody. Bottom section, ponceau S staining membrane is shown as loading controls. Shown data are the representative of two independent experiments. Parent cultivar (Taichung 65) was used as control. [See online article for color version of this figure.]
DISCUSSION
Molecular Mechanisms of DayLength Recognition Related to Phytochrome Actions in Rice
To explain how plants measure daylength, physiological models have been proposed in which relevant photoreceptors have important roles in daylength recognition. Experimental evidence from molecular genetics studies has supported both an external coincidence model and an internal coincidence model (Itoh et al., 2010; Song et al., 2010). In the external coincidence model, a particular daylength is judged to be either inductive or noninductive based on whether the given external light signal overlaps with the photosensitive phase set by an internal circadian rhythm (Pittendrigh and Minis, 1964). In the internal coincidence model, the synchronization of two distinct internal rhythms, which are differentially entrained by a given photoperiod, determines the timing of floral induction (Pittendrigh, 1972). In the external coincidence model, photoreceptors have two distinct roles: the transition of light signals and the entrainment of the internal circadian rhythm. In contrast, the roles of photoreceptors in the internal coincidence model are more complex and would be involved in distinct entrainment of the two internal rhythms.
Molecular genetics studies in Arabidopsis have revealed that both models partially fit the data on photoperiodic control of flowering. First, the abundance of the FKF1-GIGANTEA (GI) complex, which induces CO mRNA, is regulated by the coincidence of FKF1 and GI transcripts, both of which are regulated by the circadian clock and adjusted to make more FKF1-GI complexes at the end of each photoperiod under LD conditions; this mechanism seems to fit the internal coincidence model (Imaizumi et al., 2003; Sawa et al., 2007). However, the FKF1 protein functions as a blue-light receptor to transmit light signals at the end of each photoperiod only under LD conditions, which fits well with the external coincidence model. Regulated by the FKF1-GI complex, CO mRNA has a biased diurnal rhythm and is most actively transcribed at the end of the light period under LD conditions (Yanovsky and Kay, 2002; Valverde et al., 2004; Sawa et al., 2007). Furthermore, CO protein is stabilized by the actions of phyA and cry2 and promotes expression of FT (an Arabidopsis florigen); this part of the molecular mechanism again fits the external coincidence model (Valverde et al., 2004). Recently, it was reported that this cry2 action was controlled by COP1 activity (Zuo et al., 2011). It has been also proposed that FKF1 may be involved in FT expression by modifying CO protein activity in addition to its role in the transcriptional regulation of CO (Salazar et al., 2009).
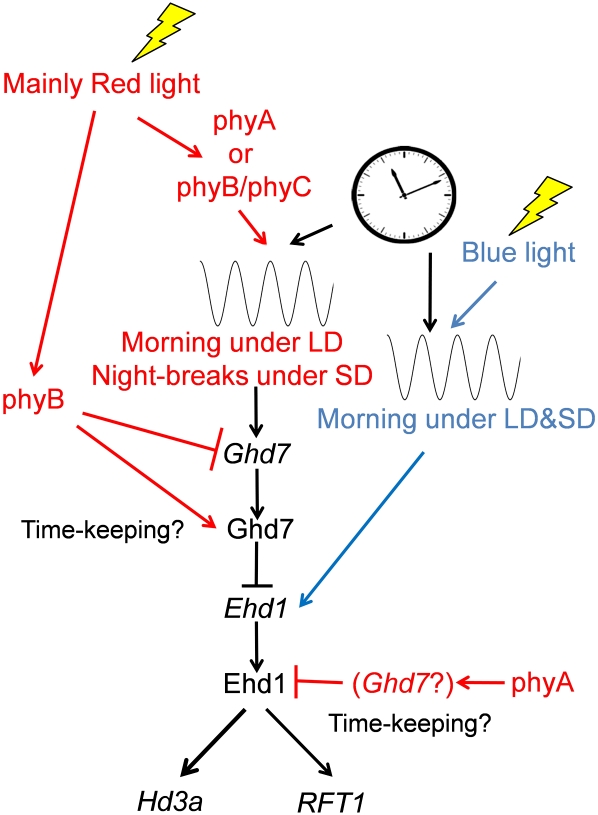
We previously showed that rice has a two-gate system to measure daylength (Itoh et al., 2010). The gate regulated by circadian clocks can enhance the perception of stimulants within a specific sensitive phase. When the stimulants are light signals and the gate phases are set around dusk or dawn, this gating mechanism becomes equivalent to the external coincidence model. When a given photoperiod differentially sets two distinct gates, both of which affect flowering time, the action of these gates becomes similar to the internal coincidence model; however, the mechanism does not completely fit an internal coincidence model because the external light signals still play critical roles. One of the gates in rice is for the induction of Ghd7 by phytochrome signals. Under LD conditions, the Ghd7 gate opens at around dawn, whereas under SD conditions, the Ghd7 gate opens at around midnight. Since our study revealed that phyA/phyA homodimer and phyB/phyC heterodimer signals induce Ghd7 mRNA (Figs. 2A and 3), the machinery of Ghd7 expression fits well with the external coincidence model. However, the gate adjustment of Ghd7 expression is complex and itself exhibits a somewhat daylength dependence. Therefore, how the gate of Ghd7 transcription is regulated remains an open question. In this work, we also revealed that Ghd7 activity is modified by phyB action. Since we have previously revealed that loss of Ghd7 function resulted in strong derepression under LD conditions (Itoh et al., 2010), we could exclude a possibility, with what other repressor of Ehd1 expression is modified by phyB. It is likely that this phyB action is also involved in the critical daylength recognition of florigen expression (Fig. 4). Another gate in rice photoperiodic flowering is for induction of Ehd1 by blue-light signals. Since the Ehd1 gate opens at around dawn regardless of daylength, this Ehd1 gate itself does not fit well with the external coincidence model. However, the LD-prominent repression by Ghd7 increases Ehd1 mRNA under SD conditions more than under LD conditions (Itoh et al., 2010). In this work, we revealed that although Ehd1 protein levels were not affected by photoperiods the Ehd1 activity to control Hd3a and RFT1 mRNA expression is modified by daylength (Fig. 5); this modification is achieved by some action of phyA (Fig. 2). Taken together, the molecular mechanisms that confer daylength recognition are rather complex and do not easily fit traditional physiological models, such as the external and internal coincidence models. With respect to phytochrome actions in rice, we revealed at least four action points of phytochromes in photoperiodic flowering (Fig. 6): (1) phyA/phyA homodimer or phyB/phyC heterodimer or both induce Ghd7 transcripts based on light signals (Fig. 2A); (2) phyB slightly represses Ghd7 transcript levels (Fig. 2A); (3) phyB enhances Ghd7 repressor activity, dependent on daylength (Figs. 2B and 4); and (4) phyA represses Ehd1 activity, dependent on daylength (Fig. 2, C and D), possibly through Ghd7 action (Fig. 2A). These events occur in addition to possible mechanisms to entrain circadian clocks by phytochrome signals (which could be minor effects, as shown by the results in Fig. 3). All of these actions of phytochromes confer the critical daylength recognition of florigen expression in rice.
Figure 6.
Newly proposed model of daylength measurement in rice. Red and blue lines indicate red- and blue-light-mediated responses, respectively. In addition to the two gate mechanisms, there are two phytochrome-dependent controls for time-keeping mechanisms in rice: LD-prominent promotion of Ghd7 repressor activity by phyB and LD-prominent inhibition of Ehd1 activity by phyA.
Flowering-Time Phenotypes and Florigen Expression in Phytochrome Mutants
In this study, we revealed that phytochromes have various action points in the regulation of florigen expression in rice. To relate the contribution of each phytochrome family member to flowering-time phenotype, we compared flowering time in each phytochrome mutant line under the same growth chamber conditions that were used for the florigen expression analysis (Supplemental Fig. S1).
phyA phyC and phyA phyB flowered much earlier under LD conditions (14.5 h) compared with other mutants and seemed to be insensitive to photoperiod (Supplemental Fig. S1). These flowering-time phenotypes were similar to that of the se5 mutant and consistent with the fact that Ghd7 expression was not induced in phyA phyC and phyA phyB, whereas Ehd1, Hd3a, and RFT1 were derepressed in these mutants regardless of daylength (Fig. 2). Indeed, in phyA phyC and phyA phyB, Hd3a and RFT1 were highly expressed under LD conditions (14.5 h) in rice seedlings around 2 weeks after germination, whereas in phyA, phyB, phyC, and phyB phyC, Hd3a and RFT1 were not expressed under these same conditions (Fig. 2).
Consistently with the expression data, phyA, phyB, phyC, and phyB phyC flowered later than phyA phyC and phyA phyB. Here, phyB and phyB phyC flowered around 10 d earlier than phyA, phyC, and wild type under LD conditions (Supplemental Fig. S1). We were unable to predict this 10-d-early flowering from the gene expression results because we examined gene expression in 2-week-old seedlings. The pattern of expression of florigen genes under LD (14.5 h) at this stage was not predictive with the 10-d-early flowering in phyB and phyB phyC mutants. In this work, we revealed that phyB represses Ehd1 expression by modifying Ghd7 activity (Fig. 4) and that Ehd1 mRNA was highly expressed under all daylength conditions examined in phyB and phyB phyC rice seedlings (Fig. 2B). Thus, these 10-d-early flowering phenotypes in phyB and phyB phyC might be associated with this regulation of florigen expression. Other independent molecular mechanisms may explain the 10-d-early flowering. For instance, it is possible that reduction of OsCOL4 mRNA in phyB (Lee et al., 2010) in later stages may accelerate flowering, although we observed that OsCOL4 expression was not reduced in young phyB seedlings (Supplemental Fig. S2). To address this question, gene expression around the timing of floral transition for each mutant line should be examined in the future.
We also revealed that phyA is required for Ehd1 to induce Hd3a and RFT1 under LD conditions (Figs. 2 and 4). Hence, the absence of phyA might induce more drastic derepression of Hd3a/RFT1 under LD conditions (>13.5 h) and earlier flowering in phyA phyB than in phyB. Note that, here, Hd3a and RFT1 were not induced much by Ehd1 in phyA under LD conditions because of the low abundance of Ehd1 mRNA (Fig. 2).
In this study, flowering time was not significantly altered in phyC under either SD (data not shown) or LD conditions (Supplemental Fig. S1). However, phyC reportedly flowered early under natural LD conditions (Takano et al., 2005). In addition, when we grew plants in a growth chamber other than those used in this study, we also observed early flowering in phyC under LD conditions (data not shown). These results suggest that PHYC may transmit other stimuli related to light signals, such as light quality or quantity, to control flowering time, in addition to photoperiod.
CONCLUSION
In this work, we identified several action points where each phytochrome family member influences the critical daylength recognition of florigen genes in rice. To our knowledge, this is the first study in which multiple action points of all members of a plant photoreceptor family have been identified in a biological genetic network. In particular, our results imply that some time-keeping molecular mechanisms exist beside the phytochrome signaling in rice, possibly independent of the circadian clock (Fig. 6). The possible action of phyB to enhance Ghd7 activity and thus to control Ehd1 expression, and the possible action of phyA to reduce Ehd1 activity and thus control Hd3a/RFT1 expression, are likely to be both daylength sensitive. In addition to elucidating how light conditions contribute to the formation of the Ghd7 and Ehd1 gates, these results raise new questions as to the molecular mechanisms by which rice phytochromes measure daylength.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
The japonica rice (Oryza sativa ‘Nipponbare’) was used as the wild type. phyA, phyB, phyC, phyB phyC, phyA phyC, and phyA phyB represent the previously described alleles phyA-2, phyB-1, phyC-1, phyB-1 phyC-1, phyA-2 phyC-1, and phyA-2 phyB-1, respectively (Takano et al., 2005). Plants were grown in growth chambers (Especmic) at 70% humidity under SD (10 h of light at 28°C and 14 h of dark at 24°C) or LD (14.5 h of light at 28°C and 9.5 h of dark at 24°C) conditions. Light was provided by a metal-halide lamp (photosynthetic photon flux density of 450 μmol m−2 s−1). For different light regimes (Figs. 3 and 4), other climate chambers (SANYO) were used. Light was provided by light-emitting diodes for red (70 μmol m−2 s−1) and blue (120 μmol m−2 s−1) light (SANYO). Flowering time was defined as the time when the first panicle emerged from the node.
Analysis of Gene Expression
Total RNA was extracted from the aboveground parts of plants by using an RNeasy plant mini kit (QIAGEN) for Figure 3 or an SDS-phenol method for Figures 2 and 4. cDNA was synthesized from 2 μg of total RNA by using dT12-18 primer (Invitrogen) and SuperScript II reverse transcriptase (Invitrogen). cDNA corresponding to 120 ng of total RNA was used as the template for quantitative analysis of gene expression. Reactions were performed with a TaqMan PCR master mix (Nippongene) in a 20-μL total volume. Data were collected by using the ABI PRISM 7700 sequence detection system in accordance with the manufacturer’s instructions. Gene-specific primer sets used in this study are listed in Supplemental Table S1.
Ehd1 Plasmid Construction and Plant Transformation
Ehd1 cDNA was amplified by using PCR with the primers 5′-GAGATATCCATGGATCACCGAGAGCTGTGG-3′ and 5′-GAGCTCCATATGGATGTGGATCATGAG-3′, and Nipponbare cDNA as the template. The PCR products were first cloned into the subcloning vector pCR BluntII-TOPO (Invitrogen) and their sequences were verified. Insertions were digested with EcoRV and SacI, and ligated into the EcoRV and SacI restriction sites of pKS221. The StrepII and 3xFLAG tags, amplified by means of PCR using the primers 5′-GACTCTAGAATGGCTAGCTG-3′ and 5′-CATGGATCCCTTATCATCATC-3′, were digested with XbaI and BamHI, and ligated into the XbaI and BamHI restriction sites of pKS221 containing the Ehd1 fragment. This insertion, containing the tags and Ehd1, was amplified by means of PCR using the primers 5′-GACTCTAGAATGGCTAGCTG-3′ and 5′-GAGCTCCATATGGATGTGGATCATGAG-3′, and cloned into the entry vector pCR 8/GW/TOPO (Invitrogen). This entry clone was used in the LR reaction with the modified pPZP2H-lac destination vector; the rice Actin1 promoter was inserted into pPZP2H-lac digested with KpnI and SacI. The LR reaction was performed as described in the manufacturer’s manual (Invitrogen). The generated binary plasmids were introduced into Agrobacterium tumefaciens strain EHA101 by use of electroporation. The empty pPZP2H-lac vector was used as a control. Plasmids were transformed into cultivar Taichung 65 according to published protocols. Several independent transgenic plants were selected based on hygromycin resistance. Plants were transplanted into soil and grown as described above. We also made tag-less Ehd1 overexpressing lines, those showed very similar flowering-time responses.
Immunoblotting
Plants grown under SD or LD conditions for 2 weeks were extracted with buffer containing 100 mm Tris-HCl (pH 8.0), 200 mm EDTA (pH 8.0), 1% 2-mercaptoethanol, and protease inhibitors (complete mini EDTA-free, Roche). Total soluble protein was obtained by 40% saturation ammonium sulfate precipitation. Five micrograms of protein was loaded on each lane in SDS-PAGE. To detect recombinant protein, anti-FLAG (monoclonal ANTI-FLAG M2-peroxidase clone M2, SIGMA-ALDRICH) were used.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers as follows: Ghd7 (EU286801), Ehd1 (AB092506), Hd3a (AB052942), RFT1 (AB062676), OsCOL4 (AK058536), PHYA (AB109891), PHYB (AB109892), and PHYC (AB18442).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Flowering time under LD conditions (14.5 h L, 9.5 h D) in phytochrome mutants.
Supplemental Figure S2. Expression levels of OsCOL4 in all single and double phytochrome mutants under various daylength conditions.
Supplemental Table S1. Primer and probe sequences used in this study.
References
- Borthwick HA. (1964) Phytochrome action and its time displays. Am Nat 98: 347–355 [Google Scholar]
- Clack T, Shokry A, Moffet M, Liu P, Faul M, Sharrock RA. (2009) Obligate heterodimerization of Arabidopsis phytochromes C and E and interaction with the PIF3 basic helix-loop-helix transcription factor. Plant Cell 21: 786–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi K, Izawa T, Fuse T, Yamanouchi U, Kubo T, Shimatani Z, Yano M, Yoshimura A. (2004) Ehd1, a B-type response regulator in rice, confers short-day promotion of flowering and controls FT-like gene expression independently of Hd1. Genes Dev 18: 926–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KA, Quail PH. (2010) Phytochrome functions in Arabidopsis development. J Exp Bot 61: 11–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanumappa M, Pratt LH, Cordonnier-Pratt MM, Deitzer GF. (1999) A photoperiod-insensitive barley line contains a light-labile phytochrome B. Plant Physiol 119: 1033–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T, Tran HG, Swartz TE, Briggs WR, Kay SA. (2003) FKF1 is essential for photoperiodic-specific light signalling in Arabidopsis. Nature 426: 302–306 [DOI] [PubMed] [Google Scholar]
- Itoh H, Nonoue Y, Yano M, Izawa T. (2010) A pair of floral regulators sets critical day length for Hd3a florigen expression in rice. Nat Genet 42: 635–638 [DOI] [PubMed] [Google Scholar]
- Izawa T. (2007) Adaptation of flowering-time by natural and artificial selection in Arabidopsis and rice. J Exp Bot 58: 3091–3097 [DOI] [PubMed] [Google Scholar]
- Izawa T, Oikawa T, Sugiyama N, Tanisaka T, Yano M, Shimamoto K. (2002) Phytochrome mediates the external light signal to repress FT orthologs in photoperiodic flowering of rice. Genes Dev 16: 2006–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa T, Oikawa T, Tokutomi S, Okuno K, Shimamoto K. (2000) Phytochromes confer the photoperiodic control of flowering in rice (a short-day plant). Plant J 22: 391–399 [DOI] [PubMed] [Google Scholar]
- Izawa T, Takahashi Y, Yano M. (2003) Comparative biology comes into bloom: genomic and genetic comparison of flowering pathways in rice and Arabidopsis. Curr Opin Plant Biol 6: 113–120 [DOI] [PubMed] [Google Scholar]
- Kim SL, Lee S, Kim HJ, Nam HG, An G. (2007) OsMADS51 is a short-day flowering promoter that functions upstream of Ehd1, OsMADS14, and Hd3a. Plant Physiol 145: 1484–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Takahashi Y, Kobayashi Y, Monna L, Sasaki T, Araki T, Yano M. (2002) Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol 43: 1096–1105 [DOI] [PubMed] [Google Scholar]
- Komiya R, Ikegami A, Tamaki S, Yokoi S, Shimamoto K. (2008) Hd3a and RFT1 are essential for flowering in rice. Development 135: 767–774 [DOI] [PubMed] [Google Scholar]
- Lee YS, Jeong DH, Lee DY, Yi J, Ryu CH, Kim SL, Jeong HJ, Choi SC, Jin P, Yang J, et al. (2010) OsCOL4 is a constitutive flowering repressor upstream of Ehd1 and downstream of OsphyB. Plant J 63: 18–30 [DOI] [PubMed] [Google Scholar]
- Liu LJ, Zhang YC, Li QH, Sang Y, Mao J, Lian HL, Wang L, Yang HQ. (2008) COP1-mediated ubiquitination of CONSTANS is implicated in cryptochrome regulation of flowering in Arabidopsis. Plant Cell 20: 292–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara K, Yamanouchi U, Wang ZX, Minobe Y, Izawa T, Yano M. (2008) Ehd2, a rice ortholog of the maize INDETERMINATE1 gene, promotes flowering by up-regulating Ehd1. Plant Physiol 148: 1425–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Kim SL, Lee S, Je BI, Piao HL, Park SH, Kim CM, Ryu CH, Park SH, Xuan YH, et al. (2008) Rice Indeterminate 1 (OsId1) is necessary for the expression of Ehd1 (Early heading date 1) regardless of photoperiod. Plant J 56: 1018–1029 [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS. (1972) Circadian surfaces and the diversity of possible roles of circadian organization in photoperiodic induction. Proc Natl Acad Sci USA 69: 2734–2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS, Minis DH. (1964) The entrainment of circadian oscillations by light and their role as photoperiodic clocks. Am Nat 98: 261–264 [Google Scholar]
- Pruneda-Paz JL, Kay SA. (2010) An expanding universe of circadian networks in higher plants. Trends Plant Sci 15: 259–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar JD, Saithong T, Brown PE, Foreman J, Locke JC, Halliday KJ, Carré IA, Rand DA, Millar AJ. (2009) Prediction of photoperiodic regulators from quantitative gene circuit models. Cell 139: 1170–1179 [DOI] [PubMed] [Google Scholar]
- Sawa M, Nusinow DA, Kay SA, Imaizumi T. (2007) FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science 318: 261–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrock RA, Clack T. (2004) Heterodimerization of type II phytochromes in Arabidopsis. Proc Natl Acad Sci USA 101: 11500–11505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YH, Ito S, Imaizumi T. (2010) Similarities in the circadian clock and photoperiodism in plants. Curr Opin Plant Biol 13: 594–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano M, Inagaki N, Xie X, Yuzurihara N, Hihara F, Ishizuka T, Yano M, Nishimura M, Miyao A, Hirochika H, et al. (2005) Distinct and cooperative functions of phytochromes A, B, and C in the control of deetiolation and flowering in rice. Plant Cell 17: 3311–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K. (2007) Hd3a protein is a mobile flowering signal in rice. Science 316: 1033–1036 [DOI] [PubMed] [Google Scholar]
- Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G. (2004) Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 303: 1003–1006 [DOI] [PubMed] [Google Scholar]
- Wu C, You C, Li C, Long T, Chen G, Byrne ME, Zhang Q. (2008) RID1, encoding a Cys2/His2-type zinc finger transcription factor, acts as a master switch from vegetative to floral development in rice. Proc Natl Acad Sci USA 105: 12915–12920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Xing Y, Weng X, Zhao Y, Tang W, Wang L, Zhou H, Yu S, Xu C, Li X, et al. (2008) Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet 40: 761–767 [DOI] [PubMed] [Google Scholar]
- Yano M, Katayose Y, Ashikari M, Yamanouchi U, Monna L, Fuse T, Baba T, Yamamoto K, Umehara Y, Nagamura Y, et al. (2000) Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 12: 2473–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovsky MJ, Kay SA. (2002) Molecular basis of seasonal time measurement in Arabidopsis. Nature 419: 308–312 [DOI] [PubMed] [Google Scholar]
- Zuo Z, Liu H, Liu B, Liu X, Lin C. (2011) Blue light-dependent interaction of CRY2 with SPA1 regulates COP1 activity and floral initiation in Arabidopsis. Curr Biol 21: 841–847 [DOI] [PMC free article] [PubMed] [Google Scholar]