Abstract
Aims
Previous studies have demonstrated the therapeutic potential for human embryonic stem cell-derived neural precursor cells (hES-NPCs) in autoimmune and genetic animal models of demyelinating diseases. Herein, we tested whether intravenous (i.v) administration of hES-NPCs would impact central nervous system (CNS) demyelination in a cuprizone model of demyelination.
Methods
C57Bl/6 mice were fed cuprizone (0.2%) for two weeks and then separated into two groups that either received an i.v. injection of hES-NPCs or i.v. administration of media without these cells. After an additional two weeks of dietary cuprizone treatment, CNS tissues were analyzed for detection of transplanted cells and differences in myelination in the region of the corpus callosum (CC).
Results
Cuprizone-induced demyelination in the CC was significantly reduced in mice treated with hES-NPCs compared with cuprizone-treated controls that did not receive stem cells. hES-NPCs were identified within the brain tissues of treated mice and revealed migration of transplanted cells into the CNS. A limited number of human cells were found to express the mature oligodendrocyte marker, O1, or the astrocyte marker, GFAP. Reduced apoptosis and attenuated microglial and astrocytic responses were also observed in the CC of hES-NPC-treated mice.
Conclusions
These findings indicated that systemically-administered hES-NPCs migrated from circulation into a demyelinated lesion within the CNS and effectively reduced demyelination. Observed reductions in astrocyte and microglial responses, and (c) the benefit of hES-NPC treatment in this model of myelin injury was not obviously accountable to tissue replacement by exogenously administered cells.
Keywords: embryonic stem cell, microglia, demyelination, differentiation, oligodendrocyte
Introduction
Cuprizone is a copper chelator that, when administered orally to rodents in lab chow, evokes central nervous system (CNS) demyelination predominantly in the corpus callosum (CC; [1]). Myelin loss during cuprizone intoxication is associated with a robust microglial response accompanied by recruitment of peripheral macrophages [2, 3], which precedes a later, more modest infiltration of T-cells into the demyelinated region [4]. In the cuprizone model of demyelination, phagocytes, brain-resident microglia and infiltrating peripheral macrophages play a central role in myelin injury [4, 5]. Cuprizone-induced demyelination differs from experimental autoimmune encephalomyelitis (EAE) models in the weakness of the T-cell response and reported absence of increased blood brain barrier permeability during demyelination [3, 6]. Furthermore, unlike autoimmune and viral models of demyelination that produce sporadic and asynchronous lesions in the CNS, cuprizone-induced myelin injury is predictable and produces peak demyelination in the CC after four consecutive weeks of treatment [1]. Thus, cuprizone treatment of mice is a system well suited for study on the potential of stem cell (SC) therapies.
Previous studies have demonstrated the potential for intravenous SC-based therapies to address CNS demyelination. For instance, in an EAE model of demyelination, it has been reported that a primary action of systemically administered SCs is anti-inflammatory [7, 8]. Pluchino and colleagues showed that intravenously injected murine neural precursor cells (NPCs) remained undifferentiated at sites of injury in the CNS and induced cell death in pro-inflammatory type 1 helper T-cells [9]. Thus, in this immune-mediated model of demyelination, SCs modify host immune responses rather than directly foster myelin repair. These findings support the clinical application of SCs for treating autoimmunity in multiple sclerosis (MS) patients [10, 11]. However, another important aspect of SC-based studies is the question of regeneration of myelin through exogenously applied SCs.
The cuprizone model provides an ideal setting to evaluate stem cell potential for preserving or repairing myelin in the absence of the autoimmune component that is inherent to immune-mediated models. A previous study by Bar-Or and others reported that intracerebral administration of murine neural progenitor cells enhanced myelination in the cuprizone model by fostering myelination by endogenous progenitor cells [12]. In this study we elected to study the effect of human embryonic stem cell-derived NPCs (hES-NPCs) in the cuprizone model of primary demyelination. Herein, we report that intravenous (i.v.) administration of hES-NPCs into mice significantly reduced cuprizone-induced demyelination.
Materials and Methods
hES-NPC Culture and Derivation
Human embryonic stem cells (hESCs; line WA09) were used to generate NPCs as previously described [13]. To allow for identification of the hES-NPCs following i.v. injection, hES-NPC monolayers were infected with lentivirus expressing dsRed prior to their in vivo administration (SIN18.PRRL.hPGK-dsRED express.WPRE) to allow for identification following i.v. injection [14–16].
Cuprizone-induced Demyelination and hES-NPC Administration
All procedures involving the use of mice were approved by the institutional animal care and use committee at The Scripps Research Institute. C57Bl/6 mice were fed cuprizone (0.2% w/w; biscyclohexanoneoxaldihydrazone, Sigma) in powdered rodent lab chow for a period of four consecutive weeks as described previously [2]. Two weeks following the initiation of the cuprizone diet, mice were injected intravenously into the tail vein with either 100ul of media used to grow hES-NPCs (n=4) or an equivalent volume that contained 5x10E5 hES-NPCs (n=6). Mice from both treatment groups were kept on the cuprizone diet for an additional two weeks following i.v. injections of either media (vehicle) or hES-NPCs. An additional group of mice that received powdered lab chow without the addition of cuprizone was also used as a non-lesion control for comparative analyses. At the four-week time point, brains were extracted, fixed in 10% formalin and paraffin embedded. Coronal brain tissue sections (3 μm thick) were stained with Luxol Fast Blue-PAS using a standard protocol [17], or processed for immunostaining (see below).
Immunohistochemistry
Immunohistochemical staining was performed as previously described [18] using the following primary antibodies: O1 (1:2000; Abcam, Cambridge, MA), DsRed (1:2000; BD Biosciences, San Jose, CA), Iba-1 (1:1000; Wako, Richmond, VA), Myelin Basic Protein (1:3000; Millipore, Billerica, MA), Human Nuclear Antigen (1:200; Millipore) and Glial Fibrillary Acidic Protein (1:1000; Dako, Carpinteria, CA).
Apoptosis Detection Assay
Detection of apoptosis was carried out using an Apoptag®Red In Situ Apoptosis Detection Kit (Millipore) according to manufacturer’s protocol. Briefly, sections were de-paraffinized in xylene and re-hydrated in decreasing concentrations of ethanol. Sections were digested with 20 μg/ml proteinase K (Invitrogen, Carlsbad, CA, USA), washed in 1x PBS and incubated with terminal deoxynucleotidyl transferase (TdT) for 1 h at 37°C. Sections were washed with provided Stop/Wash buffer, rinsed three times in 1x PBS and incubated with anti-digoxigenin conjugate for 30 min at room temperature. Sections were washed four times in 1x PBS, dehydrated through increasing concentrations of ethanol and mounted in Fluoromount-G (SouthernBiotech, Birmingham, AL, USA). Negative controls were prepared as described with 1x PBS substituted for TdT. The number of Apotag+ cells was determined in each section (n=3/subject; n=3-4 per treatment group) at 40x magnification by an experimenter who performed the analysis on coded samples.
Quantitative Evaluation of the Corpus Callosum
Coronal brain tissue sections [0.98mm to 0.26mm rostral to bregma according to the atlas of Franklin and Paxinos (2001)] were used for all analyses. For myelin quantification, antisera against myelin basic protein (MBP) was visualized by fluorescent immunostaining intensity (and therefore myelin quantity) was quantified using Image J analysis software (NIH Imaging; http://rsb.info.nih.gov.ij) as described previously [17]. Briefly, digital images were captured at the same time for all samples using identical exposure times and compensation settings. For each image the region of interest (ROI) was the field of view at 40x magnification. Analyses were performed on 3 sections/subject with a minimum 3–4 subjects/group. For quantification of GFAP+ astrocytes and Iba-1+ microglia, immunostained sections were examined at 20x magnification and digital images of the corpus callosum were collected using identical settings. Images were then processed to identify and count the number of objects in each image using automated image analysis (ImageJ). The number of immunopositive cells in at least 3 sections/subject were then averaged and the numbers calculated for 3–4 subjects/group per experimental treatment for comparison and statistical analyses.
Statistical Analyses
Grouped data were analyzed by a one-way ANOVA followed by Dunnet post-hoc test for direct intergroup comparisons. Differences were considered significant when P<0.05.
Results
hES-NPC administration attenuates cuprizone-induced demyelination
Groups of cuprizone-treated mice received either hES-NPCs (5x10E5 cells/100ul; i.v.) or an equivalent volume of media (controls) two weeks into the four week course of cuprizone. Two weeks following administration of either hES-NPCs or media, brain tissues were collected and analyzed histologically. Staining of myelin with luxol fast blue or myelin basic protein (MBP) immunohistofluorescence revealed consistent and profound loss of myelin within the CC of cuprizone-exposed mice in the control treatment group compared with cuprizone-treated mice (Figure 1A,D vs Figure 1B,E, respectively). In contrast, cuprizone-fed mice also administered hES-NPCs exhibited a marked reduction of myelin loss in the CC (Figure 1C,F). To quantitatively assess demyelination among cuprizone-treated and control groups, coronal tissue sections from each subject were stained using fluoromyelin and the relative fluorescent intensity of staining in the CC was measured (Figure 1G). This analysis confirmed that cuprizone induced a significant loss of myelin in the CC (one-way ANOVA, P=0.0002; Dunnet posthoc, P<0.001 vs. no cuprizone-treated control) and hES-NPC treatment provided a significant reduction in the demyelinating effects of cuprizone (Dunnet posthoc; P<0.001 vs. cuprizone alone) although myelin loss was not completely abated (Dunnet posthoc; P<0.05 vs. no cuprizone control).
Figure 1. Cuprizone-induced demyelination in the corpus callosum is attenuated by systemic administration of hES-derived neural precursor cells.
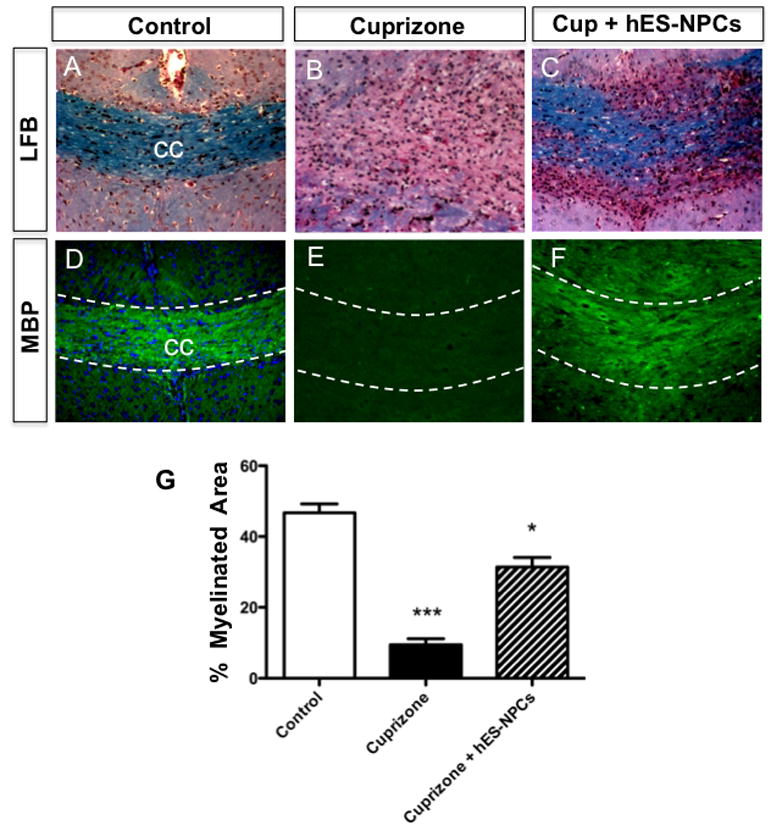
Representative images of myelin staining in the corpus callosum (CC) of coronal brain tissue sections from mice fed powdered chow without cuprizone (n=6) (A,D), mice fed cuprizone diet (n=4; B,E) or cuprizone-treated mice treated concurrently with hES-NPCs (n=6; C,F). Histological staining of myelin in adjacent tissues by Luxol Fast Blue (A-C) and immunohistofluoresent histochemistry for myelin basic protein (D-F) both demonstrated consistent patterns of myelin staining with these two techniques and revealed more myelin was present in the CC in cuprizone-treated that received hES-NPCs those treated with cuprizone alone. The data in panels D-F were quantified (G). hES-NPCs administration has a significant effect, with increased myelin content among treated mice (one-way ANOVA; P<0.0002; *** differs significantly from hES-NPC treatment and control groups (P<0.001); * differs significantly from control (P<0.05).
Detection of hES-NPCs in murine brain following i.v. administration
We sought to address the possible mechanisms by which hES-NPCs affected cuprizone-induced demyelination. First, we determined whether systemically-administered hES-NPCs entered the brains of cuprizone-fed mice. To evaluate the transmigration of hES-NPCs from peripheral circulation into the CNS, we first performed immunohistochemistry on coronal brain sections using an antibody against human nuclear antigen (hNA). Positive hNA immunostaining was intense, punctate and perinuclear (Figure 2A-C). Positive nuclei were observed with a sparse distribution in cerebral cortex, periventricular region and also the CC. Positive human nuclei immunostaining was detected only in the hES-NPC-treated mice. These results demonstrate the ability of hES-NPCs to migrate into the mouse brain.
Figure 2. Detection of human cells in cuprizone-treated mice following intravenous administration of hES-NPCs.
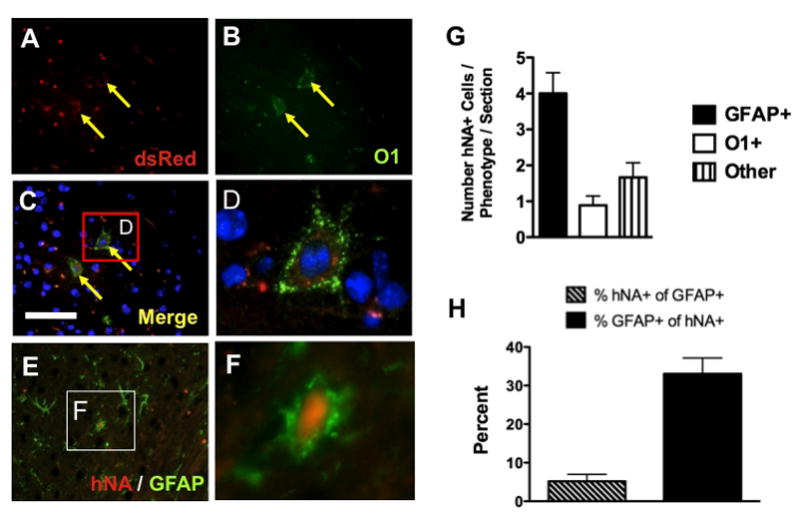
Immunohistochemical detection of hES-NPCs expressing dsRed (red, A) that also expressed the mature oligodendrocyte marker, O1 (green, B). Merged image with nuclei countered stained with DAPI (blue; C,D) and highlighted box (C) showing expression of O1 and dsRed co-localized in a hES-NPC-derived cell (D). hES-NPCs were also detected using antisera against human nuclear antigen (hNA), and, when co-labeled with antisera against glial fibrillary acidic protein (GFAP+), hES-NPC-derived cells were also identified as expressing this astrocyte marker (E), where, at higher magnification (inset in E) these cells exhibited an astrocyte morphology (F). Quantitative analysis of hES-NPC-derived cells in brain tissue sections (n=3 sections/subject; n=3–4 subjects) was performed in the rostral CC to determine the fates of these cells in these tissues as either GFAP+ (astrocytes), O1+ (oligodendrocytes) or hNA+/O1-/GFAP- cells (others) (G). Numbers of hNA+/GFAP+ cells were then calculated relative to the number of GFAP+ in each field and compared as a proportion of hES-NPC-derived cells (H).
Next, we determined whether or not these cells differentiated in situ. The cells were identified by exploiting the dsRed lentiviral labeling of the hES-NPCs, and their differentiation status was determined by evaluating expression of the mature oligodendrocyte marker O1+ (Figure 2D-F). Immunohistochemical detection of dsRed was required because the native fluorescence of the dsRed in these brain tissues was negatively affected by the formalin fixation and paraffin-embedding. Analysis of the CCs of hES-NPC treated cuprizone-fed mice identified dsRed+ cells that were also O1+ (Figure 2). The absolute number of hES-NPCs that expressed O1 was very few with a maximum ~2 cells/field of view (60x magnification) (Figure 2G). We also observed hES-NPCs in the CC that did not label with the oligodendrocyte marker, O1, but were found to co-label with the astrocyte marker glial fibrillary acidic protein (GFAP). These astrocytes were also identified by their expression of human nuclear antigen (hNA) (Figure 2E,F). The relative numbers of human-derived cells that differentiated into astrocytes was notably greater than the frequency of transplanted cells that differentiated into oligodendrocytes (Figure 2G). However, the proportion of GFAP+ cells that were derived from hES-NPCs was approximately 5% (Figure 2H), although relative to the number of hES-NPCs identified in this region of the brain, GFAP+ cells constituted the largest differentiated population (Figure 2G,H), indicating that the differentiation of hES-NPCs was not restricted to oligodendrocytes in this context. hNA+/GFAP+ cells were also identified in the cortex and occasionally along the ependymal cell layer of the lateral ventricle where we also noted an occasional hNA+ cells that did not colocalize with either GFAP or O1 markers ("other"; Figure 2G).
hES-NPC treatment attenuated apoptosis in the corpus callosum
The precise mechanism by which cuprizone evokes the specific loss of myelin in the CNS is not known [1], however the physiological response to dietary cuprizone is marked by apoptotic death of oligodendrocytes within the CC [19, 20]. To determine whether treatment with hES-NPCs modified the loss of oligodendrocytes in the CC following cuprizone-treatment, we assayed tissues from control (untreated) and cuprizone-treated groups for evidence of DNA fragmentation, a hallmark of an apoptotic form of programmed cell death (Figure 3). Quantitative analysis of apoptotic nuclei within the CC revealed a significant increase in apoptotic labeling in cuprizone-treated animals (P<0.0001), while cuprizone-fed animals that were also administered hES-NPCs did not differ from unlesioned controls in the apoptotic profile (P>0.05). These results indicated that improved myelination of the CC in hES-NPC-treated mice following cuprizone exposure was due to the protective action of the hES-NPCs rather than a regenerative function.
Figure 3. Administration of hES-NPCs to cuprizone-treated mice attenuated apoptotic loss of cells in the corpus callosum.
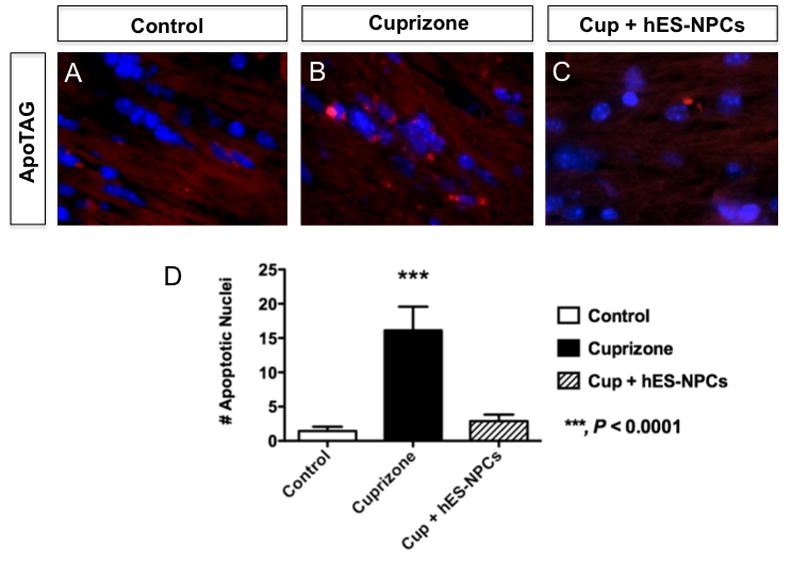
Fluorescent labeling of DNA fragmentation by ApopTag® in coronal brain tissue sections from untreated (A), and cuprizone-treated mice (B) or cuprizone-treated that had also been intravenously administered hES-NPCs (C). (D) Quantification of DAPI+ nuclei within the CC that were also positive for DNA fragmentation revealed a marked increase in the number of apoptotic cells in cuprizone treated mice (black bar), relative to untreated animals (white bar), yet cuprizone fed animals that were also administered hES-NPCs did not exhibit elevated apoptosis in the CC (hatched bar). Data represent analysis of n=4-5 subjects/treatment with n=3 sections/subject. ANOVA; ***, P<0.0001.
Attenuated Glial Responses to cuprizone following hES-NPC administration
The extent of myelination that was present in the CC of hES-NPC-treated mice seemed disproportionate to the limited number of dsRed+/O1+ cells that were observed. We postulated that, similar to previous studies [21], a primary benefit of hES-NPCs on myelination might have been to modify the glial response to myelin injury. Iba-1 was used to identify microglia in tissues from the same treatment groups. Microglia in the unlesioned CC contained sparse microglia exhibiting fine processes indicative of an unactivated state (Figure 4A). Four weeks after initiation of cuprizone treatment, Iba-1+ cells within the CC were dense and exhibited a notable thickening of their processes; reflective of an activated status (Figure 4B). Interestingly, in cuprizone-fed animals that had been treated with hES-NPCs 2 weeks previously, microglia levels were reduced compared to mice treated with cuprizone alone, although the majority of these Iba-1+ cells displayed an activated morphology (Figure 4C). These data indicate that the attenuated demyelination in hES-NPC-administered, cuprizone-treated animals was correlated with an attenuated microglial response.
Figure 4. hES-NPCs administration attenuated glial responses to cuprizone treatment.
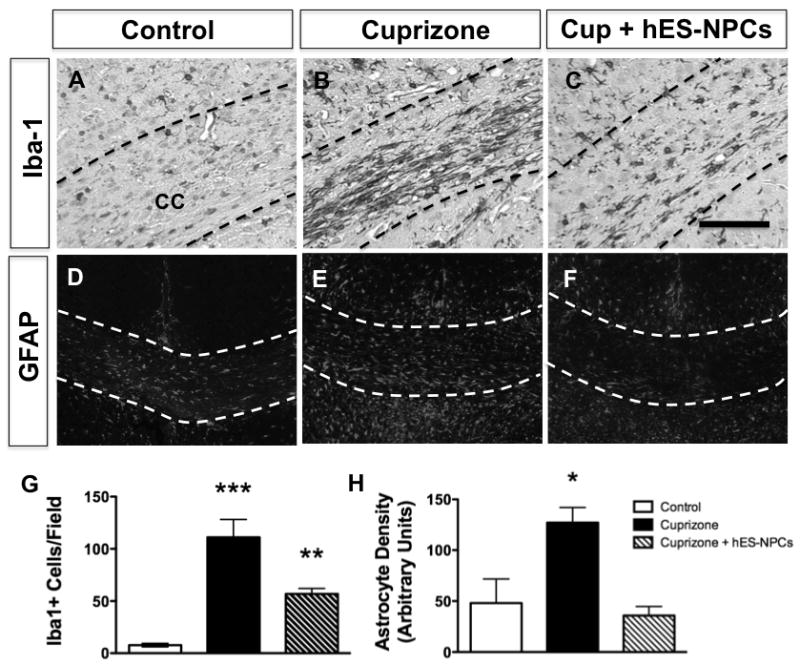
Microglial cells, identified by Iba-1 immunostaining, within the corpus callosum of control (unlesioned) mice (A) were increased in abundance by cuprizone treatment (B) but not cuprizone treatment was coincident with hES-NPCs administration (C). Similarly, astrocytes were identified using immunostaining for GFAP in sections from untreated (D), cuprizone-treated (E) and cuprizone-treated mice that also received hES-NPCs (F). Quantification of the numbers of Iba-1+ cells (G) and GFAP+ astrocytes (H) within the corpus callosum of these mice (n=3 sections/subject with n=4–6/treatment group) determined that cuprizone induced a robust microglial and astrocyte responses that were significantly attenuated by co-treatment with hES-NPCs. G:**, P<0.01; ***, P<0.001; H: *, P=0.017. Scale bars = 100 μm.
Immunostaining for glial fibrillary acidic protein (GFAP), to identify astrocytes, revealed that a robust astrocyte response was observed in and around the CC of animals treated with cuprizone (Figure 4D-F). Increased GFAP+ responses were observed in the CC, dorsal fornix and lateral septal nucleus (immediately ventral to the CC) in all animals given cuprizone, but the magnitude of astrocytosis in cuprizone-treated mice not given hES-NPCs was significantly greater than in either control or hES-NPCs-treated animals (Figure 4H; ANOVA, P=0.017). Hence, intravenous administration of hES-NPCs influenced both microglial and astrocytic responses in the CC folllowing cuprizone treatment.
Discussion
In this study we have shown that intravenous administration of hES-NPCs reduced the demyelinating effect of cuprizone treatment in mice. Our findings build upon several previous studies describing the potential benefit of using oligodendrocyte progenitors to repair the brains of genetically demyelinated animals [22–25] or in experimental models of demyelination [9, 21, 26, 27]. Moreover, this study is also complemented by recent studies by others that have shown systemic delivery of progenitor cells can be a viable strategy to provide therapeutic benefit in models of neurologic disease [28–33]. To our knowledge, this is the first demonstration that systemic administration of human progenitor cells modified a non-T-cell-mediated form of myelin injury.
An important observation in our study was that hES-NPCs administered into the blood stream were capable of migrating into the CNS and differentiating into oligodendrocytes and astrocytes. From our detailed examination of the rostral corpus callosum, in coronal brain tissue sections, we estimate that less than 1% (approximately 0.2%) of hES-NPCs migrated into the brains of cuprizone-treated mice [34]. This approximation is limited by several assumptions; namely, a homogeneous distribution of cells throughout the CNS and reliance upon expression of marker proteins for identification of transplanted cells in histological sections. Our preliminary estimation suggests that a very small proportion of hES-NPCs migrated or survived in the CNS of treated mice. Nevertheless, cuprizone-induced pathology was markedly attenuated by intravenous treatment with hES-NPCs.
Although we did observe integration and differentiation of human cells into both O1+ oligodendrocytes and GFAP+ astrocytes within the CC, the numbers of these cells were low and thus we hypothesize that the primary benefit of hES-NPCs may not have been directly accountable to myelin replacement by the transplanted cell themselves. Our findings are consistent and support the conclusions of Einstein et al. (2009) that demonstrated intracerebroventicular (i.c.v.) administration of murine neural progenitor cells to cuprizone-treated animals promoted myelination of the CC through the differentiation of endogenous progenitors rather than differentiation of the transplanted cells [12]. Our findings complement this previous study by demonstrating similar effects of human neural progenitor cells in the cuprizone model and also show that i.v. rather than i.c.v. administration can be used to achieve therapeutic benefit. Together, these reports on neural precursor cells in the cuprizone model support additional recent studies on the restorative functions of transplanted neural stem or neural progenitor cells in vivo, which have indicated that de novo replacement of cells is not a primary mode of therapeutic benefit [9, 35]. These previous studies have elucidated a central role for cell-to-cell contact as an important mediator of SCs. For instance, integration of donor cells into the murine CNS requires cellular contacts involving the formation of gap junctions [35]. Indeed, previous work has also demonstrated that rescue of host cells by donor neural stem cells contributes to the restorative effects of SCs in neurodegenerative models [35, 36]. In the context of demyelinating injury as well, immunomodulation by SCs rather than de novo myelination by these donor cells accounted for the abrogation of myelin pathology in other studies [9, 21]. Thus, our data would suggest that hES-NPC treatment provided benefit through the support of pre-existing oligodendrocytes within the CC, rather then by promoting regeneration. The high degree of conservation between human and mouse in myelin proteins limits our ability to precisely quantify and therefore determine the relative contribution, if any, of hES-NPCs toward the myelin content of the CC of treated mice. Additional study would be required to resolve any regenerative influence of hES-NPCs on endogenous progenitor cells.
Another relevant finding of our study was that progenitor cells were capable of extravasation from peripheral circulation into the brain parenchyma in mice treated with cuprizone. This result is consistent with several recent studies by others, which have also shown that i.v.-administered progenitor cells can migrate to areas of injury within the CNS. Indeed, one study reported that intravenous injection of GFP+ microglia, derived from murine ES cells, migrated from the blood stream into the hippocampus and CC [37]. Our use of human ES-derived neural precursors in this non-T cell mediated model of CNS demyelination is also consistent with a recent report by Pluchino et al. (2009) demonstrating that human neural progenitor cells can ameliorate demyelination in other species.
Another observation made in our study was that hES-NPC treatment attenuated both astrocytosis and microgliosis in the CC during cuprizone treatment. Astrocytes are known to play positive and inhibitory roles on myelination through the production of growth factors. Hence, the differential response to cuprizone-induced injury in hES-NPC-treated mice may have contributed to the observed differences in myelination with hES-NPC administration. Studies on the role of microglia in myelin injury have also yielded conflicting results, some of which support a role for microglia in myelin injury while others suggest an important role in myelin repair. This dichotomy has led to the idea that the precise function of microglia depends upon the type of injury and its context [38]. In experimental autoimmune models of demyelination, for instance, microglia are important participants in demyelination wherein depletion of microglia can prevent the development of inflammatory demyelination [39] and chronically activated microglia are associated with persistent myelin injury [17, 19].
At present it is unclear how hES-NPC administration in our study amended the microglial, or astrocytic, responses to cuprizone intoxication. It is probable that the observed reduction in microglial response in hES-NPC treated mice was a result of lessened demyelination in the CC of these animals. A second possibility is that hES-NPCs may have influenced the microglial response to cuprizone treatment as a primary action. This latter scenario is based on previous observations that human mesenchymal stem cells secrete Th2 cytokines that are anti-inflammatory [40] and can promote myelin repair [41]. For instance, the pro-inflammatory cytokine tumour necrosis factor (TNF), which is produced by microglia during cuprizone-induced demyelination, is also required for the efficient differentiation of oligodendrocytes progenitors during the remyelination process [20]. Previous work has also shown that cytokine activation of microglia can promote the differentiation of oligodendrocytes from neural progenitor cells [42]. Thus, if hES-NPC treatment modified the inflammatory environment of the CC during cuprizone treatment, then hES-NPCs could have prevented demyelination or fostered early myelin repair by modulating microglia responses. Additional study will be required to define the effect of hES-NPCs on microglial reactivity or functions during cuprizone treatment.
In summary, we have demonstrated hES-NPCs can ameliorate cuprizone-induced myelin injury. We have shown that systemically-administered hES-NPCs exhibited an appropriate homing ability into injured CNS tissue within the mouse, leading to the amelioration of demyelinating disease. This study supports the potential use of SC-derived neural cells as a strategy to foster myelination in the injured brain.
Acknowledgments
We thank Debbie Bishop and Bandana Shrestha for technical assistance. This work was supported by NIH grant R01 AI027028 and P01 AI077607 (to J.L.W.), the Burnham Institute for Medical Research transitional funds (to A.V.T.) and start-up funding from the University of Connecticut Health Center (to S.J.C.). R.B. was partially supported by a California Institute for Regenerative Medicine (CIRM) training grant. S.J.C. was supported, in part, through a Career Transition Award from the National Multiple Sclerosis Society (TA 3021 A1/1).
References Cited
- 1.Matsushima GK, Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain pathology (Zurich, Switzerland) 2001;11:107–16. doi: 10.1111/j.1750-3639.2001.tb00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hiremath MM, Saito Y, Knapp GW, Ting JP, Suzuki K, Matsushima GK. Microglial/macrophage accumulation during cuprizone-induced demyelination in C57BL/6 mice. Journal of neuroimmunology. 1998;92:38–49. doi: 10.1016/s0165-5728(98)00168-4. [DOI] [PubMed] [Google Scholar]
- 3.McMahon EJ, Suzuki K, Matsushima GK. Peripheral macrophage recruitment in cuprizone-induced CNS demyelination despite an intact blood-brain barrier. Journal of neuroimmunology. 2002;130:32–45. doi: 10.1016/s0165-5728(02)00205-9. [DOI] [PubMed] [Google Scholar]
- 4.Remington LT, Babcock AA, Zehntner SP, Owens T. Microglial recruitment, activation, and proliferation in response to primary demyelination. The American journal of pathology. 2007;170:1713–24. doi: 10.2353/ajpath.2007.060783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMahon EJ, Cook DN, Suzuki K, Matsushima GK. Absence of macrophage-inflammatory protein-1alpha delays central nervous system demyelination in the presence of an intact blood-brain barrier. J Immunol. 2001;167:2964–71. doi: 10.4049/jimmunol.167.5.2964. [DOI] [PubMed] [Google Scholar]
- 6.Kondo A, Nakano T, Suzuki K. Blood-brain barrier permeability to horseradish peroxidase in twitcher and cuprizone-intoxicated mice. Brain Res. 1987;425:186–90. doi: 10.1016/0006-8993(87)90499-9. [DOI] [PubMed] [Google Scholar]
- 7.Pluchino S, Martino G. The therapeutic use of stem cells for myelin repair in autoimmune demyelinating disorders. Journal of the neurological sciences. 2005;233:117–9. doi: 10.1016/j.jns.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 8.Cassiani-Ingoni R, Muraro PA, Magnus T, Reichert-Scrivner S, Schmidt J, Huh J, Quandt JA, Bratincsak A, Shahar T, Eusebi F, Sherman LS, Mattson MP, Martin R, Rao MS. Disease progression after bone marrow transplantation in a model of multiple sclerosis is associated with chronic microglial and glial progenitor response. Journal of neuropathology and experimental neurology. 2007;66:637–49. doi: 10.1097/nen.0b013e318093f3ef. [DOI] [PubMed] [Google Scholar]
- 9.Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalini A, Bergami A, Furlan R, Comi G, Constantin G, Martino G. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436:266–71. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- 10.Freedman MS. Bone marrow transplantation: does it stop MS progression? Journal of the neurological sciences. 2007;259:85–9. doi: 10.1016/j.jns.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 11.Metz I, Lucchinetti CF, Openshaw H, Garcia-Merino A, Lassmann H, Freedman MS, Atkins HL, Azzarelli B, Kolar OJ, Bruck W. Autologous haematopoietic stem cell transplantation fails to stop demyelination and neurodegeneration in multiple sclerosis. Brain. 2007;130:1254–62. doi: 10.1093/brain/awl370. [DOI] [PubMed] [Google Scholar]
- 12.Einstein O, Friedman-Levi Y, Grigoriadis N, Ben-Hur T. Transplanted neural precursors enhance host brain-derived myelin regeneration. J Neurosci. 2009;29:15694–702. doi: 10.1523/JNEUROSCI.3364-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bajpai R, Coppola G, Kaul M, Talantova M, Cimadamore F, Nilbratt M, Geschwind DH, Lipton SA, Terskikh AV. Molecular stages of rapid and uniform neuralization of human embryonic stem cells. Cell Death Differ. 2009;16:807–25. doi: 10.1038/cdd.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bajpai R, Terskikh AV. Genetic modification of human embryonic stem cells. In: Loring J, editor. Human Stem Cell Manual. New York, NY: Academic Press; 2007. [Google Scholar]
- 15.Hao E, Tyrberg B, Itkin-Ansari P, Lakey JR, Geron I, Monosov EZ, Barcova M, Mercola M, Levine F. Beta-cell differentiation from nonendocrine epithelial cells of the adult human pancreas. Nature medicine. 2006;12:310–6. doi: 10.1038/nm1367. [DOI] [PubMed] [Google Scholar]
- 16.Terskikh AV, Ershler MA, Drize NJ, Nifontova IN, Chertkov JL. Long-term persistence of a nonintegrated lentiviral vector in mouse hematopoietic stem cells. Experimental hematology. 2005;33:873–82. doi: 10.1016/j.exphem.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Crocker SJ, Whitmire JK, Frausto RF, Chertboonmuang P, Soloway PD, Whitton JL, Campbell IL. Persistent macrophage/microglial activation and myelin disruption after experimental autoimmune encephalomyelitis in tissue inhibitor of metalloproteinase-1-deficient mice. The American journal of pathology. 2006;169:2104–16. doi: 10.2353/ajpath.2006.060626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feuer R, Mena I, Pagarigan RR, Harkins S, Hassett DE, Whitton JL. Coxsackievirus B3 and the neonatal CNS: the roles of stem cells, developing neurons, and apoptosis in infection, viral dissemination, and disease. The American journal of pathology. 2003;163:1379–93. doi: 10.1016/S0002-9440(10)63496-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mason JL, Toews A, Hostettler JD, Morell P, Suzuki K, Goldman JE, Matsushima GK. Oligodendrocytes and progenitors become progressively depleted within chronically demyelinated lesions. The American journal of pathology. 2004;164:1673–82. doi: 10.1016/S0002-9440(10)63726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nature neuroscience. 2001;4:1116–22. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- 21.Pluchino S, Gritti A, Blezer E, Amadio S, Brambilla E, Borsellino G, Cossetti C, Del Carro U, Comi G, t Hart B, Vescovi A, Martino G. Human neural stem cells ameliorate autoimmune encephalomyelitis in non-human primates. Annals of neurology. 2009;66:343–54. doi: 10.1002/ana.21745. [DOI] [PubMed] [Google Scholar]
- 22.Warrington AE, Barbarese E, Pfeiffer SE. Differential myelinogenic capacity of specific developmental stages of the oligodendrocyte lineage upon transplantation into hypomyelinating hosts. Journal of neuroscience research. 1993;34:1–13. doi: 10.1002/jnr.490340102. [DOI] [PubMed] [Google Scholar]
- 23.Givogri MI, Galbiati F, Fasano S, Amadio S, Perani L, Superchi D, Morana P, Del Carro U, Marchesini S, Brambilla R, Wrabetz L, Bongarzone E. Oligodendroglial progenitor cell therapy limits central neurological deficits in mice with metachromatic leukodystrophy. J Neurosci. 2006;26:3109–19. doi: 10.1523/JNEUROSCI.4366-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schiff R, Rosenbluth J, Dou WK, Liang WL, Moon D. Distribution and morphology of transgenic mouse oligodendroglial-lineage cells following transplantation into normal and myelin-deficient rat CNS. The Journal of comparative neurology. 2002;446:46–57. doi: 10.1002/cne.10192. [DOI] [PubMed] [Google Scholar]
- 25.Windrem MS, Schanz SJ, Guo M, Tian GF, Washco V, Stanwood N, Rasband M, Roy NS, Nedergaard M, Havton LA, Wang S, Goldman SA. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell. 2008;2:553–65. doi: 10.1016/j.stem.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben-Hur T, van Heeswijk RB, Einstein O, Aharonowiz M, Xue R, Frost EE, Mori S, Reubinoff BE, Bulte JW. Serial in vivo MR tracking of magnetically labeled neural spheres transplanted in chronic EAE mice. Magn Reson Med. 2007;57:164–71. doi: 10.1002/mrm.21116. [DOI] [PubMed] [Google Scholar]
- 27.Politi LS, Bacigaluppi M, Brambilla E, Cadioli M, Falini A, Comi G, Scotti G, Martino G, Pluchino S. Magnetic-resonance-based tracking and quantification of intravenously injected neural stem cell accumulation in the brains of mice with experimental multiple sclerosis. Stem cells (Dayton, Ohio) 2007;25:2583–92. doi: 10.1634/stemcells.2007-0037. [DOI] [PubMed] [Google Scholar]
- 28.Shen CC, Lin CH, Yang YC, Chiao MT, Cheng WY, Ko JL. Intravenous implanted neural stem cells migrate to injury site, reduce infarct volume, and improve behavior after cerebral ischemia. Curr Neurovasc Res. 2010;7:167–79. doi: 10.2174/156720210792231822. [DOI] [PubMed] [Google Scholar]
- 29.Chao YX, He BP, Tay SS. Mesenchymal stem cell transplantation attenuates blood brain barrier damage and neuroinflammation and protects dopaminergic neurons against MPTP toxicity in the substantia nigra in a model of Parkinson's disease. Journal of neuroimmunology. 2009;216:39–50. doi: 10.1016/j.jneuroim.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Hall AA, Guyer AG, Leonardo CC, Ajmo CT, Jr, Collier LA, Willing AE, Pennypacker KR. Human umbilical cord blood cells directly suppress ischemic oligodendrocyte cell death. Journal of neuroscience research. 2009;87:333–41. doi: 10.1002/jnr.21857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–94. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- 32.Fujiwara Y, Tanaka N, Ishida O, Fujimoto Y, Murakami T, Kajihara H, Yasunaga Y, Ochi M. Intravenously injected neural progenitor cells of transgenic rats can migrate to the injured spinal cord and differentiate into neurons, astrocytes and oligodendrocytes. Neuroscience letters. 2004;366:287–91. doi: 10.1016/j.neulet.2004.05.080. [DOI] [PubMed] [Google Scholar]
- 33.Song M, Kim Y, Ryu S, Song I, Kim SU, Yoon BW. MRI tracking of intravenously transplanted human neural stem cells in rat focal ischemia model. Neurosci Res. 2009;64:235–9. doi: 10.1016/j.neures.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–47. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- 35.Jaderstad J, Jaderstad LM, Li J, Chintawar S, Salto C, Pandolfo M, Ourednik V, Teng YD, Sidman RL, Arenas E, Snyder EY, Herlenius E. Communication via gap junctions underlies early functional and beneficial interactions between grafted neural stem cells and the host. Proceedings of the National Academy of Sciences of the United States of America; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ourednik J, Ourednik V, Lynch WP, Schachner M, Snyder EY. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat Biotechnol. 2002;20:1103–10. doi: 10.1038/nbt750. [DOI] [PubMed] [Google Scholar]
- 37.Tsuchiya T, Park KC, Toyonaga S, Yamada SM, Nakabayashi H, Nakai E, Ikawa N, Furuya M, Tominaga A, Shimizu K. Characterization of microglia induced from mouse embryonic stem cells and their migration into the brain parenchyma. Journal of neuroimmunology. 2005;160:210–8. doi: 10.1016/j.jneuroim.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 38.Muzio L, Martino G, Furlan R. Multifaceted aspects of inflammation in multiple sclerosis: the role of microglia. Journal of neuroimmunology. 2007;191:39–44. doi: 10.1016/j.jneuroim.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 39.Heppner FL, Greter M, Marino D, Falsig J, Raivich G, Hovelmeyer N, Waisman A, Rulicke T, Prinz M, Priller J, Becher B, Aguzzi A. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nature medicine. 2005;11:146–52. doi: 10.1038/nm1177. [DOI] [PubMed] [Google Scholar]
- 40.Yagi H, Soto-Gutierrez A, Navarro-Alvarez N, Nahmias Y, Goldwasser Y, Kitagawa Y, Tilles AW, Tompkins RG, Parekkadan B, Yarmush ML. Mol Ther. 2010. Reactive Bone Marrow Stromal Cells Attenuate Systemic Inflammation via sTNFR1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bai L, Lennon DP, Eaton V, Maier K, Caplan AI, Miller SD, Miller RH. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009;57:1192–203. doi: 10.1002/glia.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Molecular and cellular neurosciences. 2006;31:149–60. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]


